Introduction
Non-Hodgkin's lymphoma (NHL) consists of many
histologically and biologically unique lymphoid malignancies
(1). In Western countries, 85% of
NHL is of B-cell origin (2).
Unfortunately, little progress has been made in improving the
survival of NHL patients receiving standard therapy, largely due to
insensitivity or resistance of the cancer cells to treatment. Many
patients have short complete remission or early relapse after
treatment, which shortens survival and causes tremendous
psychological and physical pain.
Recently, in vitro studies have shown that
chemotherapy response depends on activation of the apoptosis
cascade (3). Moreover, the most
important factor in chemotherapy resistance is suppression of the
apoptosis pathway, which leads to disease recurrence in NHL
patients. Imbalances in apoptosis regulation are associated with
the abnormal activation of growth signal transduction pathways, and
constitutive activation of these pathways, including NF-κB and
STAT3, has been shown to occur in NHL tissue and cell lines.
Activation of these pathways is thought to be the major cause of
cancer cell resistance to chemotherapeutics via downstream
alterations in apoptosis pathway regulation (4,5).
Accumulating data suggest that aldehyde
dehydrogenase 1A1 (ALDH1A1) is also involved in the chemotherapy
resistance of tumor cells (6–8).
ALDH1A1 is overexpressed in a variety of solid tumors and
leukemias, and it is a newly discovered cancer stem cell (CSC)
marker (9–11). Hodgkin's lymphoma cells with high
expression of ALDH1A1 possess the characteristics of stem cells
(12). Notably, p-STAT3 plays a
role in maintaining CSC characteristics in colon cancer (13), whereas NF-κB plays a similar role in
pancreatic cancer (14). Recently,
we demonstrated that knockdown or inhibition of ALDH1A1 increases
chemosensitivity in diffuse large B-cell lymphoma (DLBCL) Farage
cells, potentially via modulation of NF-κB/STAT3 signaling
(15); however, in contrast, Fujita
et al demonstrated by immunohistochemistry that ALDH1 is not
expressed in DLBCL (16).
Subsequently, we found that ALDH1A1 confers chemoresistance in
DLBCL Pfeiffer cells, and that its expression is associated with
poor prognosis in DLBCL patients (17).
In the present study, we analyzed ALDH1A1
expression in human NHL patient samples, and we assessed the
relationship between ALDH1A1 expression and B-cell NHL
patient prognosis. Furthermore, we choose the Raji cell line, a
Burkitt's lymphoma cell line as a model since Raji cells with
mutant p53, constitutively activated NF-κB and increased BCL-2
expression which are commonly present in patients with NHL and are
considered a source of chemotherapy failure in patients whose
disease are chemoresistant in B-cell NHL (18). we used the Raji cell line to explore
the role of ALDH1A1 in chemotherapy resistance, via modulation of
NF-κB/STAT3 signaling and apoptosis, in B-cell NHL.
Patients and methods
Patient characteristics
The samples were obtained from 112 patients treated
in the Xiang-Ya Hospital of Central South University (Hunan, China)
after being diagnosed with B-cell NHL according to the WHO (2008)
classification, and was confirmed by pathological histology, from
2013 to 2014. Indolent lymphoma defined follicular lymphoma,
marginal zone lymphoma, mucosa associated lymphoid tissue type,
unclassified small B cell lymphoma. Progressive lymphoma contained
diffuse large B cells, mantle cells and Burkitt's lymphoma. For
comparison, we obtained samples from 24 healthy donors as the
normal controls. All patients were enrolled following approval from
the Ethics Committee, and they all provided informed consent. From
each patient, 3- to 5-ml peripheral blood samples were collected in
sterile tubes containing anticoagulant (heparin sodium) before they
received treatment. Mononuclear cells (MNCs) were enriched by
density centrifugation over Ficoll-Paque (TBD Science, Tianjin,
China) and stored at −80°C.
RNA isolation and real-time PCR
Cells were lysed, and the total RNA was extracted
with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's instructions. Τotal RNA (1 µg) was used in cDNA
synthesis. Reverse transcription of RNA was carried out with a
PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan).
Synthesized cDNA was subjected to quantitative real-time (qRT)-PCR
for the detection of ALDH1A1 and GAPDH using the
SYBR-Green fluorescence-based Assay kit (Takara). The following
primers were used: 5′-TGTTAGCTGATGCCGACTTG-3′ and
5′-TTCTTAGCCCGCTCAACACT-3′ for ALDH1A1; and
5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for
GAPDH (Sangon Biotech, Shanghai, China). The following
amplification conditions were used: pre-denaturation at 95°C for 30
sec, 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C
for 34 sec, and elongation at 72°C for 60 sec, and a final
extension at 72°C for 10 min. The relative quantification (RQ) of
ALDH1A1 was calculated based on the threshold cycle (Ct)
values as follows: RQ = 2−ΔΔCt, where ΔΔCt =
[Ct(ALDH1A1) - Ct(GAPDH)] sample (patients) - [Ct
(ALDH1A1) - Ct(GAPDH)] sample (healthy controls). The
qRT-PCR was performed using the ABI 7500 Fast Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA).
Cell culture
Raji cells [human Burkitt's lymphoma cell line, ATCC
CCL-86) (ATCC; American Type Culture Collection, Manassas, VA,
USA)] were grown in RPMI-1640 medium containing 10% fetal bovine
serum (FBS) and 100 U/ml penicillin/streptomycin and incubated at
37°C in 5% CO2.
Chemical treatment and cell survival
rate assay
Cells (5,000) were seeded on a 96-well plate in a
volume of 100 µl. CHOP treatment was performed using
cyclophosphamide, vincristine, adriamycin and prednisone at a
clinical ratio of 80/5.5/0.16/11.1, respectively, with dosages
ranging from 5–1,280 ng/ml (19).
