Introduction
The 2017 Cancer Statistics revealed that colorectal
cancer (CRC) was the third most common cancer in the US (1). The common causes of CRC-related deaths
include late tumor diagnosis and rapid progression. Despite
substantial progress achieved in interventional radiology, surgery,
regional and systemic therapy for CRC in recent years, the overall
5-year survival rate remains unsatisfactory (2). Population screening programs for
identification of patients with early CRC stages is important for
increasing the survival of patients. At present, the precise
mechanisms of carcinogenesis in CRC are still unknown. Therefore,
seeking novel diagnostic biomarkers and therapeutic strategies is
necessary and urgent for the early detection of CRC before the
onset of metastasis in order to improve the survival rate of
patients with CRC.
MicroRNAs (miRNAs) are a class of small-non-coding
RNA molecules (−18–25 nucleotides in length). It has been
demonstrated that miRNAs could regulate gene expression at the
post-transcriptional level, and regulate several cellular
biological processes, such as apoptosis, proliferation and
migration. It has been reported that dysregulated miRNAs may play
important roles in the occurrence and development of CRC (3–5).
Presently, circulating miRNAs could be stably detected in plasma or
serum, as well as in other body fluids (6,7).
Therefore, miRNAs may act as potential biomarkers for diagnostic
and prognostic evaluation of CRC. In recent years, miR-485-5p has
received much attention as a functional miRNA. Previous studies
reported that miR-485-5p was significantly downregulated and
overexpression of miR-485-5p could suppress cell proliferation,
metastasis and promote cell apoptosis in many cancers (8–11).
Thus, miR-485-5p may function as a potential tumor suppressor.
However, the expression of miR-485-5p in CRC and its role in CRC
carcinogenesis are unknown.
Cluster of differentiation 147 (CD147) which belongs
to the immunoglobulin superfamily (IgSF) is a glycosylated cell
surface transmembrane protein, also known as basigin (BSG).
Previous studies have demonstrated that aberrant expression of
CD147 was observed in many cancers, and the increased expression of
CD147 in CRC was associated with poor prognosis (12–18).
In our previous studies, we found that CD147 significantly
contributed to tumor growth and metastasis in CRC (19,20).
In the present study, we confirmed the differential
expression of miR-485-5p in CRC cancer tissues and CRC cell lines,
and demonstrated that miR-485-5p could regulate cell proliferation,
migration and invasion by targeting CD147 in vitro.
miR-485-5p may play an important role in the pathogenesis of CRC as
a tumor suppressor, and serve as a potential diagnostic and
therapeutic target in CRC.
Materials and methods
Clinical tissue specimens
Forty-seven pairs of CRC tissue specimens and
adjacent non-tumor (ANT) tissues were obtained from Nanjing First
Hospital Affiliated to Nanjing Medical University. The tumor
tissues and non-tumor tissues were snap-frozen following surgery
and immediately stored at −80°C until use. The present study was
approved by the Medical Ethics Committee of Nanjing First Hospital
Affiliated to Nanjing Medical University, and all patients provided
written informed consents. Immunohistochemistry was performed using
Histostain®-SP kits (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer's instructions. Immunopositivity was evaluated
independently by two pathologists.
Plasmid constructs and generation of
stable cell clones
To construct the miR-485-5p overexpression vector,
the miR-485-5p precursor_RNA (85 bp) was synthesized by Synbio
Technologies (Suzhou, China), and then cloned into pcDNA3.1(+) in
NheI and EcoR sites (Invitrogen; Thermo Fisher
Scientific, Inc.), and named pcDNA3.1-miR-485-5p. The
anti-miR-485-5p and anti-miR-NC were synthesized by RiboBio Co.,
Ltd., (Guangzhou, China). We used TargetScan (http://www.targetscan.org/) to find the predicted
target of miR-485-5p. The sequence alignment confirmed that the
seed sequence of miR-485-5p was complementary to the 3′UTR of CD147
(BSG) in positions 229–257, 429–463 and 510–534. The wild-type and
mutant 3′untranslated region (UTR) (822 bp) of target gene BSG
(CD147) were synthesized by Synbio Technologies, and then cloned
into pmirGLO in NheI and SalI sites, named
CD147/3′UTR-WT and CD147/3′UTR-Mut. To construct the CD147
expression vector, the CD147 DNA (828 bp) was synthesized by Synbio
Technologies, and then cloned into pcDNA3.1(+) in NheI and
XhoI sites (Invitrogen; Thermo Fisher Scientific, Inc.), and
named pcDNA3.1-CD147. All constructs were further confirmed by
sequencing.
Cell lines and culture conditions
Cell lines HCT116, HT29, HCT8 (human colon cancer
cell lines) and FHC (normal human intestinal epithelial cell line)
were obtained from Shanghai Cell Collection, Chinese Academy of
Sciences. All aforementioned cell lines were maintained in
Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA). The cells were incubated in a
humidified incubator at 37°C with 5% CO2.
RNA extraction and real-time
quantitative PCR (RT-qPCR)
Total RNA was isolated from tissues and cell lines
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Reverse transcription
was performed using Prime-Script RT reagent kit (Takara Bio, Inc.,
Otsu, Japan). Quantitative PCR was performed on the cDNA using
specific primers (Sangon, Shanghai, China) for CD147 and β-actin
(as an internal control), which are listed in Table I. The expression of miR-485-5p was
detected by Stem-loop reverse transcriptase polymerase chain
reactions (RT-PCR). The primers for miR-485-5p and U6 snRNA were
purchased from RiboBio Co., Ltd. All reactions were carried out on
the Applied Biosystems 7500 Sequence Detection System (Applied
Biosystems, Foster City, CA, USA). Relative expression levels were
calculated as ratios normalized against those of β-actin or U6
snRNA. Comparative quantification was determined using the
2−ΔΔCt method. Each sample was prepared in triplicate
and all reactions were triplicated independently to ensure the
reproducibility of the results.
 | Table I.Primers of CD147 and β-actin for
real-time PCR. |
Table I.
Primers of CD147 and β-actin for
real-time PCR.
| Target | Primers |
|---|
| CD147 | Sense:
5′-CCATGCTGGTCTGCAAGTCAG-3′ |
|
| Antisense:
5′-CCGTTCATGAGGGCCTTGTC-3′ |
| β-actin | Sense:
5′-CTGGAACGGTGAAGGTGACA-3′ |
|
| Antisense:
5′-AAGGGACTTCCTGTAACAACGCA-3′ |
Stable cell line generation
The transfected CRC cells in six-well plates were
selected with G418 (1,000 µg/ml). Two weeks later, few cells
survived, and G418 was reduced to 500 µg/ml. A stable cell line
which could stably express miR-485-5p was established, and the
expression of miR-485-5p was assessed by Stem-loop RT-PCR.
CCK-8 assay
Cell growth viability was assessed using Cell
Counting Kit-8 (CCK-8) (Beyotime Institute of Biotechnology,
Shanghai, China). The cells were seeded in a 96-well plate at a
density of 1×104 cells/well and incubated for 24, 48,72
and 96 h, respectively. Following the addition of 10 µl of CCK-8
into the medium, each plate was assessed at 450 nm using the
microplate reader Tecan M200 PRO (Tecan Austria GmbH,
Untersbergstr, Austria).
