Introduction
Cancer is a leading cause of death worldwide and
both the numbers of cancer cases and cancer-related deaths are
expected to continue to rise. There are currently an estimated 17
million deaths worldwide due to cancer per year (1), with colon, lung, breast, liver and
stomach cancer being responsible for most cancer-related deaths.
Colorectal cancer (CRC) is the second most frequent cancer in
Europe (2) and the second most
common cause of cancer-related deaths in the United States
(3). CRC was also the leading cause
of cancer-related deaths among women and the third leading cause
among men in Japan as of 2013 and its incidence continues to
increase (4). Surgical resection of
the primary tumor and regional lymph nodes is an important
treatment strategy for CRC and 5-year survival rates of 92% of
patients in stage I, 85% in stage II and 72% in stage III have been
reported following complete resection (5,6).
However, recurrence occurred in 17.3% of these patients and distant
metastases were the major cause of death in CRC patients, with a
5-year survival rate of only 19% in stage IV patients with distant
metastases.
It is necessary to identify the genes responsible
for CRC in order to identify new therapeutic targets. Multiple
receptor tyrosine kinases and their growth factor ligands have
recently been reported to play important roles in cancer
progression and metastasis (2).
Platelet-derived growth factor receptors (PDGFRs) belong to a
family of cell surface type III receptor tyrosine kinases and have
been reported to increase proliferation and migration in several
malignant tumors (7–11). CRC tissue expresses PDGFR-α and
PDGFR-β (12) and these factors
were revealed to stimulate invasion and liver-metastasis formation
in mice (13). Crenolanib is a
highly selective PDGFR inhibitor (14) and low micromolar concentrations in
plasma were achieved with no significant myelosuppression in a
phase I study in patients with advanced cancer (15).
The present study examined the correlation between
the expression of PDGFR-β in CRC tissues and
clinicopathological factors and also examined the possible use of
PDGFR inhibitors for the treatment of CRC.
Materials and methods
Clinical tissue samples for the
analysis of PDGFR-β
A total of 194 patients with CRC were registered and
underwent resection of CRC and any distant metastases at Osaka
International Cancer Institute from 2009 to 2013. None of the
patients received chemotherapy or radiotherapy prior to surgery and
none died of any other cancer. Primary CRC specimens and adjacent
normal colorectal mucosa were obtained from the patients after
obtaining their informed written consent, in accordance with the
ethical guidelines of the Osaka International Cancer Institute. The
surgical specimens were fixed in formalin, processed through graded
ethanols, embedded in paraffin, sectioned and stained with
hematoxylin and eosin (H&E). The degree of histological
differentiation, lymphatic and venous invasion was examined. Pieces
of all specimens were also frozen in liquid nitrogen immediately
after resection and kept at −80°C for RNA extraction. After
surgery, the patients underwent follow-up blood examinations to
assess tumor markers (serum carcinoembryonic antigen and cancer
antigen 19-9) and imaging examinations (including abdominal
ultrasonography, computed tomography and chest X-rays) every 3–6
months. Patients with stage III and stage IV lesions with no
residual tumor (R0)-operation received adjuvant postoperative
chemotherapy according to the Japanese Society for Cancer of the
Colon and Rectum (JSCCR) guidelines (5), following informed patient consent. The
clinicopathological factors were assessed according to the tumor
node metastasis (TNM) classification of the International Union
Against Cancer (UICC) (16). The
Review Board and Animal Research Committee of the Osaka
International Cancer Institute approved the present study and
written informed consents for the study were obtained from all
participants according to the ethics guidelines of the Osaka
International Cancer Institute.
RNA preparation and expression
analysis
Total RNA was prepared using an RNA Purification kit
(Qiagen GmbH, Hilden, Germany). Reverse transcription was performed
with a Transcriptor First Strand cDNA Synthesis kit (Roche
Diagnostics, Tokyo, Japan). A 92-bp PDGFR-β fragment was
amplified. Two human PDGFR-β oligonucleotide primers were
designed for the polymerase chain reaction (PCR) as follows:
forward 5′-CAACTTCGAGTGGACATACCC-3′ and reverse,
5′-AGCGGATGTGGTAAGGCATA-3′. PCR was also performed using primers
specific for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
gene, to normalize gene expression levels. The GAPDH primers
(forward, 5′-AGCCACATCGCTCAGACAC-3′ and reverse
5′-GCCCAATACGACCAAATCC-3′) produced a 66-bp amplicon. cDNA from the
Human Reference Total RNA (Clontech Laboratories; Takara Bio USA,
Inc., Palo Alto, CA, USA) and RNA extracted from NTERA-2 cancer
cells were studied concurrently as positive controls. Quantitative
assessment was performed by real-time reverse
transcription-polymerase chain reaction (RT-PCR) using a Universal
ProbeLibrary platform (Roche Diagnostics) and a FASTStart TaqMan
Probe Master (Roche Diagnostics) for the cDNA amplification of the
target genes (Table I). The
expression ratios of PDGFR-β mRNA copies in tumor and normal
tissues were calculated after normalization against GAPDH mRNA
expression.
 | Table I.Primer sequences corresponding to
universal probe libraries. |
Table I.
