Introduction
Fos-related antigen 1 (Fra-1) protein forms
activator protein-1 complexes in association with members of the
JUN family, which drives the expression of genes involved in
various biological processes, including cell proliferation,
differentiation, transformation, and invasiveness, in several
cancer cell lines (1–3). Fra-1 is usually absent in normal
epithelial cells but is upregulated in various cancers, such as
lung, breast, colon, prostate, brain, head and neck, esophagus,
ovary, and nasopharynx cancers (3–13).
Increased Fra-1 expression has been shown to be correlated with
tumor stage in esophageal squamous cell carcinoma (8), and high levels of Fra-1 expression are
associated with severe malignancy in breast cancer progression
(13); thus, Fra-1 is recognized as
a prognostic factor for certain cancers (2,8).
Colorectal cancer is currently the most common
gastrointestinal malignancy, and it remains the third most common
cancer and second leading cause of cancer-related death in
developed countries (14). Although
surgical resection is the first choice of treatment for colorectal
cancer, radiation therapy and chemotherapy are also essential
interventions in colorectal cancer treatment. In addition, many
patients with local recurrences are not eligible for surgical
resection, and they are frequently referred for radiotherapy.
However, the results of conventional photon radiotherapy are still
far from satisfactory, with many studies in the literature
reporting a 50% 1-year survival rate and a 10% 3-year survival rate
(15). Thus, the role of photon
radiotherapy is often described as mere pain control (16). Carbon-ion (C-ion) beam therapy is
well known for its high linear energy transfer (LET), and it has
some unique advantages over photon irradiation, including more
accurate dose distribution (17–19), a
high rate of double-strand breaks of the DNA chain (20,21),
and high relative biologic effectiveness of tumor cell killing
(22–24). Thus, C-ion radiotherapy is expected
to become a promising alternative to surgery for colorectal cancer
treatment. Previous research has shown that C-ion radiotherapy may
be a safe and effective treatment option for locally recurrent
rectal cancer and may serve as an alternative to surgery (25–31).
The radiation dose required for tumor control varies
widely among human tumors and depends on a range of factors, such
as inherent cellular radiosensitivity, repair and repopulation
phenomena, and tumor hypoxia (32–34).
Since resistance to radiation is one of the reasons for treatment
failure, the identification of key factors involved in cancer
radioresistance is important for developing an effective method of
chemoradiotherapy. A previous study reported that downregulation of
Fra-1 reduced the radioresistance of a prostate cancer cell line,
PC-3, after treatment with 4-Gy photon beam irradiation (35). However, there are no published
studies of the role of Fra-1 in radioresistance to X-ray or C-ion
radiation for colorectal cancer cells.
Herein, we used two human colon cancer cell lines,
SW620 and SW480, and demonstrated that Fra-1 has a role in the
radioresistance to both X-ray and C-ion radiation.
Materials and methods
Cell culture and reagents
Cells of the human colon cancer cell lines SW620 and
SW480 were purchased from ATCC (Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Nissui, Tokyo, Japan)
supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT,
USA), and penicillin/streptomycin (Gibco, Carlsbad, CA, USA).
Irradiation
Cells were irradiated with X-rays or C-ions at room
temperature. X-rays were produced by a PANTAK HF-320S generator
(Shimadzu Corp., Kyoto, Japan), at 200 kVp and 20 mA, and filtered
with 0.5 mm Al and 0.5 mm Cu (36).
C-ions were accelerated by the Heavy-Ion Medical Accelerator in
Chiba at the National Institute of Radiological Sciences, Chiba,
Japan (37). The initial energy of
the C-ion beams was 290 MeV/nucleon, and the LET value was 80
keV/µm with a monoenergetic beam (20). An outline of the experimental
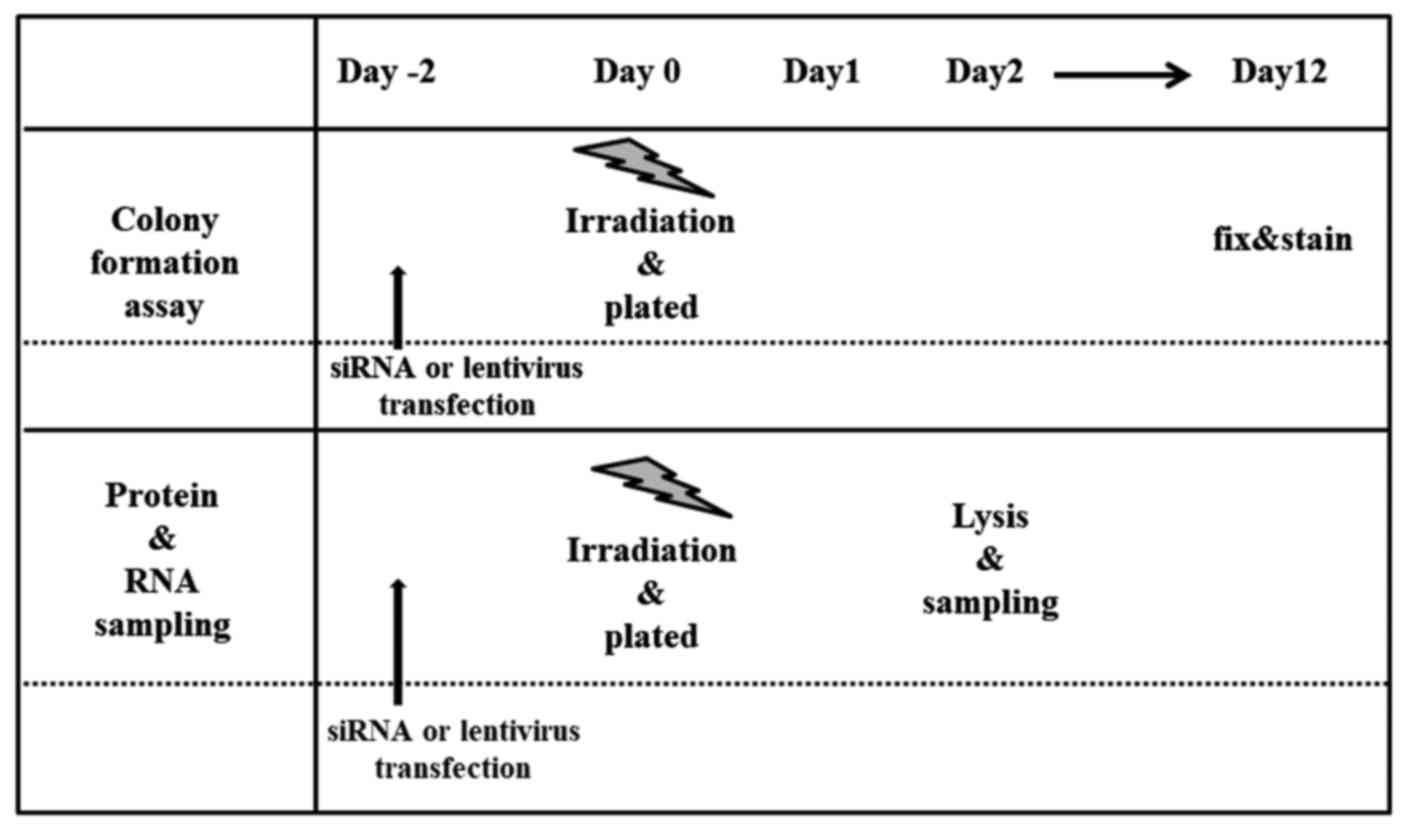
procedures after irradiation is shown in Fig. 1.