Cytotoxicity was assessed using the Cell Counting Kit-8 (CCK-8)
assay according to the manufacturer's instructions (Dojindo,
Kumamoto, Japan). Forty-eight hours after drug treatment, CCK-8 was
added to each well, and the OD at 450 nm of each sample was
determined using a microplate spectrophotometer (Bio-Rad, Hercules,
CA, USA).
Colony formation assay
Cells were seeded into 6-well culture plates at a
density of 1,000 cells/well in triplicate, with 2 ml of
methylcellulose (stem cell) mixture containing Dulbecco's modified
Eagle's medium (DMEM) and FBS supplemented with 10% fetal bovine
serum (FBS), with or without 400 ng/ml CHOP. The numbers of
colonies were counted on day 14.
ALDH1A1 knockdown by shRNA
Lentiviral vectors expressing short hairpin RNAs
(shRNA) against ALDH1A1 (GenBank accession no. NM_000689)
were obtained from GeneChem Co., Ltd. (Shanghai, China), and
synthesized with the following strand sequences: forward,
5′-tcgGGCTAAGAAGTATATCCTTctcgagAAGGATATACTTCTTAGCCcgttttttc-3′ and
reverse,
5′-TCGAGAAAAAAcgGGCTAAGAAGTATATCCTTCTCGAGAAGGATATACTTCTTAGCCCGA-3′.
The lentivirus was transfected according to the Lentiviral Vector
Particle operation manual instructions as previously described
(15). Validation of the knockdown
was performed at the protein level by western blotting, and at the
messenger RNA (mRNA) level by relative qRT-PCR.
Detection of active caspase-3 and
−9
Caspase activity was assayed using the Caspase
Colorimetric Assay kit (KeyGen Biotech, Nanjing, China) according
to the manufacturer's protocol. Briefly, cells were harvested and
lysed for 30 min. Then, 50 µl samples were mixed with reaction
buffer and the caspase-3/-9 substrate and incubated for 4 h at 37°C
in the dark. The percentage of A405 values for the test samples vs.
those for the control samples indicated the percentage of caspase
activity.
Flow cytometric assay for
apoptosis
Cell apoptosis was assayed using the Annexin V/FITC
apoptosis detection kit (Beijing Biosea Biotechnology Co., Ltd.,
Beijing, China) according to the manufacturer's protocol. Data
acquisition and analysis were performed using a flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Western blotting
Western blotting was carried out as previously
described (15). Total protein (20
µg) was loaded per well. The following antibodies and dilutions
were used: anti-ALDH1A1 (1:500; Abcam, Cambridge, MA, USA),
anti-NF-κB (1:1,000), anti-p-NF-κB (1:1,000), anti-STAT3 (1:1,000),
anti-p-STAT3 (1:1,000), anti-BCL-2 (1:1,000), anti-BAX (1:1,000),
anti-caspase-3 (1:1,000), anti-caspase-9 (1:1,000) (all from Cell
Signaling Technology, Inc., Danvers, MA, USA). Anti-GAPDH (1:2,000;
Goodhere Biotechnology Co., Ltd., Hangzhou, China) served as a
loading control.
Statistical analysis
All data shown represent the results of at least
three independent experiments. The calculations were analyzed using
the Statistical Package for the Social Sciences (SPSS) software.
For analysis of the survival data, the Kaplan-Meier model was
applied, and the log-rank test was performed. The association
between ALDH1A1 expression and clinicopathological features
was studied using the χ2 test. Differences between the
results of experimental treatments and the average cloning number
were assessed by one-way analysis of variance. Differences were
two-tailed and considered significant at values of P<0.05. The
diagrams were generated using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
