Introduction
Pituitary tumor is a common neuroendocrine system
tumor, the morbidity of which accounts for 10–15% of all central
nervous system tumors. Moreover, the morbidity increases annually,
as is shown in epidemiological investigations (1). A majority of pituitary tumors are
benign tumors that grow slowly, with no typical early symptoms in
clinic. However, a small number of these tumors can induce certain
central nervous system symptoms since the excessive growth has
compressed the intracranial structures (2). Furthermore, some tumors belong to
functional pituitary adenomas, which cause endocrine function
disorders as a result of the abnormal secretion of hormones.
Consequently, this has led to numerous complications (3). Early tumor diagnosis is of vital
importance to improve prognosis for cancer patients, while the
early diagnosis of pituitary tumor, not only improves patient
quality of life, but can also effectively prolong the life of
patients (4).
miRNA generally acts on various types of oncogenes
(including proto-oncogenes and tumor suppressor genes) through
direct or indirect pathways, and thereby participates in tumor
pathogenesis. It is indicated in current research that, ~50% of
miRNAs are related to tumor pathogenesis. Such miRNAs, which are
located in tumor-associated fragile sites or particular tumor
growth-associated genome domains, have dual identities (oncogenes
or tumor suppressor genes) (5).
Cancer miRNAs possess certain properties of oncogenes, which can
specifically bind with the mRNA of tumor suppressor genes, and
inhibit or degrade the expression of tumor suppressor genes. It can
lead to silencing of targeted oncogenes, and thus indirectly
promote growth or genesis of some tumors. In addition, such miRNAs
are mostly excessively expressed in tumors, which means that they
have elevated expression levels in the event of tumor genesis. In
other words, their expression levels are upregulated in the event
of tumor genesis. Anticancer miRNAs can specifically bind with
cancer mRNA, and inhibit or degrade the expression of oncogenes
(6). Therefore, they can inhibit
transcription of targeted oncogenes and eventually inhibit tumor
growth or genesis. Different from the former, the expression levels
of such miRNAs are reduced (6). The
discovery of cancer miRNA and anticancer miRNA has led to medical
workers investigating the association of miRNA with tumor (7). This provides insight into the
investigation of the specific pathogenesis, diagnosis and treatment
of pituitary tumor (7).
Pituitary tumor is a kind of benign tumor that does
not metastasize under general conditions, but ~30% invades the
surrounding structures (8). Such
tumors can hardly be radically treated, and total resection is
difficult, leading to a high postoperative recurrence rate. CD147,
which is also referred to as the extracellular matrix
metalloproteinase inducer (EMMPRIN), is a cell surface adhesion
molecule. It plays a role in stimulating the production of matrix
metalloproteinases (MMPs) and affects tumor invasion and
metastasis. In the subfamily of MMPs, MMP-9 is most closely
associated with the invasion of pituitary tumor (9). Tumor angiogenesis is closely related
to tumor growth and metastasis. Vascular endothelial growth factor
(VEGF2) is one of the pro-angiogenic factors known currently to
have the strongest effect, and it plays an important role in tumor
formation (10).
From the point of view of cell apoptosis, out of
control growth and excessive proliferation of tumor cells occurs
owing to the fact that that death cells cannot be eliminated
normally since the tumor apoptotic mechanism is inhibited (11). p38MAPK pathway is an important
pathway involved in the initiation of cell apoptosis, which can
exert biological effects after being activated by the extracellular
stimulus, thus regulating various cell functions (12). It mainly mediates physiological
functions such as differentiation, proliferation and apoptosis
(11). Therefore, clarifying its
mechanism of action during tumor genesis, development and treatment
outcome is necessary.
NF-κB is a family of transcription factors existing
in eukaryotic cells with extensive distribution and multiple
effects. It is one of the most important intracellular nuclear
transcription factors that participate in the expression and
regulation of multiple genes, which is the symbol of activated
cells (13). NF-κB is verified in
research to participate in inflammatory reaction and immune
response of the body. In addition, it is involved in
pathophysiological processes, such as cell proliferation,
differentiation and apoptosis (14). Furthermore, the nuclear factor NF-κB
is associated with tumor invasion and metastasis. NF-κB controls
DNA transcription and the binding of the fixed nucleotide sequence
in the promoter region of the gene. It is involved in
pathophysiological processes including immune reaction,
inflammatory reaction, and cell apoptosis. Thus, it promotes a
series of important vital activities, such as cell proliferation
and differentiation, tumor formation and metastasis (15). The aim of the present study was to
clarify how microRNA-16 expression affects the proliferation and
survival of pituitary tumor and reveal its potential mechanism.
Materials and methods
Human tissue samples and reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Thirty-six patients with pituitary tumor and 8
healthy volunteers were selected, and the peripheral blood was
collected and centrifuged at 2,000 × g for 20 min. Serum was
collected and saved at −80°C. Total RNA was extracted using RNAiso
Plus according to the manufacturer's instructions (Takara, Japan).
cDNA was reverse-transcribed using the One Step
PrimeScript® miRNA cDNA Synthesis kit (Takara) and
PrimeScript® RT Master Mix Perfect Real Time (Takara),
according to the manufacturer's instructions. RT-qPCR reactions
were performed using SYBR® Premix Ex Taq™ II (Perfect
Real Time; Takara) by an Applied Biosystems 7500 Fast Real-Time PCR
system.
Written informed consent was obtained from the
patients. The study was approved by the Ethics Committee of
Tangshan Gonren Hospital, Tangshan, China.
Cells and overexpression of
miR-16
Human pituitary cancer HP75 cells were incubated
with low-glucose Dulbecco's modified Eagle's medium complete medium
(Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum
(FCS, Harlan, Madison, WI, USA) in a humidified chamber with 5%
CO2 at 37°C. HP75 cells were seeded in 6-well plates
(~70% confluent), and 50 nM of miR-16 mimics (miR-16) and negative
control RNA mimics (Ribobio, Guangzhou, China) were transfected
using Lipofectamine 3000 (Invitrogen, Guangzhou, China). HP75 cells
were treated with 5 µM of PDTC (NF-κB inhibitor) for 24 h after
transfection.
MTT proliferation assay
HP75 cells were seeded in a 96-well plate after
transfection for 48 h. Then, MTT (5 mg/ml in sodium chloride) was
added to the cells for the last 2 h of incubation and cell
viability was measured at 570 nm using a microplate reader (Tecan
M1000, Invitrogen, Carlsbad, CA, USA) after being dissolved in
DMSO.
Flow cytometry
The anticancer effect of dihydroartemisinin on
apoptosis of tumor cells was examined using a FACSCalibur flow
cytometer (BD Biosciences, San Jose, CA, USA). HP75 cells were
seeded in a 6-well plate after transfection for 48 h. After rinsing
in PBS three times, HP75 cells were resuspended with Annexin V-FITC
(5 µl, KeyGen Biotech Co., Ltd.) in the dark for 30 min followed by
PI dye (5 µl, KeyGen Biotech Co., Ltd., Jiangsu, China). Apoptosis
rate of HP75 cells was measured by FACSCalibur flow cytometer (BD
Biosciences).
Western blot assay
The anticancer effect of dihydroartemisinin on
apoptosis of tumor cells was examined using a FACSCalibur flow
cytometer (BD Biosciences). HP75 cells were seeded in a 6-well
plate after transfection for 48 h. The cells were then collected
and lysed in Laemmli buffer to extract total proteins. Protein
content was measured using the bicinchoninic acid protein assay kit
method (Beyotime Institute of Biotechnology, Jiangsu, China).
