Introduction
Colorectal cancer (CRC) is the third most common
cancer and the second leading cause of cancer-related deaths in
industrialized countries (1).
Conversly, in Japan CRC is one of the most common types of cancer
and the second leading cause of cancer-related deaths. In 2012,
134,575 new cases of CRC occured, while 48,785 patients succumbed
to this disease in 2014, accounting for 13.3% of all cancer-related
deaths in Japan that year (2). In
the past decade, the development of several effective cytotoxic
drugs and molecular targeted drugs and their combinations, has
notably improved the prognosis of patients with metastatic CRC
(mCRC) (3–5). However, many patients present with
disease progression due to chemoresistance.
For the purpose of overcoming the limitations of
mCRC treatment, we previously conducted a phase II study for the
unresectable CRC using five human leukocyte antigen
(HLA-A*24:02)-restricted peptides (6), three derived from oncoantigens [kinase
of the outer chloroplast membrane 1, KOC1 (7); translocase of outer mitochondrial
membrane 34, TOMM34 (8); and ring
finger protein 43, RNF43 (9)] and
two derived from vascular endothelial growth factor receptors
[VEGFR1 (10) and VEGFR2 (11)]. In that study, we demonstrated a
significant improvement in patient survival 20 months after the
beginning of the vaccination. However, it is extremely difficult to
evaluate the clinical benefit of treatment because patients with
advanced cancer and with poor immunity are generally admitted to
enroll into clinical trials during the initial stage of drug
development (12). Therefore, it is
necessary to identify predictive biomarkers that facilitate the
selection of patients who are likely to respond well, either before
treatment or early in the course of treatment (13). Although CEA, CA19-9 and CRP are
included as predictive and prognostic biomarkers in CRC (14–16),
they are not necessarily adapted to immunotherapy. To this purpose,
we have previously reported candidate biomarkers of efficacy for
such peptide-vaccine treatments (17–19).
In spite of the fact that both cellular and humoral
immune responses are important for the induction of potent
antitumor immunity, most peptide-vaccine studies have focused only
to cellular responses (20). Under
these circumstances, it was reported that immunoglobulin G (IgG)
responses were identified as a remarkable biomarker for predicting
the overall survival (OS) of vaccinated patients (20,21).
In the present study, the relationship between OS and potential
biomarkers of efficacy, including cytotoxic T lymphocyte (CTL) and
IgG responses to the vaccinated peptides, were investigated in
patients with advanced CRC.
Materials and methods
Patients and eligibility criteria
The detailed protocol of this phase II study was
previously described (6). Briefly,
patients were eligible for enrollment if they were histologically
confirmed as advanced CRC, were chemotherapy-naïve, had adequate
functioning of critical organs and had a life expectancy of ≥3
months. Written informed consent was obtained from all patients
prior to their entry into the present study. This study was
approved by the Institutional Ethics Review Boards of Yamaguchi
University (H20-102) and was registered in the UMIN Clinical Trials
Registry as UMIN000001791.
This was a single-arm trial, non-randomized and
HLA-A status double-blind study to evaluate the clinical benefits
of cancer vaccination treatment for advanced CRC. The therapy
consisted of a cocktail of five therapeutic HLA-A*24:02 restricted
epitope-peptides: RNF43-721 (NSQPVWLCL) (9), TOMM34-299 (KLRQEVKQNL) (8), KOC1 (IMP-3)-508 (KTVNELQNL) (22), VEGFR1-1084 (SYGVLLWEI) (23) and VEGFR2-169 (RFVPDGNRI) (11) in addition to oxaliplatin-containing
chemotherapy.
The cocktail containing 3 mg of each of the five
peptides was mixed with 1.5 ml of incomplete Freund's adjuvant
(IFA, Montanide ISA51; Seppic, Paris, France) and administered
subcutaneously into the thigh or axilla region on day 1 of each
week for 13 weeks and then the vaccination schedule was reduced to
once every 2 weeks.
Oxaliplatin-containing regimens were administered
concurrently with the peptide vaccinations. Briefly, mFOLFOX6
(24) consisted of oxaliplatin (85
mg/m2) with leucovorin (400 mg/m2), followed
by a FU (400 mg/m2) bolus, and then 2,400
mg/m2 continuous infusion without bevacizumab. This
treatment was repeated biweekly. XELOX (25) consisted of oxaliplatin (130
mg/m2) on day 1, followed by oral capecitabine (1,000
mg/m2) twice daily on day 1 through 14 of a 21-day cycle
without bevacizumab.
Sample collection
Complete blood counts and serum chemistry tests were
performed before the treatment and every two weeks thereafter. A
total of 15 ml of blood were drawn before each course and then
peripheral-blood mononuclear cells (PBMCs) and blood plasma were
isolated by means of Ficoll-Conray density gradient centrifugation.
The evaluation of PBMCs viability was performed by Vi-CEL™ XR
(Beckman Coulter, Inc., Brea, CA, USA). PBMCs and plasma were
preserved in liquid nitrogen until examination.