High levels of ALDH1A1 expression are
associated with an unfavorable prognosis in NHL patients
Quantitative real-time (qRT)-PCR analysis of
ALDH1A1 expression was conducted in peripheral blood samples
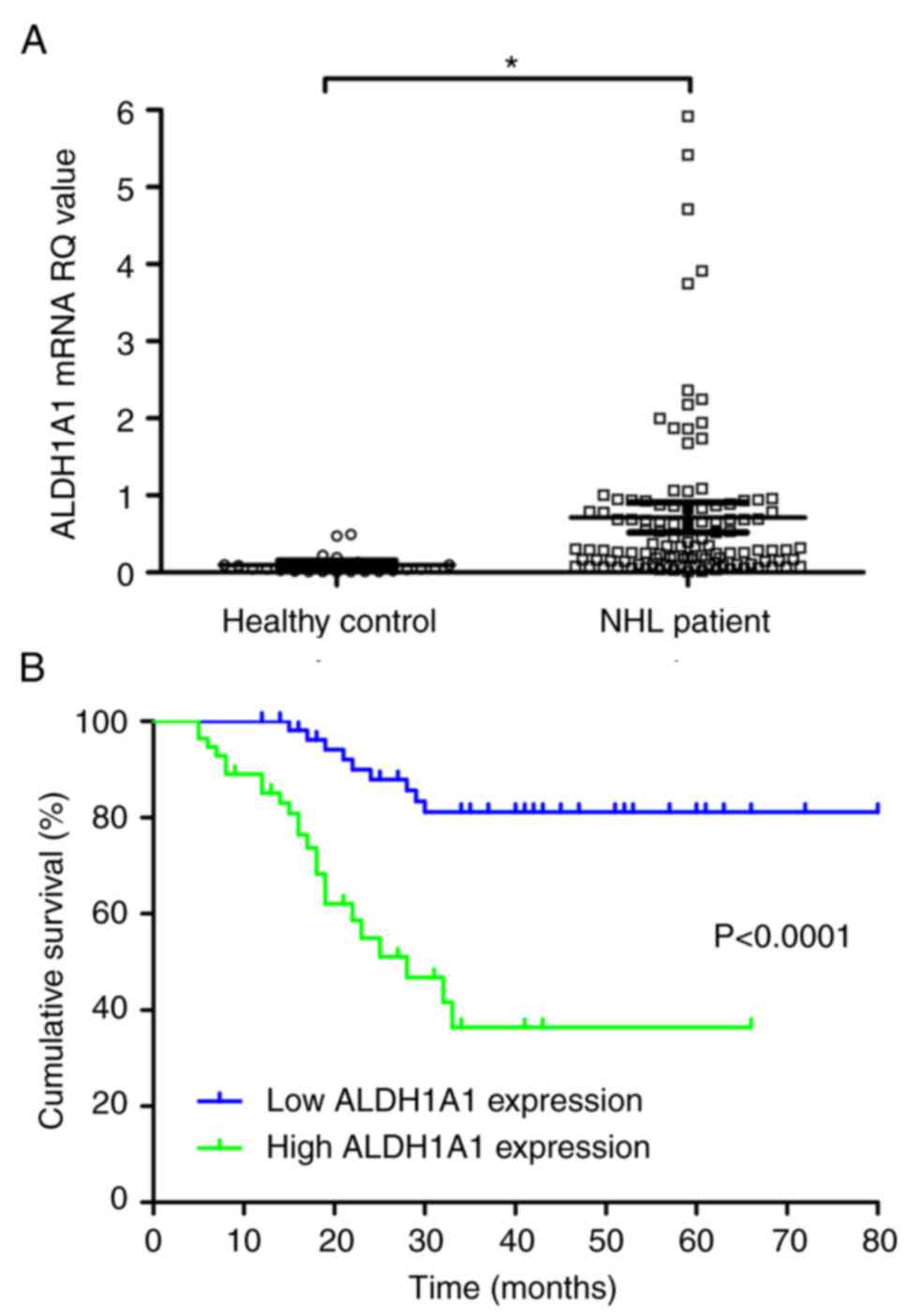
from 112 NHL and 24 healthy control patients. The median relative
quantification (RQ) values of ALDH1A1 in NHL and control
patients were 0.326 (range, 0.010–5.918) and 0.041 (range,
0.010–0.492), respectively; thus, ALDH1A1 levels were
significantly higher in NHL patients than in controls (P<0.05;
Fig. 1A).
Next, we used the median RQ value of ALDH1A1
to separate the patients into a high ALDH1A1 group
(>0.326) or a low ALDH1A1 group (<0.326). The 56
patients with high expression had a median ALDH1A1 RQ of
0.846 (range, 0.336–5.92), whereas those with low expression had a
median ALDH1A1 RQ of 0.148 (range, 0.010–0.316). Baseline
ALDH1A1 levels were correlated with patient lactate
dehydrogenase (LDH) levels (P=0.014), performance status (PS)
(P=0.011), Ann Arbor stage (P>0.05), International Prognostic
Index (IPI) score (P>0.05), and lymphoma category (P=0.001), but
not with other factors (Table I).
Importantly, patients in the high ALDH1A1 group showed
shorter cumulative survival than those in the low ALDH1A1
group (P<0.0001; Fig. 1B). The
expression of ALDHA1 in indolent lymphoma was significantly lower
than in progressive lymphoma. Although in the experimental group of
42 cases of indolent lymphoma patient's data showed that ALDH1A1
difference in expression related to difference cumulative survival
rate (P=0.031), but in multivariate analysis ALDH1A1 was not an
independent prognostic indicator (P=0.053). The number of indolent
lymphoma patients was too small to get a convinced conclusion.
Whether ALDH1A1 was also suitable for inactive lymphoma is unknown
and required more evidence to validate it. Moreover, among all NHL
patients, IPI score, lymphoma category and ALDH1A1 levels
were independent prognostic indicators (Table II).
 | Table I.Correlation between ALDH1A1
expression and clinicopathological parameters in 112 NHL
patients. |
Table I.
Correlation between ALDH1A1
expression and clinicopathological parameters in 112 NHL
patients.
|
| Low expression | High
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
features | N | % | N | % | χ2 | P-value |
|---|
| Age (years) |
|
|
|
| 0.801 | 0.502 |
|
≤60 | 45 | 52.33 | 41 | 47.67 |
|
|
|
>60 | 11 | 42.31 | 15 | 57.69 |
|
|
| Sex |
|
|
|
| 1.885 | 0.239 |
|
Female | 24 | 58.54 | 17 | 41.46 |
|
|
|
Male | 32 | 45.07 | 39 | 54.93 |
|
|
| B symptom |
|
|
|
| 0.237 | 0.703 |
|
Negative | 32 | 51.61 | 30 | 48.39 |
|
|
|
Positive | 23 | 46.94 | 26 | 53.06 |
|
|
| LDH |
|
|
|
| 7.009 | 0.014 |
|
Normal | 36 | 62.07 | 22 | 37.93 |
|
|
|
High | 20 | 37.04 | 34 | 62.96 |
|
|
| PS |
|
|
|
| 7.467 | 0.011 |
|
<2 | 42 | 51.22 | 28 | 48.78 |
|
|
| ≥2 | 14 | 33.33 | 28 | 66.67 |
|
|
| Ann Arbor
stage |
|
|
|
| 14.756 | 0.000 |
|
I–II | 33 | 71.74 | 13 | 28.26 |
|
|
|
III–IV | 23 | 34.85 | 43 | 65.15 |
|
|
| IPI score |
|
|
|
| 14.583 | 0.000 |
|
0–2 | 42 | 65.63 | 22 | 34.36 |
|
|
|
3–5 | 14 | 29.17 | 34 | 70.84 |
|
|
| Lymphoma
category |
|
|
|
| 12.341 | 0.001 |
|
Indolent | 30 | 71.43 | 12 | 28.57 |
|
|
|
Progressive | 26 | 37.14 | 44 | 62.86 |
|
|
 | Table II.Multivariate analysis of factors
contributing to overall survival in NHL patients. |
Table II.
