Introduction
Gastric cancer (GC) is the fourth most common cancer
and advanced GC has a poor prognosis and high mortality rate
worldwide (1,2). GC progresses through a multistep
process, whereby cells acquire a series of genetic and epigenetic
alterations in key growth regulatory genes that endow them with
proliferative and survival advantages (3,4). Early
stage GC patients usually do not present with specific symptoms.
Thus, most GC patients are diagnosed at an advanced stage with
lymph node or organ metastasis, resulting in a low 5-year survival
rate, ranging from 20 to 30% (5,6).
Conventional treatments for GC include surgery, radiotherapy and
chemotherapy. However, there is a need for more efficacious
therapies and treatment strategies.
Heterogeneous nuclear ribonucleoproteins (hnRNPs)
are a large family of RNA-binding nuclear proteins in mammalian
cells. There are more than 20 members in hnRNPs (7–9), all
of which are associated with precursor mRNAs and many of which
influence pre-mRNA processing and other functions of mRNA. The
best-characterized protein in this family is hnRNPA1 (10,11),
which is aberrantly expressed in various cancers (12,13).
Liu et al (14) found that
hnRNPA1 protein was overexpressed in lung cancer (LC), whereas
knockdown of hnRNPA1 inhibited cell viability and colony formation
of LC cells and arrested the cell cycle in the G0/G1 phase.
Furthermore, another study revealed the upregulated expression and
aberrant cytoplasmic localization of hnRNPA1 in cervical squamous
cell cancer (15). Recently,
hnRNPA1 has emerged as a plausible GC biomarker (16). However, the role and molecular
mechanism of hnRNPA1 in GC invasion and migration are not well
defined.
Based on these previous studies, we hypothesized
that hnRNPA1 is important in the development of GC. Therefore,
bioinformatics combined with in vivo and in vitro
experiments were performed to characterize the effect of hnRNPA1 on
the invasive biological behavior of GC.
Materials and methods
Cell lines and tissue specimens
Mouse anti-hnRNPA1 (4B10; cat. no. sc-32301) and
rabbit anti-E-cadherin (H-108; cat. no. sc-7870) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit
anti-vimentin (Ag0489; cat. no. 65039-1-Ig) and mouse anti-human
GAPDH (cat. no. HRP-60004) were purchased from ProteinTech Group,
Inc. (Wuhan, China). Rabbit anti-Snail (cat. no. ab110490) was
purchased from Abcam (Cambridge, UK). Human gastric carcinoma cell
lines AGS (American Type Culture Collection, Rockville, MD, USA)
and BGC-823 cell lines (Beijing Institute of Cancer Research,
Beijing, China) were maintained in Dulbecco's modified Eagle's
medium (DMEM) basic media containing 10% fetal calf serum (FCS;
Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C and 5%
CO2. Seven pairs of GC tissues and normal gastric
tissues were collected. In radical resection of GC, the surgeon
usually resects at least 5 cm away from the tumor as the cut edge
to ensure that the boundary is pathologically negative.
Subsequently, the tissue is stained with hematoxylin and eosin
(H&E) and the pathologist judges whether the cut edge has been
invaded by tumor cells. The tissue we used to perform western
blotting was chosen by this criterion and it was clinically
pathologically confirmed. All tissue slides used to perform H&E
staining and IHC analysis were examined independently by two
experienced pathologists. All patients signed informed consents for
the use of their tissues and the present study was approved by the
Institutional Review Board of the Gannan Medical University.
RNA isolation and quantitative
real-time PCR
The cells were harvested and total RNA was extracted
using TRIzol reagent (Gibco-BRL; Thermo Fischer Scientific,
Gaithersburg, MD, USA) as previously described (17,18).
The primer sequences in RT-PCR were as follows: hnRNPA1 forward,
5′-TGCCCAGAAAATGAAAAAGG-3′ and reverse, 5′-GTGTATGTGGCAATGCGTTC-3′
(201 bp); GAPDH forward, 5′-GTCAACGGATTTGGTCGTATTG-3′ and reverse,
5′-CTCCTGGAAGATGGTGATGGG-3′ (216 bp); c-jun forward,
5′-CCCCAAGATCCTGAAACAGA-3′ and reverse, 5′-CCGTTGCTGGACTGGATTAT-3′
(168 bp); Survivin forward, 5′-TGTCTTGAAAGTGGCACCAG-3′ and reverse,
5′-GCCTTCTTCCCCCTCACTT-3′ (154 bp); SNAI1 forward,
5′-TTTACCTTCCAGCAGCCCTA-3′ and reverse, 5′-CCCACTGTCCTCATCTGACA-3′
(207 bp); Cyclin D1 forward, 5′-CGTGGCCTCTAAGATGAAGG-3′ and
reverse, 5′-CTGGCATTTTGGAGAGGAAG-3′ (185 bp); ZEB1 forward,
5′-CGCTTTACCTCTCTGAAAGAACA-3′ and reverse,
5′-TTACACCCAGACTGCGTCAC-3′ (170 bp).