Assessement of peptide-specific CTL
responses
Antigen-specific T-cell responses were assessed by
enzyme-linked ImmunoSpot (ELISpot) assay following in vitro
sensitization, as previously described (26). Briefly, frozen PBMCs derived from
the patient were thawed concurrently and viability was confirmed as
>90%. PBMCs (5×105/ml) were cultured with 10 mg/ml of
the candidate peptide and 100 IU/ml of interleukin (IL)-2
(Novartis, Emeryville, CA, USA) at 37°C for 2 weeks. Peptide was
added into the culture on day 0 and 7. To monitor antigen-specific
immune responses, ELISpot assays were performed with the human
IFN-γ ELISpot PLUS kit (Mabtech AB, Nacka Strand, Sweden). Briefly,
96-well plates with nitrocellulose membranes (Millipore, Molshelm,
France) were pre-coated with primary anti-IFN-γ antibody (1-D1K) at
4°C overnight. The plates were then pre-reacted with RPMI-1640
medium containing 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc, Waltham, MA, USA).
For the HLA-matched group, vaccine peptide (10
µg/ml)-pulsed or HIV-specific peptide (RYLRDQQLL, 10 µg/ml)-pulsed
(as the control) HLA-A*24:02-positive TISI cells (IHWG Cell and
Gene Bank, Seattle, WA, USA) (2×104/well) were used as
stimulators and incubated for 24 h with responder cells (from
2×104/well to 2.5×103/well in triplicate, in
a total of 200 µl/well), at different responder/stimulator ratios
as indicated. Stimulation with phorbol 12-myristate 13-acetate
(PMA, 25 ng/ml) plus ionomycin (500 pM) (both from Sigma-Aldrich,
St. Louis, MO, USA) was used as a positive control for T-cell
activity.
For the HLA-unmatched group, responder cells
(2×104/well) and each peptide (10 µg/ml) or HIV-specific
peptide (10 µg/ml), in a total of 200 µl/well, were cultured in
triplicate without stimulators for 24 h again using the combination
of PMA and ionomycin as a positive control.
For subsequent measurements, these cell mixtures
were treated with biotinylated secondary anti-IFN-γ antibody
(7-B6-1) and incubated for 2 h. Then the plates were incubated with
HRP-reagent and stained with TMB (Mabtech AB). Protein spots were
quantified with an auto-analysis system, ImmunoSPOT S4 (Cellular
Technology Ltd., Cleveland, OH, USA). Antigen-specific T-cell
responses were estimated as previously described (27), and classified into four grades (−,
+, ++ and +++) according to an algorithm described in a previous
study (28). Antigen-specific
T-cell responses classified (+) or more, were defined as CTL
positive.
Assessement of peptide-specific IgG
responses
The levels of anti-peptide IgGs were assessed using
the Luminex system (Austin, TX, USA), as previously reported
(29). In brief, 100 µl of 1:100
diluted plasma were incubated with 25 µl of peptide-coupled
color-coded beads for 2 h at room temperature on a plate shaker.
After incubation, the mixture was washed with a vacuum manifold
apparatus and incubated with 100 µl of biotinylated goat anti-human
IgG (γ-chain specific) for 1 h at room temperature. The plate was
then washed, followed by the addition of 100 µl
streptavidin-phycoerythrin (PE)/well and incubated for an
additional 30 min at room temperature on a plate shaker. The bound
beads were washed three times followed by the addition of 100 µl of
Tween-phosphate buffered saline (PBS) into each well. Each sample
(50 µl) was then analyzed using the Luminex system. Samples with
antigen-specific IgG values at least 4-fold higher than those prior
to treatment, were defined as being IgG positive.
Peptide-binding assays
The binding of peptides to the HLA-A*24:02 molecule
was determined by acid stripping and a reconstitution assay, as
previously described by Zeh et al (30) with minor modifications. Briefly,
C1R-A24 cells were exposed to pH 3.3 citrate phosphate buffer and
were then reconstituted with graded concentrations of peptide and
0.1 µM human β2-microglobulin (M-4890; Sigma-Aldrich) in Dulbecco's
modified Eagle's medium (DMEM) containing 0.25% bovine serum
albumin (BSA). A FITC-labeled mAb 17A12 (31) was used to detect properly-folded and
peptide-bound HLA-A*24:02 molecules. The fluorescence intensity was
determined by FACScan (Becton-Dickinson Japan, Tokyo, Japan). Both
high- and low-binding control peptides, HER2-63 TYLPTNASL and
Met149 RVWE SATPL, respectively, were included in each assay and
used to normalize variations between experiments. Peptide affinity
was calculated as previously described (32).
Statistical analysis
The Mann-Whitney U-test was used to compare the
median values of two independent parametric continuous variables.