siRNA transfection
The cells were transiently transfected with siRNA
specific for Fra-1 using Lipofectamine 2000 Reagent (Invitrogen,
Carlsbad, CA, USA), as previously described (8). The sequences of the Fra-1 siRNA were
as follows: Sense, agaaaucugggcugcagcgagagau, and antisense,
aucucucgcug cagcccagauuucu. Fra-1 protein expression was evaluated
by western blotting, and Fra-1 downregulation was confirmed at 48 h
after siRNA transfection by comparison with the Fra-1 expression
level of the cells transfected with scrambled negative control
siRNA (Invitrogen).
Lentivirus production and
transduction
The coding sequence of the human FRA1 gene was
amplified from cDNA derived from SW480 cells by PCR using a
gene-specific primer set: Sense,
ggggacaagtttgtacaaaaaagcaggcttcaccatgttccgagacttcggggaacccggcccg,
and antisense,
ggggaccactttgtacaagaaagctgggtctcacaaagcgaggagggttggagagccaag. The
PCR fragment was introduced into a pDONR221 vector for cloning of
the gene, in accordance with the instructions for Gateway Cloning
Technology (Invitrogen), and confirmed by sequencing. Then, this
gene was transferred by LR recombination from its entry clone into
a pLenti7.3V5-DEST vector containing Emerald Green Fluorescent
Protein (EmGFP). pLenti7.3/V5-GW/lacZ was the construct for the
negative control. Lentiviral stocks were produced in 293FT cells in
accordance with a modification of the manufacturer's protocol
(Invitrogen). Briefly, 18 µl of FuGENE 6 was diluted in 0.6 ml of
Opti-MEM I medium, and then 1.5 µg of plasmid DNA and 4.5 µg of
packaging mix (Applied Biological Materials Inc., Richmond, BC,
Canada) were added to this medium. These transfection complexes
were incubated at room temperature and added to 5 ml of Opti-MEM I
containing 6×106 cells. After incubation at 37°C for 8
h, the culture medium was replaced with 5 ml of DMEM supplemented
with 10% heat-inactivated FBS. Virus-containing supernatants were
harvested 48 h after transfection and then centrifuged at 3,000 rpm
at 4°C for 15 min and passed through a 0.45-µm Millex-HV filter to
remove debris. The virus was precipitated at 4°C overnight by
adding 3.3 ml of cold 40% PEG6000, to concentrate the virus, and
then suspended in 100 µl of phosphate-buffered saline (PBS). Then,
1×105 cells were transduced by 10 µl of the virus
preparation in the presence of 6 µg/ml hexadimethrine bromide
(Polybrene) for 48 h. Fra-1 protein expression was evaluated by
western blotting, and upregulation of Fra-1 was confirmed at 48 h
after lentivirus transfection by comparison with the Fra-1
expression level of the cells transfected with the negative
control.
Colony formation assay
Cell survival curves were determined by a colony
formation assay as previously described, with some modifications
(38). Briefly, cell cultures at
70% confluence were rinsed with PBS and detached with 0.1%
trypsin/PBS. Cell numbers were determined with a hemocytometer.
Cells were plated in triplicate onto 60-mm diameter plastic dishes
and incubated for 12 days, whereupon the colonies were fixed and
stained with 1% methylene blue in 30% methanol. Colonies consisting
of more than 50 cells were scored as surviving colonies.
For the radiosensitivity analysis, non-irradiated
cells or cells irradiated with X-rays at 1, 2, 4, 6, or 8 Gy or
C-ions at 0.5, 1, 2, 3, or 4 Gy were used. The cells were
trypsinized and counted immediately after irradiation. Eighty cells
for non-irradiated cells or 150, 300, 1,500, or 3,000 cells for
X-ray irradiation at 1, 2, 4, 6, or 8 Gy or C-ion irradiation at
0.5, 1, 2, 3, or 4 Gy were plated onto 60-mm diameter dishes,
respectively. The surviving fraction was normalized to that of the
non-irradiated control.
To assess the clonogenicity of the Fra-1
siRNA-transfected or lentivirus-transfected cells, cells were
treated with siRNA or lentivirus vector for 48 h before
irradiation. Immediately after irradiation, the cells were
trypsinized, and the same numbers of cells as that used in the
radiosensitivity analysis were plated onto dishes containing fresh
media; colony-forming assays were then performed.
Protein sampling and western
blotting
Non-irradiated or irradiated cells were trypsinized
and counted immediately after irradiation, and the same number of
non-irradiated and irradiated cells was plated onto dishes
containing fresh media. Two days after irradiation, cells were
lysed with RIPA lysis buffer containing PMSF and sodium
orthovanadate (Santa Cruz Biotechnology, Dalla, TX, USA) and then
used for the western blotting.
For the proteasome inhibitor treatment, two patterns
of schedule were used: 1) 10 nM of epoxomicin (proteasome
inhibitor; Peptide Institute Inc., Osaka, Japan) was added to the
culture media immediately before cell irradiation, and the cells
were cultured for 48 h in a 5% CO2 incubator at 37°C and
then lysed, or 2) 10 nM of epoxomicin was added to the culture
media 48 h after irradiation, and the cells were cultured in a 5%
CO2 incubator at 37°C for 24 h. The cells were then
lysed with RIPA lysis buffer and used for the western blotting.
Immunoblotting was performed as previously described
(37). Primary antibodies for human
Fra-1 (Santa Cruz Biotechnology) and GAPDH (Trevigen, Bristol, UK)
with horseradish peroxidase-conjugated anti-mouse IgG or
anti-rabbit IgG (Amersham Biosciences, Buckinghamshire, UK) were
used for this study. Protein bands were detected by enhanced
chemiluminescence and imaged using a Lumino image analyzer
(LAS4000; Fujifilm, Tokyo, Japan).
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed
on a LightCycler 480 with Probes Master (Roche Diagnostics, Basel,
Switzerland) as previously described (38). The Universal Probe Library (UPL;
Roche Diagnostics) probes and primer sequences for the Fra-1
(FOSL1) and GAPDH genes were as follows: FOSL1
(UPL probe: 26) sense, aggaactgaccgacttcctg, and antisense,
cagctctaggcgctccttc; GAPDH (UPL probe: 60) sense,
agccacatcgctcagaca, and antisense, gcccaatacgaccaaatcc.