Transient siRNA transfection
Knockdown of hnRNPA1 expression was performed by
transfecting cells with small interfering RNA (siRNA) duplexes
(sense strand: 632-GCCACAACTGTGAAGTTAGAA-653, synthesized by
GenePharma Co., Shanghai, China) using Lipofectamine 2000
(Invitrogen; Thermo Fischer Scientific). Scrambled RNA (scr-siRNA)
was used as a negative control. Subsequently, 48 h
post-transfection, western blot analysis was performed.
Constructs and generation of stable
transfectants
cDNA corresponding to full-length hnRNPA1 was
obtained by RT-PCR amplification of normal human testis cDNA with
primers specific to hnRNPA1. PCR aliquots were subcloned into the
pcDNA3.1 mammalian expression vector (Invitrogen; Thermo Fischer
Scientific). pcDNA 3.1 or pcDNA3.1-hnRNPA1 were transfected into
BCG823 and AGS cells. Transfects were cultured by RPMI-1640 medium
supplemented with Geneticin (G418; Calbiochem; Merck KGaA,
Darmstadt, Germany) to generate stable cell lines.
Western blot analysis and
immunofluorescence
Whole cell lysates were prepared as previously
described (19,20). For western blot analysis, 30 µg
whole protein lysates were used to detect the indicated protein.
For the immunofluorescence assay, the cells on a cover glass were
fixed and incubated with primary antibodies followed by Texas Red
(cat. no. SAB3700022; Sigma-Aldrich, Darmstadt, Germany) or
fluorescein isothiocyanate-conjugated secondary antibodies (cat.
no. PA1-85440; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Nuclei were stained with Hoechst 33258 nuclear staining dye.
Coverslips were washed, mounted and then analyzed using a
fluorescence microscope.
Anchorage-independent cell growth
assay
Scr-siRNA and hnRNPA1-siRNA cells (5×103)
were seeded into medium with 0.35% agar, plated in triplicate on
plates containing 0.7% agar base. Colonies were stained with
Coomassie Blue (cat. no. sc-24972; Santa Cruz Biotechnology, Inc.).
Colonies containing at least 50 cells were counted using Photoshop
software.
EdU incorporation assay
Each group of isolated tumor cells was seeded onto
96-well plates in triplicate and incubated for 48 h in normal
medium, and then for an additional 2 h in medium containing 50 µM
5-ethynyl-2′-deoxyuridine (EdU; Guangzhou RiboBio, Co., Ltd.,
Guangzhou, China). After being washed, fixed and permeabilized, the
cells were incubated with 1X Apollo reaction cocktail (Guangzhou
RiboBio Co., Ltd.) for 30 min. DNA was incubated with Hoechst 33342
nuclear staining dye for 30 min and visualized with an inverted
fluorescence microscope (Leica DM5500; Leica, Stuttgart, Germany).
Five random fields were imaged at a ×100 magnification for each EdU
experiment. Captured images were processed and analyzed with ImageJ
software (National Institutes of Health, Bethesda, MD, USA). The
number of EdU-positive cells was identified by Hoechst 33342 nuclei
staining and expressed as a percentage of the total number of cells
in each field.
Cell migration and invasion
assays
Cell migration was assessed by wound healing assay
as previously described (21).
Briefly, indicated cells with 24-h culture were wounded with a
pipette tip. The media was changed to remove cell debris and images
were captured after 60 h. The cell invasion was assessed using the
Matrigel Invasion Chamber (BD Biosciences, San Jose, CA, USA)
according to the manufacturer's instructions. Twenty-four hour
post-siRNA transfection, the cells were re-suspended in serum-free
media and placed on each Transwell membrane filter insert, with the
lower chamber filled with complete medium. After 24-h incubation,
invaded cells were stained with crystal violet and counted under a
microscope.
Construction of lentiviral vectors
with hnRNPA1 short hairpin RNA
An hnRNPA1 RNAi lentiviral vector
(pGCSIL-hnRNPA1-shRNA) was constructed (Shanghai GeneChem Co.,
Ltd., Shanghai, China) to investigate the effect of knockdown of
hnRNPA1 expression on the metastasis of GC in vivo.
Double-stranded oligonucleotides encoding human hnRNPA1-vshRNA
(NM_002136,
5′-GCCACAACTGTGAAGTTAGAACTCGAGTTCTAACTTCACAGTTGTGGCTTTTT-3′) were
annealed and inserted into the pGCSIL-GFP short hairpin RNA (shRNA)
expression vector. A GFP-lentiviral vector (pGCSIL-GFP) was used as
the negative control.