Pearson's Chi-square or Fisher's exact test were used to compare
categorical variables. OS was determined by the Kaplan-Meier
analysis, with differences between survival curves assessed by the
log-rank test. The Cox proportional hazards regression model was
used for univariate and multivariate analyses to identify
combinations of factors that had a significant impact on survival,
such as CEA, CA19-9 and CRP (14–16).
All baseline parameters in the survival and proportional hazards
regression analysis were analyzed as dichotomous variables using
the overall mean values as cut-off levels. All statistical
calculations were carried out using JMP® 11 (SAS
Institute Inc., Cary, NC, USA). A two-sided significance level of
5% was considered statistically significant.
Results
Patients
Among the 89 patients who could be evaluated in the
present study, 87 received mFOLFOX6 and two received XELOX. None of
the patients was treated with bevacizumab. The peptide vaccination
was administered to all patients. Forty-eight patients had at least
one allele of HLA-A*24:02 and 41 had no HLA-A*24:02 allele. The
characteristics of the 89 patients are summarized in Table I. The baseline characteristics were
generally well balanced between the HLA-matched and HLA-unmatched
groups. There was no significant difference in the incidence of
injection-site reaction in the two groups. The overall response
rate was 66.7 and 58.5% in the HLA-matched and HLA-unmatched
groups, respectively.
 | Table I.Patient's characteristics. |
Table I.
Patient's characteristics.
|
| HLA-A*2402 |
|
|---|
|
|
|
|
|---|
|
Characteristics | Matched (n=48) | Unmatched
(n=41) | P-value |
|---|
| Sex |
|
| N.S.a |
|
Male | 25 | 20 |
|
|
Female | 23 | 21 |
|
| Age, median
(IQR) | 67 (58.3–71.8) | 65 (59.5–69) | N.S.b |
| Primary lesion |
|
| N.S.a |
|
Colon | 28 | 31 |
|
|
Rectum | 20 | 10 |
|
| No. of unresectable
sites |
|
| N.S.a |
| 1 | 34 | 26 |
|
| 2 | 9 | 11 |
|
| 3 | 5 | 4 |
|
| Chemotherapy |
|
| N.S.a |
|
mFOLFOX6 | 2 | 0 |
|
|
XELOX | 46 | 41 |
|
| Injection site
reaction |
|
| N.S.a |
| Grade 0
or 1 | 20 | 21 |
|
| Grade 2
or 3 | 28 | 20 |
|
| Best overall
response |
|
| N.S.a |
| CR | 1 | 0 |
|
| PR | 29 | 24 |
|
| SD | 15 | 16 |
|
| PD | 3 | 1 |
|
| Response rate | 30/45 (66.7%) | 24/41(58.5%) |
|
Immune responses to the peptides
Both IgG and T-cell responses specific to the
vaccine peptides were analyzed using plasma samples obtained from
the 89 patients both before and after the vaccination. For
monitoring humoral responses, the levels of peptide-specific IgGs
reactive to each of the five different peptides were determined by
bead-based multiplex assay. Although there was a difference in
degree, the values of five peptide-specific IgGs were significantly
increased compared to the levels before the vaccination (Table II). The IgG response to the TOMM34
peptide was significantly higher in the HLA-A*24:02-unmatched group
than the matched group, although the biological significance of
this is unclear. There were no significant differences in the IgG
positive rates between the HLA-matched and HLA-unmatched groups for
the other peptides (Table
III).
 | Table II.Changes of peptide-specific IgG
values before and after the vaccinations. |
Table II.
Changes of peptide-specific IgG
values before and after the vaccinations.
| Peptides | Before
vaccination | After
vaccination |
P-valuea |
|---|
| RNF43, median
(IQR) | 16.3 | 110 | <0.0001 |
|
| (0–36.3) | (18.8–1056.5) |
|
| TOMM34, median
(IQR) | 19.5 | 54.8 | <0.0001 |
|
| (0–37.6) | (19.3–1145) |
|
| KOC1, median
(IQR) | 14 | 19.8 | 0.038 |
|
| (0–29.6) | (0–60.9) |
|
| VEGFR1, median
(IQR) | 0 | 0 | 0.014 |
|
|
| (0–15.1) |
|
| VEGFR2, median
(IQR) | 17.5 | 58.5 | <0.0001 |
|
| (0–37.8) | (21.3–1522.1) |
|
 | Table III.CTL induction and IgG production for
five peptides. |
Table III.