Multivariate analysis of factors
contributing to overall survival in NHL patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<60
vs. ≥60) | 1.597
(0.756–3.376) | 0.220 | – | – |
| Sex (female vs.
male) | 1.568
(0.725–3.391) | 0.253 | – | – |
| B symptom (negative
vs. positive) | 1.352
(0.676–2.705) | 0.393 | – | – |
| LDH (normal vs.
high) | 1.670
(0.823–3.387) | 0.155 | – | – |
| PS (<2 vs.
≥2) | 2.694
(1.334–5.440) | 0.006 | 1.175
(0.520–2.657) | 0.698 |
| Ann Arbor stage
(I–II vs. III–IV) | 2.880
(1.29–6.428) | 0.010 | 0.694
(0.230–2.096) | 0.517 |
| IPI score (0–2 vs.
3–5) | 5.135
(2.340–11.266) | 0.000 | 3.814
(1.262–11.523) | 0.018 |
| Lymphoma
category | 4.905
(1.719–13.993) | 0.003 | 3.714
(1.254–11.005) | 0.018 |
| ALDH1A1 | 0.196
(0.089–0.431) | 0.000 | 0.393
(0.160–0.968) | 0.042 |
Inhibition of ALDH1A1 resensitizes
Raji cells to the CHOP regimen
Next, we performed western blot analysis and showed
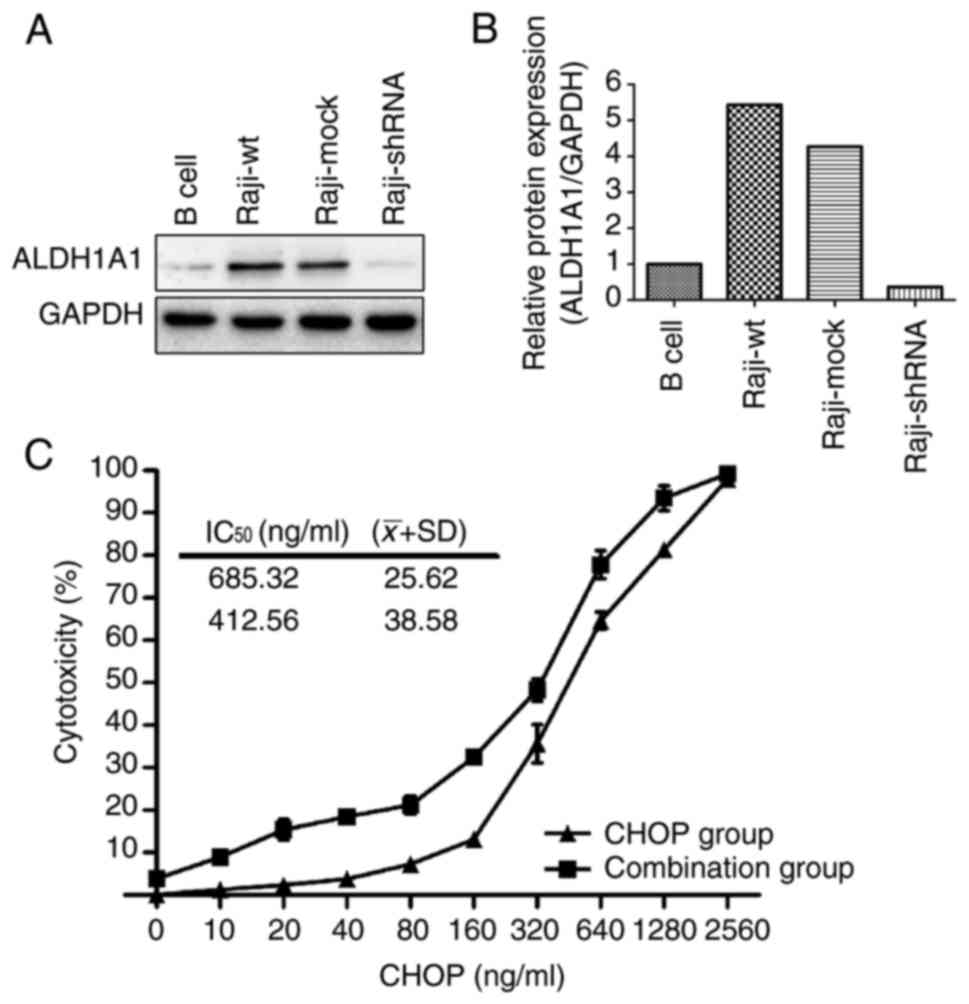
that ALDH1A1 expression was higher in human Burkitt's lymphoma Raji
cells than in human B-cells (negative control) (Fig. 2A and B). To determine whether
ALDH1A1 mediates resistance to CHOP chemotherapy in Raji cells, the
ALDH1A1 inhibitor DEAB was applied to Raji cells in combination
with CHOP treatment, and the resulting cytotoxicity was assessed.
The cytotoxicity in the combination group (CHOP plus DEAB) was
higher than in the CHOP group alone at each concentration (Fig. 2C). Moreover, the IC50
values of the Raji cells to the CHOP regimen decreased from
685±25.62 to 412.56±38.58 ng/ml (P=0.013) in the presence of DEAB
(Fig. 2C).