In vivo metastasis assays
Four- to 6-week-old BALB/c-nu/nu nude mice were
obtained from the Laboratory Animal Unit, Southern Medical
University (Baiyun, China). Four hundred thousand cells from each
group (pcDNA3.1 shRNA and pcDNA3.1-hnRNPA1 shRNA lentiviral) were
injected into mice through the tail vein (n=3 for each group).
Following 35 days, the mice were sacrificed, the lungs were
harvested and images were captured. Tissue sections were attained
by traditional methods and H&E, IHC and qPCR assays were
performed. All experiments performed followed the internationally
recognized guidelines, as well as the local and national
regulations for animal experiments. Institutional Review Board of
the Gannan Medical University approved the experiments.
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent experiments, using SPSS
16.0 version (SPSS, Inc., Chicago, IL, USA). Groups with different
treatments were compared using the two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
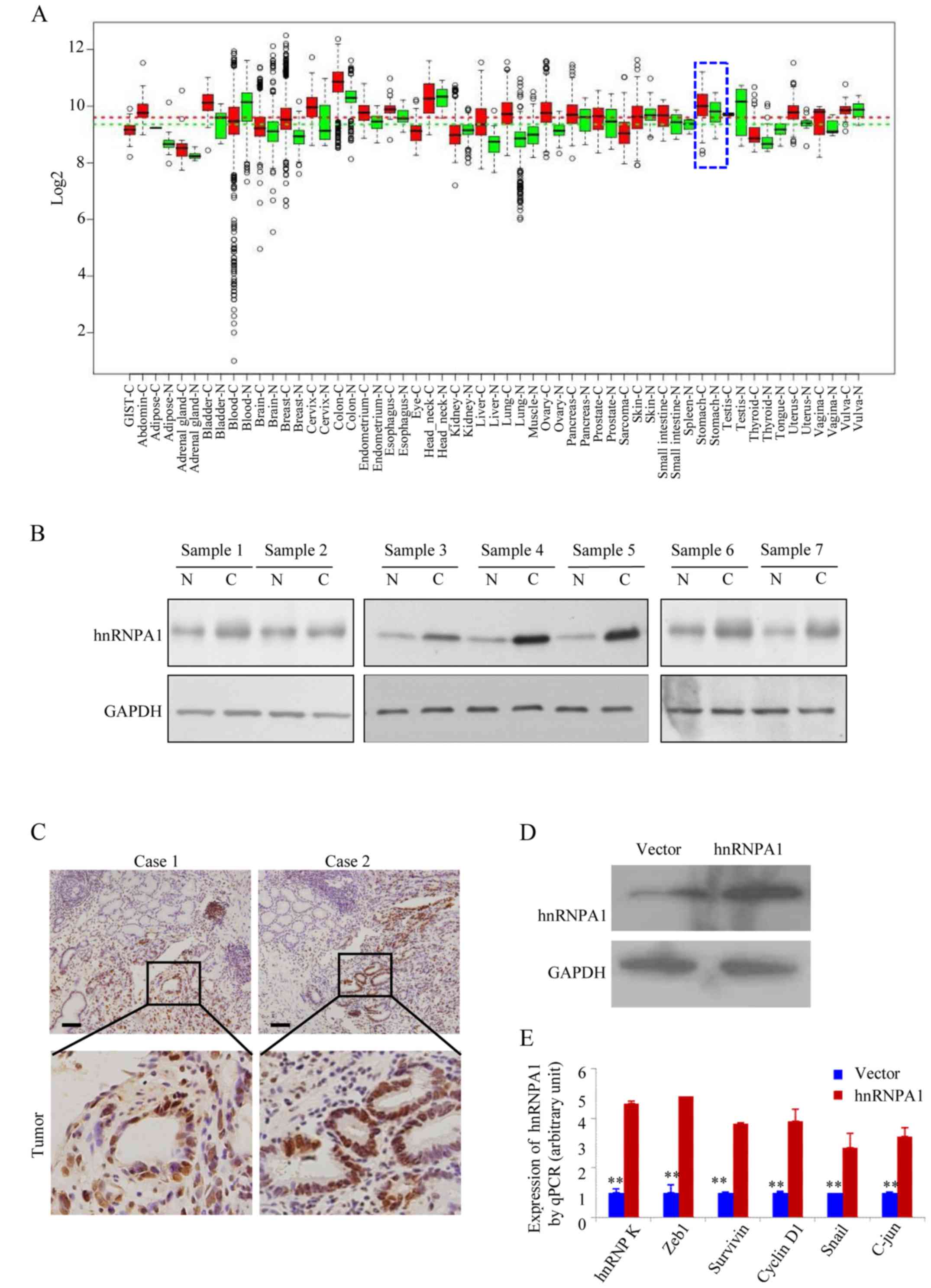
HnRNPA1 expression is higher in human
GC tissues
In the GENT database (available at http://medicalgenome.kribb.re.kr/GENT/
or http://genome.kobic.re.kr/GENT/),
hnRNPA1 is upregulated in adrenal gland, bladder, breast, cervix
uteri, colon, endometrium, esophagus, liver, lung, ovary, pancreas,
prostate, small intestine, thyroid, uterus and stomach cancer
compared with corresponding normal tissues (Fig. 1A). This finding indicated that
hnRNPA1 may be associated with various types of cancer including
gastric cancer. We examined the expression of hnRNPA1 in seven
pairs of human GC tissues and matched non-cancerous colonic mucosa
by western blotting. As displayed in Fig. 1B, all of the cancer tissues
exhibited a higher expression level of hnRNPA1 in relation to their