In vitro invasion assay
For the invasion assays, Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) was polymerized in Transwell chambers for
45 min at 37°C. After the Matrigel was solidified, 1×105
transfected CRC cells were seeded in the top chamber with
serum-free medium, and the bottom chamber was filled with DMEM
containing 10% FBS.
After 24 h, the cells on the lower surface of the
membrane were fixed with alcohol and stained with crystal violet,
and then the number of cells were counted under a light microscope.
Each assay was carried out in triplicate and repeated three
times.
In vivo proliferation assay
All BALB/c nude mice were purchased from the
Comparative Medical Center of Yangzhou University and maintained
under specific pathogen-free conditions. The animal experiments
were approved by the Animal Care Committee of Nanjing Medical
College (acceptance no. SYXK20160006). The miR-485-5p transfected
HT29 cells were trypsinized to a single cell suspension, and then
subcutaneously injected into the flank area of adult (6–8-week-old)
athymic male nude mice. The developed tumors were examined weekly
and measured in two dimensions, and the tumor volume was calculated
according to the formula: (width)2 × length × 0.5. The
mice were euthanized 42 days post-inoculation. Animal experiments
were performed in accordance with the Institutional Guidelines for
Animal Care by the Nanjing Medical University, Jiangsu, China.
Luciferase assay
The 293T and HT29 cells were seeded in 96-well
plates, respectively. Co-transfection with either the empty vector
or pcDNA3.1-miR-485-5p and the luciferase reporter comprised of the
3′UTR of CD147, CD147/3′UTR-NC or CD147/3′UTR-Mut, using
Lipofectamine 2000 (Invitrogen-Life Technologies, CA, USA) was
performed. After 16 h the old medium was removed and DMEM which
contained 5% FBS was added. Then, the cells were harvested 48 h
after transfection and luciferase activity was assessed as
chemiluminescence using the Dual-Luciferase reporter assay system
(Tecan Austria GmbH, Untersbergstr, Austria) according to the
manufacturer's protocol.
Western blot analysis
The transfected CRC cells were harvested and lysed
by three cycles of freeze/thaw at −80°C. Total protein was
separated by 10% SDS-PADE gels, and transferred to a polyvinylidene
difluoride (PVDF) membrane. The membrane was blocked with 5%
skimmed milk powder (soluble in TBST buffer solution) at 4°C for 2
h, and then the membrane was incubated with mouse anti-CD147
primary antibodies (1:5,000; cat. no. Ab108317) and GAPDH
(1:10,000; cat. no. ab181602,) at room temperature for 2 h,
followed by secondary antibodies goat anti-rabbit IgG-HRP
(1:10,000; cat. no. Ab6721; Abcam, Inc., Cambridge, UK) for 1 h at
room temperature. The proteins were visualized by ECL detection
system (GE ImageQuant LAS 4000; GE Healthcare Bio-Sciences,
Uppsala, Sweden).
Statistical analysis
The SPSS 15.0 software (SPSS, Inc., Chicago, IL,
USA) was used for general statistical. Experimental data are
presented as the mean ± standard deviation (SD) and analyzed by
Student's t-test or one-way analysis of variance (ANOVA), and the
criterion for statistical significance was considered to be
P<0.05.
Results
miR-485-5p is downregulated in CRC
tissues and cell lines
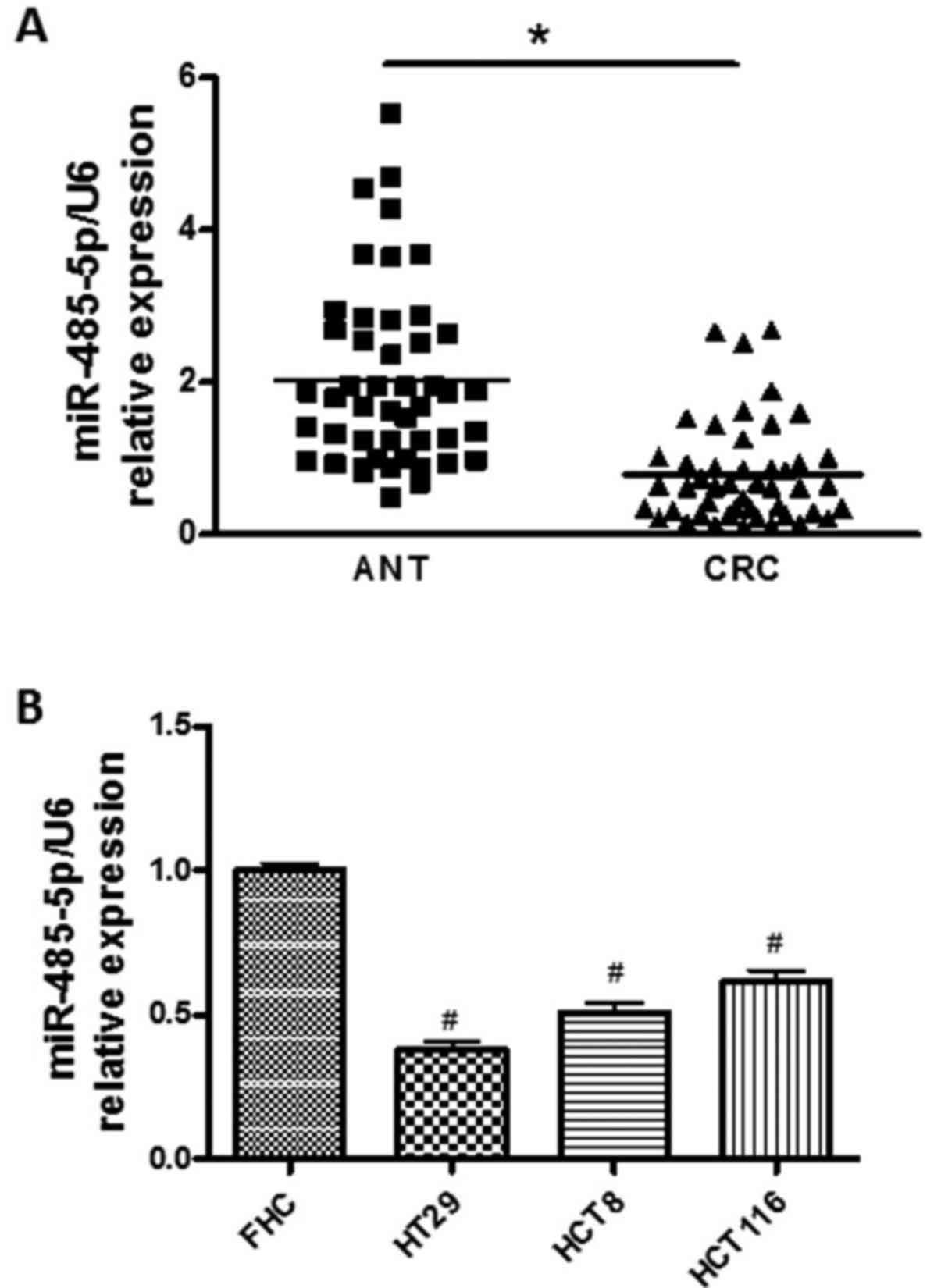
To identify the expression of miR-485-5p in the CRC,
forty-seven pairs of CRC and ANT tissues were assessed by RT-qPCR.