Primer sequences corresponding to
universal probe libraries.
| Primer | Sequence (5′-3′) | UPL no. | Applications |
|---|
| PDGFR-β | F:
CAACTTCGAGTGGACATACCC | 28 | PDGFR-β |
| PDGFR-β | R:
AGCGGATGTGGTAAGGCATA |
| RT-PCR |
| GAPDH | F:
AGCCACATCGCTCAGACAC | 60 | GAPDH |
| GAPDH | R:
GCCCAATACGACCAAATCC |
| RT-PCR |
Immunohistochemistry
Twenty-one formalin-fixed, paraffin-embedded CRC
surgical specimens were selected randomly for immunohistochemical
detection of PDGFR-β. After deparaffinization and blocking, the
sections were incubated with primary anti-PDGFR-β rabbit polyclonal
antibody (cat. no. 4564; Cell Signaling Technology Inc. Danvers,
MA, USA) at a dilution of 1:50 overnight at 4°C. The signal was
detected using Vectastain Universal Elite kit (Vector Laboratories,
Burlingame, CA, USA). Diaminobenzidine was used for color
modification. All sections were counterstained with
hematoxylin.
Culture of CRC cell lines
The colorectal tumor cell lines, HCT116, DLD-1 and
RKO gifted by Dr Bert Vongelstein (Johns Hopkins University,
Baltimore, MD, USA), were cultured in Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific Inc., Waltham, MA, USA), 1% GlutaMAX-I
(Thermo Fisher Scientific Inc.), 1%
penicillin/streptomycin/amphotericin B (Wako Pure Chemical
Industries, Ltd., Osaka, Japan). The cells were kept at 37°C in a
humidified atmosphere containing 5% CO2.
Primary culture of CRC cells
CRC tissue was minced into 1-mm pieces using
scissors, dissociated with 1 mg/ml collagenase (C6885;
Sigma-Aldrich, St. Louis, MO, USA) in DMEM (Sigma-Aldrich) and
shaken using a BioShaker BR-13FP (Taitec Co, Saitama, Japan) at 6 ×
g for 15 min at 37°C. The dissociated tissue was filtered through
custom-made filters (Sansho Co. Ltd., Tokyo, Japan). The collected
cells were then centrifuged at 400 × g for 5 min at room
temperature and the cell pellet was resuspended in 2 ml culture
medium (modified embryonic stem cell culture medium containing
fibroblast growth factor 2 and transforming growth factor-β).
Suspended primary culture cells (603iCC and 821iCC) were seeded on
plates coated with 0.03% Matrigel (Corning Inc., Corning, NY, USA)
in DMEM/F12 (Sigma-Aldrich) and the medium was changed every two
days. After the cells had spread over more than 50% of the plate,
they were passaged using Accutase (Nacalai Tesque, Kyoto, Japan)
for 3–5 min and checked at 1-min intervals. The primary culture
cells were then collected and resuspended in the medium and seeded
on a Matrigel-coated plate for passage.
Small interfering RNA inhibition of
cultured cells
CRC cell lines (HCT116, DLD-1 and RKO) and primary
cultured cells were used. For small interfering RNA (siRNA)
inhibition, double-stranded RNA duplexes targeting human
PDGFR-β were purchased as a Validated Stealth RNAi kit
(Thermo Fisher Scientific Inc.) and a negative control siRNA (cat.
no. 12935-112; Stealth RNAi Negative Control, Med GC Duplex; Thermo
Fisher Scientific Inc.). CRC cell lines were transfected with siRNA
at a concentration of 20 nM using lipofectamine RNAiMAX (Thermo
Fisher Scientific Inc.), incubated in glucose-free Opti-MEM (Thermo
Fisher Scientific Inc.) and analyzed.
Cell proliferation assay in vitro
PDGFR-β knockdown cells (PDGFR-β siRNA),
negative control cells (NC siRNA) and wild-type cells (WT) were
seeded on 96-well plates. The cell proliferation was analysed using
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). The values are presented as the means ± standard
deviation (SD) from all independent experiments performed six
times.
Cell invasion assay in vitro
The cells (5×104; PDGFR-β siRNA and NC
siRNA) suspended with DMEM (Sigma-Aldrich) were seeded on 24-well
insert chambers [Corning® BioCoat™ Matrigel®
Invasion Chamber (cat.no. 354480); Corning] and DMEM supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific Inc.)
was added to each well. The cells were kept at 37°C in a humidified
atmosphere containing 5% CO2 for 24 h. The cells on the
lower surface of the membrane were stained with DAPI
(ProLong® Gold; Thermo Fisher Scientific Inc.) and
counted by four parts of the membrane. The values are presented as
the means ± SD from all independent experiments performed in
triplicate.
Drug-sensitivity assay in vitro
The cells were harvested using 0.25% Trypsin-EDTA
(Thermo Fisher Scientific Inc.). Primary cultured cells
(1×104/well) and cell lines (5×103/well) were
added to 96-well plates and exposed to crenolanib (Selleck
Chemicals LLC, Houston, TX, USA) and PDGFR-α antibody (MAB322-500;
R&D Systems, Abingdon, UK) 72 h later. The percentage of viable
cells was determined after 96 h using a TACS XTT Cell Proliferation
assay (Trevigen, Gaithersburg, MD, USA).
