Introduction
Osteosarcoma is the most common malignant bone tumor
affecting bone growth, particularly in children and adolescents
(1). Osteosarcoma is closely
associated with lung metastasis, causing death in ~15–25% of
osteosarcoma patients (2). The
survival rate for patients with metastatic disease or with tumor
recurrence is <20% (3). Despite
developments in osteosarcoma therapy, the relative 5-year survival
rate of osteosarcoma patients remains only 60–70% due to high
malignancy, invasion and metastasis (4). Therefore, it is of great importance to
clarify the mechanism of osteosarcoma and develop new therapeutic
strategies to prevent metastasis in osteosarcoma and improve
patient prognosis.
Glycoprotein non-metastatic melanoma protein B
(GPNMB), also known as osteoactivin, encodes the type I
transmembrane proteins of 572 amino acids (5). GPNMB contains an extracellular domain,
a transmembrane region and a cytoplasmic domain (6,7). GPNMB
is expressed in numerous normal tissues, such as bone, the
hematopoietic system and skin and it correlates with many
biological processes, such as tissue regeneration, inflammation,
cell proliferation, adhesion and migration (8). Several studies have reported that
GPNMB is also expressed in malignant tissues and influences the
metastasis of tumor cells (6,9–11).
Furthermore, GPNMB level is reportedly elevated in several
malignant cancers, such as uveal melanoma (12), prostate (13) and lung cancer (14). Halim et al (15) reported that GPNMB overexpression is
prevalent in osteosarcoma. However, the potential impact of GPNMB
on the progression of osteosarcoma remains unclear.
The phosphatidylinositol 3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway
is a major signaling cascade. The PI3K/Akt/mammalian target of the
mTOR signaling pathway regulates downstream of the receptor
tyrosine kinases, including insulin-like growth factor-1 receptor
(IGF-1R) (16). This pathway plays
a pivotal role in a variety of biological activities that regulate
cell growth, survival and migration (17). In addition, researchers have
confirmed that abnormalities in the PI3K/Akt/mTOR signaling pathway
are involved in the carcinogenesis of various cancers, including
osteosarcoma (18,19). This pathway is reportedly activated
in osteosarcoma and its suppression could inhibit the proliferation
and invasion of osteosarcoma cells (19). Ono et al (20) demonstrated that the extracellular
fragment of GPNMB has neuroprotective effects and activates
PI3K/Akt pathway. However, whether the PI3K/Akt/mTOR pathway is
involved in the effects of GPNMB on osteosarcoma remains unclear.
The present study was conducted to clarify the role of GPNMB in
osteosarcoma. To understand the possible mechanisms involved, the
effect of GPNMB on the PI3K/Akt/mTOR signaling pathway was
explored. The results of the present study provided a prospective
therapeutic target for osteosarcoma.
Materials and methods
Cell lines and clinical specimens
The normal human fetal osteoplastic cell line (hFOB)
and human osteosarcoma cell lines SaOS2, 143B, MG63 and U2OS were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific) at 37°C in a humidified 5% CO2
atmosphere. Twenty paired osteosarcoma and adjacent normal tissues
were received from patients in the Second Affiliated Hospital of
Xi'an Jiaotong University from January to March, 2017 (Xi'an,
China). All patients provided informed written consent and the
study was approved by the Institutional Research Ethics Committee
of Xi'an Jiaotong University.
Cell transfection
MG63 and U2OS cells were seeded into 6-well plates
(1×105 cells/well) and grown for 24 h. Subsequently,
MG63 and U2OS cells were transfected with 50 µM of GPNMB siRNAs,
siGPNMB-1 and siGPNMB-2 or negative control siRNAs, siNC-1 and
siNC-2 (all from Thermo Fisher Scientific, Inc.) for 48 h using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific). The
transfection efficiency was detected using qPCR and western blot
analysis.