corresponding non-cancerous controls (Fig. 1B). Higher expression levels of
hnRNPA1 protein in GC tissues from two patients were also confirmed
by IHC (Fig. 1C). To assess the
effect of constitutive hnRNPA1 expression on the malignant behavior
of GC cells in vitro, we established stable transfectants
with hnRNPA1-sense and vector plasmids (Fig. 1D) and confirmed the induced
expression of five major oncogenes involved in the proliferation
and transformation (Fig. 1E). The
mRNA expression of endogenous ZEB1, survivin, cyclin D1, Snail and
c-jun genes was upregulated in the stable hnRNPA1 transfectants of
AGS cells. These results indicated that hnRNPA1 is overexpressed
and is involved in GC tumorigenesis.
HnRNPA1 enhances the malignant
biological behavior of GC cells
The soft agar assay was performed as
anchorage-independent growth factor to evaluate hnRNPA1 function in
malignant transformation (22). The
siRNA-knockdown efficiency was confirmed by western blotting
(Fig. 2A). As expected, hnRNPA1
downregualtion significantly inhibited the colony forming capacity
of both BCG823 and AGS GC cells (Fig.
2B and C). The EdU incorporation assays revealed that hnRNPA1
supression was significantly lower than that of AGS cells
containing scr-siRNA (Fig. 2D). In
order to elevate hnRNPA1 downregulation on the metastatic potential
of GC cells, wound healing assay was performed. Compared with the
cells transfected with scr-siRNA, those transfected with
hnRNPA1-siRNA exhibited significantly decreased migration (Fig. 2E). To examine cell invasion in
vitro, we used matrix-coated cell culture inserts. After the
downregulation of hnRNPA1, the invasiveness of BCG823 and
AGS cells decreased by ~2.5- and ~6-fold, respectively, compared
with the control cells (Fig. 2F).