As shown in Fig. 1A, the expression
levels of miR-485-5p were downregulated in CRC tissues compared
with ANT tissues (P<0.05). The miR-485-5p expression in CRC cell
lines (HCT116, HT29, HCT8) were also detected and compared to the
level of normal human intestinal epithelial cell line (FHC). As
shown in Fig. 1B, the expression
levels of miR-485-5p in all CRC cell lines (HCT116, HT29, HCT8)
were lower than that of the FHC cell line, and miR-485-5p
expression in the HT29 cells was relatively the lowest.
miR-485-5p inhibits CRC cell
proliferation and invasion in vitro
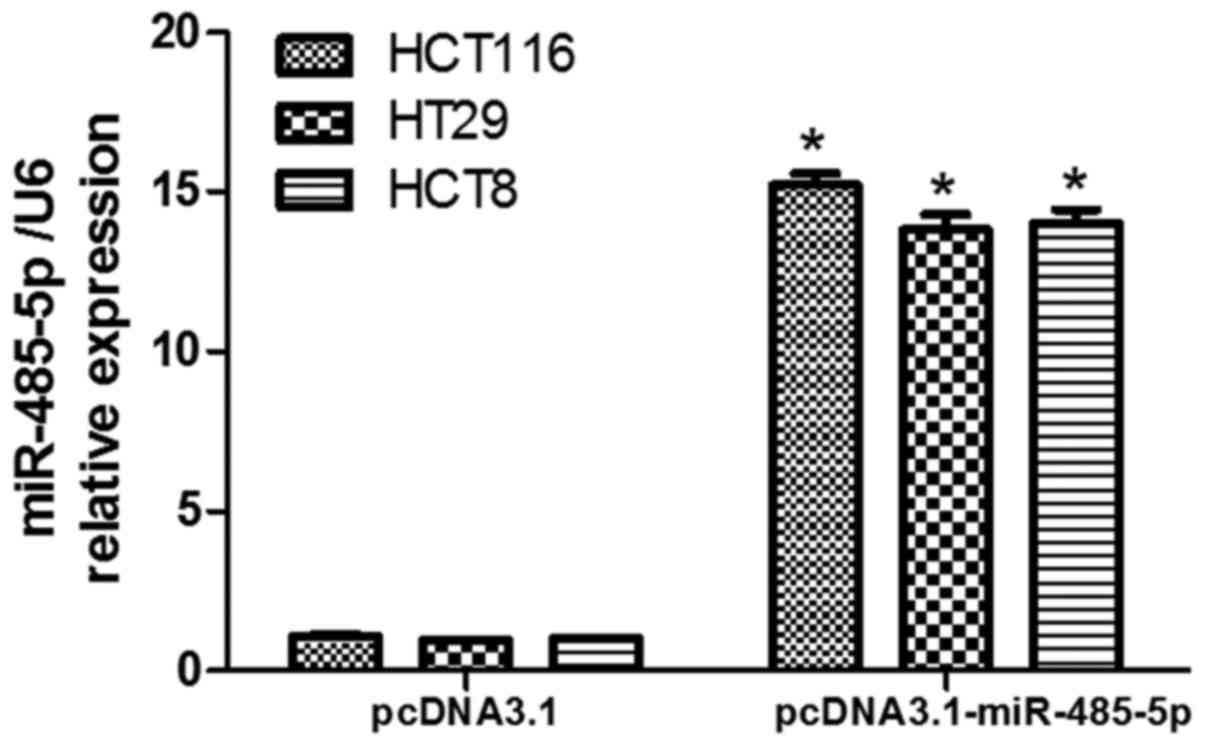
We then investigated the effects of miR-485-5p on
cell proliferation and invasion in CRC cells. First, we transfected
miR-485-5p overexpression vector pcDNA3.1-miR-485-5p into HCT116,
HT29 and HCT8 cells to increase miR-485-5p expression, and the
stably transfected cell lines were separated by G418. The results
revealed that the expression of miR-485-5p in CRC cells transfected
with the miR-485-5p overexpression vector was increased compared