MTT assay
The effect of GPNMB on cell viability was assessed
by an MTT assay, as previously reported (21). The cells were seeded into 96-well
plates at 1×104 cells/well. Subsequently, 20 µl of MTT
(5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added
at 24, 48 and 72 h and incubated for 4 h. The supernatant was
aspirated. Dimethyl sulfoxide (DMSO; 200 µl; Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China) was added to
dissolve formazan crystals. Absorbance at 490 nm was detected using
a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA).
5′-Bromo-2′-deoxyuridine assay
A 5′-bromo-2′-deoxyuridine (BrdU) assay was
performed to assess the effect of GPNMB on cell proliferation, as
previously described (22). Cells
(1×105 cells/ml) grown on coverslips were incubated with
BrdU (Sigma-Aldrich; Merck KGaA) for 40 min and stained with
anti-BrdU antibody (1:200; cat. no. sc-70443; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) for 2 h. Images were captured
under an MC 100 microscope (Carl Zeiss, Oberkochen, Germany),
assessing the percentages of BrdU in six random fields.
Transwell assay
To prevent proliferation, the cells were cultured in
10 g/ml of mitomycin C (Sigma-Aldrich; Merck KGaA) for 2 h
(23). Then, a Transwell invasion
assay was performed using an invasion chamber coated with Matrigel
(BD Biosciences, San Jose, CA, USA). The lower chamber was filled
with 600 µl of medium. Cells (1×105 cells/well) were
plated into the upper Transwell chambers in serum-free medium for
24 h at 37°C. The cells on the surface of the upper chamber were
wiped with a cotton swab. After fixing with methanol for 20 min and
staining with 0.1% crystal violet for 30 min, the invasive cells on
the surface of the bottom membrane were determined by counting five
random 100X fields under an MC 100 microscope (Carl Zeiss). The
migration assay was the same as the invasion assay, with the
exception of the upper chamber which was not coated with Matrigel.
Each experiment was performed in triplicate.
Quantitative real-time PCR (qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The synthesis of cDNA
was performed with the PrimeScript™ RT reagent kit
(Takara Biotechnology, Co., Ltd., Dalian, China). The qPCR was
carried out using SYBR-Green Premix (Takara Biotechnology, Co.,
Ltd.). The Bio-Rad CFX96 Touch qPCR system (Bio-Rad Laboratories,
Hercules, CA, USA) was used to analyze the signal. The primers for
qRT-PCR were as follows: GPNMB forward, 5′-ACAAGGAATACAACCCAATA-3′
and reverse, 5′-ATAGCCACTCCAGCACA-3′; GAPDH forward,
5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′. The expression of mRNAs was normalized
to GAPDH. The relative expression was calculated using the
2−ΔΔCt method.
Western blot analysis
Proteins were resolved in 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then the
separated proteins were transferred to polyvinylidene fluoride
membranes (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, the membranes were blocked in 5% bovine serum albumin
(BSA) for 2 h and probed with primary antibodies, including rabbit
anti-GPNMB (1:1,000; cat. no. ab98856), rabbit anti-p-Akt (1:1,000;
ab38449), rabbit anti-Akt (1:500; cat. no. ab8805), rabbit
anti-p-mTOR (1:1,000; cat. no. ab109268) and rabbit anti-mTOR
(1:2,000; cat. no. ab2732; all from Abcam, Cambridge, MA, USA)
overnight at 4°C. Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:5,000; cat. no. ab6721; Abcam). The immunoblots were
visualized with an enhanced chemiluminescence system (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.). Densitometry
analysis was performed using Image-Pro Plus 6.0 software (Media
Cybernetics Inc., Rockville, MD, USA).
Statistical analysis
The data were analyzed using SPSS 22.0 software (IBM
Corp., Armonk, NY, USA) and are presented as the mean ± SD.