Statistical analysis
PDGFR-β expression levels in CRC and normal
colorectal mucosa, and the relationships between PDGFR-β
expression levels and clinicopathological factors were analysed
using Wilcoxon's rank sum and χ2 tests. Kaplan-Meier
survival curves were plotted and compared using the generalized
log-rank test. Prognostic factors were identified by univariate and
multivariate analyses using a Cox proportional hazards regression
model. In vitro assay results were analysed using Wilcoxon's
rank test. All test results were analysed using JMP software
version 11.2 (SAS Institute, Cary, NC, USA). A P value of <0.05
was considered to indicate a statistically significant
difference.
Results
Expression of PDGFR-β in clinical
tissue specimens
We determined PDGFR-β mRNA expression levels
in primary CRC and adjacent normal colorectal mucosa by
quantitative RT-PCR. PDGFR-β mRNA expression levels were
calculated as PDGFR-β/GAPDH expression for each sample
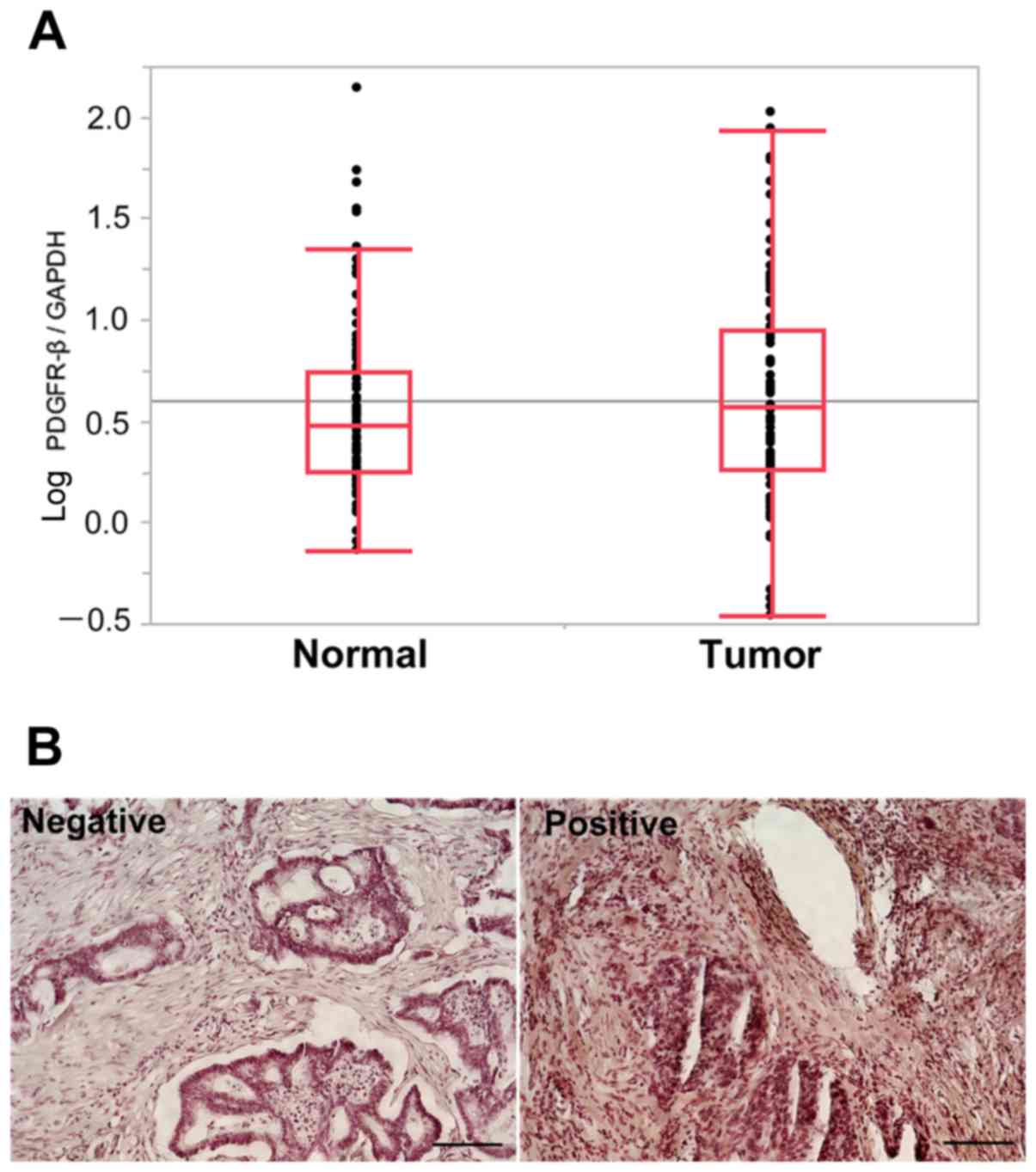
(Fig. 1A). There was no significant
difference in PDGFR-β mRNA expression levels between tumor
and normal tissues. The median PDGFR-β/GAPDH mRNA expression
ratio in tumor tissue was 3.01 (range, 0.16–105.97). Patients were
then divided into high- and low-expression groups according to the
median calculated PDGFR-β expression level.
Immunohistochemical detection of
PDGFR-β expression
PDGFR-β protein staining was observed in the
cytoplasm and cellular membrane of cancer cells (Fig. 1B). All sections were examined
independently for protein expression and scored as positive when
>50% of tissues in the examined area were stained. Among the 21
CRC specimens, five exhibited higher expression of the PDGFR-β
protein and 16 lower expression in cancer tissues (data not
shown).