Proteins (50 µg) were separated by 10% SDS-PAGE and transferred
onto PVDF membrane (0.45 mm, Millipore, Billerica, MA, USA). The
membrane was blocked with 5% non-fat milk in TBST for 2 h and
incubated overnight with the corresponding primary antibodies:
anti-p27 (sc-528, 1:500, Santa Cruz Biotechnology), anti-Bax
(sc-6236, 1:500, Santa Cruz Biotechnology), anti-NF-κB (sc-7151,
1:500, Santa Cruz Biotechnology), anti-MMP-9 (sc-10737, 1:500,
Santa Cruz Biotechnology), anti-VEGFR2 (9698, 1:2,000, Cell
Signaling Technology, Inc.), anti-p53 (2527, 1:2,000, Cell
Signaling Technology, Inc.) and anti-GAPDH (sc-25778, 1:2,000,
Santa Cruz Biotechnology) at 4°C. Then, the membrane was incubated
with secondary antibody (sc-2004, 1:5,000, Santa Cruz
Biotechnology) at 37°C for 1 h and was determined with ImageJ
software (open source, http://rsb.info.nih.gov/ij/index.html).
Caspase-3 and −9 activity assay
HP75 cells were seeded in a 6-well plate after
transfection for 48 h. The cells were then collected and lysed in
Laemmli buffer for the extraction of total proteins. Protein
content was measured using the bicinchoninic acid protein assay kit
method (Beyotime Institute of Biotechnology). Proteins (20 µg) were
used to measure caspase-3 and −9 activity with Ac-DEVD-pNA
for caspase-3 or Ac-LEHD-pNA for caspase-9. Caspase-3 and −9
activity was measured using a microplate reader (Tecan M1000) at
405 nm.
Statistical analysis
Statistical significance was calculated employing
analysis of variance (one-way ANOVA, Tukey's multiple comparison
test). Data were presented as mean ± standard deviation (SD). A
statistical significance was defined as P<0.05.
Results
MicroRNA-16 expression of pituitary
tumor patients
Firstly, 36 patients with pituitary tumor and 8
healthy volunteers were selected, and serum was collected and used
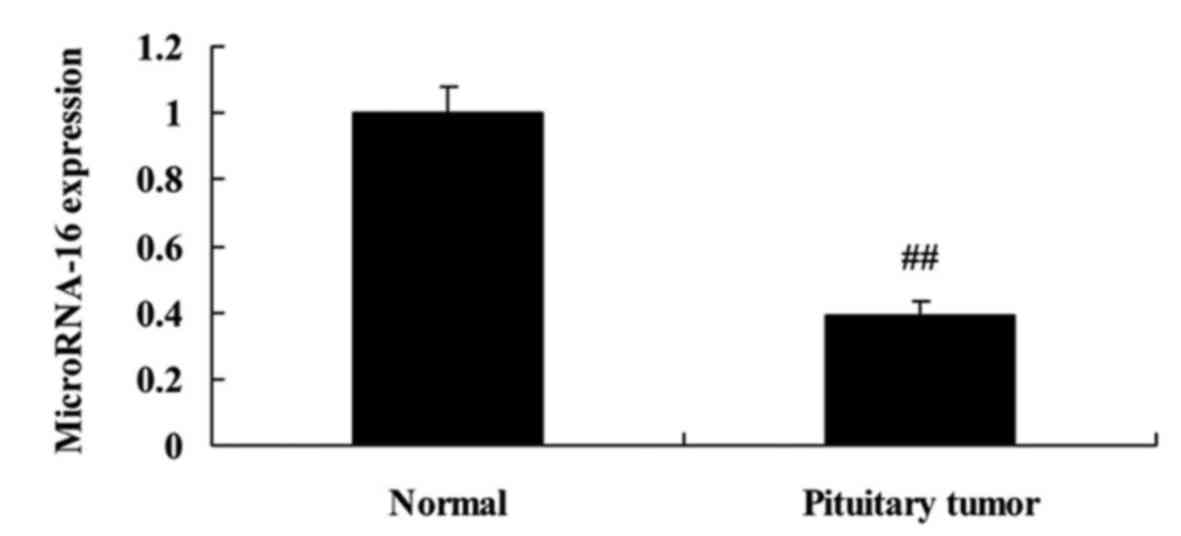
to analyze microRNA-16 expression of pituitary tumor patients. As
shown in Fig. 1, microRNA-16
expression of pituitary tumor patients was observably declined,
compared with the normal group. The differences were statistically
significant (P<0.05).
Survival rate of pituitary tumor
patients with microRNA-16 expression
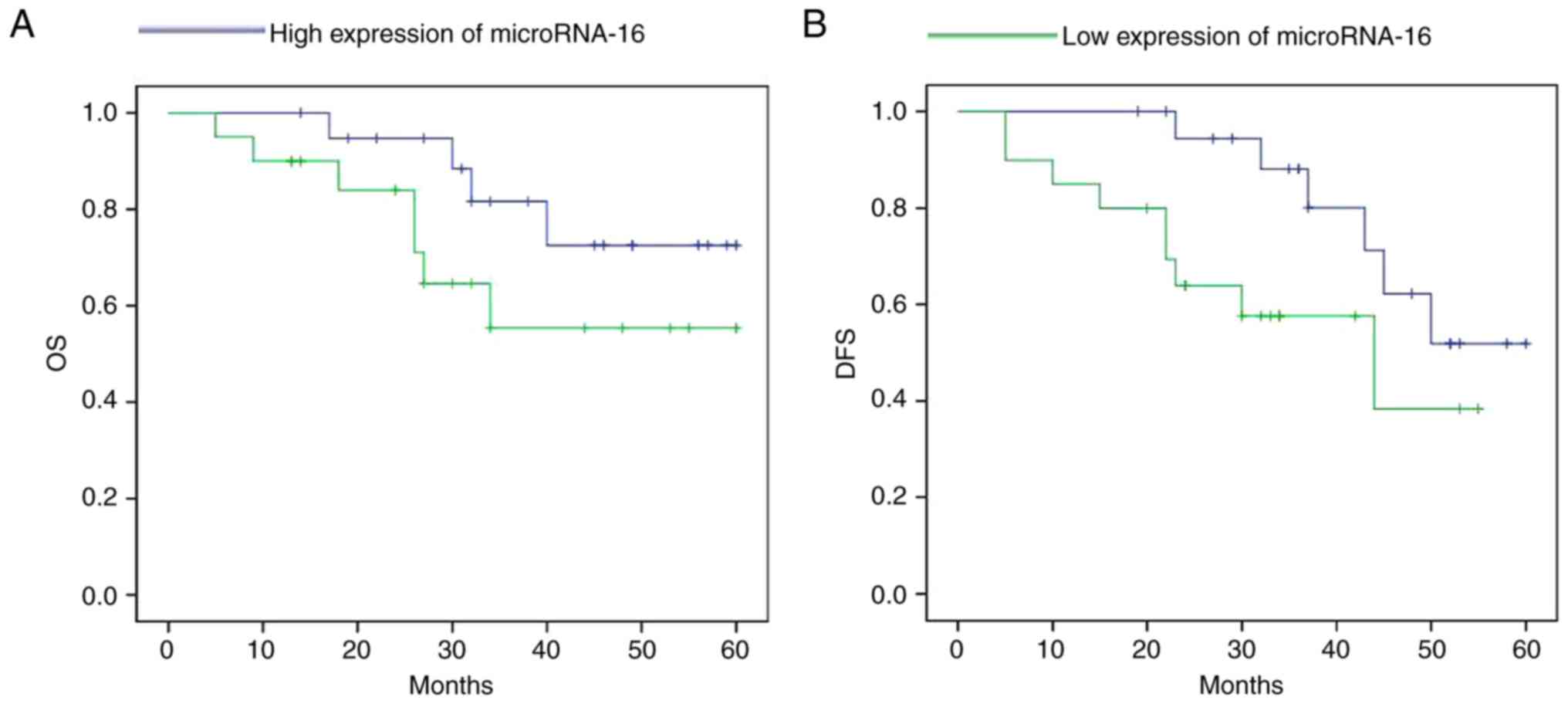
The high expression of microRNA-16 shows longer
survival (overall survival, OS and disease-free survival, DFS) in
pituitary tumor patients, compared to a low expression microRNA-16
in pituitary tumor patients (Fig.