with the control transfected cells, respectively (P<0.05,
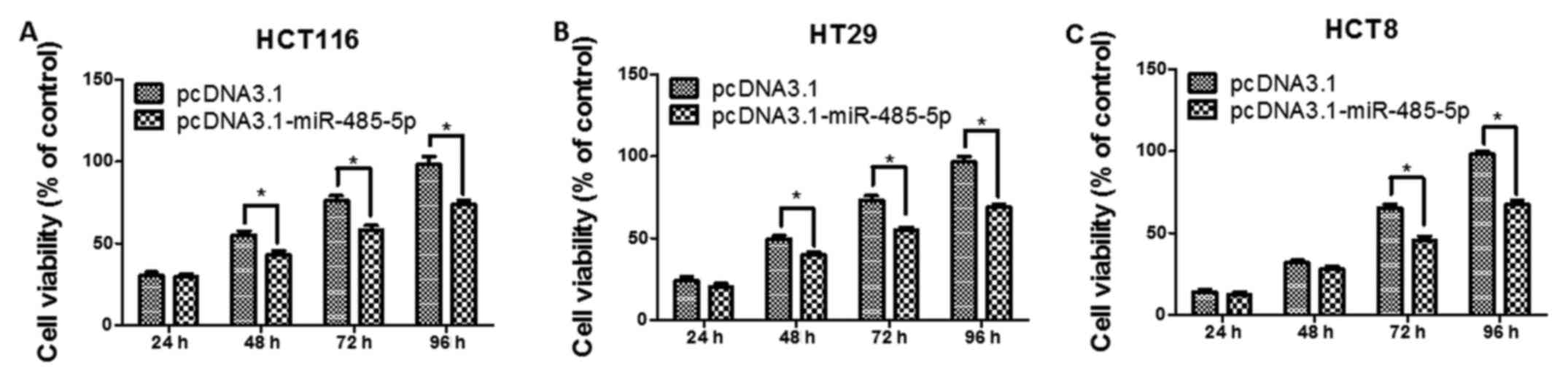
Fig. 2). Second, we performed CCK-8
assays in transfected CRC cells, and the results revealed that the
proliferation of CRC cells transfected with miR-485-5p
overexpression vector was significantly decreased compared with the
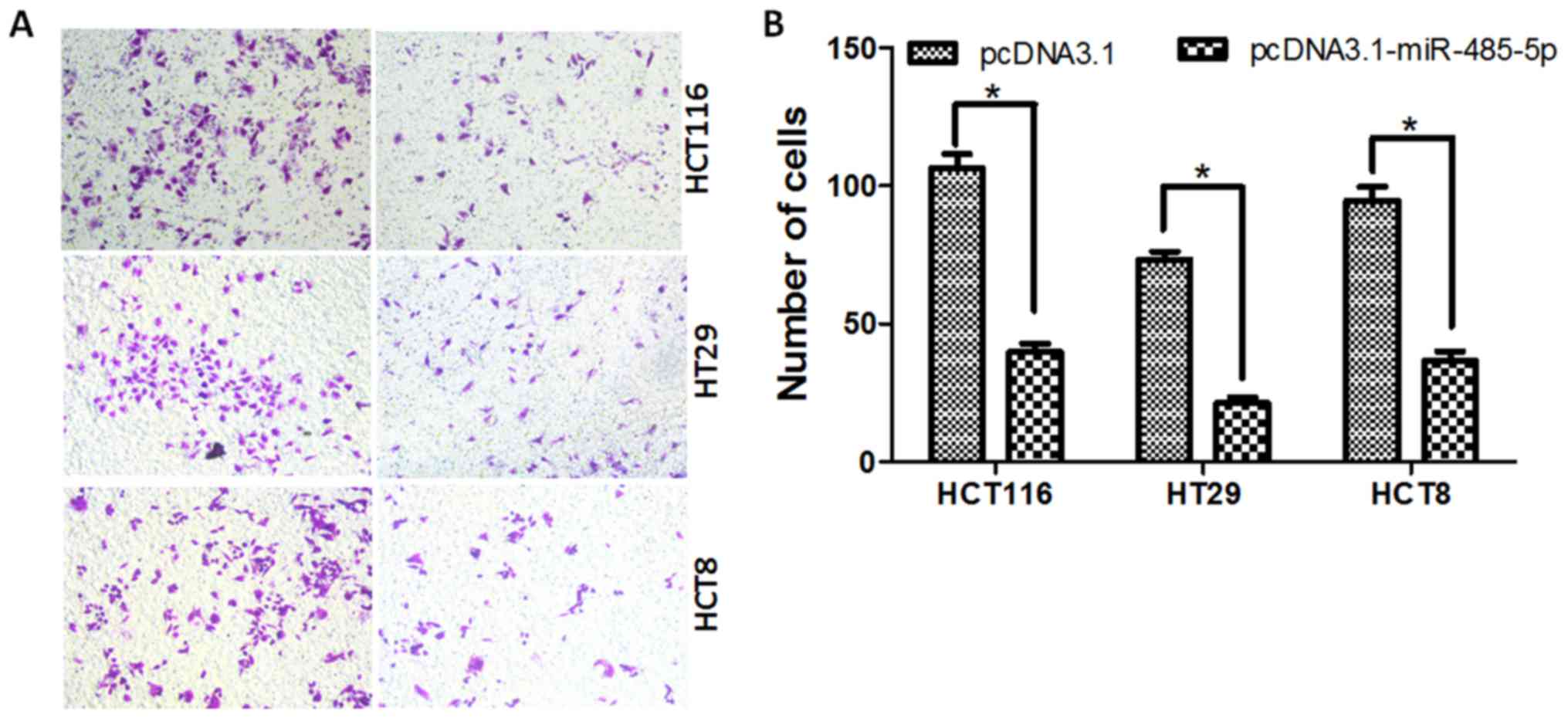
control transfected cells (P<0.05, Fig. 3). Moreover, the results of the cell
invasion assay revealed that overexpression of miR-485-5p could
reduce the invasion rate of HT29, HCT116 and HCT8 cells compared
with the control, respectively (Fig.
4).
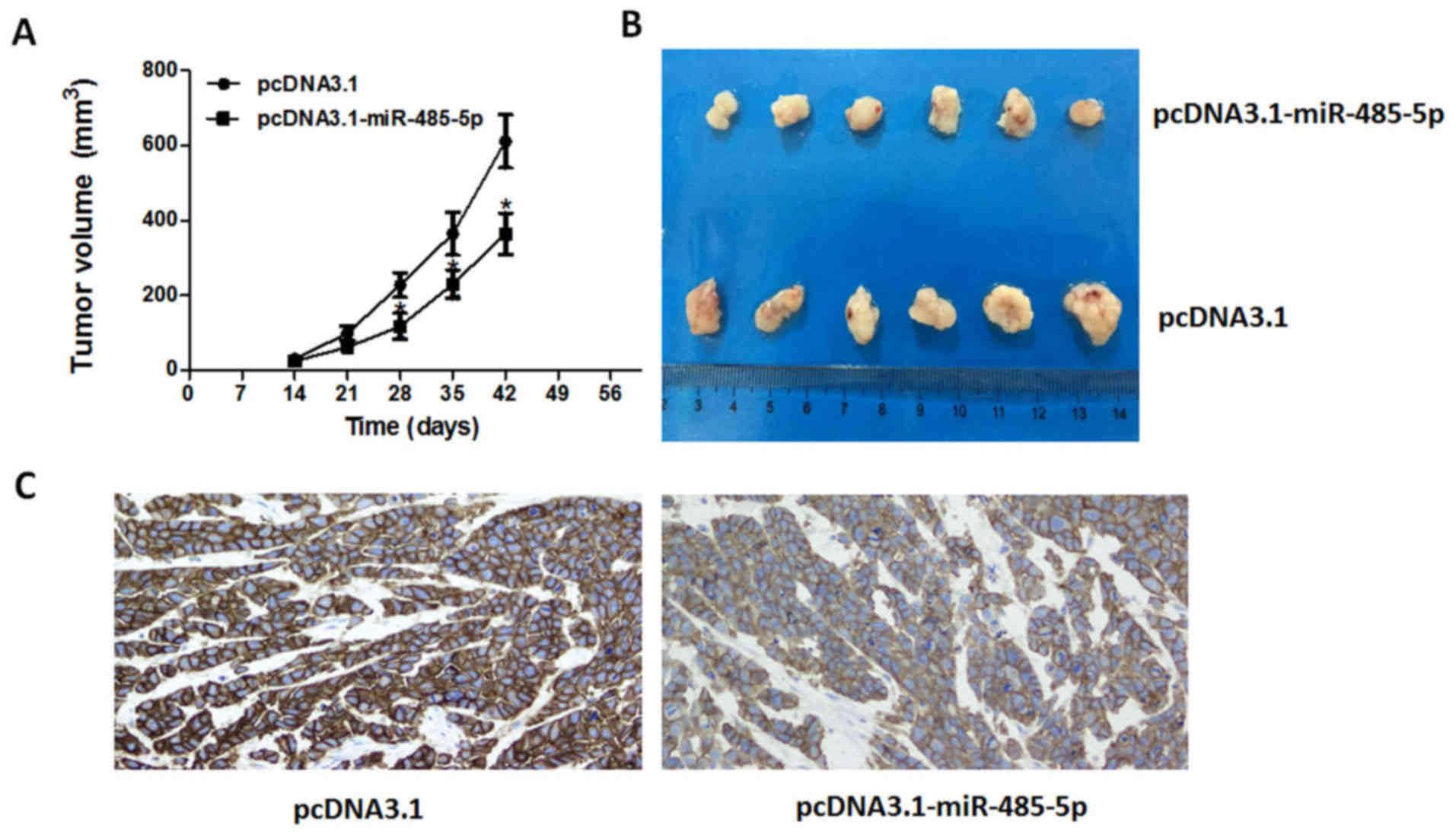
miR-485-5p inhibits CRC tumor growth
in vivo
To investigate whether the overexpression of
miR-485-5p could inhibit CRC development in vivo, we
established the CRC xenograft models with transfected HT29 cells.
The results revealed that overexpression of miR-485-5p could
significantly inhibit the growth of tumors during the same
observation period compared with the control group in HT29
xenograft models, as shown in Fig. 5A
and B.