The frequency of high PDGFR-β expression was in
accordance with the results for PDGFR-β mRNA expression. The
RT-PCR confirmed that all five of the tumors with high protein
expression levels, also had higher PDGFR-β mRNA expression
levels, whereas 12 of the 16 tumors with low protein expression had
lower mRNA levels, indicating that high expression of
PDGFR-β mRNA was associated with PDGFR-β protein expression
(P=0.003; χ2 test). We concluded that PDGFR-β
mRNA and protein levels were associated in patients with CRC.
Expression of PDGFR-β and
clinicopathological characteristics
We divided the samples into two groups according to
the PDGFR-β expression status for clinicopathological
evaluation. The relationships between the clinicopathological
factors and PDGFR-β expression status in the 194 patients
are summarized in Table II.
PDGFR-β expression was not significantly correlated with any
of the examined clinicopathological factors.
 | Table II.Clinicopathological factors and
PDGFR-β mRNA expression in 194 CRC patients. |
Table II.
Clinicopathological factors and
PDGFR-β mRNA expression in 194 CRC patients.
| Factors | Low expression
(n=97) | High expression
(n=97) | P-value |
|---|
| Age (years) |
|
| 0.196a |
|
<66 | 43 | 52 |
|
|
≥66 | 54 | 45 |
|
| Sex |
|
| 0.665a |
|
Male | 55 | 52 |
|
|
Female | 42 | 45 |
|
| Histological
grade |
|
| 0.516a |
|
Well-mod | 91 | 93 |
|
|
Otherb | 6 | 4 |
|
| Tumor invasion |
|
| 0.602a |
|
T1-2 | 7 | 9 |
|
|
T3-4 | 90 | 88 |
|
| Lymph node
metastasis |
|
| 0.378a |
| N0 | 41 | 35 |
|
|
N1-2 | 56 | 62 |
|
| Lymphatic
invasion |
|
| 0.662a |
|
Absent | 55 | 58 |
|
|
Present | 42 | 39 |
|
| Vascular
invasion |
|
| 1.000a |
|
Absent | 22 | 22 |
|
|
Present | 75 | 75 |
|
| Surgical
resection |
|
| 0.830a |
| R0 | 84 | 85 |
|
|
R1-2 | 13 | 12 |
|
Relationship between PDGFR-β
expression and prognosis
The median patient follow-up time was 3.78 years.
Disease-free survival (DFS) was evaluated in 169 patients with R0
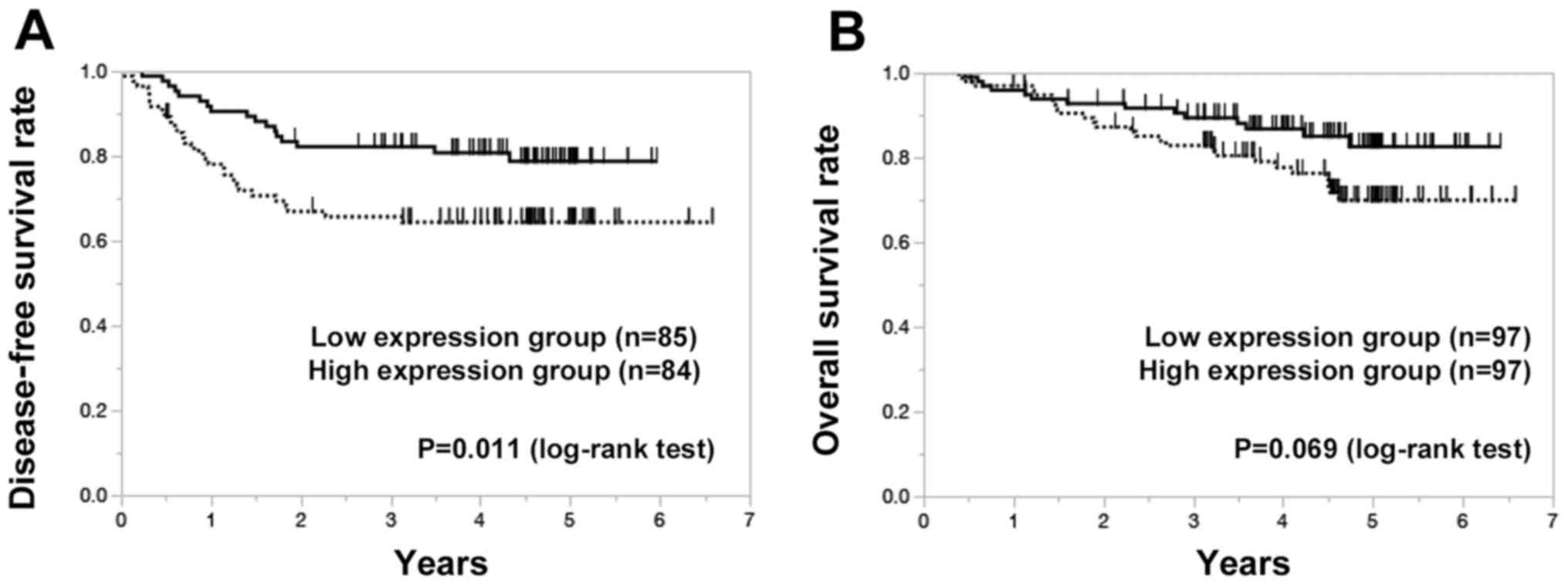
resection. Patients in the high-PDGFR-β expression group had
lower disease-free survival (DFS) compared with the low-expression
group (P=0.011) (Fig. 2A).