These data indicated that hnRNPA1 downregulation inhibited the
malignant biological behavior of GC cells.
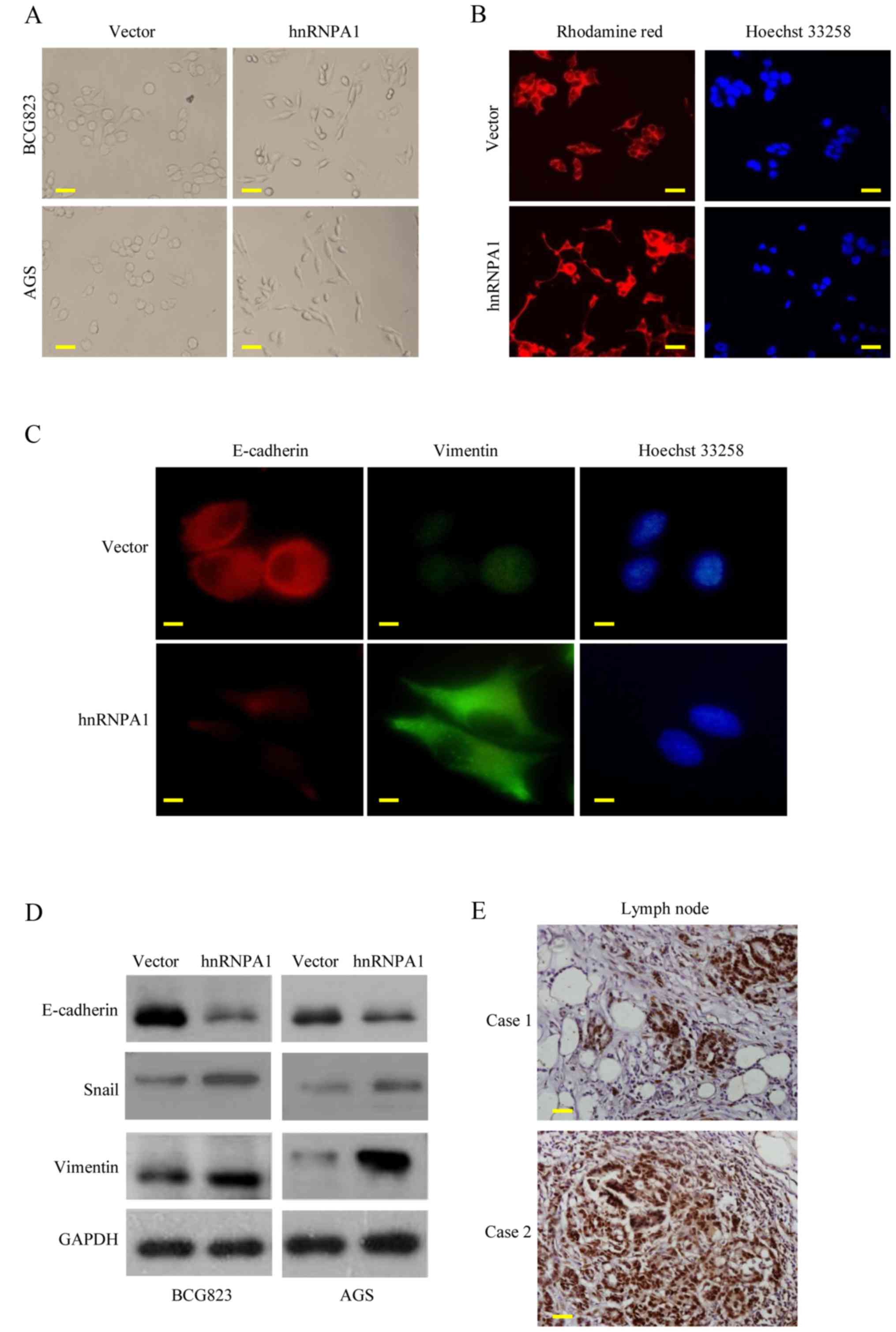
HnRNPA1 upregulation promotes
metastasis through EMT in GC
EMT is the key process driving cancer metastasis
(23,24). To evaluate the role of hnRNPA1 in
EMT, we examined the morphologic features of GC cells. HnRNPA1
overexpression induced the loss of cell-cell contact and a
cobblestone-like phenotype. The cells became elongated,
spindle-shaped and scattered. In contrast, empty vector
transfectants had a round or flat morphology with a short
cytoplasmic process (Fig. 3A).
Furthermore, compared with vector-expressing cells, F-actin
staining of the hnRNPA1-overexpressing cells was observed
throughout the cytoplasm and at the rim zone of the protrusion.
Concurrently, filopodia and lamellipodia were present on the
hnRNPA1-overexpressing cell membrane surfaces and were required for
actin polymerization and involved in the invasion and metastasis of
cancer cells (Fig. 3B).
Concurrently, the expression of EMT markers was assessed.
E-cadherin expression was decreased, while vimentin expression was
increased in the hnRNPA1-overexpressing cells, as determined by
immunofluorescent analysis (Fig.
3C). HnRNPA1 overexpression was also confirmed by western blot
analysis. EMT induction was demonstrated by a shift from the
expression of epithelial markers (E-cadherin) to mesenchymal
markers (vimentin and Snail) (Fig.
3D). To further confirm these findings in vivo, we
detected the hnRNPA1 expression of regional lymph nodes in
metastatic cancer tissues from two patients. High levels of hnRNPA1
in the nucleus of the cancer cells were investigated (Fig. 3E). These findings demonstrated that
hnRNPA1 induced EMT and facilitated GC cell invasion.