Knockdown or inhibition of ALDH1A1
reduces clonogenic capacity and increases apoptotic activity in
Raji cells
To further determine the mechanisms of ALDH1A1
action, we performed loss-of-function studies, via ALDH1A1
inhibition or knockdown, in Raji cells. First, we confirmed
successful shRNA-mediated knockdown of ALDH1A1 by western blot
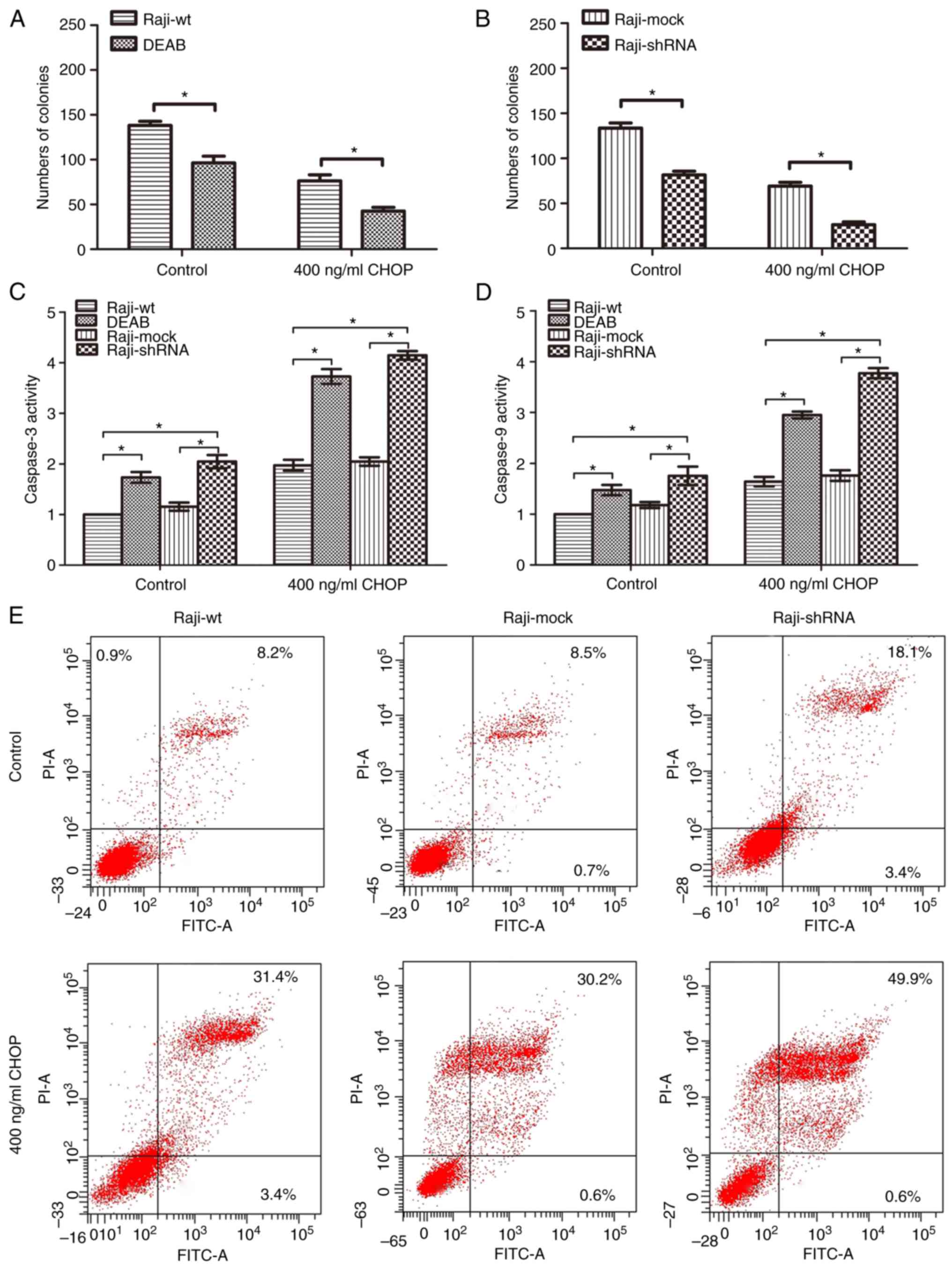
analyses (Fig. 2A and B). In colony
formation assays, the numbers of colonies in the DEAB treatment
group was significantly less than those of the Raji-wt control
group, both in the absence or presence of CHOP treatment (Fig. 3A). Similarly, the numbers of
colonies in the Raji-shRNA group was significantly less than those
in the Raji-mock group, both in the absence or presence of CHOP
treatment (Fig. 3B). These data
demonstrated that ALDH1A1 loss-of-function reduced the clonogenic
capacity of Raji cells.
Regarding apoptotic effects, colorimetric caspase
assays showed that caspase-3 activity was increased in the DEAB and
shRNA groups compared with those of their respective control
groups, both in the absence or presence of CHOP treatment (Fig. 3C). Consistent with these data,
caspase-9 activity was increased in the DEAB and shRNA groups
compared with those of their respective control groups, both in the
absence or presence of CHOP treatment (Fig. 3D). Finally, Annexin V and PI
staining revealed an increased apoptosis rate in the shRNA group
compared those of the wt or mock groups (Fig. 3E). Moreover, the shRNA group showed
an even greater apoptosis rate after 400 ng/ml CHOP treatment for
24 h (Fig. 3E).
Knockdown or inhibition of ALDH1A1
decreases NF-κB/STAT3 signaling and increases pro-apoptosis
signaling
Following ALDH1A1 knockdown, we also observed
decreased levels of total NF-κB and STAT3, and phospho-NF-κB and
-STAT3, compared with those in the control groups (Raji-wt and
Raji-mock) (Fig. 4A and B, left
panel and graph). Moreover, ALDH1A1 knockdown reduced BCL-2 levels
and increased BAX, caspase-3 and −9 levels compared with those in
the control groups (Raji-wt and Raji-mock) (Fig. 4A and B, right panel and graph).
Inhibition of ALDH1A1 showed similar results (data not shown).