Statistical analysis was performed using one-way ANOVA with
subsequent SNK-q testing for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
GPNMB expression is upregulated in
osteosarcoma
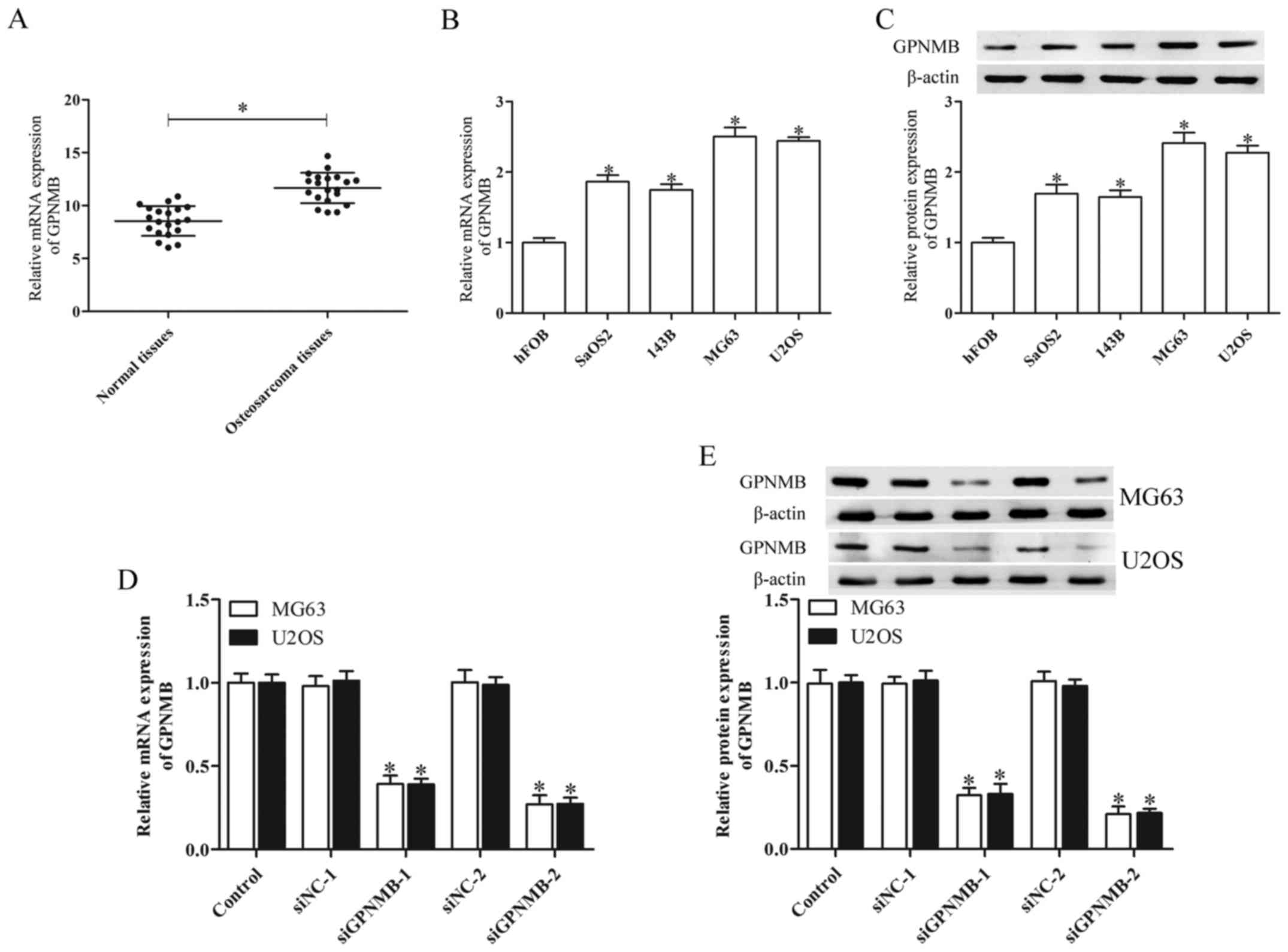
The expression of GPNMB in human osteosarcoma
tissues and human osteosarcoma cell lines was determined by qPCR
and western blot analysis. The human osteosarcoma tissues derived
from 20 patients demonstrated significantly higher GPNMB mRNA
levels than the adjacent non-cancerous tissues (Fig. 1A). The mRNA and protein expression
of GPNMB in the osteosarcoma cell lines SaOS2, 143B, MG63 and U2OS
was upregulated in comparison with normal human fetal osteoplastic
cells (hFOBs) (Fig. 1B and C). The
MG63 and U2OS cells indicated higher GPNMB mRNA and protein
expression than the SaOS2 and 143B cells. Therefore, these two cell
lines were chosen for further study.