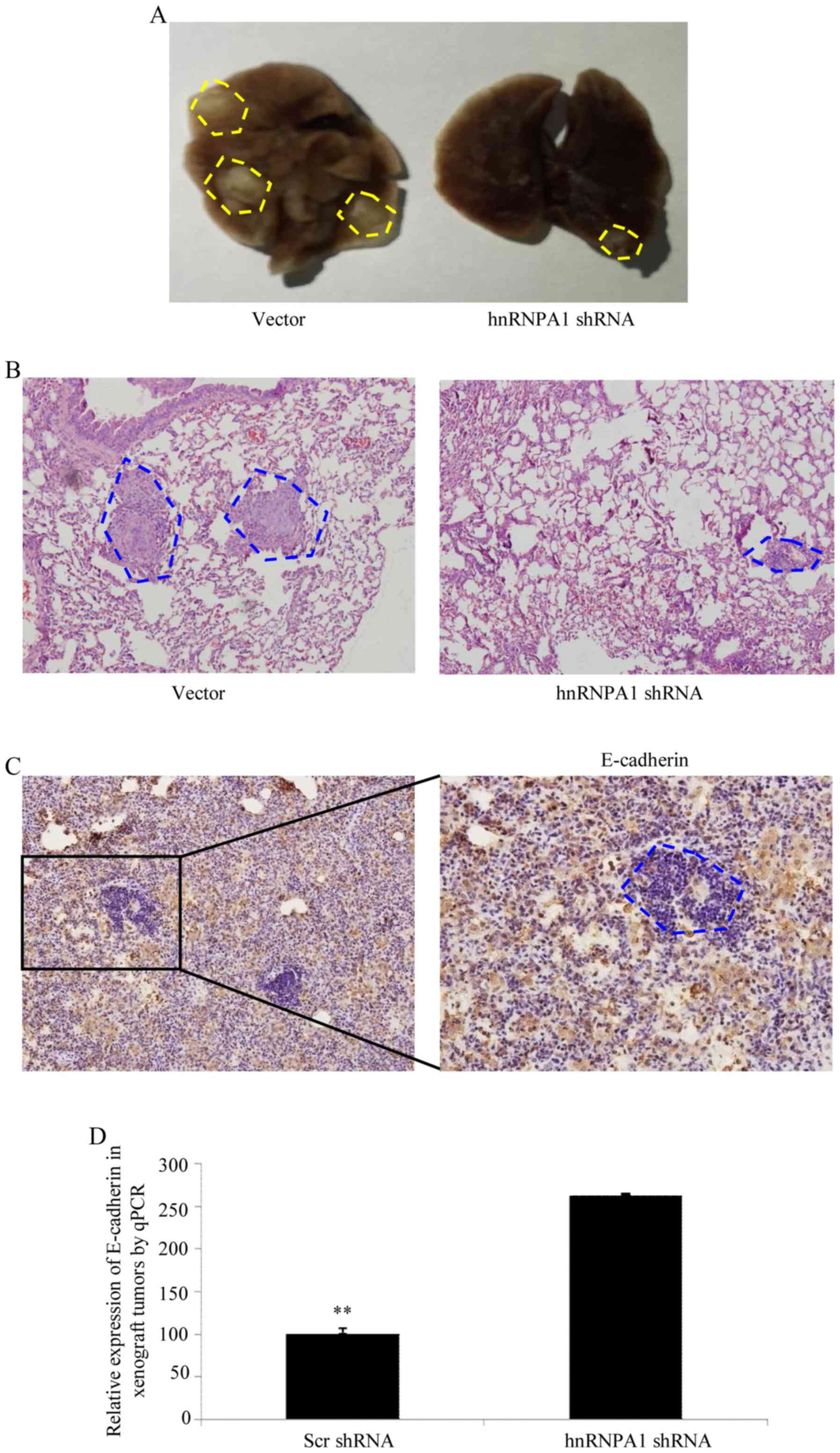
HnRNPA1 induces EMT and metastasis in
GC in vivo
To verify the aforementioned results in vivo,
shRNA-mediated silencing of hnRNPA1 was performed in BCG823 cells
and an orthotopic lung cancer model was established in mice by tail
vein injections (Fig. 4A). Compared
with scr-shRNA transfection in BCG823 cells, cells transfected with
hnRNPA1-shRNA exhibited a significant decrease in visible lung
tumors, which correlated with a higher number of metastatic loci
(Fig. 4B). The expression of
E-cadherin in tumors was determined by IHC (Fig. 4C). Furthermore, orthotopic xenograft
tumors with depletion of the hnRNPA1 expression exhibited increased
E-cadherin levers compared with scr-shRNA at the mRNA level
(Fig. 4D). Collectively, these
results demonstrated that hnRNPA1 is important in EMT and
metastasis progression in GC.
Discussion
The present study characterized the role of hnRNPA1
in GC cell growth and invasion both in vivo and in
vitro. Elevated levels of hnRNPA1 protein were detected in most
GC tissues compared to normal tissues. Silencing of hnRNPA1
resulted in reduced cell growth, invasion, migration and reversal
of EMT in GC cells. Collectively, these findings indicated that
hnRNPA1 plays a pivotal role in GC invasion and metastasis.
HnRNPA1 is known to be involved in cancer
progression and metastasis (25,26).
However, few studies have focused on its role in GC. In the present
study, we found that GC tissues had higher levels of hnRNPA1
compared with normal tissues and hnRNPA1 knockdown significantly
inhibited anchorage-dependent growth in GC cells. These data
indicated that hnRNPA1 was important to cell growth and progression
of GC.
EMT is a crucial phenotypic conversion during cancer
progression (27,28) and is a process by which cancer cells
lose their polarized epithelial structures and acquire plastic and
high motile mesenchymal properties (29,30).
Clinicopathological studies revealed that EMT strongly correlated
with poor histological differentiation, destruction of tissue
integrity and metastasis (31,32).