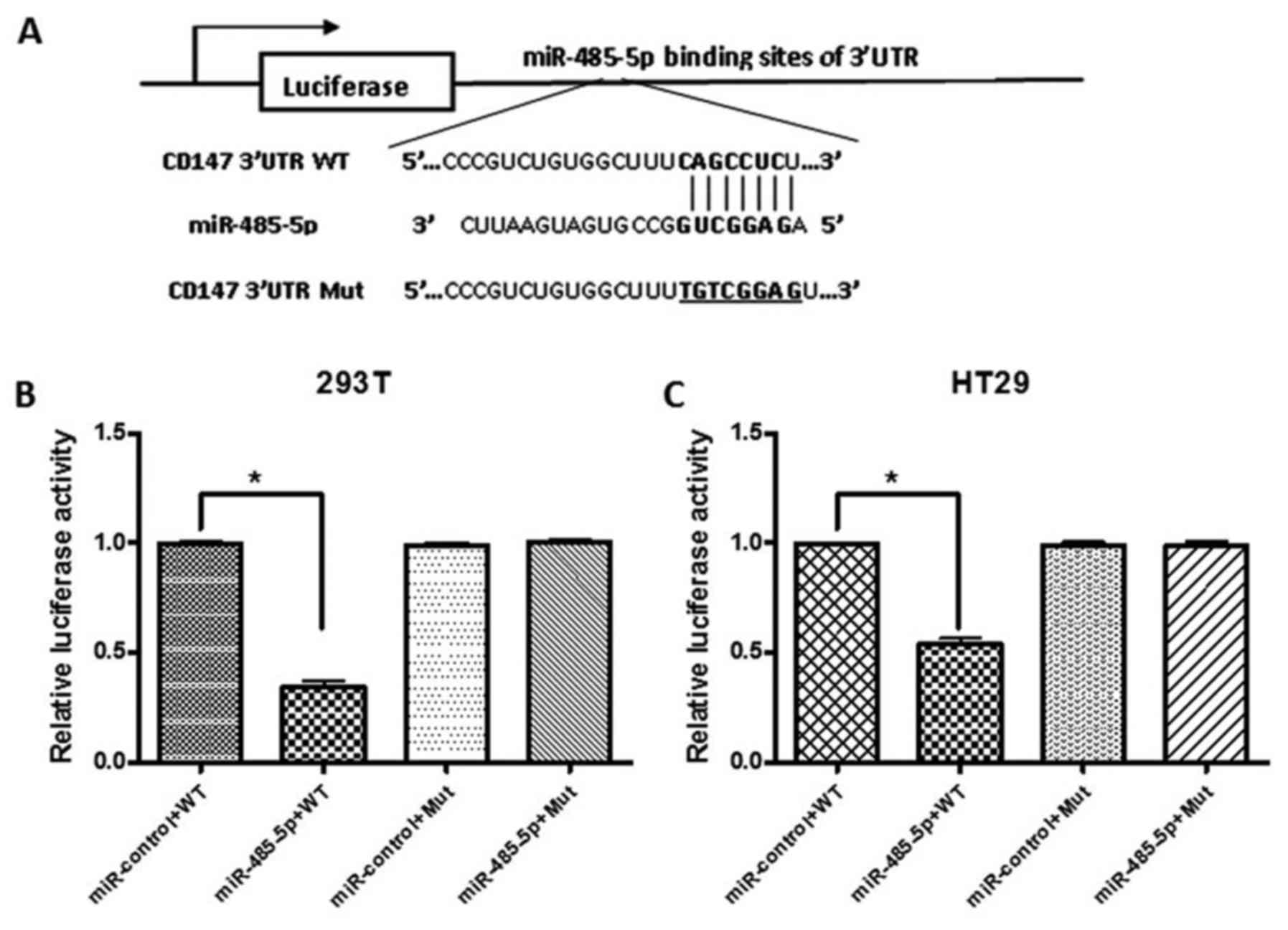
CD147 is a target gene of
miR-485-5p
We then investigated the mechanisms by which
miR-485-5p inhibited CRC progression. Bioinformatics analysis
revealed that the CD147 3′UTR was the target of miR-485-5p, so the
CD147/3′UTR-WT and CD147/3′UTR-Mut with a substitution of
nucleotides within the miR-485-5p binding site were constructed
(Fig. 6A). The luciferase reporter
assays were performed to ascertain whether miR-485-5p directly
targeted CD147. Co-transfection of 293T and HT29 cells with
CD147/3′UTR-WT and pcDNA3.1-miR-485-5p caused a significant
decrease in the luciferase activity compared with the control, and
the luciferase activity of the CD147/3′UTR-Mut group was rescued
(Fig. 6B and C, P<0.05). The
results confirmed that CD147 was the target gene of miR-485-5p in
CRC cells.
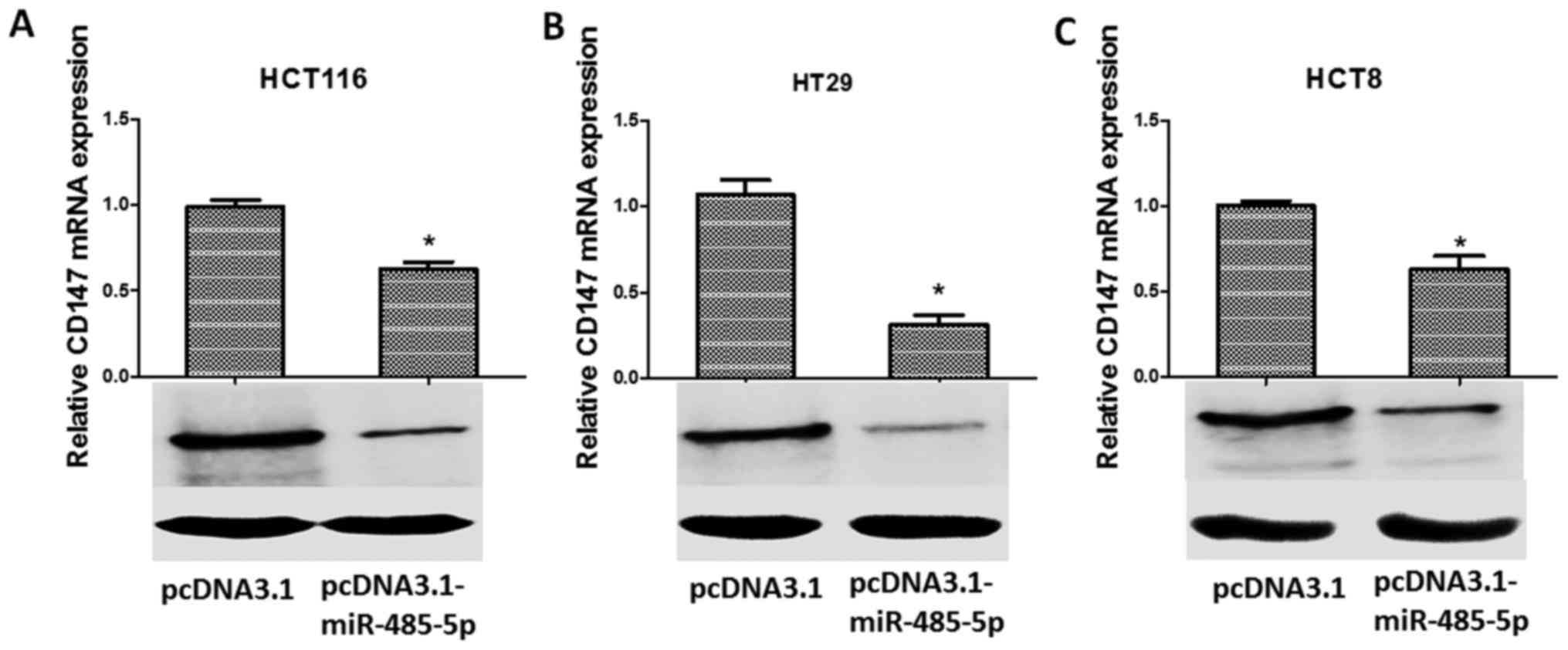
miR-485-5p negatively regulates CD147
gene expression
In order to explore the relationship between CD147
and miR-485-5p, the expression of CD147 in transfected CRC cells
was detected by RT-PCR and western blotting. The results revealed
that overexpression of miR-485-5p in CRC cells significantly
inhibited the expression of CD147 at the mRNA level (Fig. 7A-C, upper panels; P<0.05) and
protein level (Fig. 7A-C, lower
panels). The results revealed that the upregulation of miR-485-5p