According to univariate analysis, lymph node metastasis
(P<0.001), positive lymphatic invasion (P=0.019), positive
vascular invasion (P=0.003) and high PDGFR-β expression
(P=0.019) were significantly correlated with DFS (Table III). Multivariate regression
analysis indicated that high PDGFR-β expression (P=0.040),
lymph node metastasis (P<0.001) and vascular invasion (P=0.010)
were independent predictors of DFS.
 | Table III.Univariate and multivariate analyses
of disease-free survival in CRC patients after R0 resection. |
Table III.
Univariate and multivariate analyses
of disease-free survival in CRC patients after R0 resection.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years,
<66/≥66) | 1.020 | 0.571–1.831 |
0.946 |
|
|
|
| Sex
(male/female) | 1.137 | 0.637–2.061 |
0.665 |
|
|
|
| Histological grade
(otherb/well-mod) | 0.973 | 0.159–3.152 |
0.970 |
|
|
|
| Tumor invasion
(T3-4/T1-2) | 2.512 | 0.776–15.397 |
0.141 |
|
|
|
| Lymph node
metastasis (N1-2/N0) | 8.320 | 3.609–24.114 |
<0.001a | 6.979 | 2.948–20.568 |
<0.001a |
| Lymphatic invasion
(present/absent) | 2.082 | 1.122–4.102 |
0.019a | 1.234 | 0.656–2.466 |
0.523a |
| Vascular invasion
(present/absent) | 3.702 | 1.397–12.316 |
0.003a | 3.170 | 1.279–10.559 |
0.010a |
| PDGFR-β
expression (high/low) | 2.015 | 1.119–3.743 |
0.019a | 1.851 | 1.027–3.443 |
0.040a |
According to univariate analysis, overall survival
(OS) was significantly lower in patients with T3/4 tumor invasion
(P=0.004), lymph node metastasis (P<0.001), positive lymphatic
invasion (P=0.038) and positive vascular invasion (P=0.005).
Multivariate regression analysis indicated that T3/4 tumor invasion
(P=0.030) and lymph node metastasis (P=0.002) were independent
predictors of OS (Table IV). The
5-year OS rates of patients with high and low PDGFR-β
expression were 70 and 83%, respectively (P=0.069) (Fig. 2B), after a median follow-up of 4.31
years.
 | Table IV.Univariate and multivariate analyses