Therefore, EMT is considered to be an exclusive phenotypic switch
for the invasion and metastasis of cancers including GC (33). EMT-gene signature included a
decrease of epithelial molecules such as E-cadherin, an increase of
mesenchymal molecules such as vimentin and an alteration of the
localization and/or function of some transcription factors such as
ZEB1 (33) and Snail (34). A previous study (27) demonstrated that hnRNPA1 promoted
breast cancer progression through the induction of EMT and provided
potential targets to develop anticancer therapies. In the present
study, we revealed that GC cells with high hnRNPA1 expression
exhibited amoeboid morphology and shifted changes of the EMT
markers, indicating that hnRNPA1 may be a potent inducer of EMT and
promote the invasion and metastasis in GC. Furthermore, recent
studies have hypothesized that mesenchymal-epithelial transition
(MET) was essential for the successful seeding and outgrowth of
distant metastasis. Therefore, we investigated the expression of
EMT markers in metastatic lung nodules from xenograft tumor models
by IHC and found that MET was essential for hnRNPA1-induced lung
metastasis as expected.
In conclusion, our results revealed a novel and
unexpected regulatory function of hnRNPA1. HnRNPA1 may be a
mediator or trigger of EMT and may cause dissemination and
metastatic phenotypes in GC. Thus, the data of the present study
provided evidence that hnRNPA1 played a vital role in GC growth and
progression, and as such, may serve as a therapeutic target for
GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the NSFC of
Jiangxi Province.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
YC, JL, WW and LX performed the experiments, JW, SL
and ZG designed the study, HZ and ZG prepared and wrote the study.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houghton J, Fox JG and Wang TC: Gastric
cancer: Laboratory bench to clinic. J Gastroenterol Hepatol.
17:495–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strickler JG, Zheng J, Shu Q, Burgart LJ,
Alberts SR and Shibata D: p53 mutations and microsatellite
instability in sporadic gastric cancer: When guardians fail. Cancer
Res. 54:4750–4755. 1994.PubMed/NCBI
|
|
4
|
Zheng L, Wang L, Ajani J and Xie K:
Molecular basis of gastric cancer development and progression.
Gastric Cancer. 7:61–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasako M, Sano T, Yamamoto S, Kurokawa Y,
Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai
K, et al: D2 lymphadenectomy alone or with para-aortic nodal
dissection for gastric cancer. N Engl J Med. 359:453–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HH, Chang JG, Lu RM, Peng TY and Tarn
WY: The RNA binding protein hnRNP Q modulates the utilization of
exon 7 in the survival motor neuron 2 (SMN2) gene. Mol Cell Biol.
28:6929–6938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostareck-Lederer A, Ostareck DH, Cans C,
Neubauer G, Bomsztyk K, Superti-Furga G and Hentze MW:
c-Src-mediated phosphorylation of hnRNP K drives translational
activation of specifically silenced mRNAs. Mol Cell Biol.
22:4535–4543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM,
Shi YH, Zhou J and Fan J: Overexpression of HnRNP A1 promotes tumor
invasion through regulating CD44v6 and indicates poor prognosis for
hepatocellular carcinoma. Int J Cancer. 132:1080–1089. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sueoka E, Goto Y, Sueoka N, Kai Y, Kozu T
and Fujiki H: Heterogeneous nuclear ribonucleoprotein B1 as a new
marker of early detection for human lung cancers. Cancer Res.
59:1404–1407. 1999.PubMed/NCBI
|
|
11
|
Loh TJ, Moon H, Cho S, Jang H, Liu YC, Tai
H, Jung DW, Williams DR, Kim HR, Shin MG, et al: CD44 alternative
splicing and hnRNP A1 expression are associated with the metastasis
of breast cancer. Oncol Rep. 34:1231–1238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu C, Guo J, Liu Y, Jia J, Jia R and Fan
M: Oral squamous cancer cell exploits hnRNP A1 to regulate cell
cycle and proliferation. J Cell Physiol. 230:2252–2261. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torosyan Y, Dobi A, Glasman M, Mezhevaya
K, Naga S, Huang W, Paweletz C, Leighton X, Pollard HB and
Srivastava M: Role of multi-hnRNP nuclear complex in regulation of
tumor suppressor ANXA7 in prostate cancer cells. Oncogene.
29:2457–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Zhou Y, Lou Y and Zhong H:
Knockdown of HNRNPA1 inhibits lung adenocarcinoma cell
proliferation through cell cycle arrest at G0/G1 phase. Gene.