could downregulate the expression of CD147. Moreover, the
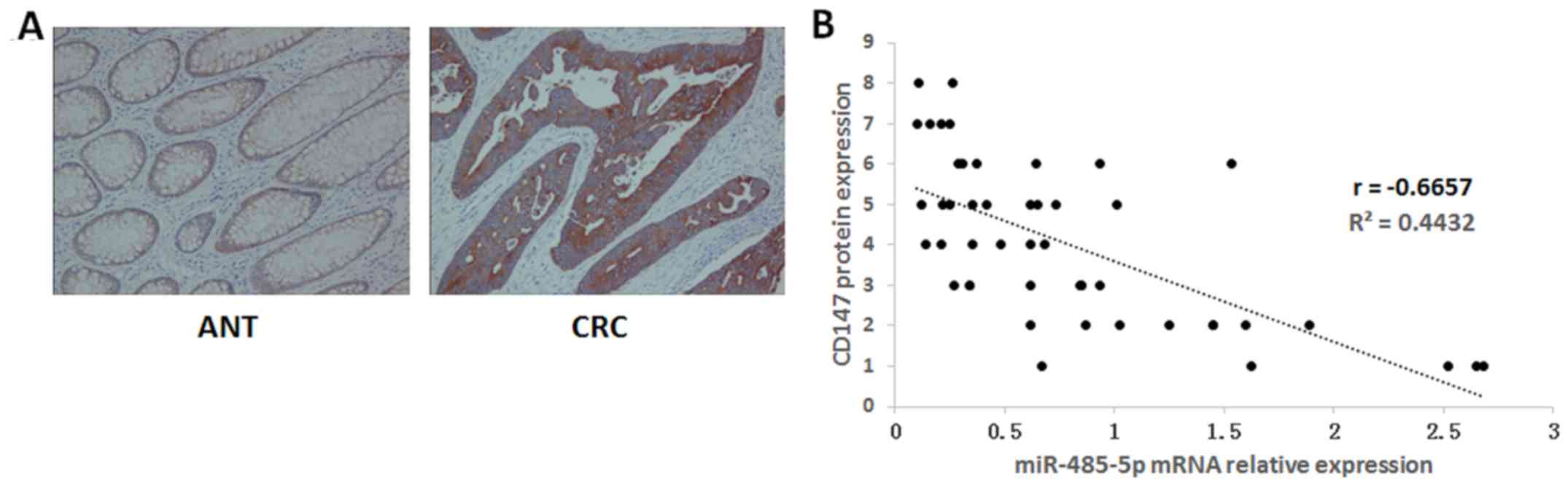
expression of the CD147 protein in forty-seven pairs of CRC and ANT
tissues was detected by immunohistochemistry. The results revealed
that the positive rate of CD147 was much higher in CRC (35/47) than
that in ANT (15/47) (Fig. 8A). In
addition, we detected the expression of miR-485-5p mRNA in CRC and
ANT tissues by RT-PCR. The correlation analyses revealed that the
expression of the CD147 protein was inversely correlated with the
levels of miR-485-5p expression in CRC tissues (Fig. 8B), suggesting that miR-485-5p could
negatively regulate CD147 expression in CRC tissues.
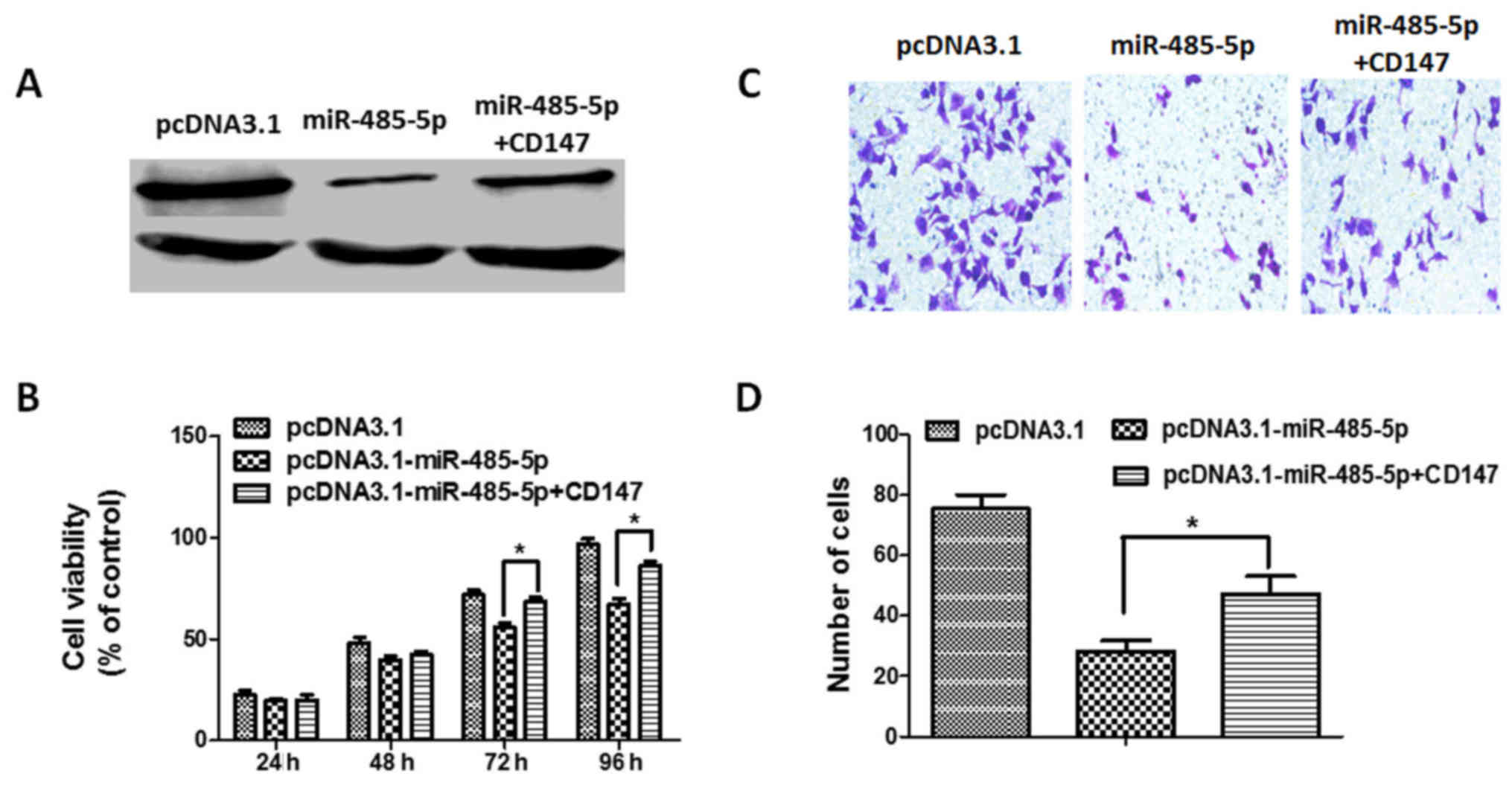
miR-485-5p regulates CRC cell
proliferation and invasion by downregulating CD147
In order to confirm that CD147 is the target gene of
miR-485-5p, rescue experiments with CD147 in miR-485 overexpressed
CRC cells were performed. We first constructed the CD147 expression
vector pcDNA3.1-CD147, and co-transfected it with either the empty
vector or pcDNA3.1-miR-485-5p in HT29 cells, and confirmed the
efficiency of transfection by western blotting (Fig. 9A). Next, we performed a CCK-8 assay
in HT29 cells, and the results indicated that CD147 overexpression
partly reversed the inhibition of cell proliferation by miR-485
overexpression (Fig. 9B).