CTL induction and IgG production for
five peptides.
|
|
| CTL positive rate
(%) | IgG positive
rate |
|---|
|
|
|
|
|
|---|
| Peptide | A*24:02 binding
affinity | All | HLA matched | HLA unmatched |
P-valuea | All | HLA matched | HLA unmatched |
P-valuea |
| RNF43 | −6.28 | 29/79 | 15/38 | 13/41 | 0.491 | 33/89 | 15/48 | 18/41 |
0.273 |
|
|
| (35.4%) | (39.5%) | (31.7%) |
| (37.1%) | (31.3%) | (43.9%) |
|
| TOMM34 | −3.76 | 20/79 | 8/41 | 12/38 | 0.301 | 27/89 | 7/48 | 20/41 | <0.001 |
|
|
| (25.3%) | (19.5%) | (31.6%) |
| (30.4%) | (14.9%) | (48.8%) |
|
| KOC1 | −5.85 | 28/80 | 14/42 | 12/38 | 1.000 | 3/89 | 0/48 | 3/41 |
0.094 |
|
|
| (32.5%) | (33.3%) | (31.6%) |
| (3.4%) | (0%) | (7.3%) |
|
| VEGFR1 | −7.82 | 45/83 | 28/44 | 16/44 | 0.049 | 2/89 | 0/48 | 2/41 |
0.209 |
|
|
| (53.0%) | (63.6%) | (41.0%) |
| (2.3%) | (0%) | (4.9%) |
|
| VEGFR2 | −7.17 | 45/84 | 31/44 | 13/40 | <0.001 | 30/89 | 13/48 | 17/41 |
0.181 |
|
|
| (52.4%) | (70.5%) | (32.5%) |
| (33.7%) | (27.1%) | (41.5%) |
|
T-cell responses to the vaccine
peptides were also determined by ELISpot
The CTL-positive rates for VEGFR1 and VEGFR2 were
significantly higher in the HLA-matched group than the
HLA-unmatched group and exceeded 50% (Table III). Peptides with higher binding
affinity to HLA-A*24:02 tended to be associated with higher CTL
responses. Overall, there was no evident correlation between
positive IgG responses and CTL responses in this analysis (Table III).
Immune responses to the peptides and
clinical outcome
We also investigated whether IgG or CTL responses
correlated with patient survival. First, the relationship between
the IgG responses to three of the peptides (TOMM34, RNF43 and
VEGFR2) and OS in HLA-matched and HLA-unmatched groups was
investigated. In the HLA-matched group, OS in patients with
increased IgG responses to VEGFR2 were significantly longer
(P=0.0339) than for those with no IgG response (Fig. 1C). Conversely, there was no
difference in OS for patients with increased IgG response to VEGFR2
in the HLA-unmatched group (Fig.
1C). There were no differences in OS based on IgG responses to
TOMM34 and RNF43 in either the HLA-matched or -unmatched groups
(Fig. 1A and B). In regard to CTLs,
univariate analysis revealed no significant association of CTL
induction (in response to any of the peptides) with OS (Table IV).
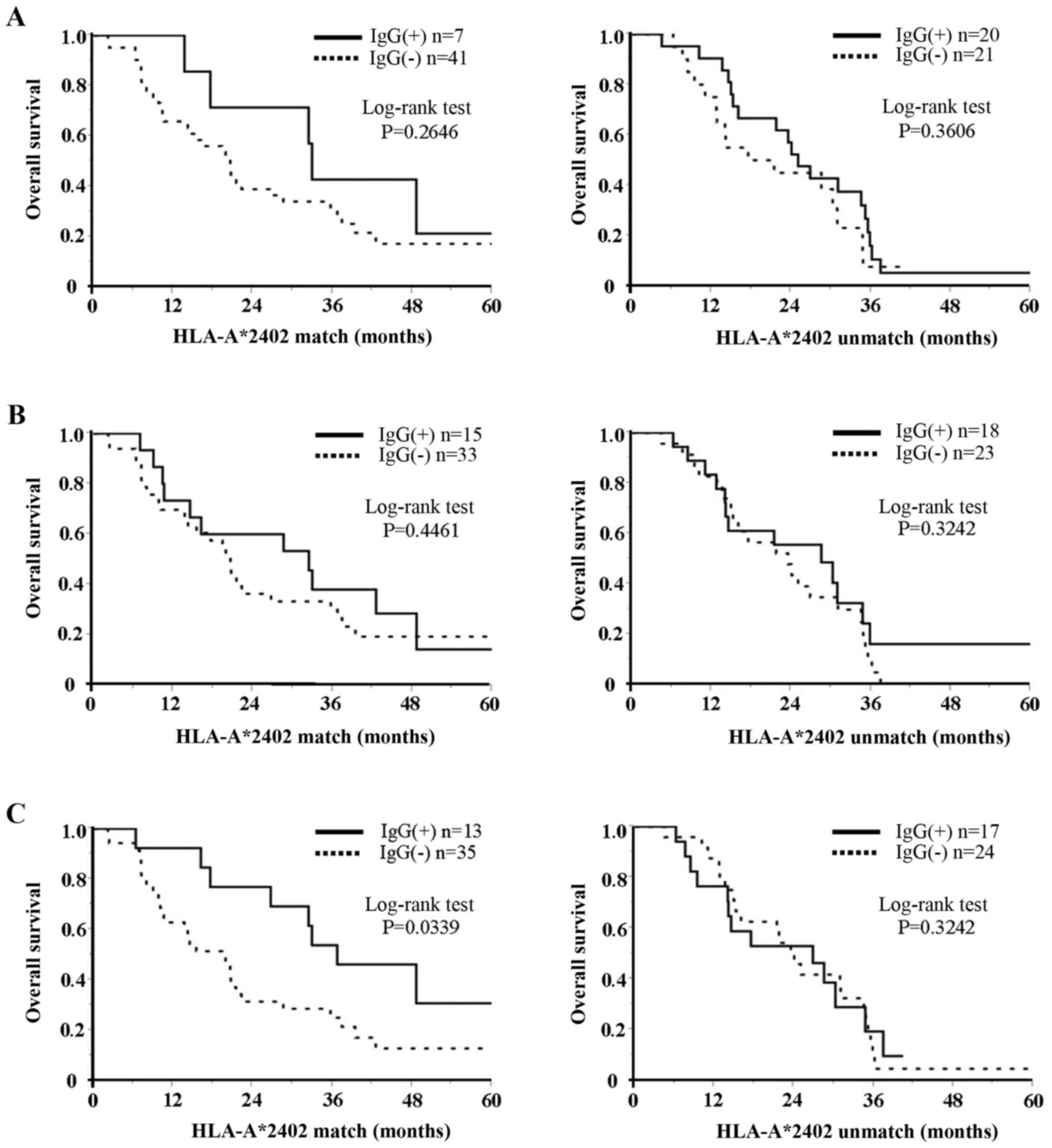 | Figure 1.Relation between IgG response to
three peptides and OS in two HLA-matched and HLA-unmatched groups.
(A) TOMM34, (B) RNF43 and (C) VEGFR2. As to HLA-matched group, OS
in patients with increased IgG response to VEGFR2 was significantly
(P=0.0339) longer than that in patients with negative IgG response.
Conversely, there was no difference in OS in patients with