To investigate the functions of GPNMB in relation to
osteosarcoma, we separately silenced its expression in the MG63 and
U2OS cells via GPNMB siRNA transfection. A high inhibitory GPNMB
mRNA and protein level was found in the MG63 and U2OS cells
transfected with GPNMB siRNA (P<0.05; Fig. 1D and E). Of the two GPNMB siRNAs
(siGPNMB-1 and siGPNMB-2), siGPNMB-2 was the most efficient and
thus was chosen for further experiments.
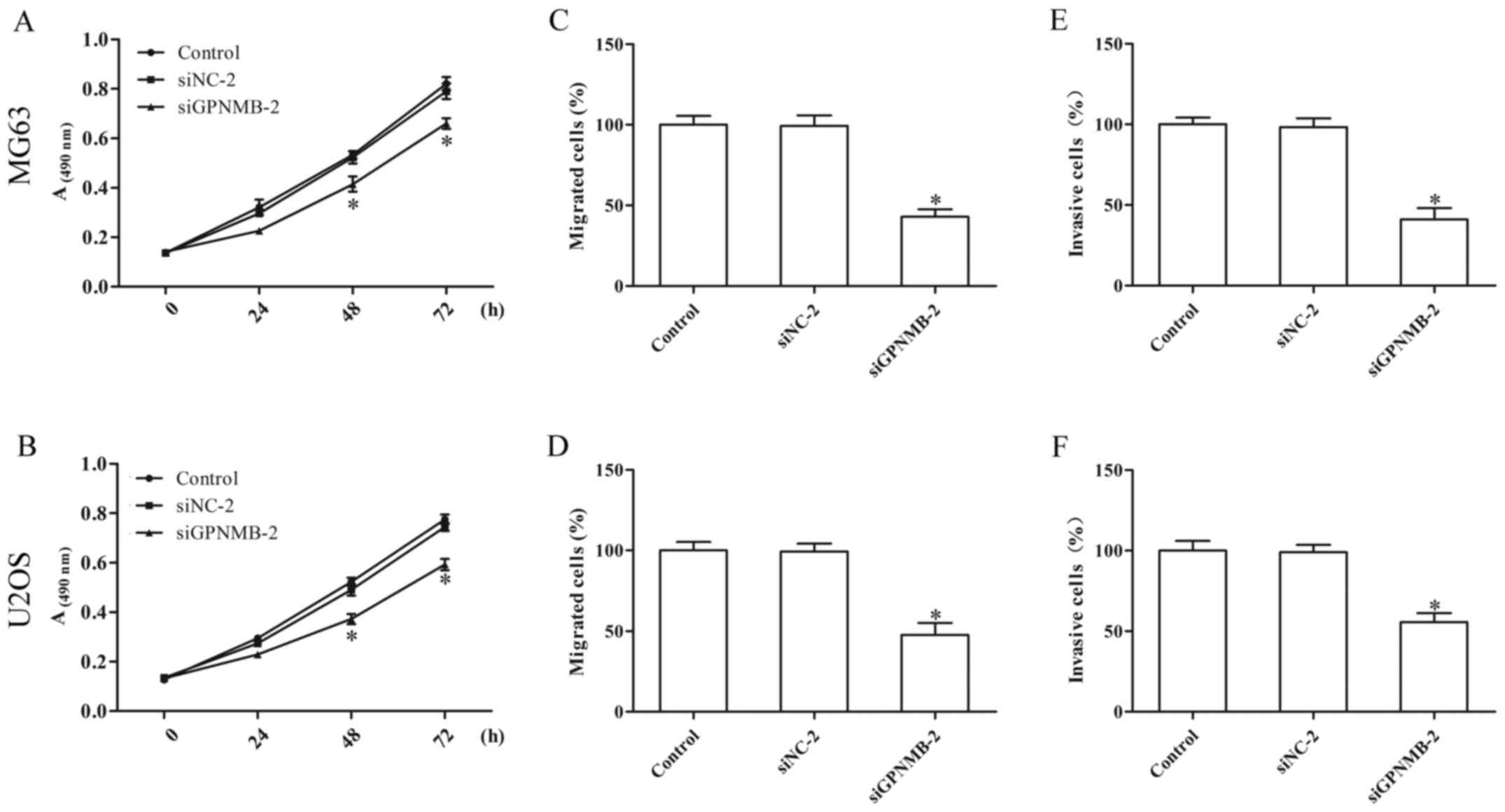
GPNMB silencing inhibits proliferation
and metastasis of osteosarcoma cells
Knockdown of GPNMB in the MG63 and U2OS cells
resulted in reduced cell proliferation (Fig. 2A and B). The Transwell assay
indicated that GPNMB silencing notably inhibited the migration
(Fig. 2C and D) and invasion
(Fig. 2E and F) of the MG63 and
U2OS cells. These results indicated that GPNMB may promote the
progression of osteosarcoma.