2).
MicroRNA-16 upregulation affects cell
proliferation and apoptosis of HP75 cells
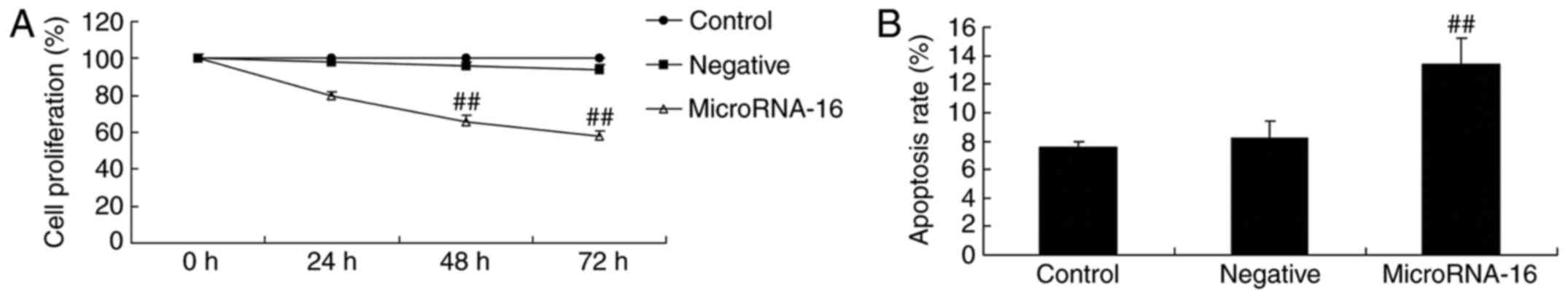
In order to predict the upstream microRNA-16 effects
on cell proliferation and apoptosis of HP75 cells, miR-16 mimics
and mimics-NC were transiently transfected into HP75 cells.
microRNA-16 mimics decreased cell proliferation and induced
apoptosis of HP75 cells in a dose-dependent manner, compared to the
mimics-NC group (Fig. 3).
MicroRNA-16 upregulation affects
caspase-3/8 activities in HP75 cells
To investigate the role of microRNA-16 in the
regulation of caspase-3/8 activities of HP75 cells, caspase-3/8
activities were measured using caspase-3 and −8 activity kits.
Additionally, Fig. 4A shows that
microRNA-16 mimics effectively increased caspase-3/8 activities in
HP75 cells, compared to the mimics-NC group.
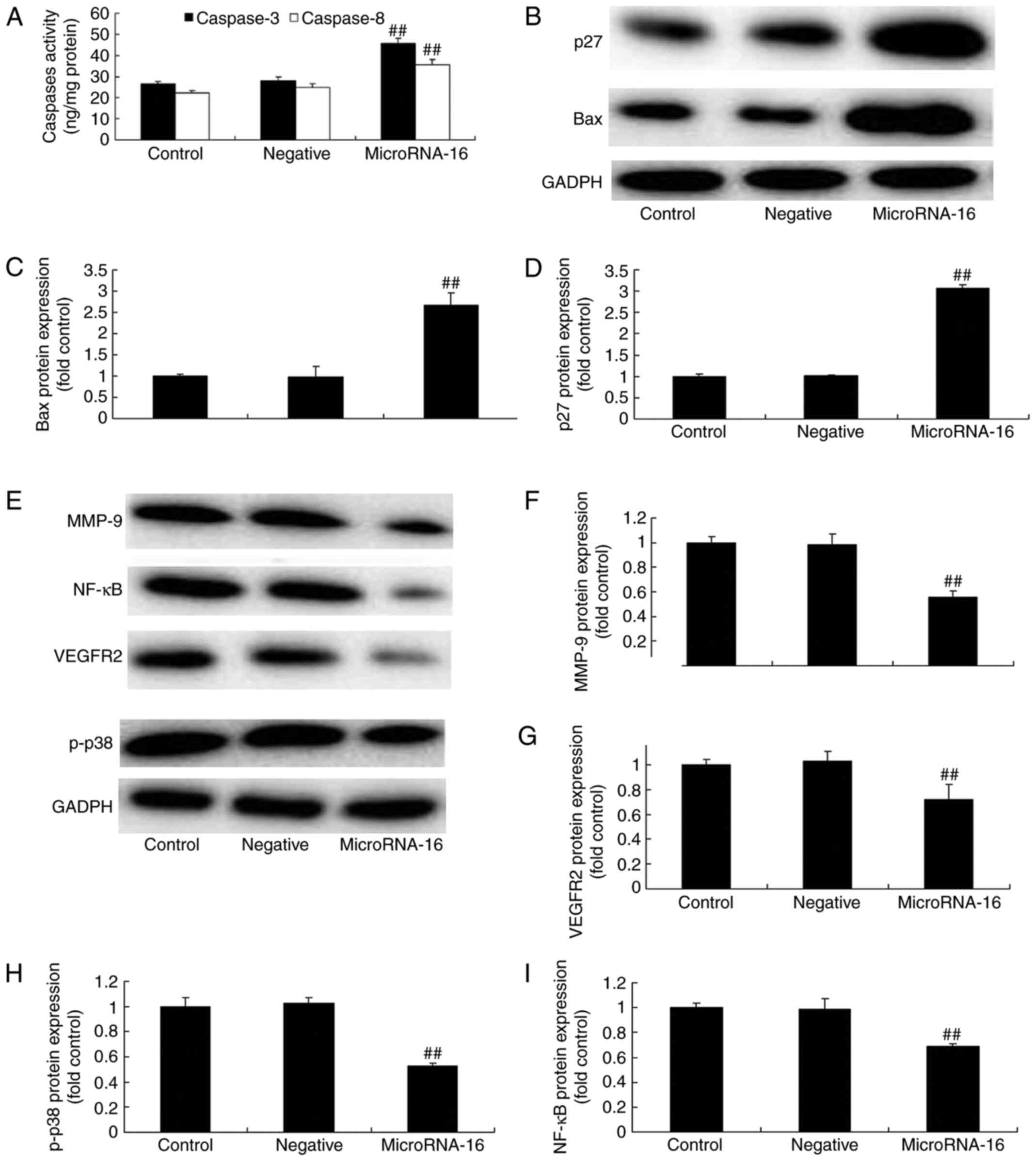 | Figure 4.MicroRNA-16 upregulation affects
caspase-3/8 activities, p27/Bax protein expression and p27 and Bax
protein expression in HP75 cells. MicroRNA-16 upregulation affects
(A) caspase-3/8 activities, (B) p27 and Bax protein expression
using western blot analysis and statistical analysis of (C) Bax and
(D) p27 protein expression, (E) p-p38MAPK, NF-κB, MMP-9 and VEGFR2
protein expression using western blot analysis and statistical
analysis of (F) MMP-9, (G) VEGFR2, (H) p-p38MAPK and (I) NF-κB
protein expression in HP75 cells. Control, control group. Negative,
negative control group. MicroRNA-16, microRNA-16 upregulation
group. Repeat times (n=3). ##P<0.01 versus normal
group. |
MicroRNA-16 upregulation affects p27
and Bax protein expression in HP75 cell
To confirm the prediction of microRNA-16 mechanism
on pituitary tumor, we measured p27, Bax protein expression in HP75
cells. Our results indicated a significant increase of p27, Bax
protein expression in HP75 cells after microRNA-16 upregulation,
compared to the mimics-NC group (Fig.