Statistical analysis
Statistical analyses were performed using unpaired
Student's t-tests or Mann-Whitney U-tests. P-values of
<0.05 was considered to indicate a statistically significant
difference.
Results
Role of Fra-1 in radioresistance
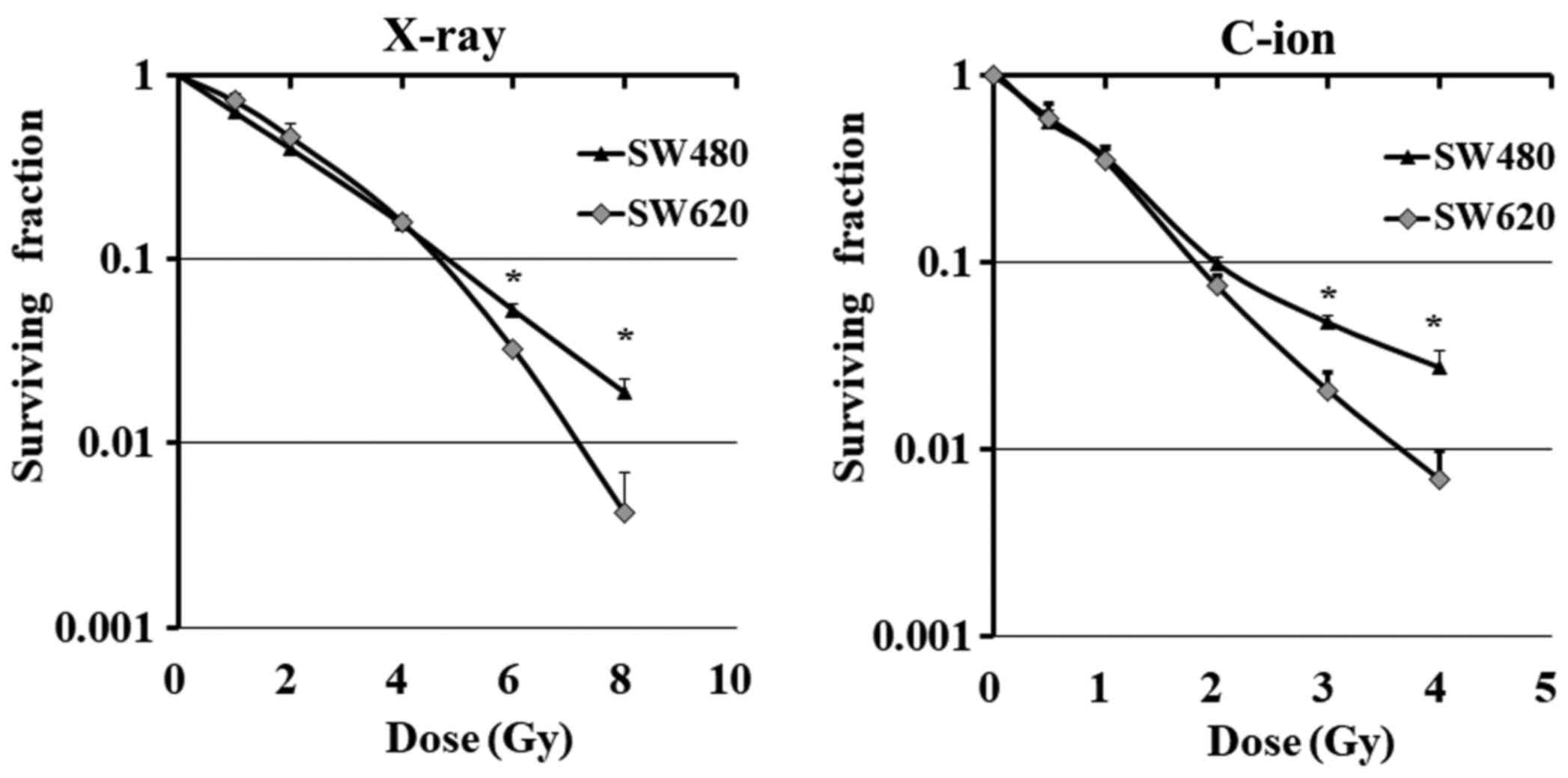
Surviving fractions of SW620 and SW480 cells were
determined after X-ray or C-ion irradiation. Sensitivity to X-ray
or C-ion irradiation differed between the cell lines; SW620 showed
lower surviving fractions than SW480 at doses greater than 6 Gy for
X-ray or 3 Gy for C-ion radiation (Fig.
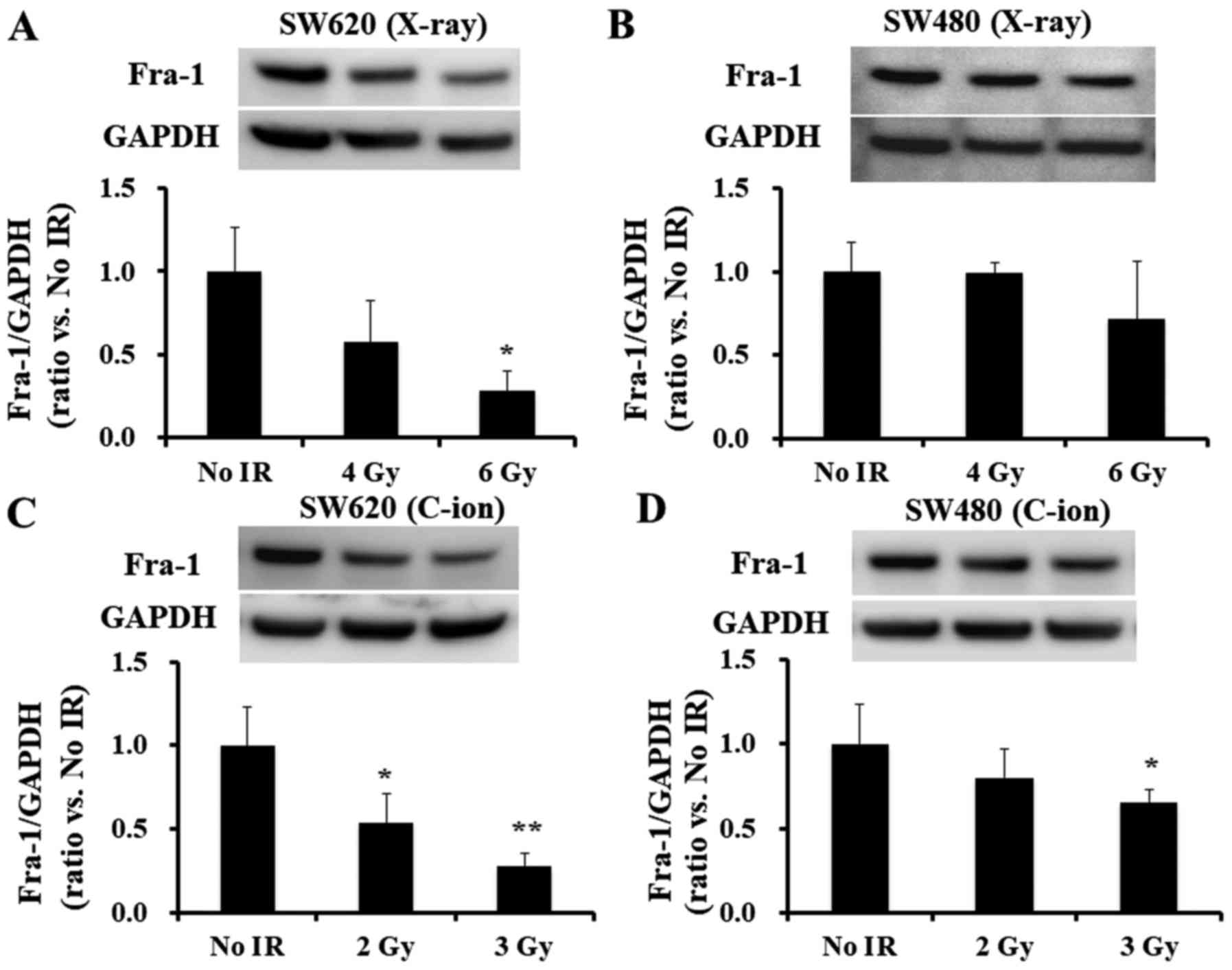
2). Of note, SW620 cells showed a greater decrease in Fra-1
after 6 Gy for X-ray or 3 Gy for C-ion irradiation than SW480 cells
(Fig. 3A and B for X-ray and
Fig. 3C and D for C-ion
irradiation, respectively).