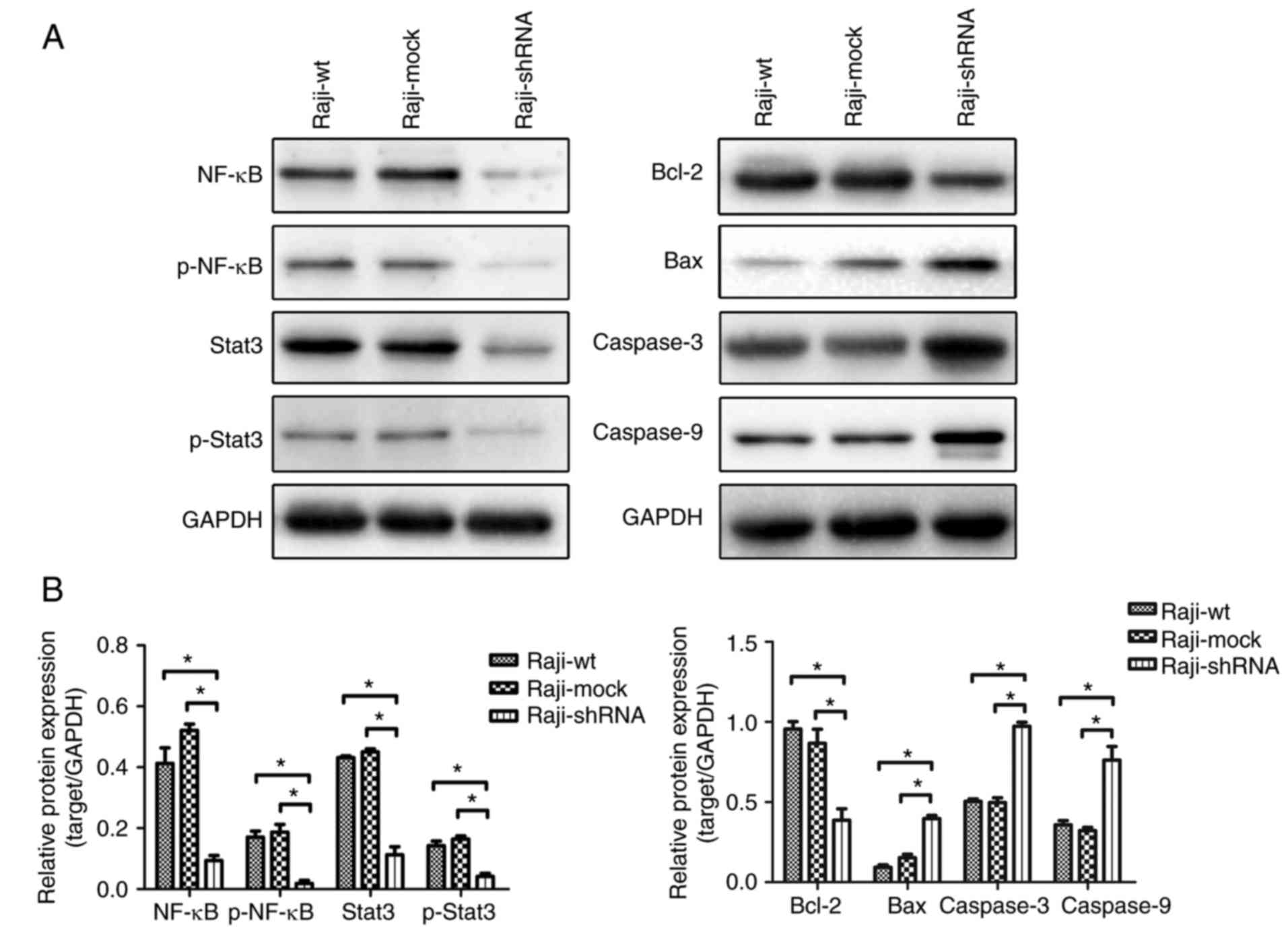 | Figure 4.Western blotting showed the total
Stat3, NF-κB and phosphorylation-Stat3, phosphorylation-NF-κB (A),
Bcl-2, Bax, caspase-3, caspase-9 (A) expressed in Raji cells.
Knockdown or inhibition of ALDH1A1 had concordant trend. Both of
them significantly decreased the total Stat3, NF-κB and
phosphorylation-Stat3, phosphorylation-NF-κB, Bcl-2 levels and
increased Bax, Caspase-3, Caspase-9 protein (B). |
Discussion
There is increasing evidence that ALDH1A1 expression
is associated with poor prognosis in a variety of cancers. In a
meta-analysis of 38 studies involving 6,057 patients, ALDH1A1
expression was significantly associated with lymph node metastasis,
histological differentiation and clinical stage in lung and breast
cancer (20). In the present study,
ALDH1A1 was differentially expressed in peripheral blood
samples from 112 NHL patients, compared with those in controls, and
the median RQ level was 0.3263. Importantly, we further showed that
high ALDH1A1 expression was associated with elevated levels
of LDH, higher frequencies of >2 ECOG performance status and
stage III/IV disease, higher IPI scores and a more invasive
lymphoma category. Moreover, the overall survival time was
significantly shorter in the high ALDH1A1 expression group
than in the low ALDH1A1 expression group. Multivariate
survival analysis further showed that the IPI score, lymphoma
pathologic type and ALDH1A1 expression level were
independent prognostic factors.
ALDH1A1 is now recognized as a CSC marker that is
associated with malignant behavior and drug resistance in tumor
cells. For instance, among Hodgkin's lymphoma cells, there is a
subset of clonal CD27+/ALDH1A1high cells that
are thought to be the initiating cells for HL (21). In addition, ALDH1 expression is
higher in Epstein-Barr virus (EBV)-associated T/natural killer
(NK)-cell lymphoproliferative disorder in children and young adults
(TNKLPDC) than in extranodal nasal NK/T-cell lymphoma, and it is
correlated with the biological characteristics of stem cells
(22). There is also a subset of
clonogenic ALDH+ cells in mantle cell lymphoma that are
associated with multiple drug resistance (23). Our previous study demonstrated that
ALDH1A1 mediates resistance of DLBCL cell lines Farage and Pfeiffer
cells to CHOP treatment, which included cyclophosphamide,
doxorubicin, vincristine and prednisone (15,17).