increased IgG response to VEGFR2 in the HLA-unmatched group. There
was no difference in OS in patients with increased IgG response to
TOMM34 and RNF43 in both HLA-matched and HLA-unmatched groups. IgG,
immunoglobulin G; OS, overall survival; HLA, human leukocyte
antigen; TOMM34, translocase of outer mitochondrial membrane 34;
RNF43, ring finger protein 43; VEGFR2, vascular endothelial growth
factor receptor 2; IgG(+), positive response; IgG(−) negative
response. |
 | Table IV.Univariate analysis of CTL induction
of five peptides for overall survival using Cox regression
model. |
Table IV.
Univariate analysis of CTL induction
of five peptides for overall survival using Cox regression
model.
|
| HLA-A*2402 |
|---|
|
|
|
|---|
|
| Matched (n=48) | Unmatched
(n=41) |
|---|
|
|
|
|
|---|
| Peptides | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| RNF43 | 1.08 | 0.50–2.18 | 0.83 | 0.78 | 0.37–1.57 | 0.489 |
| TOMM34 |
1.11 | 0.44–2.43 | 0.807 | 1.28 | 0.59–2.63 | 0.514 |
| KOC1 |
0.85 | 0.39–1.75 | 0.677 | 0.92 | 0.40–1.92 | 0.827 |
| VEGFR1 |
0.93 | 0.47–1.88 | 0.827 | 0.95 | 0.46–1.91 | 0.896 |
| VEGFR2 |
1.14 | 0.56–2.50 | 0.721 | 0.79 | 0.37–1.59 | 0.522 |
Multivariate analysis of prognostic
factors
We also performed multivariate analysis by Cox
proportional hazards regression to assess the significance of the
conventional prognostic factors CEA, CA19-9 and CRP levels as well
as the IgG response to VEGFR2 which was also included as covariate.
This analysis demonstrated that the IgG response to VEGFR2 was the
most significant predictor for OS in the HLA-A*24:02-matched group
[P=0.04; HR, 1.99; 95% confidence interval (CI), 1.04–4.08;
Table V].
 | Table V.Univariate and multivariate analysis
of biomarkers for OS using Cox regression model. |
Table V.
Univariate and multivariate analysis
of biomarkers for OS using Cox regression model.
|
|
| HLA-A*2402 matced
group (n=48) |
|---|
|
|
|
|
|---|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Cut off | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| CEA | ≥2 × ULN | 1.23 | 0.63–2.56 | 0.55 |
|
|
|
| CA19-9 | ≥2 × ULN | 1.12 | 0.54–2.19 | 0.76 |
|
|
|
| CRP (mg/dl) | ≥1 | 1.59 | 0.72–3.28 | 0.24 |
|
|
|
| VEGFR2_IgG | <4 × PTV | 2.31 | 1.09–5.45 | 0.03 | 1.99 | 1.04–4.08 | 0.04 |
Discussion
Recent developments in tumor immunology have led to
the identification of numerous antigens and their epitope-peptides
that are recognized by tumor-reactive and MHC class I-restricted
CTLs (33). Cancer vaccines are
acknowledged as a reliable treatment (34), however their clinical benefits have
been limited (35). Therefore, to
overcome this problem, it is necessary to investigate biomarkers
for estimating the clinical responses to immunotherapy.