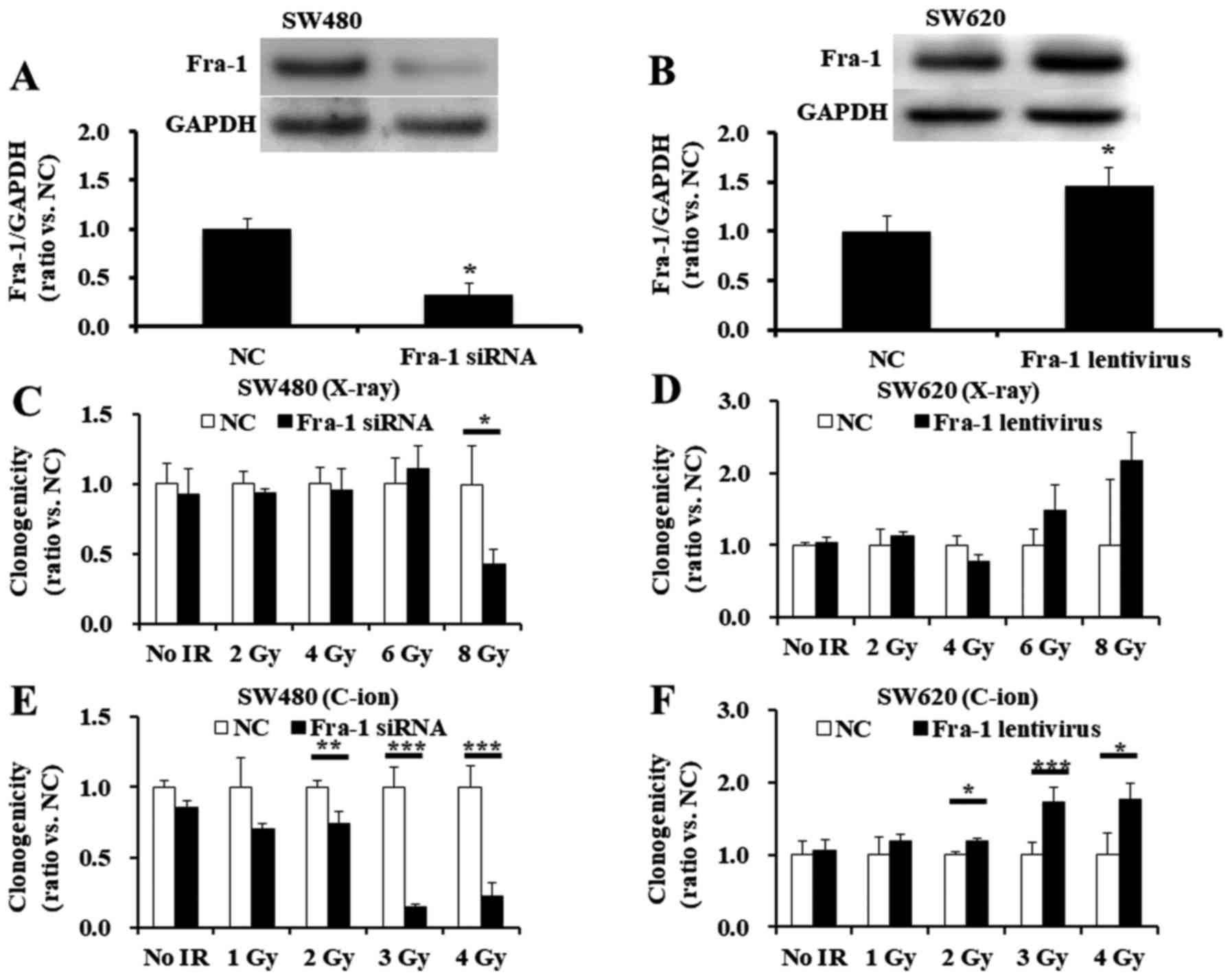
To investigate a possible association between Fra-1
downregulation and cellular radiosensitivity, we first treated
SW480 cells with Fra-1 siRNA. The effectiveness of Fra-1 reduction
with siRNA transfection is shown in Fig. 4A. Downregulation of Fra-1 in
siRNA-treated SW480 cells showed increased radiosensitivity to 8-Gy
X-ray radiation (Fig. 4C) and to
2-, 3-, or 4-Gy C-ion radiation (Fig.
4E), compared with that of the scrambled negative
control-treated SW480 cells.
To further clarify the significance of Fra-1 in
radioresistance, we next overexpressed Fra-1 in SW620 cells via
transfection with a lentivirus vector. Fra-1 induction with
lentivirus transfection is shown in Fig. 4B. Further, overexpression of Fra-1
in lentivirus-transfected SW620 cells tended to increase the
resistance to X-ray radiation (Fig.
4D) and significantly enhanced the resistance to C-ion
radiation at doses greater than 2 Gy (Fig. 4F). Overall, the results indicate
that Fra-1 has some role in radioresistance to X-ray or C-ion
radiation for SW480 and SW620 cells.