Consistent with these previous studies, in the present study, we
have similar conclusions. ALDH1A1 was upregulated in Raji cells,
and inhibition of ALDH1A1 activity by DEAB increased the
sensitivity of Raji cells to CHOP drugs. Furthermore,
shRNA-mediated knockdown of ALDH1A1 decreased clonogenic
ability, and increased apoptotic activity, in Raji cells. Next we
should verify the conclusion in another non-aggressive NHL cell
line to validate the effect of ALDH1A1.
NF-κB plays an important role in the development of
B-cell NHL, and constitutive NF-κB activation is a major cause of
drug resistance in relapse-refractory DLBCL patients. In a study
using co-cultured engineered CD20-specific T cells with Raji cells,
the T cells exerted antitumor activity against, and decreased the
levels of p-STAT3 and BCL-2 in Raji cells potentially via
inhibition of the NF-κB pathway (24). Moreover, invasive B-cell NHL is
characterized by constitutive activation of NF-κB signaling; thus,
targeting NF-κB is an attractive therapeutic strategy (25). NF-κB and STAT3 pathways interact
with each other, and there are complex regulatory mechanisms
between these signaling pathway networks. For instance,
transglutaminase (TG2)/NF-κB and interleukin-6 (IL6)/STAT3
signaling cascades interact to promote autophagy and survival in
mantle cell lymphoma, and blocking these pathways increases
antitumor activity (26).
Similarly, in DLBCL inhibition of an NF-κB/IL10/STAT3 autocrine
loop is the main mechanism of drug-induced apoptosis (27). In the present study, we found that
the levels of NF-κB/STAT3 pathway members and BCL-2 were decreased
following ALDH1A1 knockdown, and concomitantly, the levels
of the apoptosis-related proteins BAX, caspase-3 and −9 were
increased.
In summary, the present study demonstrated that
ALDH1A1 was associated with poor prognosis in NHL, and importantly,
our data suggested that ALDH1A1 may be an independent prognostic
indicator and a new molecular biomarker for diagnosis in NHL.
Furthermore, we showed that inhibition of ALDH1A1 increased the
sensitivity of NHL cells to chemotherapeutic drugs, further
supporting the validity of ALDH1A1 as a potential therapeutic
target in NHL treatment. Several inhibitors of ALDH1A1 could be
used in the clinic (28); however,
disulfiram (DSF), which is an oral drug that was formerly used in
the treatment of chronic alcoholism, is particularly attractive
since its antitumor effects have been confirmed in prostate,
breast, lung and glioma (29–32).
Recently, DSF has been applied in a phase II clinical study to
treat prostate and lung cancer (ClinicalTrials.gov Identifier: NCT01118741,
NCT00312819). Thus, these clinical studies by others provide
evidence of the feasibility, and the present study provides
evidence of the theoretical basis, for the promising use of ALDH1A1
inhibitors to treat NHL patients.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81570200).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiu BC and Weisenburger DD: An update of
the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma.
4:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Advani A, Coiffier B, Czuczman MS,
Dreyling M, Foran J, Gine E, Gisselbrecht C, Ketterer N, Nasta S,
Rohatiner A, et al: Safety, pharmacokinetics, and preliminary
clinical activity of inotuzumab ozogamicin, a novel immunoconjugate
for the treatment of B-cell non-Hodgkin's lymphoma: Results of a
phase I study. J Clin Oncol. 28:2085–2093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maxwell SA and Mousavi-Fard S:
Non-Hodgkin's B-cell lymphoma: Advances in molecular strategies
targeting drug resistance. Exp Biol Med. 238:971–990. 2013.
View Article : Google Scholar
|
|
4
|
Huang X, Meng B, Iqbal J, Ding BB, Perry
AM, Cao W, Smith LM, Bi C, Jiang C, Greiner TC, et al: Activation
of the STAT3 signaling pathway is associated with poor survival in
diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol.
31:4520–4528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bohers E, Mareschal S, Bouzelfen A,
Marchand V, Ruminy P, Maingonnat C, Ménard AL, Etancelin P,
Bertrand P, Dubois S, et al: Targetable activating mutations are
very frequent in GCB and ABC diffuse large B-cell lymphoma. Genes
Chromosomes Cancer. 53:144–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duong HQ, Hwang JS, Kim HJ, Kang HJ, Seong
YS and Bae I: Aldehyde dehydrogenase 1A1 confers intrinsic and
acquired resistance to gemcitabine in human pancreatic
adenocarcinoma MIA PaCa-2 cells. Int J Oncol. 41:855–861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schäfer A, Teufel J, Ringel F, Bettstetter
M, Hoepner I, Rasper M, Gempt J, Koeritzer J, Schmidt-Graf F, Meyer
B, et al: Aldehyde dehydrogenase 1A1-a new mediator of resistance
to temozolomide in glioblastoma. Neuro Oncol. 14:1452–1464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shien K, Toyooka S, Yamamoto H, Soh J,
Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, et al:
Acquired resistance to EGFR inhibitors is associated with a
manifestation of stem cell-like properties in cancer cells. Cancer
Res. 73:3051–3061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alamgeer M, Ganju V, Szczepny A, Russell
PA, Prodanovic Z, Kumar B, Wainer Z, Brown T, Schneider-Kolsky M,
Conron M, et al: The prognostic significance of aldehyde
dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage
non-small cell lung cancer. Thorax. 68:1095–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Beça FF, Caetano P, Gerhard R,
Alvarenga CA, Gomes M, Paredes J and Schmitt F: Cancer stem cells
markers CD44, CD24 and ALDH1 in breast cancer special histological
types. J Clin Pathol. 66:187–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen M, Bunaciu RP, Congleton J, Jensen
HA, Sayam LG, Varner JD and Yen A: Interferon regulatory factor-1
binds c-Cbl, enhances mitogen activated protein kinase signalingand
promotes retinoic acid-induced differentiation of HL-60 human
myelo-monoblastic leukemia cells. Leuk Lymphoma. 52:2372–2379.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeda J, Mamat S, Tian T, Wang Y, Luo W,
Rahadiani N, Aozasa K and Morii E: Reactive oxygen species and
aldehyde dehydrogenase activity in Hodgkin lymphoma cells. Lab
Invest. 92:606–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neradugomma NK, Subramaniam D, Tawfik OW,
Goffin V, Kumar TR, Jensen RA and Anant S: Prolactin signaling
enhances colon cancer stemness by modulating Notch signaling in a
Jak2-STAT3/ERK manner. Carcinogenesis. 35:795–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Salnikov AV, Bauer N,
Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J,
Schemmer P, Werner J and Herr I: Triptolide reverses
hypoxia-induced epithelial-mesenchymal transition and stem-like
features inpancreatic cancer by NF-κB downregulation. Int J Cancer.