Furthermore, the invasion assay indicated that CD147 overexpression
partly overcame the inhibition of cell invasion by miR-485-5p
overexpression (Fig. 9C and D).
Additionally, immunohistochemistry staining was performed in
implanted tumors to detect the protein expression of CD147. As
shown in Fig. 5C, the CD147 protein
expression in the miR-485-5p overexpression group was significantly
decreased compared with the control group. Collectively, the
results revealed that CD147 downregulation by miR-485-5p
contributed to the inhibition of CRC cell proliferation and
invasion.
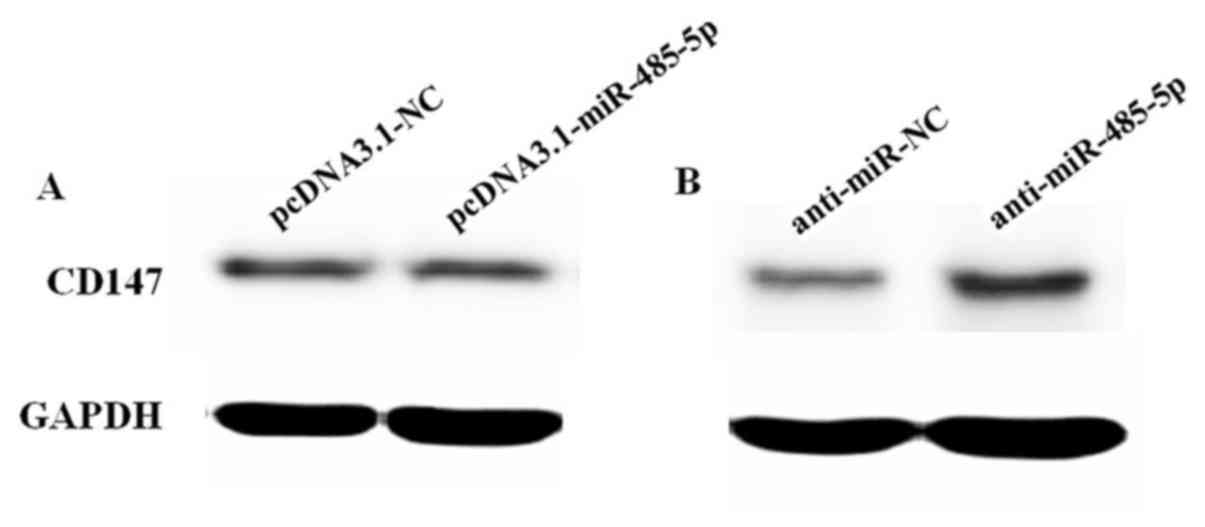
Furthermore, we transfected pcDNA3.1-miR-485-5p into
FHC cells (normal human intestinal epithelial cell line) to
investigate the effects on CD147 expression. The results revealed
that the expression of CD147 exhibited no significant alteration
when the expression of miR-485-5p was increased (Fig. 10A). Then, we transfected
anti-miR-485-5p into FHC cells. The results revealed that the
expression of CD147 in cells transfected with anti-miR-485-5p was
increased compared with the control (Fig. 10B).
Discussion
Colorectal cancer has become the third most common
cancer in the US (1). In
economically developing countries, the morbidity and mortality of
CRC is rising, especially in China (21). There is great clinical value in
identifying novel diagnostic or prognostic biomarkers that could
improve the outcome of this disease.
Recently, research revealed that miRNAs may play
important roles in the tumorigenesis and development of CRC. To
date, the effect of miR-485-5p in human cancer has not been
extensively reported. Recently, Chen et al revealed that
miR-485-5p inhibited bladder cancer metastasis by targeting high
mobility group AT-hook 2 (HMGA2) (11). Lou et al demonstrated that
overexpression of miR-485-5p suppressed mitochondrial respiration
and potential for cell migration and invasion in vitro, and
also inhibited spontaneous metastasis of breast cancer cells in
vivo. The suppression of mitochondrial respiration and cell
invasion could be partially relieved by restoration of PGC-1α
expression, suggesting that miR-485-5p inhibited breast cancer cell
migration and invasion by inhibiting PGC-1α expression (22). Kang et al reported that
upregulation of miR-485-5p could decrease the expression of Flot1
in gastric cancer cells, and ectopic expression of Flot1 partially
reversed the inhibitory effect of enforced miR-485-5p expression on
the malignant phenotypes of gastric cancer cells, suggesting that
miR-485-5p could be a potential prognostic marker and function as a
tumor suppressor in human gastric cancer by post-transcriptionally
targeting Flot1 (10). Guo et
al demonstrated that overexpression of miR-485-5p mimics could
inhibit, while its antisense oligos promoted cell proliferation and
invasion, and the dual-luciferase reporter gene assays and western
blotting further revealed that stanniocalcin 2 was a direct target
of miR-485-5p (23). In the present
study, we demonstrated that the expression of miR-485-5p was lower
in CRC tissues and cell lines than that in the normal controls.
Overexpression of miR-485-5p in HCT116, HT29 and HCT8 cells
significantly suppressed the proliferation and invasion capability
of CRC cells in vitro. Moreover, overexpression of
miR-485-5p could also inhibit the CRC tumor growth in
vivo.
Previous studies found that the expression of CD147
was overexpressed in CRC and was associated with poor prognosis
(17–19). In the present study, we used a dual
luciferase system to ascertain that CD147 was the target gene of
miR-485-5p. We found that CD147 mRNA and protein were suppressed
when miR-485-5p was overexpressed in CRC cells. In addition, the
expression of CD147 was negatively correlated to miR-485-5p in CRC
tissues. Our results revealed that overexpression of miR-485-5p
could inhibit the proliferation and invasion of CRC cells in
vitro, and inhibit CRC tumor growth in vivo. In
addition, CD147 contained the binding site for miR-485-5p, and
overexpression of miR-485-5p could significantly suppress the
expression of CD147.