of overall survival in CRC patients. |
Table IV.
Univariate and multivariate analyses
of overall survival in CRC patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years,
<66/≥66) | 1.426 | 0.759–2.727 |
0.270 |
|
|
|
| Sex
(male/female) | 0.930 | 0.496–1.764 |
0.822 |
|
|
|
| Histological grade
(otherb/well-mod) | 1.600 | 0.386–4.441 |
0.463 |
|
|
|
| Tumor invasion
(T3-4/T1-2) | NA | 2.252–2.252 |
0.004 | NA | NA | 0.030 |
| Lymph node
metastasis (N1-2/N0) | 7.033 | 2.806–23.552 |
<0.001a | 5.403 | 2.094–18.417 | 0.002 |
| Lymphatic invasion
(present/absent) | 2.059 | 1.038–4.446 |
0.038 | 1.225 | 0.606–2.691 | 0.585 |
| Vascular invasion
(present/absent) | 3.989 | 1.438–16.548 |
0.005 | 2.733 | 0.974–11.10 | 0.057 |
| PDGFR-β
expression (high/low) | 1.818 | 0.958–3.591 |
0.068 |
|
|
|
Effect of PDGFR-β inhibition in CRC
cell growth and invasion
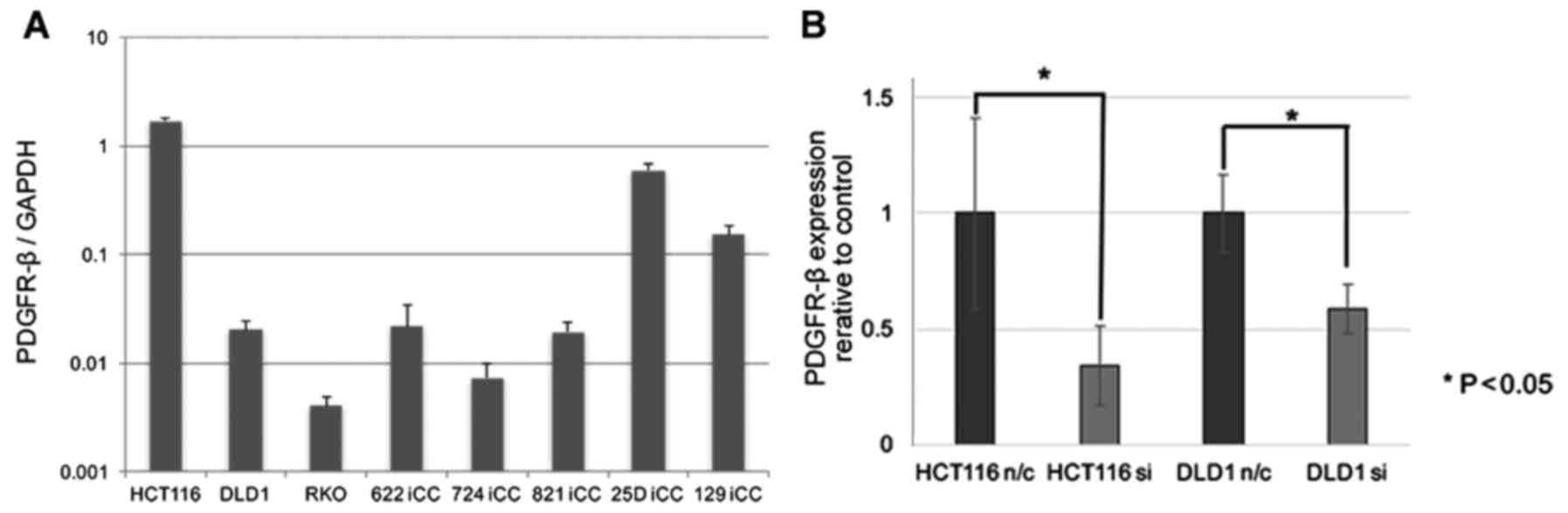
The expression of the PDGFR-β gene was
evaluated in three CRC cell lines and six primary cultured CRC
cells and all cells expressed PDGFR-β (Fig. 3A). CRC cell lines, HCT116 and DLD1
were subjected to siRNA knockdown. The biological role of
PDGFR-β in vitro was analyzed in CRC, in which
PDGFR-β expression was knocked down. Significant suppression
of endogenous PDGFR-β expression by siRNA was confirmed by
real-time RT-PCR (Fig. 3B). To
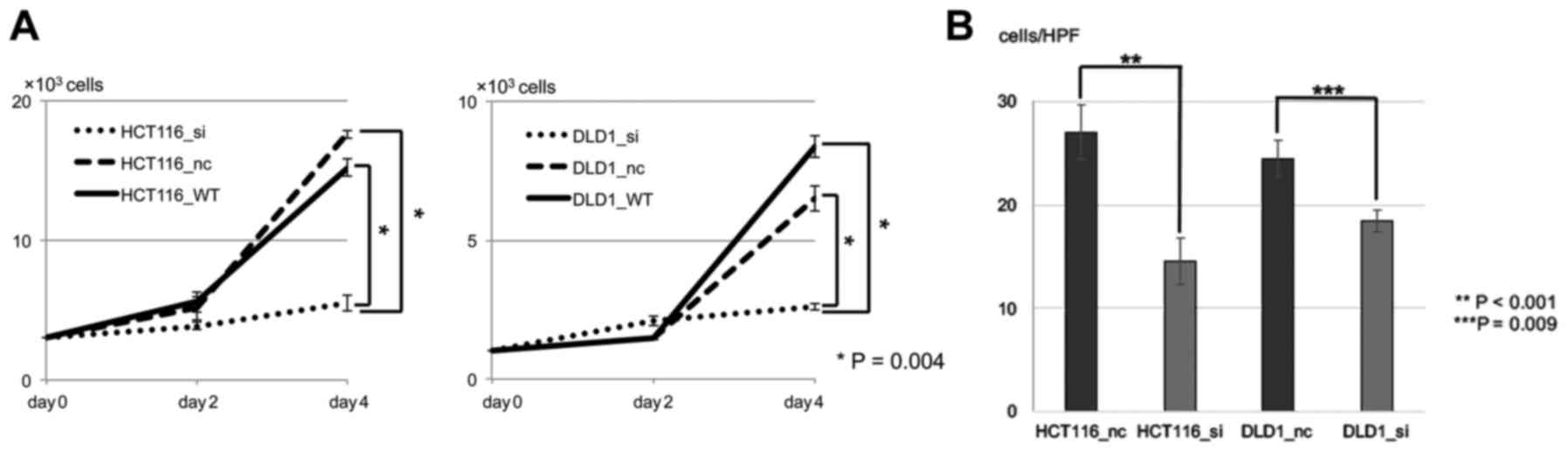
determine the proliferative properties, the cells were seeded and
cultured. There were significant differences in the numbers between
the wild-type or negative control and PDGFR-β siRNA
(P<0.05) in both CRC cell lines (Fig. 4A). There was no significant change
between the negative control and the wild-type. In addition, in
order to determine the invasive properties, an invasion assay was
performed. There were significant differences in numbers between
negative control and PDGFR-β siRNA (P<0.05) in both CRC
cell lines (Fig. 4B).