Fra-1 levels in irradiated SW620 cells
were downregulated by protein degradation through a proteasome
pathway
To identify the molecular mechanisms modulating
Fra-1 levels after irradiation, we first compared the changes of
Fra-1 protein and the corresponding FOSL1 transcript levels in
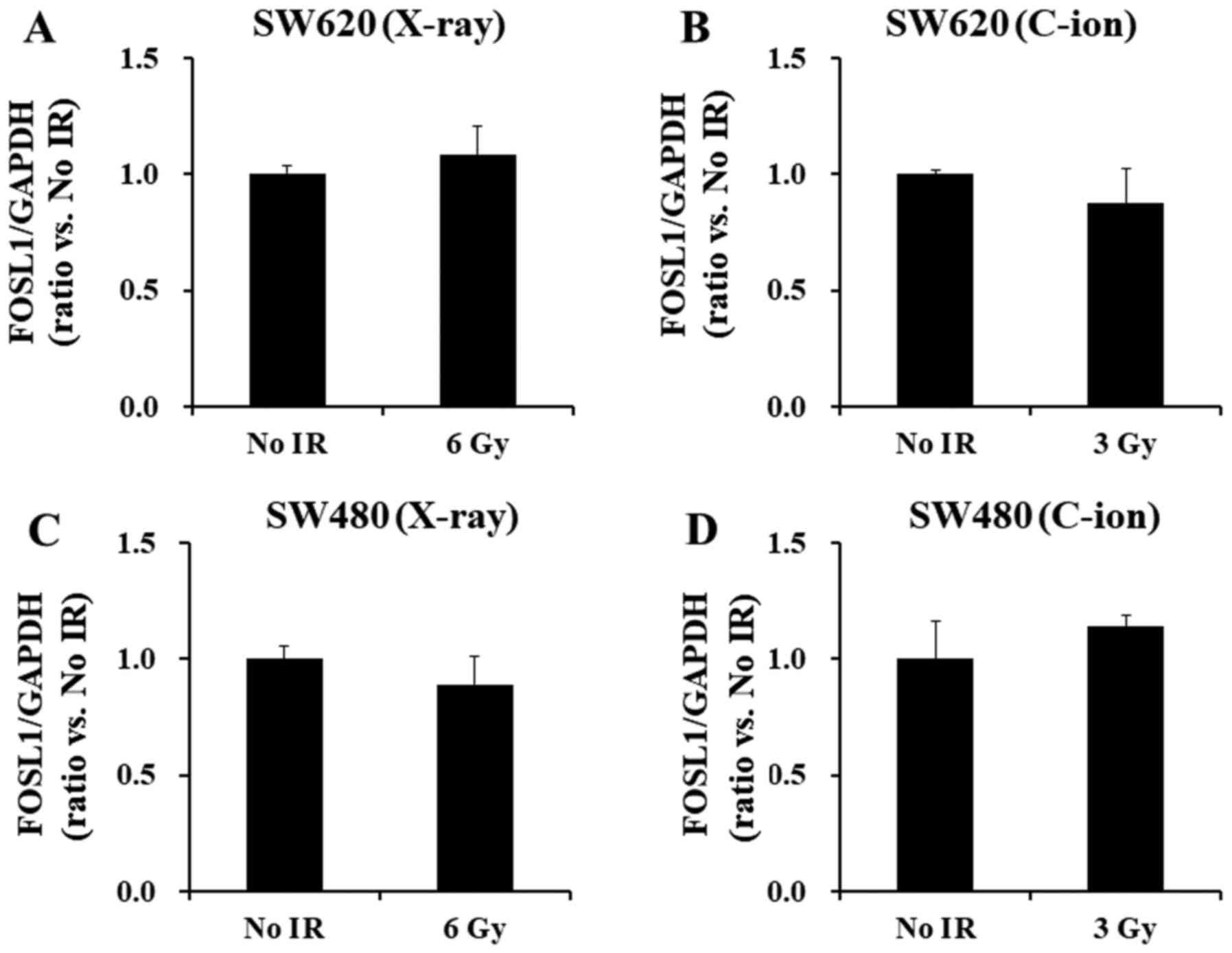
SW620 and SW480 cells after X-ray or C-ion irradiation. Although
Fra-1 protein levels significantly decreased after irradiation with
6-Gy X-ray or 3-Gy C-ion radiation (Fig. 5A-D), no alteration in the Fra-1
transcript, FOSL1, levels was found for either the SW620 (Fig. 5A and B) or the SW480 cell lines
(Fig. 5C and D), which indicates
discrepancies between the reduction of Fra-1 protein and transcript
levels in the irradiated cells.
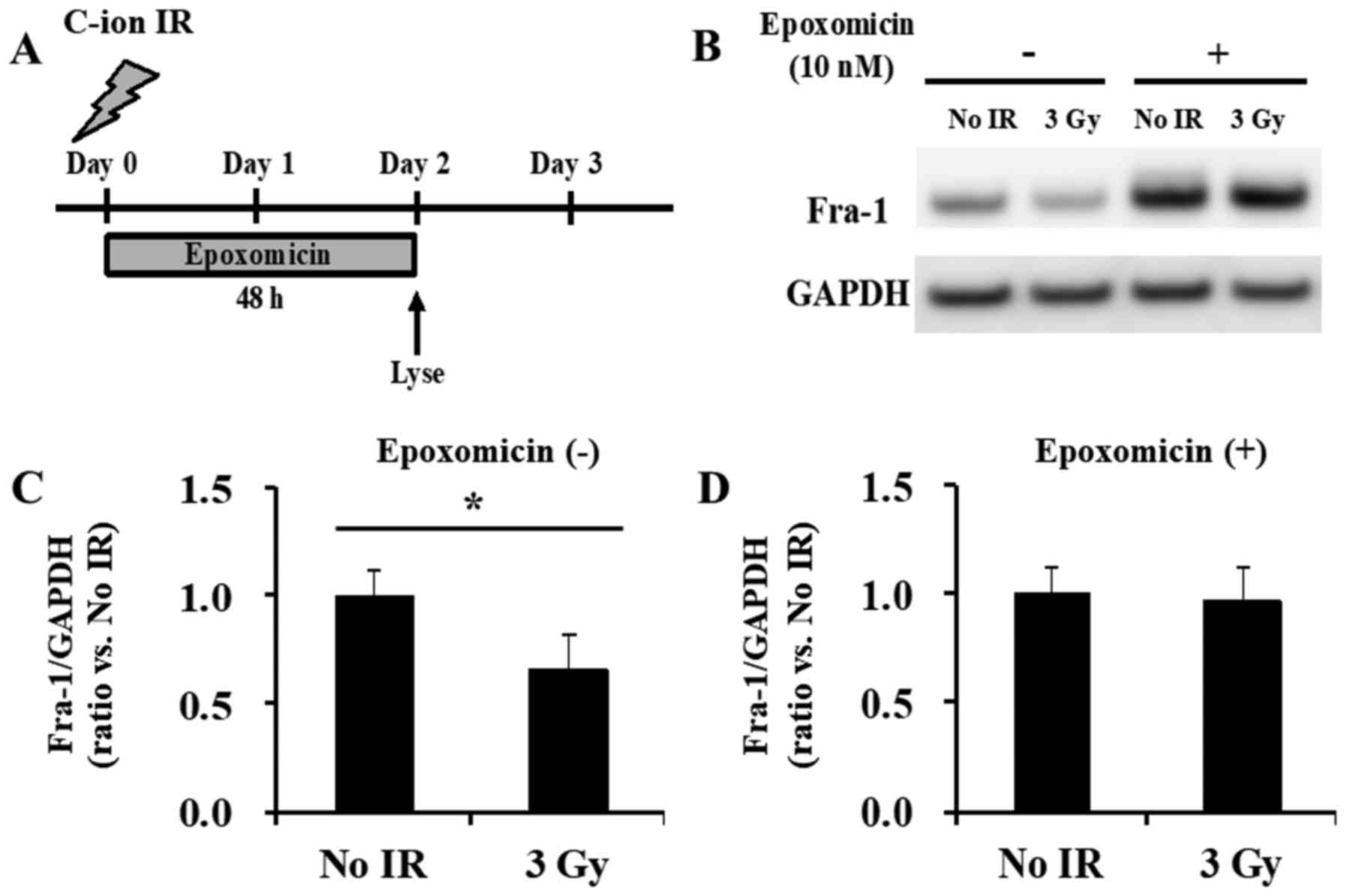
The expression of Fra-1 protein is known to be
highly regulated by proteasomal degradation (39). To clarify whether proteasomal
degradation was involved in the reduction of the Fra-1 protein
levels observed in the irradiated cells, C-ion-irradiated SW620
cells were further studied, because a clear discrepancy between the
Fra-1 protein and transcript levels was observed in these cells
(Figs. 3C and 5B). Pre-treatment of SW620 cells with the