576:791–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qing S, Tulake W, Ru M, Li X, Yuemaier R,
Lidifu D, Rouzibilali A, Hasimu A, Yang Y, Rouziahong R, et al:
Proteomic identification of potential biomarkers for cervical
squamous cell carcinoma and human papillomavirus infection. Tumour
Biol. 39:10104283176975472017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hermann R, Hensel F, Müller EC, Keppler M,
Souto-Carneiro M, Brändlein S, Müller-Hermelink HK and Vollmers HP:
Deactivation of regulatory proteins hnRNP A1 and A2 during SC-1
induced apoptosis. Hum Antibodies. 10:83–90. 2001.PubMed/NCBI
|
|
17
|
Guo Z, Zhang W, Xia G, Niu L, Zhang Y,
Wang X, Zhang Y, Jiang B and Wang J: Sp1 upregulates the four and
half lim 2 (FHL2) expression in gastrointestinal cancers through
transcription regulation. Mol Carcinog. 49:826–836. 2010.PubMed/NCBI
|
|
18
|
Wu Y, Guo Z, Zhang D, Zhang W, Yan Q, Shi
X, Zhang M, Zhao Y, Zhang Y, Jiang B, et al: A novel colon cancer
gene therapy using rAAV-mediated expression of human shRNA-FHL2.
Int J Oncol. 43:1618–1626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Guo Z, Jiang B, Niu L, Xia G,
Wang X, Cheng T, Zhang Y and Wang J: Identification of a functional
p53 responsive element within the promoter of XAF1 gene in
gastrointestinal cancer cells. Int J Oncol. 36:1031–1037.
2010.PubMed/NCBI
|
|
20
|
Guo Z, Zhou Y, Evers BM and Wang Q: Rictor
regulates FBXW7-dependent c-Myc and cyclin E degradation in
colorectal cancer cells. Biochem Biophys Res Commun. 418:426–432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Jiang B, Guo Z, Sardet C, Zou B,
Lam CS, Li J, He M, Lan HY, Pang R, et al: Four-and-a-half LIM
protein 2 promotes invasive potential and epithelial-mesenchymal
transition in colon cancer. Carcinogenesis. 31:1220–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin KH, Kang MK, Kim RH, Christensen R
and Park NH: Heterogeneous nuclear ribonucleoprotein G shows tumor
suppressive effect against oral squamous cell carcinoma cells. Clin
Cancer Res. 12:3222–3228. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gumireddy K, Li A, Gimotty PA,
Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L and Huang
Q: KLF17 is a negative regulator of epithelial-mesenchymal
transition and metastasis in breast cancer. Nat Cell Biol.
11:1297–1304. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang
SY, Liu CJ, Teng SC and Wu KJ: Direct regulation of TWIST by
HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park WC, Kim HR, Kang DB, Ryu JS, Choi KH,
Lee GO, Yun KJ, Kim KY, Park R, Yoon KH, et al: Comparative
expression patterns and diagnostic efficacies of SR splicing
factors and HNRNPA1 in gastric and colorectal cancer. BMC Cancer.
16:3582016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou B, Wang Y, Jiang J, Jiang H, Song J,
Han T, Shi J and Qiao H: The long noncoding RNA colon
cancer-associated transcript-1/miR-490 axis regulates gastric
cancer cell migration by targeting hnRNPA1. IUBMB Life. 68:201–210.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonomi S, di Matteo A, Buratti E, Cabianca
DS, Baralle FE, Ghigna C and Biamonti G: HnRNP A1 controls a
splicing regulatory circuit promoting mesenchymal-to-epithelial
transition. Nucleic Acids Res. 41:8665–8679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tauler J, Zudaire E, Liu H, Shih J and
Mulshine JL: hnRNP A2/B1 modulates epithelial-mesenchymal
transition in lung cancer cell lines. Cancer Res. 70:7137–7147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaudhury A, Hussey GS, Ray PS, Jin G, Fox
PL and Howe PH: TGF-beta-mediated phosphorylation of hnRNP E1
induces EMT via transcript-selective translational induction of
Dab2 and ILEI. Nature Cell Biol. 12:286–293. 2010.PubMed/NCBI
|
|
30
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratz L, Laible M, Kacprzyk LA,
Wittig-Blaich SM, Tolstov Y, Duensing S, Altevogt P, Klauck SM and
Sültmann H: TMPRSS2:ERG gene fusion variants induce TGF-β signaling
and epithelial to mesenchymal transition in human prostate cancer
cells. Oncotarget. 8:25115–25130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandra Mangalhara K, Manvati S, Saini SK,
Ponnusamy K, Agarwal G, Abraham SK and Bamezai RNK:
ERK2-ZEB1-miR-101-1 axis contributes to epithelial-mesenchymal
transition and cell migration in cancer. Cancer Lett. 391:59–73.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bucay N, Bhagirath D, Sekhon K, Yang T,
Fukuhara S, Majid S, Shahryari V, Tabatabai Z, Greene KL, Hashimoto
Y, et al: A novel microRNA regulator of prostate cancer
epithelial-mesenchymal transition. Cell Death Differ. 24:1263–1274.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu
C, Liu D, Zheng M, Sun J, Feng H, et al: Tumor-derived CXCL5
promotes human colorectal cancer metastasis through activation of
the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer.
16:702017. View Article : Google Scholar : PubMed/NCBI
|