Effect of crenolanib on CRC cell
viability
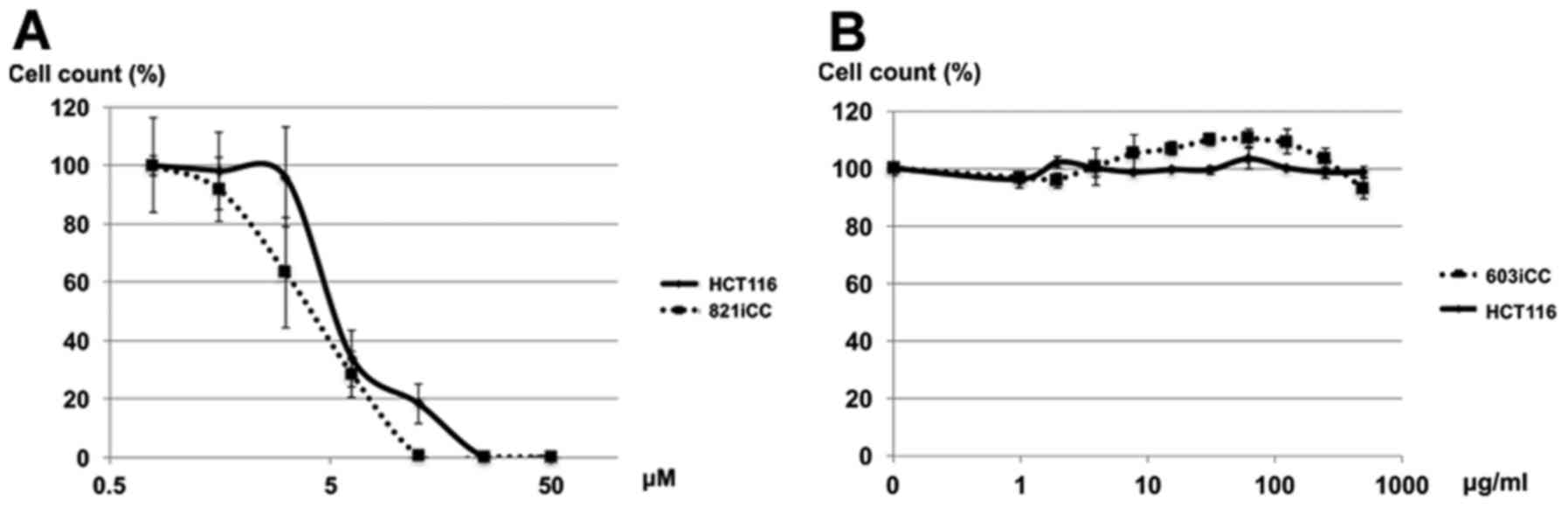
Human CRC cell lines and primary cultured cells were
both sensitive to crenolanib, according to the proliferation assay
(Fig. 5A), however they were not
sensitive to PDGFR-α antibody (Fig.
5B).
Discussion
The results of the present study revealed that high
PDGFR-β expression in cancer tissue was an independent
marker of poor prognosis relating to recurrence in patients with
CRC. High PDGFR-β expression levels were also associated with
shorter survival in patients with ovarian cancer and renal cell
carcinoma (11,17). Although high PDGFR-β
expression levels were not significantly associated with OS in the
present study, OS was relatively lower in the high-expression
group. To the best of our knowledge, this findings represented the
first evidence for PDGFR-β as a significant predictor of CRC
prognosis relating to recurrence after curative resection. These
results indicated the possible involvement of a PDGFR-β-dependent
pathway in the progression and metastasis of CRC.
In biological assessment, the present study revealed
that PDGFR-β expression was related to tumor malignancy in
CRC cell lines. The in vivo study revealed that siRNA
inhibition of PDGFR-β resulted in a significant reduction in
cell growth and invasion of CRC cell lines (P<0.05).
Furthermore, PDGFR has recently been reported as a possible new
therapeutic target in several solid tumors, such as breast cancer,
gastrointestinal stromal tumor, lung cancer and rhabdomyosarcoma
(8,18–20). A
PDGFR inhibitor decreased TGF-β-induced migration in human cells
in vitro and suppressed tumor growth in vivo in a
mouse hepatocarcinoma model (21).
PDGFR-β was also expressed in mesenchymal-like CRC cell lines in
vitro and was related to tumor invasion and liver metastasis
formation in mice (13). PDGFR-α
antibody did not inhibit the proliferation of CRC, while the PDGFR
inhibitor crenolanib inhibited CRC cell proliferation. These
findings indicated that PDGFR-β inhibitor inhibited cell
proliferation and that crenolanib may be a promising new treatment
for CRC through the inhibition of PDGFR-β.
The present study had some limitations. Notably, it
was a retrospective study with a relatively small sample size,
which may have limited its ability to detect a significant
relationship between PDGFR-β expression and OS. High
PDGFR-β expression was an independent prognostic factor in
DFS, however a PDGFR-β-dependent pathway in the progression and
metastasis of CRC was not clarified. Further studies with larger
samples are needed to confirm these findings.
In conclusion, PDGFR-β may be a useful prognostic
indicator and a potential therapeutic target in patients with
CRC.
Acknowledgements
We thank Ms. Aya Ito for her technical
assistance.
Glossary
Abbreviations
Abbreviations:
|
PDGFR
|
platelet-derived growth factor
receptor
|
|
CRC
|
colorectal cancer
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Thun MJ, DeLancey JO, Center MM, Jemal A
and Ward EM: The global burden of cancer: Priorities for
prevention. Carcinogenesis. 31:100–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Cervantes A, Nordlinger B
and Arnold: ESMO Guidelines Working Group: Metastatic colorectal
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25 Suppl 3:iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Cancer society, . Colorectal
cancer: What are the key statistics about colorectal cancer?