proteasome inhibitor epoxomicin and continued treatment for another
48 h after irradiation (Fig. 6A)
blocked the Fra-1 degradation of these irradiated cells compared
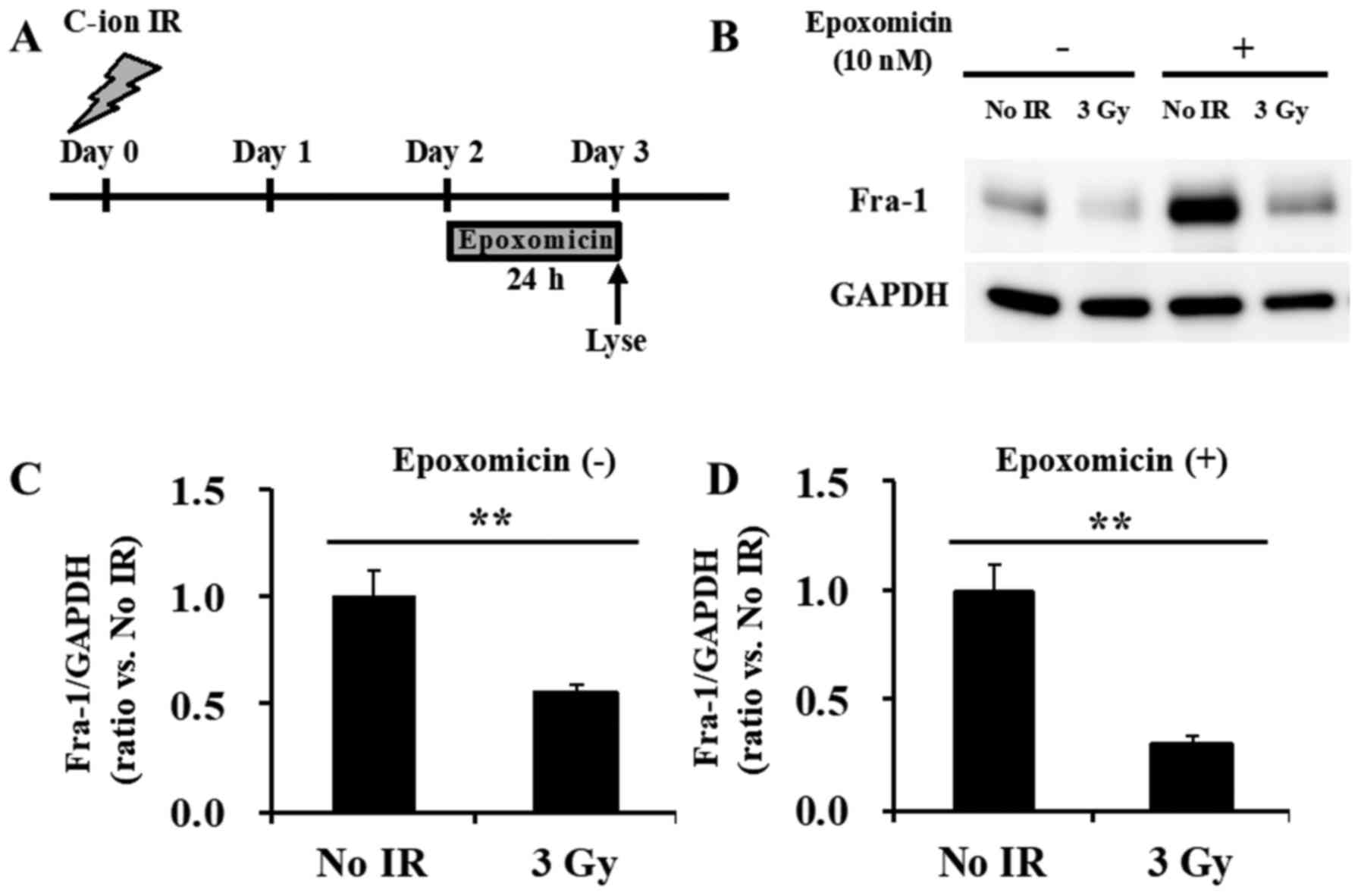
with that of the non-epoxomicin-treated SW620 cells (Fig. 6B-D). Of note, epoxomicin treatment
from 48 h after C-ion irradiation (Fig.
7A) failed to block the reduction of Fra-1 (Fig. 7B-D), which indicates that the
degradation of Fra-1 via the proteasome occurred at some time
during the first 48 h after irradiation.
Discussion
An understanding of the molecular mechanisms
involved in radioresistance is necessary for improving the clinical
outcomes of cancer radiotherapy. In this study, we demonstrated
that Fra-1 has a significant role in the radioresistance of two
colorectal cancer cell lines, SW620 and SW480. It is well known
that irradiation, especially high LET radiation such as C-ion
irradiation, induces cell cycle delay at the G2 phase, arresting
the cells at the G2 checkpoint for DNA repair and/or committing
them to undergo apoptosis (40).
Cyclin A, an important factor for the initiation of DNA
replication, is known as a transcriptional target of Fra-1
(41). Thus, greater reduction of
Fra-1 may interrupt DNA repair, which could induce cell death.