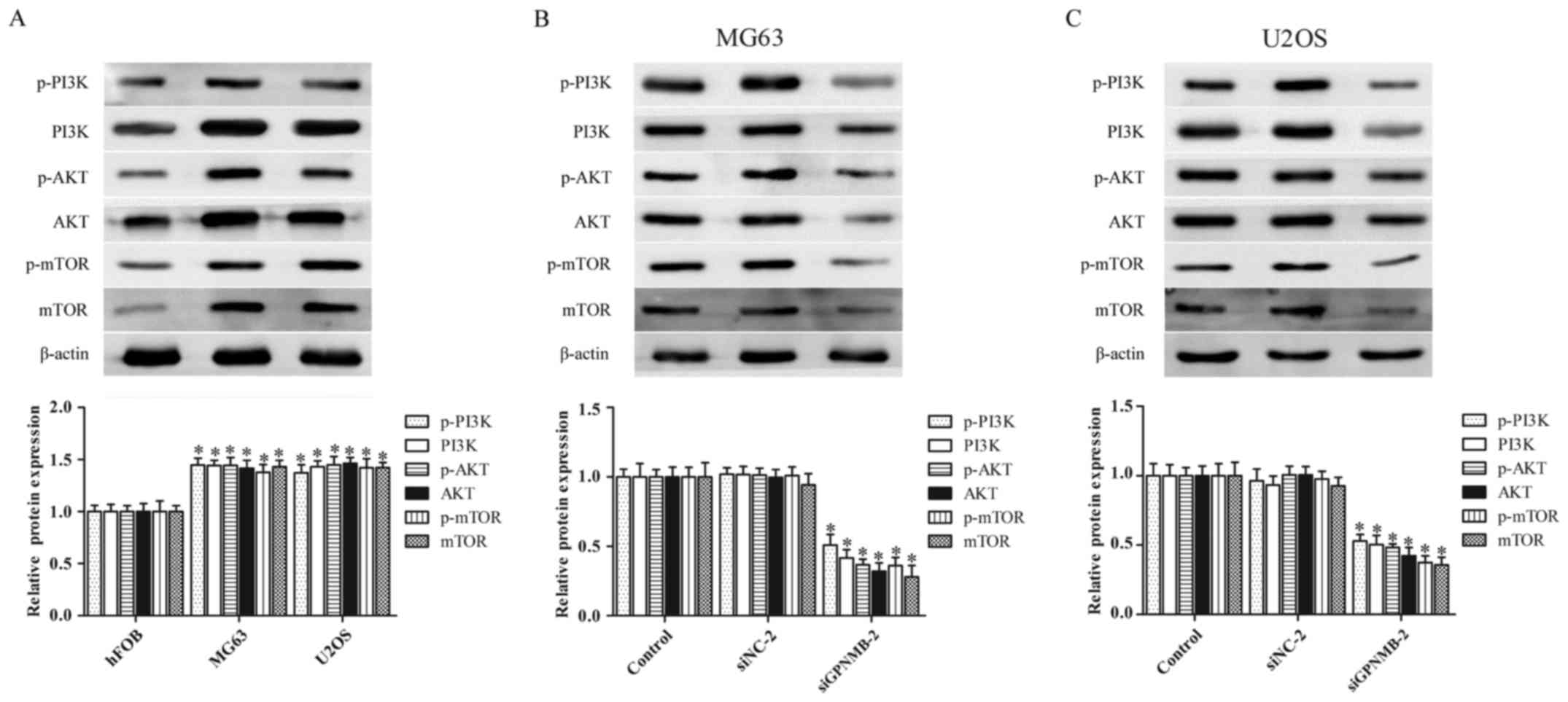
GPNMB silencing suppresses the
activity of the PI3K/Akt/mTOR signaling pathway in osteosarcoma
cells
The abnormal activation of the PI3K/Akt/mTOR
signaling pathway plays a critical role in osteosarcoma
pathogenesis (24,25). Therefore, we further evaluated
whether GPNMB affected the PI3K/Akt/mTOR signaling pathway in
osteosarcoma cells. The protein levels of p-PI3K, PI3K, p-AKT, AKT,
p-mTOR and mTOR were obviously upregulated in the MG63 and U2OS
cells (Fig. 3A), whereas the
protein levels were significantly downregulated by GPNMB siRNA in
both MG63 (Fig. 3B) and U2OS cells
(Fig. 3C). These results indicated
that the GPNMB silencing inhibited the activation of the
PI3K/Akt/mTOR signaling pathway in osteosarcoma cells.
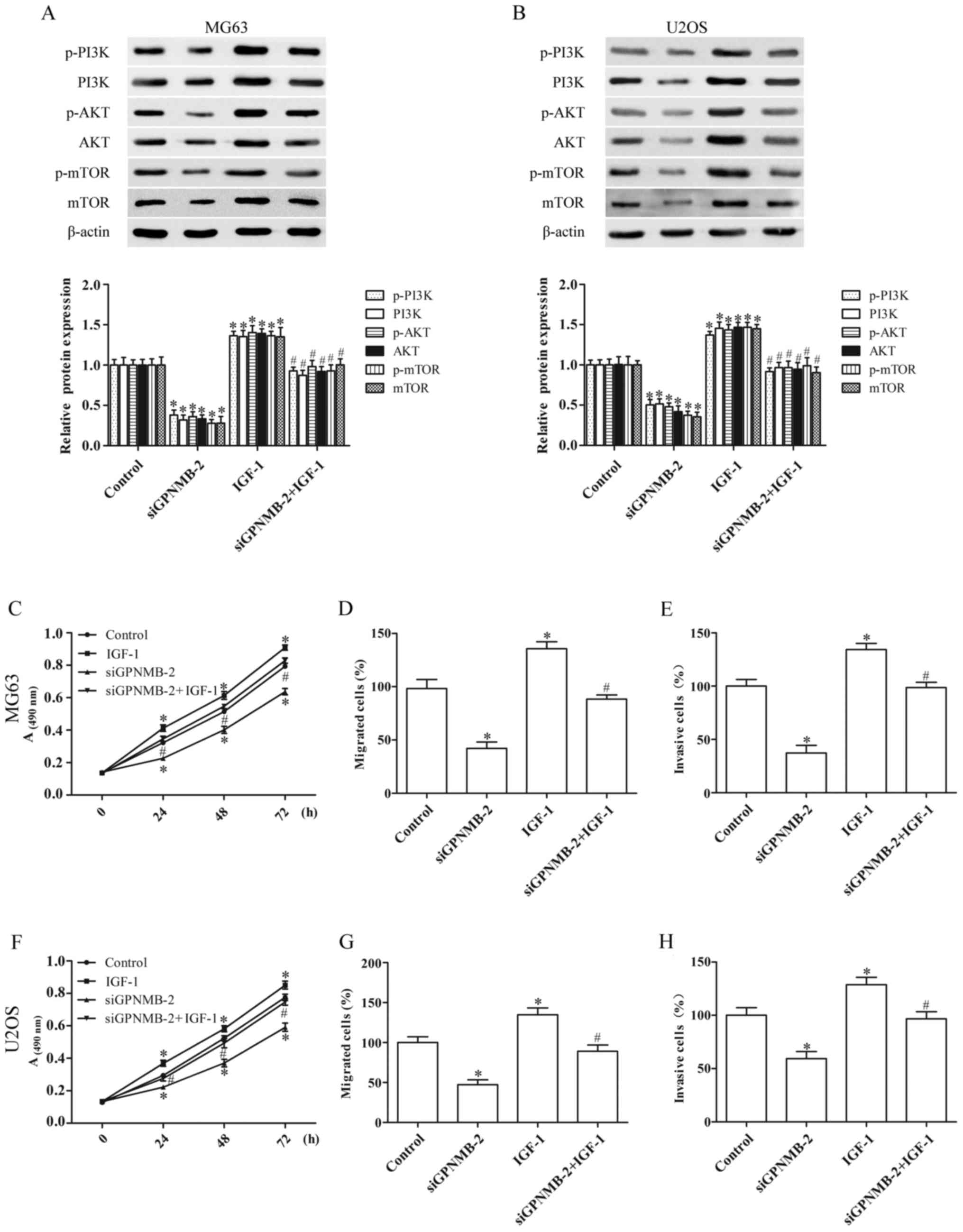
GPNMB silencing suppresses the
proliferation and metastasis of osteosarcoma cells by blocking the