In the present study, we used HLA-A*24:02-restricted
peptides and thus considered patients with HLA-A*24:02 alleles as
the treatment group and patients without HLA-A*24:02 alleles as the
control group, analyzing the relationship between OS and several
biomarkers, including CTL and IgG responses to the vaccinated
peptides. We found that the plasma levels of TOMM34 IgG, RNF43 IgG
and VEGFR2 IgG significantly increased following vaccination and
that increased VEGFR2 IgG responses correlated well with OS in the
HLA-A*24:02-matched group. Furthermore, multivariate analysis
indicated that IgG response to VEGFR2 was the most significant
predictor for OS in the HLA-A*24:02-matched group. Conversely, the
present study had a limitation of relevant across racial/ethnic
groups. The frequency of HLA types varies greatly for each human
race. HLA-A*24:02 alleles are the most frequent in Asian-Pacific
Islander group including Japan, but are ranked 4th in the European
group and 15th in the Africa-American group (36).
Most current peptide-based cancer vaccines have
focused on cellular immune responses only, although it is well
acknowledged that both cellular and humoral immune responses are
important to prompt antitumor immunity in animal models (37,38).
Several studies have reported that IgG responses may be a
remarkable biomarker for predicting OS for vaccinated patients,
although CTL responses have also shown a prognostic correlation
(20,21). Previous studies recently devised a
new regimen of peptide-based vaccination that consists of
determining IgGs responses to various vaccine candidates, prior to
administration (39–41). However, the biological roles of IgGs
specific to CTL epitope peptides are practically unknown.
CD4+ helper T-cells may recognize administreted peptides
presented on the MHC class II molecules of dendritic cells,
resulting in both the activation of helper T-cells and IgG
production. It is well known that CD4+ helper T-cells
are necessary to preserve CD8+ T-cell immunity (42). If increased levels of
peptide-specific IgG reflect activation levels of CD4+
helper T-cells, assessement of peptide-specific IgG may be useful
as an immunological biomarker to predict the clinical benefit of
cancer patients undergoing peptide vaccination.
CTL responses to VEGFR1 and VEGFR2 were
significantly higher in HLA-matched patients in the present study.
However, the OS of patients with increased CTL responses to any of
the peptides was not different to those with no CTL responses.
Certainly, cellular immune responses ought to represent important
clinical biomarkers, if suitable assay conditions were perfomed.
However, currently available assays for quantifying and
characterizing antigen-specific T-cell responses such as ELISpot,
ELISA and 51Cr release assay have insufficient
sensitivity and reproducibility for monitoring immune responses,
due to lack of universal standards (28,43,44).
The VEGFR2 IgG response appeared to play a key role
in improving OS in the present study. A possible explanation of
this phenomenon is based on the restraint of the tumor vasculature
which is crucial in immunotherapy associated with T-cell activity
(45). Several clinical studies
have reported a positive relation between patient survival and the
presence of tumor infiltrating lymphocytes (TILs) (46). Currently, it has been recognized
that the tumor vasculature organizes an important barrier against
T-cells (46). Endothelial cells
lining the blood vessels can downregurate T-cell activity and
prevent T-cells from gaining entry into the tumor through the
deregulation of adhesion molecules (47). Therefore, for clinical success, it
is important to target the tumor-endothelial barrier in order to
enhance T-cell activity. In conclusion, the present study revealed
that the VEGFR2 IgG response may be an important immunological
biomarker in patients with advanced CRC treated with a combination
therapy consisting of five therapeutic epitope peptides and
oxaliplatin-based chemotherapy. The biological role of IgGs
specific to CTL epitope peptides remains to be determined.
Acknowledgements
The authors thank Ms. Kaori Kaneyasu and Ms. Akiko
Sano for their technical support.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
HLA
|
human leukocyte antigen
|
|
KOC1
|
kinase of the outer chloroplast
membrane 1
|
|
TOMM34
|
translocase of outer mitochondrial
membrane 34
|
|
RNF43
|
ring finger protein 43
|
|
VEGFR1
|
vascular endothelial growth factor
receptor 1
|
|
CTL
|
cytotoxic T lymphocyte
|
|
IgG
|
immunoglobulin G
|
|
OS
|
overall survival
|
|
PS
|
performance status
|
|
IFA
|
incomplete Freund's adjuvant
|
|
PBMCs
|
peripheral-blood mononuclear cells
|
|
ELISpot
|
enzyme-linked immunoSpot
|
|
IL
|
interleukin
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Registry and StatisticsCancer
Information Service, National Cancer Center. Japan:
|
|
3
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chien Chang CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hazama S, Nakamura Y, Tanaka H, Hirakawa
K, Tahara K, Shimizu R, Ozasa H, Etoh R, Sugiura F, Okuno K, et al:
A phase II study of five peptides combination with
oxaliplatin-based chemotherapy as a first-line therapy for advanced
colorectal cancer (FXV study). J Transl Med. 12:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kikuchi T, Daigo Y, Katagiri T, Tsunoda T,
Okada K, Kakiuchi S, Zembutsu H, Furukawa Y, Kawamura M, Kobayashi
K, et al: Expression profiles of non-small cell lung cancers on
cDNA microarrays: Identification of genes for prediction of
lymph-node metastasis and sensitivity to anti-cancer drugs.