American cancer society website. http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-key-statisticsOctober
6–2015
|
|
4
|
Center for Cancer Control and Information
Services NCC, Japan Recent cancer statistics. 2014.http://ganjoho.jp/reg_stat/statistics/stat/summary.html03–August.
2016
|
|
5
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
Guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colvin H, Mizushima T, Eguchi H, et al:
Gastroenterological surgery in Japan: The past, the present and the
future. Ann Gastroenterol Surg. 1:5–10. 2017. View Article : Google Scholar
|
|
7
|
Ehnman M, Missiaglia E, Folestad E, Selfe
J, Strell C, Thway K, Brodin B, Pietras K, Shipley J, Östman A and
Eriksson U: Distinct effects of ligand-induced PDGFR α and PDGFRβ
signaling in the human rhabdomyosarcoma tumor cell and stroma cell
compartments. Cancer Res. 73:2139–2149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang P, Song L, Ge H, Jin P, Jiang Y, Hu W
and Geng N: Crenolanib, a PDGFR inhibitor, suppresses lung cancer
cell proliferation and inhibits tumor growth in vivo. Onco Targets
Ther. 7:1761–1768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weissmueller S, Manchado E, Saborowski M,
Morris JP IV, Wagenblast E, Davis CA, Moon SH, Pfister NT,
Tschaharganeh DF, Kitzing T, et al: Mutant p53 drives pancreatic
cancer metastasis through cell-autonomous PDGF receptor β
signaling. Cell. 157:382–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi Y, Bardsley MR, Toyomasu Y,
Milosavljevic S, Gajdos GB, Choi KM, Reid-Lombardo KM, Kendrick ML,
Bingener-Casey J, Tang CM, et al: Platelet-derived growth factor
receptor-α regulates proliferation of gastrointestinal stromal
tumor cells with mutations in KIT by stabilizing ETV1.
Gastroenterology. 149:420–432.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frödin M, Mezheyeuski A, Corvigno S,
Harmenberg U, Sandström P, Egevad L, Johansson M and Östman A:
Perivascular PDGFR- β is an independent marker for prognosis in
renal cell carcinoma. Br J Cancer. 116:195–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mezheyeuski A, Lindh Bradic M, Guren TK,
Dragomir A, Pfeiffer P, Kure EH, Ikdahl T, Skovlund E, Corvigno S,
Strell C, et al: Survival-associated heterogeneity of
marker-defined perivascular cells in colorectal cancer. Oncotarget.
7:41948–41958. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steller EJ, Raats DA, Koster J, Rutten B,
Govaert KM, Emmink BL, Snoeren N, van Hooff SR, Holstege FC, Maas
C, et al: PDGFRB promotes liver metastasis formation of
mesenchymal-like colorectal tumor cells. Neoplasia. 15:204–217.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith CC, Lasater EA, Lin KC, Wang Q,
McCreery MQ, Stewart WK, Damon LE, Perl AE, Jeschke GR, Sugita M,
et al: Crenolanib is a selective type I pan-FLT3 inhibitor. Proc
Natl Acad Sci USA. 111:5319–5324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis NL, Lewis LD, Eder JP, Reddy NJ, Guo
F, Pierce KJ, Olszanski AJ and Cohen RB: Phase I study of the
safety, tolerability, and pharmacokinetics of oral CP-868,596, a
highly specific platelet-derived growth factor receptor tyrosine
kinase inhibitor in patients with advanced cancers. J Clin Oncol.
27:5262–5269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH GM and Wittekind C: TNM
Classification of Malignant Tumors. 7th. Wiley-Blackwell; Oxford:
2010
|
|
17
|
Corvigno S, Wisman GB, Mezheyeuski A, van
der Zee AG, Nijman HW, Åvall-Lundqvist E, Östman A and Dahlstrand
H: Markers of fibroblast-rich tumor stroma and perivascular cells
in serous ovarian cancer: Inter- and intra-patient heterogeneity
and impact on survival. Oncotarget. 7:18573–18584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinrich MC, Griffith D, McKinley A,
Patterson J, Presnell A, Ramachandran A and Debiec-Rychter M:
Crenolanib inhibits the drug-resistant PDGFRA D842V mutation
associated with imatinib-resistant gastrointestinal stromal tumors.
Clin Cancer Res. 18:4375–4384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gril B, Palmieri D, Qian Y, Anwar T,
Liewehr DJ, Steinberg SM, Andreu Z, Masana D, Fernández P, Steeg PS
and Vidal-Vanaclocha F: Pazopanib inhibits the activation of
PDGFRβ-expressing astrocytes in the brain metastatic
microenvironment of breast cancer cells. Am J Pathol.
182:2368–2379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heske CM, Yeung C, Mendoza A, Baumgart JT,
Edessa LD, Wan X and Helman LJ: The role of PDGFR-β activation in
acquired resistance to IGF-1R blockade in preclinical models of
rhabdomyosarcoma. Transl Oncol. 9:540–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gotzmann J, Fischer AN, Zojer M, Mikula M,
Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H and
Mikulits W: A crucial function of PDGF in TGF-beta-mediated cancer
progression of hepatocytes. Oncogene. 25:3170–3185. 2006.
View Article : Google Scholar : PubMed/NCBI
|