134:2489–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song YH, Zhong MZ, Gan PP, Yi PY, Tang YH,
Liu YP, Jiang JQ and Li L: ALDH1A1 mediates resistance of diffuse
large B cell lymphoma to the CHOP regimen. Tumour Biol.
35:11809–11817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita S, Morii E, Rahadiani N, Wada N,
Hori Y, Ikeda JI and Aozasa K: Significance of aldehyde
dehydrogenase 1 expression in stromal cells of diffuse large B-cell
lymphoma. Exp Ther Med. 2:591–594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang J, Liu Y, Tang Y, Li L, Zeng R, Zeng
S and Zhong M: ALDH1A1 induces resistance to CHOP in diffuse large
B-cell lymphoma through activation of the JAK2/STAT3 pathway. Onco
Targets Ther. 9:5349–5360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson WH, Teruya-Feldstein J, Fest T,
Harris C, Steinberg SM, Jaffe ES and Raffeld M: Relationship of
p53, bcl-2, and tumor proliferation to clinical drug resistance in
non-Hodgkin's lymphomas. Blood. 89:601–609. 1997.PubMed/NCBI
|
|
19
|
Maxwell SA, Li Z, Jaye D, Ballard S,
Ferrell J and Fu H: 14-3-3zeta mediates resistance of diffuse large
B cell lymphoma to an anthracycline-based chemotherapeutic regimen.
J Biol Chem. 284:22379–22389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Wang Y, Ju X, Lan J, Zou H, Li S,
Qi Y, Jia W, Hu J, Liang W, et al: Clinicopathological significance
of ALDH1A1 in lung, colorectal, and breast cancers: A
meta-analysis. Biomark Med. 9:777–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones RJ, Gocke CD, Kasamon YL, Miller CB,
Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M, et
al: Circulating clonotypic B cells in classic Hodgkin lymphoma.
Blood. 113:5920–5926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng SB, Ohshima K, Selvarajan V, Huang G,
Choo SN, Miyoshi H, Shimizu N, Reghunathan R, Chua HC, Yeoh AE, et
al: Epstein-Barr virus-associated T/natural killer-cell
lymphoproliferative disorder in children and young adults has
similar molecular signature to extranodal nasal natural
killer/T-cell lymphoma but shows distinctive stem cell-like
phenotype. Leuk Lymphoma. 56:2408–2415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan SK, Meade B, Wang Q, Merchant AA,
Kowalski J and Matsui W: Mantle cell lymphoma activation enhances
bortezomib sensitivity. Blood. 116:4185–4191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang L, Yu K, Du J, Ni W, Han Y, Gao S,
Li H, Wu J, Zheng Y and Tan Y: Inhibition of p38 MAPK activity in
B-NHL Raji cells by treatment with engineered CD20-specific T
cells. Oncol Lett. 2:753–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pham LV, Tamayo AT, Yoshimura LC, Lo P,
Terry N, Reid PS and Ford RJ: A CD40 Signalosome anchored in lipid
rafts leads to constitutive activation of NF-kappaB andautonomous
cell growth in B cell lymphomas. Immunity. 16:37–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Chen Z, Miranda RN, Medeiros LJ
and McCarty N: TG2 and NF-κB signaling coordinates the survival of
mantle cell lymphoma cells via IL6-mediated autophagy. Cancer Res.
76:6410–6423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ezell SA, Mayo M, Bihani T, Tepsuporn S,
Wang S, Passino M, Grosskurth SE, Collins M, Parmentier J, Reimer C
and Byth KF: Synergistic induction of apoptosis by combination of
BTK and dual mTORC1/2 inhibitors in diffuse large B cell lymphoma.
Oncotarget. 5:4990–5001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koppaka V, Thompson DC, Chen Y, Ellermann
M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD and
Vasiliou V: Aldehyde dehydrogenase inhibitors: A comprehensive
review of the pharmacology, mechanism of action, substrate
specificity, and clinical application. Pharmacol Rev. 64:520–539.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Haffner MC, Zhang Y, Lee BH,
Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, et
al: Disulfiram is a DNA demethylating agent and inhibits prostate
cancer cell growth. Prostate. 71:333–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JY, Cho Y, Oh E, Lee N, An H, Sung D,
Cho TM and Seo JH: Disulfiram targets cancer stem-like properties
and the HER2/Akt signaling pathway in HER2-positive breast cancer.
Cancer Lett. 379:39–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Wang L, Cui W, Yuan X, Lin L, Cao
Q, Wang N, Li Y, Guo W, Zhang X, et al: Targeting ALDH1A1 by
disulfiram/copper complex inhibits non-small cell lung cancer
recurrence driven by ALDH-positive cancer stem cells. Oncotarget.
7:58516–58530. 2016.PubMed/NCBI
|
|
32
|
Paranjpe A, Zhang R, Ali-Osman F, Bobustuc
GC and Srivenugopal KS: Disulfiram is a direct and potent inhibitor
of human O6-methylguanine-DNA methyltransferase (MGMT)
in brain tumor cells and mouse brain and markedly increases the
alkylating DNA damage. Carcinogenesis. 35:692–702. 2014. View Article : Google Scholar : PubMed/NCBI
|