Oncogene. 22:2192–2205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimokawa T, Matsushima S, Tsunoda T,
Tahara H, Nakamura Y and Furukawa Y: Identification of TOMM34,
which shows elevated expression in the majority of human colon
cancers, as a novel drug target. Int J Oncol. 29:381–386.
2006.PubMed/NCBI
|
|
9
|
Uchida N, Tsunoda T, Wada S, Furukawa Y,
Nakamura Y and Tahara H: Ring finger protein 43 as a new target for
cancer immunotherapy. Clin Cancer Res. 10:8577–8586. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olofsson B, Korpelainen E, Pepper MS,
Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M,
Alitalo K, et al: Vascular endothelial growth factor B (VEGF-B)
binds to VEGF receptor-1 and regulates plasminogen activator
activity in endothelial cells. Proc Natl Acad Sci USA.
95:11709–11714. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wada S, Tsunoda T, Baba T, Primus FJ,
Kuwano H, Shibuya M and Tahara H: Rationale for antiangiogenic
cancer therapy with vaccination using epitope peptides derived from
human vascular endothelial growth factor receptor 2. Cancer Res.
65:4939–4946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagorsen D and Thiel E: Clinical and
immunologic responses to active specific cancer vaccines in human
colorectal cancer. Clin Cancer Res. 12:3064–3069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hazama S, Takenouchi H, Tsunedomi R, Iida
M, Suzuki N, Iizuka N, Inoue Y, Sakamoto K, Nakao M, Shindo Y, et
al: Predictive biomarkers for the outcome of vaccination of five
therapeutic epitope peptides for colorectal cancer. Anticancer Res.
34:4201–4205. 2014.PubMed/NCBI
|
|
14
|
Bacolod MD and Barany F: Molecular
profiling of colon tumors: The search for clinically relevant
biomarkers of progression, prognosis, therapeutics, and
predisposition. Ann Surg Oncol. 18:3694–3700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giessen-Jung C, Nagel D, Glas M, Spelsberg
F, Lau-Werner U, Modest DP, Schulz C, Heinemann V, Di Gioia D and
Stieber P: Preoperative serum markers for individual patient
prognosis in stage I–III colon cancer. Tumour Biol. 36:7897–7906.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozawa T, Ishihara S, Kawai K, Nozawa H,
Yamaguchi H, Kitayama J and Watanabe T: Prognostic significance of
preoperative serum carbohydrate antigen 19-9 in patients with stage
IV colorectal cancer. Clin Colorectal Cancer. 15:e157–e163. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kijima T, Hazama S, Tsunedomi R, Tanaka H,
Takenouchi H, Kanekiyo S, Inoue Y, Nakashima M, Iida M, Sakamoto K,
et al: MicroRNA-6826 and −6875 in plasma are valuable non invasive
biomarkers that predict the efficacy of vaccine treatment against
metastatic colorectal cancer. Oncol Rep. 37:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitahara M, Hazama S, Tsunedomi R,
Takenouchi H, Kanekiyo S, Inoue Y, Nakajima M, Tomochika S,
Tokuhisa Y, Iida M, et al: Prediction of the efficacy of
immunotherapy by measuring the integrity of cell-free DNA in plasma
in colorectal cancer. Cancer Sci. 107:1825–1829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shindo Y, Hazama S, Suzuki N, Iguchi H,
Uesugi K, Tanaka H, Aruga A, Hatori T, Ishizaki H, Umeda Y, et al:
Predictive biomarkers for the efficacy of peptide vaccine
treatment: based on the results of a phase II study on advanced
pancreatic cancer. J Exp Clin Cancer Res. 36:362017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mine T, Sato Y, Noguchi M, Sasatomi T,
Gouhara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, et
al: Humoral responses to peptides correlate with overall survival
in advanced cancer patients vaccinated with peptides based on
pre-existing, peptide-specific cellular responses. Clin Cancer Res.
10:929–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suda T, Tsunoda T, Daigo Y, Nakamura Y and
Tahara H: Identification of human leukocyte antigen-A24-restricted
epitope peptides derived from gene products upregulated in lung and
esophageal cancers as novel targets for immunotherapy. Cancer Sci.