Fra-1 is known to be involved in various biological
processes (1,2). In order to avoid the potential
pathological effects of Fra-1 overexpression, the stability of the
Fra-1 protein is highly regulated by phosphorylation-dependent
proteasomal degradation (39).
Thus, Fra-1 is usually absent in normal epithelial cells, but it is
upregulated in several cancers (2).
Several studies have suggested that the stability of the Fra-1
protein is regulated by phosphorylation upon ERK-MAPK pathway
activation (39,42–44).
The upstream signaling effectors, such as proteins encoded by
oncogenic KRAS, found in colon carcinoma cell lines have been shown
to result in constitutive ERK activation, followed by Fra-1
accumulation (38). To clarify
whether Fra-1 phosphorylation status is involved in the Fra-1
degradation of irradiated cells, we also determined whether
irradiation reduced the phospho-Fra-1 (p-Fra-1) levels in SW620
cells, as Fra-1 de-phosphorylation causes Fra-1 to become unstable
and be degraded via the proteasome. Treatment of irradiated SW620
cells with epoxomicin for 48 h upon irradiation clearly blocked the
downregulation of Fra-1 (Fig. 6);
therefore, we hypothesized that the remaining undegraded Fra-1
contained many of the dephosphorylated Fra-1 proteins, which were
destined to be degraded via the proteasome but remained because we
had blocked this proteasome function. However, the levels of
p-Fra-1 in the epoxomicin-treated SW620 cells were unchanged even
after irradiation; the remaining undegraded Fra-1 following
epoxomicin treatment did not contain dephosphorylated Fra-1
proteins, and most of them were phosphorylated Fra-1 (data not
shown). These results indicate that the dephosphorylation of Fra-1
is not the trigger of proteasomal degradation upon irradiation. We
also intended to check the levels of ubiquitinated Fra-1, but we
could not detect the ubiquitination of Fra-1 protein (data not
shown). Thus far, we have not yet discovered how irradiation leads
to Fra-1 degradation via the proteasome, without the involvement of
dephosphorylation or ubiquitination of Fra-1, and further studies
are required to solve this question.
In conclusion, we found that Fra-1 has a role in the
radioresistance to X-ray or C-ion irradiation. To our knowledge,
this is the first study indicating the role of Fra-1 in the
radioresistance of colorectal cancer cells. In addition, we
observed Fra-1 degradation within 48 h after irradiation. It would
be of interest to further study whether Fra-1 level could be a
candidate as an early response marker to reflect the effectiveness
of radiotherapy.
Acknowledgements
This work was supported in part by JSPS KAKENHI
grant no. 22591394 for TI and grant no. 23791467 for MF from the
Japan Society for the Promotion of Science. We thank Yoshimi Shoji
for technical assistance. We would like to thank Enago (www.enago.jp) for the English language review.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Fra-1
|
Fos-related antigen 1
|
|
C-ion
|
carbon ion
|
|
RBE
|
relative biologic effectiveness
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
Eferl R and Wagner EF: AP-1: A
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young MR and Colburn NH: Fra-1 a target
for cancer prevention or intervention. Gene. 379:1–11. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu YC, Lam KY, Law S, Wong J and
Srivastava G: Identification of differentially expressed genes in
esophageal squamous cell carcinoma (ESCC) by cDNA expression array:
Overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC.
Clin Cancer Res. 7:2213–2221. 2001.PubMed/NCBI
|
|
4
|
Ramos-Nino ME, Scapoli L, Martinelli M,
Land S and Moss-man BT: Microarray analysis and RNA silencing link
fra-1 to cd44 and c-met expression in mesothelioma. Cancer Res.
63:3539–3545. 2003.PubMed/NCBI
|
|
5
|
Hapke S, Kessler H, Luber B, Benge A,
Hutzler P, Höfler H, Schmitt M and Reuning U: Ovarian cancer cell
proliferation and motility is induced by engagement of integrin
alpha(v)beta3/Vitronectin interaction. Biol Chem. 384:1073–1083.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kustikova O, Kramerov D, Grigorian M,
Berezin V, Bock E, Lukanidin E and Tulchinsky E: Fra-1 induces
morphological transformation and increases in vitro invasiveness
and motility of epithelioid adenocarcinoma cells. Mol Cell Biol.
18:7095–7105. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diesch J, Sanij E, Gilan O, Love C, Tran
H, Fleming NI, Ellul J, Amalia M, Haviv I, Pearson RB, et al:
Widespread FRA1-dependent control of mesenchymal
transdifferentiation programs in colorectal cancer cells. PLoS One.
9:e889502014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Usui A, Hoshino I, Akutsu Y, Sakata H,
Nishimori T, Murakami K, Kano M, Shuto K and Matsubara H: The
molecular role of Fra-1 and its prognostic significance in human
esophageal squamous cell carcinoma. Cancer. 118:3387–3396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Hart J, McLeod HL and Wang HL:
Differential expression of the AP-1 transcription factor family
members in human colorectal epithelial and neuroendocrine
neoplasms. Am J Clin Pathol. 124:11–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zerbini LF, Wang Y, Cho JY and Libermann
TA: Constitutive activation of nuclear factor kappaB p50/p65 and
Fra-1 and JunD is essential for deregulated interleukin 6
expression in prostate cancer. Cancer Res. 63:2206–2215.
2003.PubMed/NCBI
|
|
11
|
Mann B, Gelos M, Siedow A, Hanski ML,
Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ and
Hanski C: Target genes of beta-catenin-T
cell-factor/lymphoid-enhancer-factor signaling in human colorectal
carcinomas. Proc Natl Acad Sci USA. 96:pp. 1603–1608. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fung LF, Lo AK, Yuen PW, Liu Y, Wang XH
and Tsao SW: Differential gene expression in nasopharyngeal
carcinoma cells. Life Sci. 67:923–936. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belguise K, Kersual N, Galtier F and
Chalbos D: FRA-1 expression level regulates proliferation and
invasiveness of breast cancer cells. Oncogene. 24:1434–1444. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada S, Shinoto M, Endo S, Yasuda S,
Imada H, Kamada T and Tsujii H: Carbon ion radiotherapy for
patients with locally recurrent cancer. Proceedings of NIRS-IMP
Joint Symposium on Carbon Ion Therapy and Radiation Emergency Med.
1–47. 2012.
|
|
16
|
Lingareddy V, Ahmad NR and Mohiuddin M:
Palliative reirradiation for recurrent rectal cancer. Int J Radiat
Oncol Biol Phys. 38:785–790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuji H and Kamada T: A review of update
clinical results of carbon ion radiotherapy. Jpan J Clin Oncol.
42:670–685. 2012. View Article : Google Scholar
|
|
18
|
Fokas E, Kraft G, An H and
Engenhart-Cabillic R: Ion beam radiobiology and cancer: Time to
update ourselves. Biochim Biophys Acta. 1796:216–229.