The expression of CD147 in implanted tumors was
detected by imm-unohistochemistry, and the results revealed that
the CD147 protein expression in the miR-485-5p-overexpressed groups
was significantly decreased compared with the control groups. The
results revealed that overexpression of miR-485-5p may inhibit
tumor growth by targeting CD147. In our previous study it was
demonstrated that CD147 was highly expressed in CRC cells, and
inhibition of CD147 expression reduced the ability of invasion in
CRC cells (24). The possible
mechanism involved was that CD147 silencing inhibited the secretion
of MMPs. Moreover, CD147 silencing was able to increase the
concentration of lactate thus leading to MCT1 protein reduction. In
addition, the increase in lactate concentration may reduce cell
growth or other tumor-associated biological activities.
In order to investigate the effects of
anti-miR-485-5p on CRC cell proliferation and invasion, we
transfected anti-miR-485-5p or anti-miR-NC into HT29 cells,
respectively. However, the results revealed that the inhibition of
miR-485-5p expression was not enough to affect the expression of
CD147, and had no significant effect on cell proliferation and
invasion (data not shown). Furthermore, we evaluated the effects of
miR-485 overexpression and miR-485-5p inhibition on normal human
intestinal epithelial cell line (FHC). The results revealed that
simply increasing the expression of miR-485-5p did not
significantly affect the expression of CD147. It is possible that
the expression of CD147 was very low in normal cells. However,
inhibition of miR-485-5p expression could increase the expression
of CD147, suggesting that miR485-5p was an important regulator of
CD147 expression. To the best of our knowledge, this is the first
study to identify the function of miR-485-5p in CRC, and our
results revealed that miR-485-5p may perform its effect partly
through the inhibition of CD147.
In conclusion, miR-485-5p is downregulated in CRC,
and miR-485-5p inhibits tumor development by targeting the CD147
gene in CRC. The miR-485-5p/CD147 link may provide novel diagnostic
biomarkers and therapeutic strategies for CRC.
An in depth future study by the present authors will
be performed continuing to investigate the functional mechanism of
the miR-485-5p/CD147 link, and the diagnostic and prognostic values
of circulating miR-485-5p in CRC patients.
Acknowledgements
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciombor KK, Wu C and Goldberg RM: Recent
therapeutic advances in the treatment of colorectal cancer. Annu
Rev Med. 66:83–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren A, Dong Y, Tsoi H and Yu J: Detection
of miRNA as non-invasive biomarkers of colorectal cancer. Int J Mol
Sci. 16:2810–2823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng CY, Zhan YS, Huang J and Chen YX:
MicroRNA-7 suppresses human colon cancer invasion and proliferation
by targeting the expression of focal adhesion kinase. Mol Med Rep.
13:1297–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slaby O: Non-coding RNAs as biomarkers for
colorectal cancer screening and early detection. Adv Exp Med Biol.
937:153–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang L, Cheng Q, Zhang BH and Zhang MZ:
Circulating microRNAs as biomarkers in hepatocellular carcinoma
screening: A validation set from China. Medicine (Baltimore).
94:e6032015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khoury S and Tran N: Circulating
microRNAs: Potential biomarkers for common malignancies. Biomark
Med. 9:131–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He N, Zheng H, Li P, Zhao Y, Zhang W, Song
F and Chen K: miR-485-5p binding site SNP rs8752 in HPGD gene is
associated with breast cancer risk. PLoS One. 9:e1020932014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TH, Kim YK, Kwon Y, Heo JH, Kang H,
Kim G and An HJ: Deregulation of miR-519a, 153, and 485-5p and its
clinicopathological relevance in ovarian epithelial tumours.
Histopathology. 57:734–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang M, Ren MP, Zhao L, Li CP and Deng MM:
miR-485-5p acts as a negative regulator in gastric cancer
progression by targeting flotillin-1. Am J Transl Res. 7:2212–2222.
2015.PubMed/NCBI
|
|
11
|
Chen Z, Li Q, Wang S and Zhang J:
miR-485-5p inhibits bladder cancer metastasis by targeting HMGA2.
Int J Mol Med. 36:1136–1142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallagher SM, Castorino JJ, Wang D and
Philp NJ: Monocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231. Cancer Res. 67:4182–4189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato M, Nakai Y, Nakata W, Yoshida T,
Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H and Nonomura
N: EMMPRIN promotes angiogenesis, proliferation, invasion and
resistance to sunitinib in renal cell carcinoma, and its level
predicts patient outcome. PLoS One. 8:e743132013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Omi Y, Shibata N, Okamoto T, Obara T and
Kobayashi M: The role of CD147 in the invasiveness of follicular
thyroid carcinoma cells. Thyroid. 22:383–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stenzinger A, Wittschieber D, von
Winterfeld M, Goeppert B, Kamphues C, Weichert W, Dietel M, Rabien
A and Klauschen F: High extracellular matrix metalloproteinase
inducer/CD147 expression is strongly and independently associated
with poor prognosis in colorectal cancer. Hum Pathol. 43:1471–1481.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu S, Chu D, Zhang Y, Wang X, Gong L, Han
X, Yao L, Lan M, Li Y and Zhang W: EMMPRIN/CD147 expression is
associated with disease-free survival of patients with colorectal
cancer. Med Oncol. 30:3692013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng HC, Wang W, Xu XY, Xia P, Yu M,
Sugiyama T and Takano Y: Up-regulated EMMPRIN/CD147 protein
expression might play a role in colorectal carcinogenesis and its
subsequent progression without an alteration of its glycosylation
and mRNA level. J Cancer Res Clin Oncol. 137:585–596. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, He B, Song G, Bao Q, Tang Z, Tian F
and Wang S: CD147 silencing via RNA interference reduces tumor cell
invasion, metastasis and increases chemosensitivity in pancreatic
cancer cells. Oncol Rep. 27:2003–2009. 2012.PubMed/NCBI
|
|
20
|
Pan Y, He B, Chen J, Sun H, Deng Q, Wang
F, Ying H, Liu X, Lin K, Peng H, et al: Gene therapy for colorectal
cancer by adenovirus-mediated siRNA targeting CD147 based on loss
of the IGF2 imprinting system. Int J Oncol. 47:1881–1889. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gray RT, Coleman HG, Hughes C, Murray LJ
and Cardwell CR: Statin use and survival in colorectal cancer:
Results from a population-based cohort study and an updated
systematic review and meta-analysis. Cancer Epidemiol. 45:71–81.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y
and Li Z: miR-485-3p and miR-485-5p suppress breast cancer cell
metastasis by inhibiting PGC-1α expression. Cell Death Dis.
7:e21592016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo GX, Li QY, Ma WL, Shi ZH and Ren XQ:
MicroRNA-485-5p suppresses cell proliferation and invasion in
hepatocellular carcinoma by targeting stanniocalcin 2. Int J Clin
Exp Pathol. 8:12292–12299. 2015.PubMed/NCBI
|
|
24
|
Li R, Pan Y, He B, Xu Y, Gao T, Song G,
Sun H, Deng Q and Wang S: Downregulation of CD147 expression by RNA
interference inhibits HT29 cell proliferation, invasion and
tumorigenicity in vitro and in vivo. Int J Oncol.
43:1885–1894. 2013. View Article : Google Scholar : PubMed/NCBI
|