4B-D).
MicroRNA-16 upregulation affects
p38MAPK, NF-κB, MMP-9 and VEGFR2 protein expression in HP75
cells
To confirm the mechanism of microRNA-16 on pituitary
tumor, we measured p-p38MAPK, NF-κB, MMP-9 and VEGFR2 protein
expression in HP75 cells. Our results indicated significant
reduction of p-p38MAPK, NF-κB, MMP-9 and VEGFR2 protein expression
in HP75 cells after microRNA-16 upregulation, compared to the
mimics-NC group (Fig. 4E-I). We
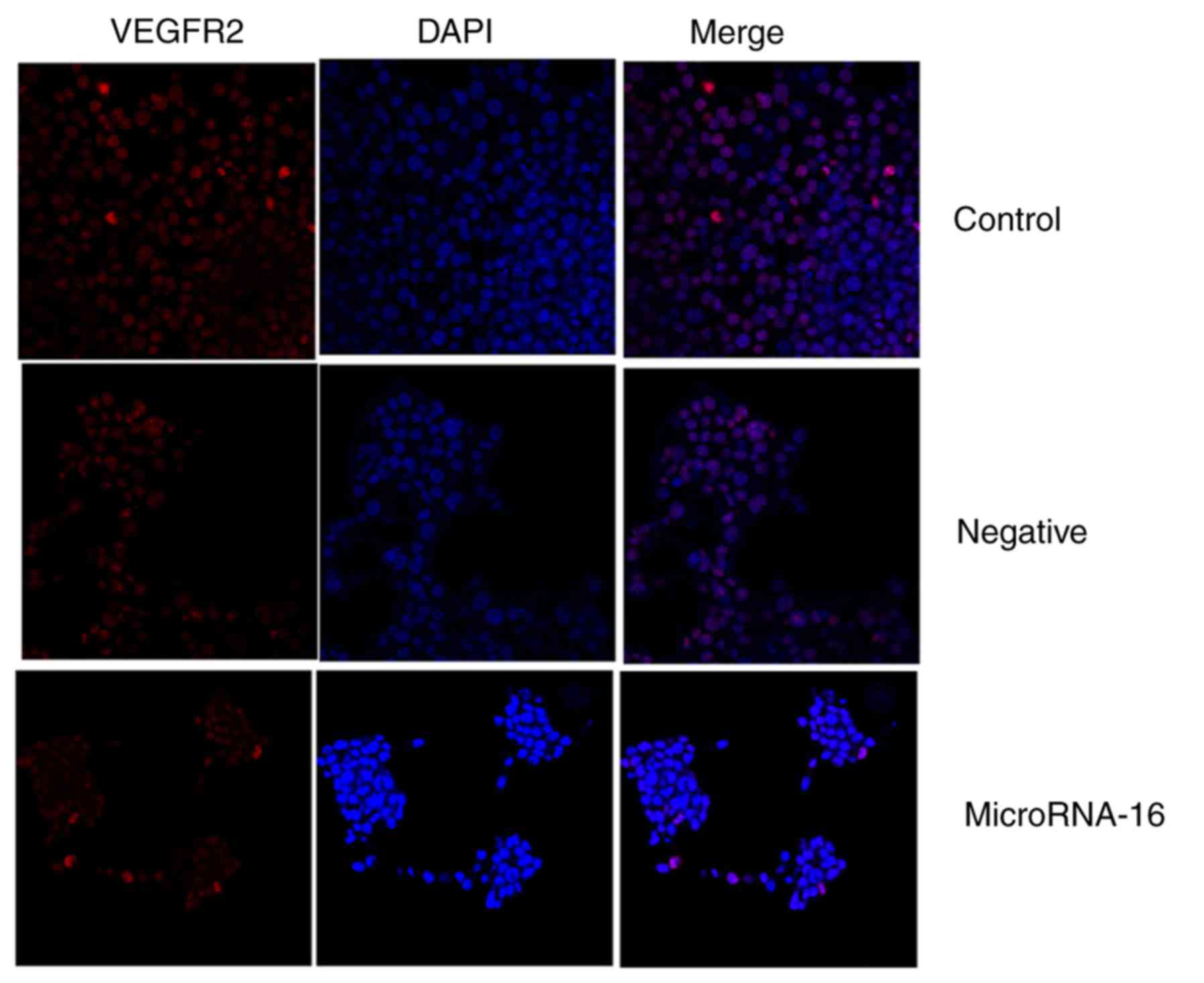
used immunohistochemistry to observe VEGFR2 protein expression in
HP75 cells after microRNA-16 upregulation. MicroRNA-16 upregulation
significantly suppressed VEGFR2 protein expression in HP75 cells,
compared to the mimics-NC group (Fig.
4E-I).
Effects of microRNA-16 overexpression
on p38MAPK, NF-κB, MMP-9 and VEGFR2 protein expression in HP75
cells following VEGFR2 suppression
To study whether VEGFR2 participated in the effects
of microRNA-16 overexpression on cell apoptosis of HP75 cells, 5 nM
of vandetanib (VEGFR2 inhibitor) was used in HP75 cells after
microRNA-16 overexpression. VEGFR2 inhibitor significantly
suppressed p-p38MAPK, NF-κB, MMP-9 and VEGFR2 protein expression of
HP75 cells after microRNA-16 overexpression, compared to the
microRNA-16 mimics group (Figs. 5
and 6A-E).