98:1803–1808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishizaki H, Tsunoda T, Wada S, Yamauchi M,
Shibuya M and Tahara H: Inhibition of tumor growth with
antiangiogenic cancer vaccine using epitope peptides derived from
human vascular endothelial growth factor receptor 1. Clin Cancer
Res. 12:5841–5849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato T, Muro K, Yamaguchi K, Bando H,
Hazama S, Amagai K, Baba H, Denda T, Shi X, Fukase K, et al:
Cediranib in combination with mFOLFOX6 in Japanese patients with
metastatic colorectal cancer: Results from the randomised phase II
part of a phase I/II study. Ann Oncol. 23:933–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki N, Hazama S, Ueno T, Matsui H,
Shindo Y, Iida M, Yoshimura K, Yoshino S, Takeda K and Oka M: A
phase I clinical trial of vaccination with KIF20A-derived peptide
in combination with gemcitabine for patients with advanced
pancreatic cancer. J Immunother. 37:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hazama S, Nakamura Y, Takenouchi H, Suzuki
N, Tsunedomi R, Inoue Y, Tokuhisa Y, Iizuka N, Yoshino S, Takeda K,
et al: A phase I study of combination vaccine treatment of five
therapeutic epitope-peptides for metastatic colorectal cancer;
safety, immunological response, and clinical outcome. J Transl Med.
12:632014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R,
et al: Multicenter, phase II clinical trial of cancer vaccination
for advanced esophageal cancer with three peptides derived from
novel cancer-testis antigens. J Transl Med. 10:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeh HJ III, Leder GH, Lotze MT, Salter RD,
Tector M, Stuber G, Modrow S and Storkus WJ: Flow-cytometric
determination of peptide-class I complex formation. Identification
of p53 peptides that bind to HLA-A2. Hum Immunol. 39:79–86. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tahara T, Yang SY, Khan R, Abish S,
Hämmerling GJ and Hämmerling U: HLA antibody responses in HLA class
I transgenic mice. Immunogenetics. 32:351–360. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Udaka K, Wiesmüller KH, Kienle S, Jung G,
Tamamura H, Yamagishi H, Okumura K, Walden P, Suto T and Kawasaki
T: An automated prediction of MHC class I-binding peptides based on
positional scanning with peptide libraries. Immunogenetics.
51:816–828. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Novellino L, Castelli C and Parmiani G: A
listing of human tumor antigens recognized by T cells: March 2004
update. Cancer Immunol Immunother. 54:187–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosenberg SA: A new era for cancer
immunotherapy based on the genes that encode cancer antigens.
Immunity. 10:281–287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maiers M, Gragert L and Klitz W:
High-resolution HLA alleles and haplotypes in the United States
population. Hum Immunol. 68:779–788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bequet-Romero M, Morera Y, Ayala-Ávila M,
Ancizar J, Soria Y, Blanco A, Suárez-Alba J and Gavilondo JV:
CIGB-247: A VEGF-based therapeutic vaccine that reduces
experimental and spontaneous lung metastasis of C57Bl/6 and BALB/c
mouse tumors. Vaccine. 30:1790–1799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng J, Cai S, Yi Y, He Y, Wang Z, Jiang
G, Li X and Du J: Prevention of spontaneous tumor development in a
ret transgenic mouse model by ret peptide vaccination with
indoleamine 2,3-dioxygenase inhibitor 1-methyl tryptophan. Cancer
Res. 69:3963–3970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Itoh K and Yamada A: Personalized peptide
vaccines: A new therapeutic modality for cancer. Cancer Sci.
97:970–976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mine T, Gouhara R, Hida N, Imai N, Azuma
K, Rikimaru T, Katagiri K, Nishikori M, Sukehiro A, Nakagawa M, et
al: Immunological evaluation of CTL precursor-oriented vaccines for
advanced lung cancer patients. Cancer Sci. 94:548–556. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sato Y, Fujiwara T, Mine T, Shomura H,
Homma S, Maeda Y, Tokunaga N, Ikeda Y, Ishihara Y, Yamada A, et al:
Immunological evaluation of personalized peptide vaccination in
combination with a 5-fluorouracil derivative (TS-1) for advanced
gastric or colorectal carcinoma patients. Cancer Sci. 98:1113–1119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Antony PA, Piccirillo CA, Akpinarli A,
Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff
CA, Overwijk WW, et al: CD8+ T cell immunity against a
tumor/self-antigen is augmented by CD4+ T helper cells
and hindered by naturally occurring T regulatory cells. J Immunol.
174:2591–2601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itoh K, Yamada A, Mine T and Noguchi M:
Recent advances in cancer vaccines: An overview. Jpn J Clin Oncol.
39:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Noguchi M, Yao A, Harada M, Nakashima O,
Komohara Y, Yamada S, Itoh K and Matsuoka K: Immunological
evaluation of neoadjuvant peptide vaccination before radical
prostatectomy for patients with localized prostate cancer.
Prostate. 67:933–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lanitis E, Irving M and Coukos G:
Targeting the tumor vasculature to enhance T cell activity. Curr
Opin Immunol. 33:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence,
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|