2009.PubMed/NCBI
|
|
19
|
Schulz-Ertner D and Tsujii H: Particle
radiation therapy using proton and heavier ion beams. J Clin Oncol.
25:953–964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuchida Y, Tsuboi K, Ohyama H, Ohno T,
Nose T and Ando K: Cell death induced by high-linear-energy
transfer carbon beams in human glioblastoma cell lines. Brain Tumor
Pathol. 15:71–76. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakano T, Suzuki Y, Ohno T, Kato S, Suzuki
M, Morita S, Sato S, Oka K and Tsujii H: Carbon beam therapy
overcomes the radiation resistance of uterine cervical cancer
originating from hypoxia. Clin Cancer Res. 12:2185–2190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M, Kase Y, Yamaguchi H, Kanai T and
Ando K: Relative biological effectiveness for cell-killing effect
on various human cell lines irradiated with heavy-ion medical
accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol
Biol Phys. 48:241–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tobias CA, Blakely EA, Alpen EL, Castro
JR, Ainsworth EJ, Curtis SB, Ngo FQ, Rodriguez A, Roots RJ,
Tenfordf T and Yang TC: Molecular and cellular radiobiology of
heavy ions. Int J Radiat Oncol Biol Phys. 8:2109–2120. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen C, Borak TB, Tsujii H and Nickoloff
JA: Heavy charged particle radiobiology: Using enhanced biological
effectiveness and improved beam focusing to advance cancer therapy.
Mutat Res. 711:150–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada S, Kamada T, Ebner DK, Shinoto M,
Terashima K, Isozaki Y, Yasuda S, Makishima H, Tsuji H, Tsujii H,
et al: Carbon-ion radiation therapy for pelvic recurrence of rectal
cancer. Int J Radiat Oncol Biol Phys. 96:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Combs SE, Kieser M, Habermehl D, Weitz J,
Jäger D, Fossati P, Orrechia R, Engenhart-Cabillic R, Pötter R,
Dosanjh M, et al: Phase I/II trial evaluating carbon ion
radiotherapy for the treatment of recurrent rectal cancer: The
PANDORA-01 trial. BMC Cancer. 12:1372012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsuzaki H, Ishihara S, Kawai K,
Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Yamada S and
Watanabe T: Late sacral recurrence of rectal cancer treated by
heavy ion radiotherapy: A case report. Surg Case Rep. 2:1092016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada S, Shinoto M, Shigeo Y, Imada H,
Kato H, Kamada T and Tsujii H: Current status and perspective of
heavy ion beam therapy for patients with pelvic recurrence after
primarily resected rectal cancer. Gan To Kagaku Ryoho.
36:1263–1266. 2009.(In Japanese). PubMed/NCBI
|
|
29
|
Mobaraki A, Ohno T, Yamada S, Sakurai H
and Nakano T: Cost-effectiveness of carbon ion radiation therapy
for locally recurrent rectal cancer. Cancer Sci. 101:1834–1839.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Habermehl D, Wagner M, Ellerbrock M,
Büchler MW, Jäkel O, Debus J and Combs SE: Reirradiation using
carbon ions in patients with locally recurrent rectal cancer at
HIT: First results. Ann Surg Oncol. 22:2068–2074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isozaki Y, Yamada S, Kawashiro S, Yasuda
S, Okada N, Ebner D, Tsuji H, Kamada T and Matsubara H: Carbon-ion
radiotherapy for isolated para-aortic lymph node recurrence from
colorectal cancer. J Surg Oncol. 116:932–938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peters LJ, Brock WA, Chapman JD and Wilson
G: Predictive assays of tumor radiocurability. Am J Clin Oncol.
11:275–287. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
West CM: Invited review: Intrinsic
radiosensitivity as a predictor of patient response to
radiotherapy. Br J Radiol. 68:827–837. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishikawa K, Koyama-Saegusa K, Otsuka Y,
Ishikawa A, Kawai S, Yasuda K, Suga T, Michikawa Y, Suzuki M,
Iwakawa M and Imai T: Gene expression profile changes correlating
with radioresistance in human cell lines. Int J Radiat Oncol Biol
Phys. 65:234–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kajanne R, Miettinen P, Tenhunen M and
Leppä S: Transcription factor AP-1 promotes growth and
radioresistance in prostate cancer cells. Int J Oncol.
35:1175–1182. 2009.PubMed/NCBI
|
|
36
|
Fujita M, Otsuka Y, Yamada S, Iwakawa M
and Imai T: X-ray irradiation and Rho-kinase inhibitor additively
induce invasiveness of the cells of the pancreatic cancer line,
MIAPaCa-2, which exhibits mesenchymal and amoeboid motility. Cancer
Sci. 102:792–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujita M, Imadome K, Shoji Y, Isozaki T,
Endo S, Yamada S and Imai T: Carbon-ion irradiation suppresses
migration and invasiveness of human pancreatic carcinoma cells
MIAPaCa-2 via Rac1 and RhoA degradation. Int J Radiat Oncol Biol
Phys. 93:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujita M, Otsuka Y, Imadome K, Endo S,
Yamada S and Imai T: Carbon-ion radiation enhances migration
ability and invasiveness of the pancreatic cancer cell, PANC-1, in
vitro. Cancer Sci. 103:677–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Basbous J, Jariel-Encontre I, Gomard T,
Bossis G and Piechaczyk M: Ubiquitin-independent-versus
ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1
transcription factors: Is there a unique answer? Biochimie.
90:296–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sasaki H, Yatagai F, Kanai T, Furusawa Y,
Hanaoka F, Zhu WG and Mehnati P: Dependence of induction of
interphase death of Chinese hamster ovary cells exposed to
accelerated heavy ions on linear energy transfer. Radiat Res.
148:449–454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Casalino L, Bakiri L, Talotta F, Weitzman
JB, Fusco A, Yaniv M and Verde P: Fra-1 promotes growth and
survival in RAS-transformed thyroid cells by controlling cyclin A
transcription. EMBO J. 26:1878–1890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vial E and Marshall CJ: Elevated ERK-MAP
kinase activity protects the FOS family member FRA-1 against
proteasomal degradation in colon carcinoma cells. J Cell Sci.
116:4957–4963. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Casalino L, De Cesare D and Verde P:
Accumulation of Fra-1 in ras-transformed cells depends on both
transcriptional autoregulation and MEK-dependent posttranslational
stabilization. Mol Cell Biol. 23:4401–4415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Basbous J, Chalbos D, Hipskind R,
Jariel-Encontre I and Piechaczyk M: Ubiquitin-independent
proteasomal degradation of Fra-1 is antagonized by Erk1/2
pathway-mediated phosphorylation of a unique C-terminal
destabilizer. Mol Cell Biol. 27:3936–3950. 2007. View Article : Google Scholar : PubMed/NCBI
|