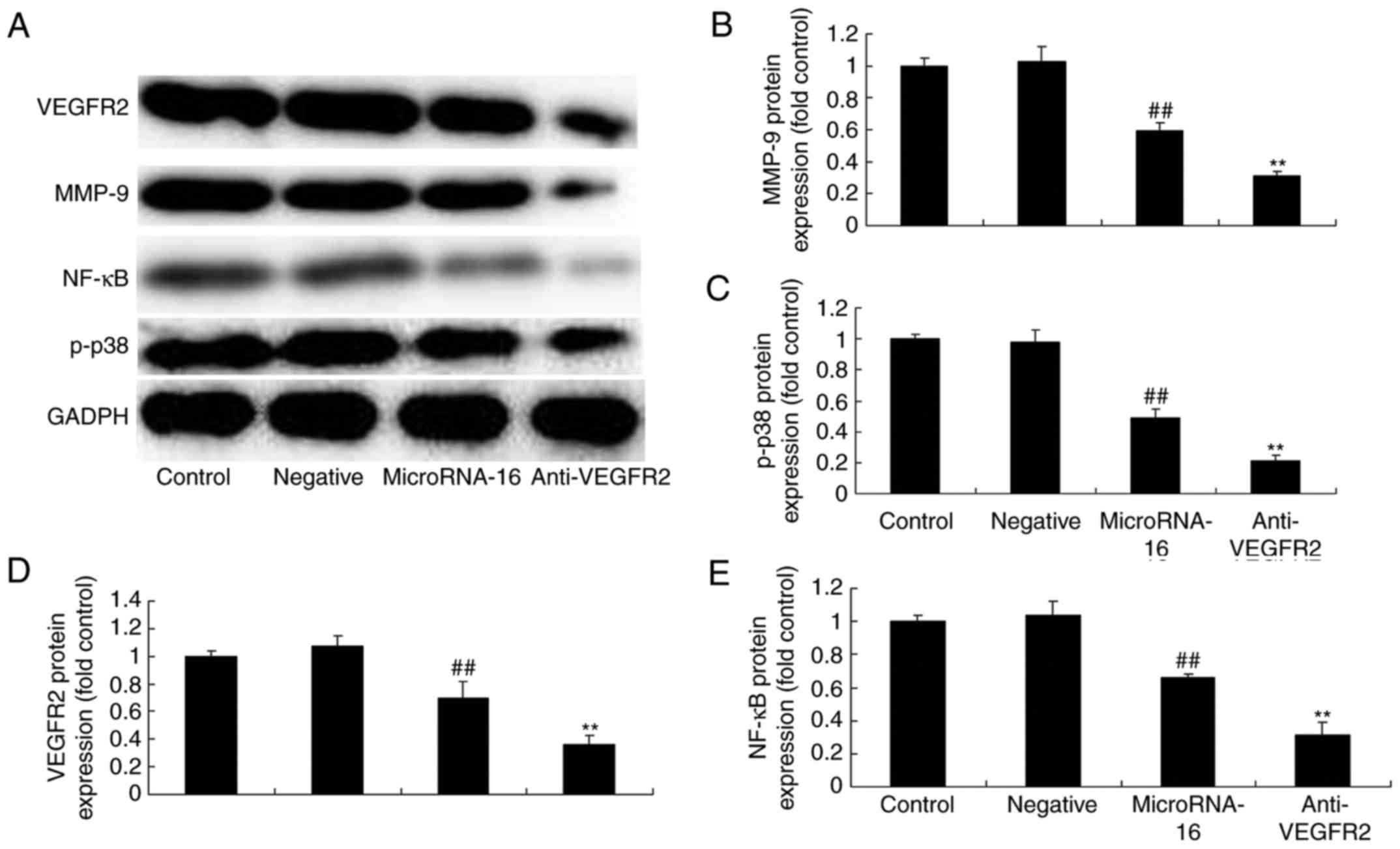 | Figure 6.MicroRNA-16 upregulation affects
p38MAPK/NF-κB/MMP-9/VEGFR2 protein expression, cell proliferation
and apoptosis, caspase-3/8 activities, and p27/Bax protein
expression in HP75 cells. MicroRNA-16 upregulation affects (A)
p-p38MAPK, NF-κB, MMP-9 and VEGFR2 protein expression, determined
using western blot analysis and statistical analysis of (B) MMP-9,
(C) VEGFR2, (D) p-p38MAPK, and (E) NF-κB protein expression, (F)
cell proliferation and (G) apoptosis, (H) caspase-3/8 activities,
(I) p27 and Bax protein expression, determined using western blot
analysis, and statistical analysis of (J) Bax, (K) p27 protein
expression in HP75 cells. Control, control group. Negative,
negative control group. MicroRNA-16, microRNA-16 upregulation
group. Anti-VEGFR2, VEGFR2 inhibitor + microRNA-16 upregulation
group. Repeat times (n=3). ##P<0.01 versus normal
group, **p<0.01 versus microRNA-16 upregulation group. |
Effects of microRNA-16 overexpression
on cell proliferation and apoptosis of HP75 cells following VEGFR2
suppression
Our results indicated that VEGFR2 inhibitor
significantly suppressed the effects of microRNA-16 overexpression
on cell proliferation reduction and apoptosis induction in HP75
cells, compared to the microRNA-16 mimics group (Fig. 6F and G).
Effects of microRNA-16 overexpression
on caspase-3/8 activities in HP75 cells following VEGFR2
suppression
To investigate whether VEGFR2 participated in the
effects of microRNA-16 overexpression on caspase-3/8 activities in
HP75 cells, caspase-3/8 activities were also measured. As shown in
Fig. 6I-K, NF-κB inhibitor
significantly increased the effects of microRNA-16 overexpression
on caspase-3/8 activities in HP75 cell, compared to the microRNA-16
mimics group (Fig. 6H).
Effects of microRNA-16 overexpression
on p27 and Bax protein expression in HP75 cells following VEGFR2
suppression
After VEGFR2 suppression, we also observed p27 and
Bax protein expression in HP75 cells following microRNA-16
overexpression. p27 and Bax protein expression in HP75 cells
following microRNA-16 overexpression was higher than those of
microRNA-16 mimics group (Fig.
6I-K).
Effects of microRNA-16 overexpression
on NF-κB, and MMP-9 protein expression in HP75 cells following
NF-κB suppression
Next, we explored the function of NF-κB and the
effects of microRNA-16 overexpression on NF-κB, and MMP-9 protein
expression in HP75 cells. As shown in Fig. 7A-C, NF-κB inhibitor suppressed NF-κB
and MMP-9 protein expression in the effects of microRNA-16
overexpression.
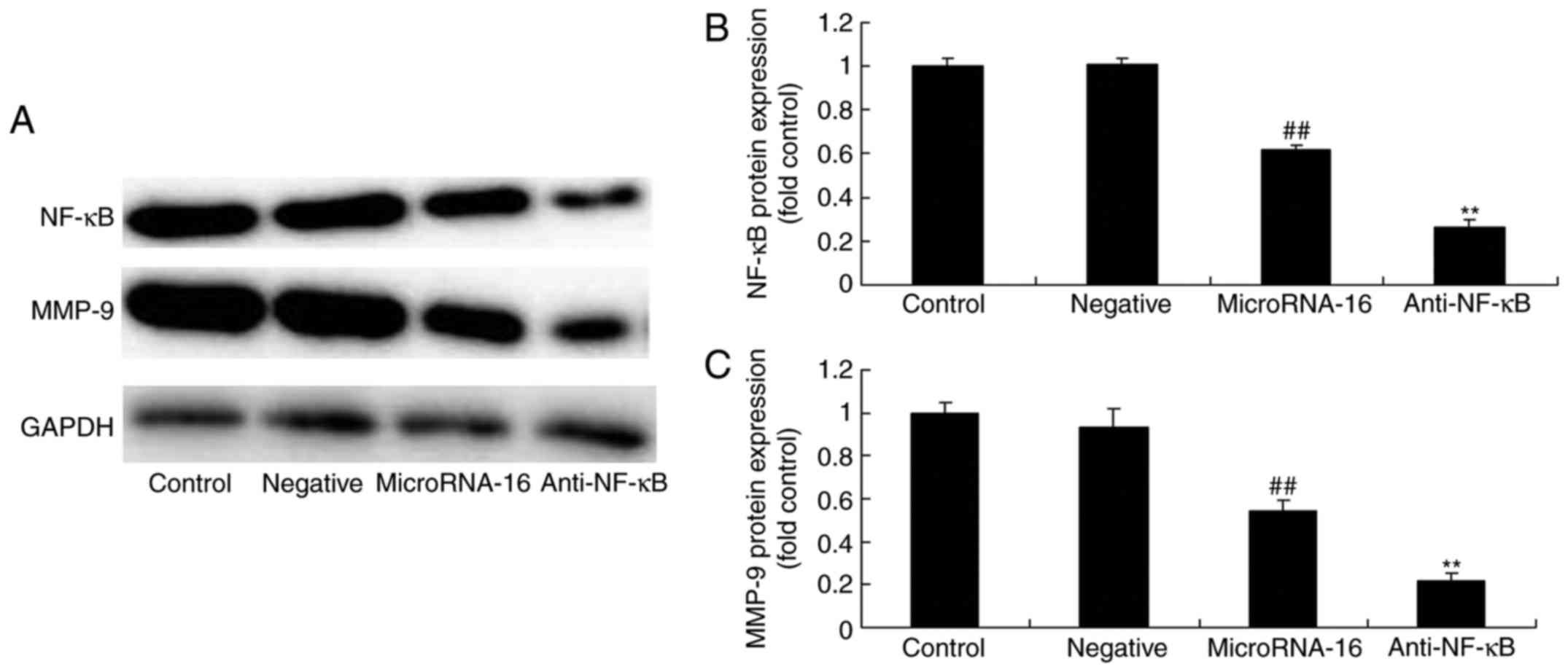 | Figure 7.After NF-κB suppression, the effects
of microRNA-16 overexpression on NF-κB/MMP-9 protein expression,
cell proliferation and apoptosis, caspase-3/8 activities, and p27
and Bax protein expression in HP75 cells were examiend. After NF-κB
suppression, the effects of microRNA-16 overexpression on NF-κB and
MMP-9 protein expression were examined using (A) western blot
analysis and statistical analysis of (B) NF-κB and (C) MMP-9
protein expression, (D) cell proliferation and (E) apoptosis, (F)
caspase-3/8 activities, (G) p27 and Bax protein expression,
determined using western blot analysis and statistical analysis of
(H) Bax, and (I) p27 protein expression in HP75 cells. Control,
control group. Negative, negative control group. MicroRNA-16,
microRNA-16 upregulation group. Anti-NF-κB, NF-κB inhibitor +
microRNA-16 upregulation group. Repeat times (n=3).
##P<0.01 versus normal group; **p<0.01 versus
microRNA-16 upregulation group. |
Effects of microRNA-16 overexpression
on cell proliferation and apoptosis of HP75 cells following NF-κB
suppression
Then, we found that the effects of microRNA-16
overexpression on the inhibition of cell proliferation and the
induction of apoptosis in HP75 cells by NF-κB inhibitor were
effectively accelerated, compared with the microRNA-16 mimics group
(Fig. 7D and E).
Effects of microRNA-16 overexpression
on caspase-3/8 activities in HP75 cells following NF-κB
suppression
However, the caspase-3/8 activities in HP75 cells
following microRNA-16 overexpression in NF-κB inhibitor were higher
than those of the microRNA-16 mimics group (Fig. 7F).
Effects of microRNA-16 overexpression
on p27 and Bax protein expression in HP75 cells following NF-κB
suppression
NF-κB inhibitor facilitated the effects of
microRNA-16 overexpression on p27 and Bax protein expression in
HP75 cells, compared with the microRNA-16 mimics group (Fig. 7G-I).
Discussion
Pituitary tumors constitute a fairly common
intracranial tumor, most of which are benign. However, some
secretory adenomas frequently result in endocrine diseases,
including Cushing syndrome, amenorrhea and gigantism (16). Some large adenomas grow in the
brain, which may compress brain tissues as a result of increased
tumor volume, leading to symptoms such as visual impairment or
headache. In addition, some tumors manifest certain characteristics
of malignancy. For instance, some pituitary tumors grow across the
sella turcica in an infiltration manner and invade the peripheral
cranial structures, which may not be completely removed through
surgery, and are susceptible to postoperative recurrence (17). Therefore, early diagnosis and
clinical treatment of pituitary tumor is essential (3,17). Our
current results show that microRNA-16 expression of pituitary tumor
patients was observably declined, compared with the normal group,
and the high expression of microRNA-16 has longer OS and DFS in
pituitary tumor patients, compared to a low microRNA-16 expression
in pituitary tumor patients.
LIN-4 was identified in caenorhabditis
elegans in 1993, and a large number of similar miRNAs were
found in succession subsequently (18). Research regarding the relationship
of miRNA with pituitary tumor has been carried out for several
years, but relatively few substantial achievements have been
attained. Nonetheless, the results definitely demonstrate that the
pathogenesis of pituitary tumor is closely associated with the
abnormal expression of miRNA (19).
In the present study, the upstream microRNA-16 decreased cell
proliferation and induced apoptosis of HP75 cells in a
dose-dependent manner. MicroRNA-16 has been shown to sensitize
breast cancer (20), ovarian cancer
(21) and hepatocellular carcinoma
(22) cells.
The expression of MMP-9 was significantly higher in
invasive pituitary tumors than in non-invasive ones, and type IV
collagen was markedly reduced in the former (9). Research suggests that in the subclass
of MMPs, MMP-9 has the closest association with pituitary tumor
invasion (9). The expression level
of MMP-9 is notably higher in invasive pituitary tumors than in
non-invasive ones, further suggesting the important role of MMP-9
in tumor invasion and metastasis (33). Therefore, MMP-9 can serve as an
indicator of tumor invasion (33).
Results of the present study indicated microRNA-16 upregulation
significantly suppressed MMP-9 protein expression in HP75 cells.
Lin et al indicated that osthole suppresses the
proliferation of human glioma cells via the upregulation of
microRNA-16 and downregulation of MMP-9 (23).
Tumor growth, invasion and metastasis require
sufficient nutrition support, and the growth of blood vessels plays
an extremely important role during this process (24). Tumor growth depends on angiogenesis,
one of the essential conditions for tumor growth, invasion and
metastasis (24). VEGF is a highly
specific mitogen for vascular endothelial cells, which is a dimer
glycoprotein with a molecular weight of 34–46 kDa (25). VEGF can exert multiple biological
effects by binding with the specific receptor on cell membrane
(26). The high VEGF expression
level in pituitary tumor may be one of the important factors
responsible for its poor prognosis. The invasion of pituitary tumor
is different from that of glioma in terms of biological behavior.
The invasion of invasive pituitary tumor is associated with the
expansive growth of the tumor (27). It has been suggested that VEGF can
upregulate uPA expression, while uPA can activate plasmin and thus
activates MMPs. Additionally, the biological behavior of invasive
pituitary tumor is related to increased MMP-9 expression and the
upregulated expression of angiogenesis regulatory factor VEGF
(9). The new vessels promote tumor
growth and invasion of the surrounding tissues. Our findings have
demonstrated that microRNA-16 upregulation significantly suppressed
VEGFR2 protein expression in HP75 cells. Yang et al, showed
that the downregulation of microRNA-16 significantly impacted the
prognosis of colorectal cancer patients by targeting VEGFR2
(28).
In recent years, great achievements have been
attained in research on factors and drugs promoting tumor cell
apoptosis, and research on p38MAPK has also achieved considerable
progress (29). p38MAPK, which
exists in a majority of cells, is a type of important signal system
for eukaryotic cells to transfer extracellular signals into cells
and thus induce a cell response (30). In addition to enhancing NF-κB
expression, activating c-jun and c-fos and participating in other
signal transduction, it may mainly influence cell metabolism
(31). Furthermore, we found that
microRNA-16 upregulation significantly suppressed p-p38MAPK and
NF-κB protein expression in HP75 cells. Chen et al reported
that microRNA-16 alleviates inflammatory pain through p38 MAPK
activation (32).
It is reported that, lowering the incidence of
inflammation-associated liver cancer and colon cancer in mouse
models results from inhibiting the activation of IKK-β-dependent
NF-κB. As previously indicated, the inflammatory response can
activate NF-κB, and the continuous activation of NF-κB can further
mediate tumorigenesis (33). NF-κB
is associated with tumor invasion and metastasis. Furthermore, it
can induce tumor cell apoptosis by inhibiting NF-κB activity of
tumor cells, and thus inhibits tumor cell growth, as is
demonstrated through experiments in vitro and in vivo
(15). In-depth research has
confirmed that NF-κB is closely related to some malignant solid
tumors, such as pancreatic and breast cancer (15). NF-κB activation affects the invasive
and metastasis capacities of tumors, and can upregulate the
expression of MMP transcription. The effect of MMPs allows for the
degradation of extracellular matrix, and thus promotes tumor
invasion. NF-κB inhibitor Bay 11–7082 can serve as a new research
method to inhibit the NF-κB pathway. Furthermore, it can be used to
inhibit MMP expression to achieve the objective of inhibiting the
invasive and metastatic capacities of tumor (13). In the present study, we found that
VEGFR2 suppression reduced the effects of microRNA-16
overexpression on p-p38, NF-κB, MMP-9 and VEGFR2 protein expression
inhibition in HP75 cells. Yang et al suggested that
microRNA-16 inhibits glioma cell growth and invasion through the
NF-κB/MMP-9 signaling pathway.
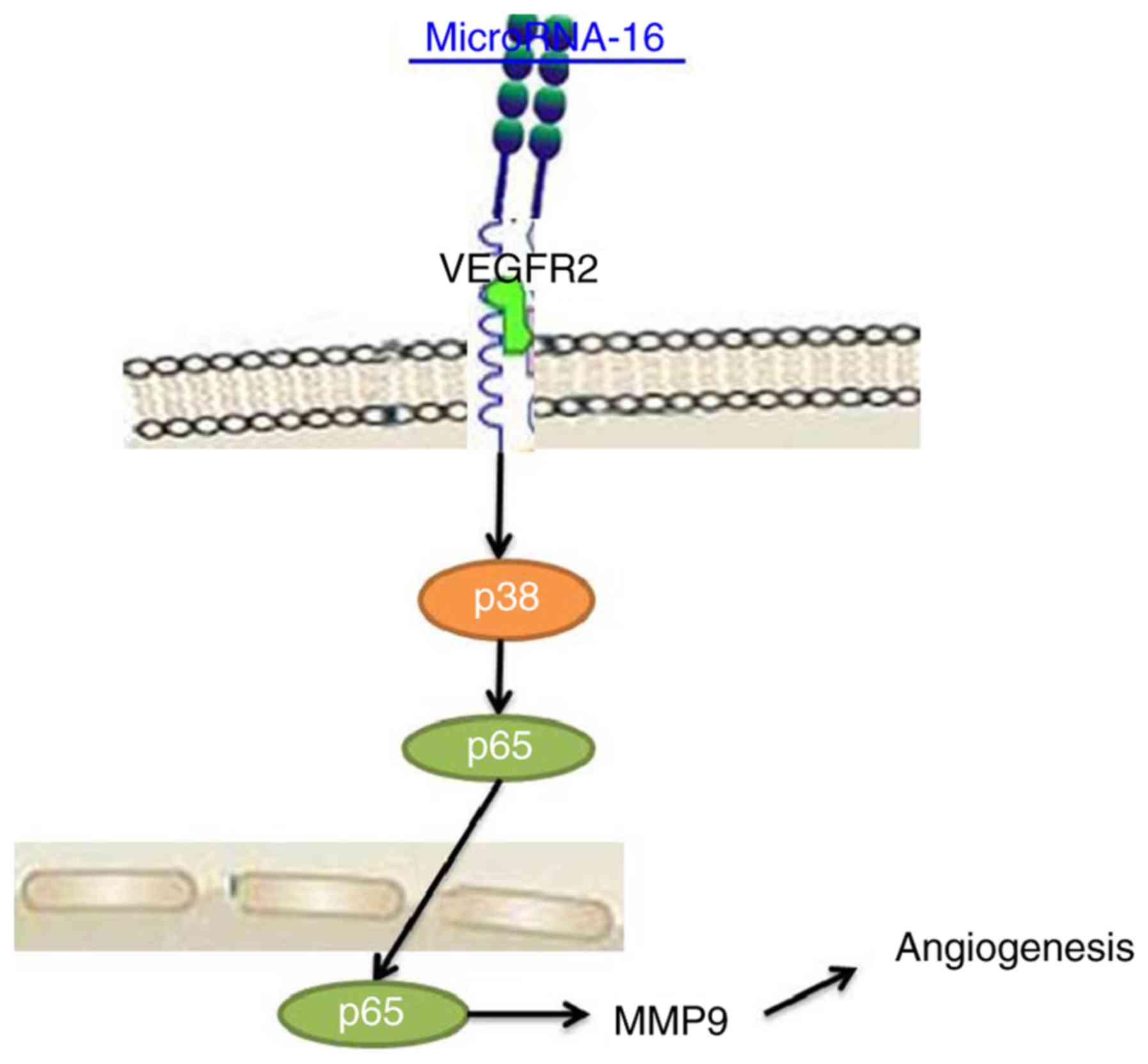
Taken together, we have demonstrated that
microRNA-16 expression suppressed cell proliferation, induced
apoptosis and reduced angiogenesis of pituitary cancer through the
VEGFR2/p38/NF-κB signaling pathway (Fig. 8). Therefore, microRNA-16 may be
necessary for pituitary cancer via the VEGFR2/p38/NF-κB signaling
pathway as a potential therapeutic in clinical application.
References
|
1
|
Gradiser M, Matovinovic Osvatic M, Dilber
D and Bilic-Curcic I: Assessment of environmental and hereditary
influence on development of pituitary tumors using dermatoglyphic
traits and their potential as screening markers. Int J Environ Res
Public Health. 13:3302016. View Article : Google Scholar :
|
|
2
|
Sibal L, Ugwu P, Kendall-Taylor P, Ball
SG, James RA, Pearce SH, Hall K and Quinton R: Medical therapy of
macroprolactinomas in males: I. Prevalence of hypopituitarism at
diagnosis. II. Proportion of cases exhibiting recovery of pituitary
function. Pituitary. 5:243–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baddour HM, Lupa MD and Patel ZM:
Comparing use of the Sonopet(®) ultrasonic bone
aspirator to traditional instrumentation during the endoscopic
transsphenoidal approach in pituitary tumor resection. Int Forum
Allergy Rhinol. 3:588–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robison NJ, Prabhu SP, Sun P, Chi SN,
Kieran MW, Manley PE, Cohen LE, Goumnerova L, Smith ER, Scott RM,
et al: Predictors of neoplastic disease in children with isolated
pituitary stalk thickening. Pediatr Blood Cancer. 60:1630–1635.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Müssnich P, Raverot G, Jaffrain-Rea ML,
Fraggetta F, Wierinckx A, Trouillas J, Fusco A and D'Angelo D:
Down-regulation of miR-410 targeting the cyclin B1 gene plays a
role in pituitary gonadotroph tumors. Cell Cycle. 14:2590–2597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sivapragasam M, Rotondo F, Lloyd RV,
Scheithauer BW, Cusimano M, Syro LV and Kovacs K: MicroRNAs in the
human pituitary. Endocr Pathol. 22:134–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Xie P and Fan H: Genomic profiling
of microRNAs and proteomics reveals an early molecular alteration
associated with tumorigenesis induced by MC-LR in mice. Environ Sci
Technol. 46:34–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knappe UJ, Hagel C, Lisboa BW, Wilczak W,
Lüdecke DK and Saeger W: Expression of serine proteases and
metalloproteinases in human pituitary adenomas and anterior
pituitary lobe tissue. Acta Neuropathol. 106:471–478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HY, Gu WJ, Wang CZ, Ji XJ and Mu YM:
Matrix metalloproteinase-9 and −2 and tissue inhibitor of matrix
metalloproteinase-2 in invasive pituitary adenomas: A systematic
review and meta-analysis of case-control trials. Medicine
(Baltimore). 95:e39042016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hui P, Xu X, Xu L, Hui G, Wu S and Lan Q:
Expression of MMP14 in invasive pituitary adenomas: Relationship to
invasion and angiogenesis. Int J Clin Exp Pathol. 8:3556–3567.
2015.PubMed/NCBI
|
|
11
|
Escós A, Risco A, Alsina-Beauchamp D and
Cuenda A: p38γ and p38δ mitogen activated protein kinases (MAPKs),
new stars in the MAPK galaxy. Front Cell Dev Biol. 4:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang M, Liu J, Ji H, Chen M, Zhao Y, Li
S, Zhang X and Li J: A Aconitum coreanum polysaccharide fraction
induces apoptosis of hepatocellular carcinoma (HCC) cells via
pituitary tumor transforming gene 1 (PTTG1)-mediated suppression of
the P13K/Akt and activation of p38 MAPK signaling pathway and
displays antitumor activity in vivo. Tumour Biol. 36:7085–7091.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grandison L, Nolan GP and Pfaff DW:
Activation of the transcription factor NF-KB in GH3 pituitary
cells. Mol Cell Endocrinol. 106:9–15. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee GS, Choi KC, Han HJ and Jeung EB: The
classical and a non-classical pathways associated with NF-kappaB
are involved in estrogen-mediated regulation of calbindin-D9k gene
in rat pituitary cells. Mol Cell Endocrinol. 277:42–50. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lasa M, Gil-Araujo B, Palafox M and Aranda
A: Thyroid hormone antagonizes tumor necrosis factor-alpha
signaling in pituitary cells through the induction of dual
specificity phosphatase 1. Mol Endocrinol. 24:412–422. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buhk JH, Jung S, Psychogios MN, Göricke S,
Hartz S, Schulz-Heise S, Klingebiel R, Forsting M, Brückmann H,
Dörfler A, et al: Tumor volume of growth hormone-secreting
pituitary adenomas during treatment with pegvisomant: A prospective
multicenter study. J Clin Endocrinol Metab. 95:552–558. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng JM, Gu JW, Kuang YQ, Ma Y, Xia X,
Yang T, Lu M, He WQ, Sun ZY and Zhang YC: Multicenter study on
adult growth hormone level in postoperative pituitary tumor
patients. Cell Biochem Biophys. 71:1239–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XH, Wang EL, Zhou HM, Yoshimoto K and
Qian ZR: MicroRNAs in human pituitary adenomas. Int J Endocrinol.
2014:4351712014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seltzer J, Ashton CE, Scotton TC, Pangal
D, Carmichael JD and Zada G: Gene and protein expression in
pituitary corticotroph adenomas: A systematic review of the
literature. Neurosurg Focus. 38:E172015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang X, Jin L, Cao P, Cao K, Huang C, Luo
Y, Ma J, Shen S, Tan M, Li X, et al: MicroRNA-16 sensitizes breast
cancer cells to paclitaxel through suppression of IKBKB expression.
Oncotarget. 7:23668–23683. 2016.PubMed/NCBI
|
|
21
|
Dwivedi SK, Mustafi SB, Mangala LS, Jiang
D, Pradeep S, Rodriguez-Aguayo C, Ling H, Ivan C, Mukherjee P,
Calin GA, et al: Therapeutic evaluation of microRNA-15a and
microRNA-16 in ovarian cancer. Oncotarget. 7:15093–15104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu WL, Wang WY, Yao WQ and Li GD:
Suppressive effects of microRNA-16 on the proliferation, invasion
and metastasis of hepatocellular carcinoma cells. Int J Mol Med.
36:1713–1719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin K, Gao Z, Shang B, Sui S and Fu Q:
Osthole suppresses the proliferation and accelerates the apoptosis
of human glioma cells via the upregulation of microRNA-16 and
downregulation of MMP-9. Mol Med Rep. 12:4592–4597. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ortiz LD, Syro LV, Scheithauer BW, Ersen
A, Uribe H, Fadul CE, Rotondo F, Horvath E and Kovacs K: Anti-VEGF
therapy in pituitary carcinoma. Pituitary. 15:445–449. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fowkes RC and Vlotides G: Hypoxia-induced
VEGF production ‘RSUMEs’ in pituitary adenomas. Endocr Relat
Cancer. 19:C1–C5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stepień T, Sacewicz M, Lawnicka H,
Krupiński R, Komorowski J, Siejka A and Stepień H: Stimulatory
effect of growth hormone-releasing hormone (GHRH(1–29)NH2) on the
proliferation, VEGF and chromogranin A secretion by human
neuroendocrine tumor cell line NCI-H727 in vitro. Neuropeptides.
43:397–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sacewicz M, Lawnicka H, Siejka A, Stepień
T, Krupiński R, Komorowski J and Stepień H: Inhibition of
proliferation, VEGF secretion of human neuroendocrine tumor cell
line NCI-H727 by an antagonist of growth hormone-releasing hormone
(GH-RH) in vitro. Cancer Lett. 268:120–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang IP, Tsai HL, Huang CW, Lu CY, Miao
ZF, Chang SF, Juo SH and Wang JY: High blood sugar levels
significantly impact the prognosis of colorectal cancer patients
through down-regulation of microRNA-16 by targeting Myb and VEGFR2.
Oncotarget. 7:18837–18850. 2016.PubMed/NCBI
|
|
29
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma
cells via ROS/MAPKs-mediated pathway. J Neurooncol. 116:221–230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peverelli E, Olgiati L, Locatelli M, Magni
P, Fustini MF, Frank G, Mantovani G, Beck-Peccoz P, Spada A and
Lania A: The dopamine-somatostatin chimeric compound BIM-23A760
exerts antiproliferative and cytotoxic effects in human
non-functioning pituitary tumors by activating ERK1/2 and p38
pathways. Cancer Lett. 288:170–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen W, Guo S and Wang S: MicroRNA-16
Alleviates inflammatory pain by targeting Ras-related protein 23
(RAB23) and inhibiting p38 MAPK activation. Med Sci Monit.
22:3894–3901. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Li Z, Chang Y, Ma L, Xu W, Li M,
Li J, Zhang W, Sun Q, An X, et al: Relationship between NF-κB,
MMP-9, and MICA expression in pituitary adenomas reveals a new
mechanism of pituitary adenomas immune escape. Neurosci Lett.
597:77–83. 2015. View Article : Google Scholar : PubMed/NCBI
|