PI3K/Akt/mTOR signaling pathway
To identify whether the effect of GPNMB silencing on
osteosarcoma cells was achieved by regulating the PI3K/Akt/mTOR
signaling pathway, the MG63 and U2OS cells were cultured with IGF-1
(3 ng/ml) (26), an agonist of
PI3K. IGF-1 activated the PI3K/Akt/mTOR signaling and abolished the
inhibition of PI3K/Akt/mTOR signaling induced by siGPNMB-2, as
evidenced by the upregulation of the protein expression of p-PI3K,
PI3K, p-AKT, AKT, p-mTOR and mTOR (Fig.
4A and B).
Furthermore, the inhibitory effect of GPNMB
silencing on the proliferation (Fig. 4C
and F), migration (Fig. 4D and
G) and invasion (Fig. 4E and H)
of the MG63 and U2OS cells was significantly reversed by IGF-1.
These results demonstrated that GPNMB silencing inhibited the
progression of osteosarcoma cells by blocking the PI3K/Akt/mTOR
signaling pathway.
Discussion
Osteosarcoma is the most common bone tumor with a
high mortality rate. In the present study, we revealed that
glycoprotein non-metastatic melanoma protein B (GPNMB) was
aberrantly overexpressed in osteosarcoma tissue and osteosarcoma
cells. The knockdown of the expression of GPNMB significantly
inhibited the progression of osteosarcoma cells by inhibiting the
PI3K/Akt/mTOR signaling pathway. Our findings indicated that GPNMB
may be a promising therapeutic target for osteosarcoma. GPNMB is a
transmembrane glycoprotein involved in various pathological
processes, including the development of cancer. It has been
reported to increase cellular survival signals, thus promoting
tumor growth (6). In addition,
GPNMB is highly expressed in various types of cancer, including
osteosarcoma (15). The results
from a pediatric preclinical testing program of solid tumor
xenografts revealed that GPNMB was primarily expressed in
osteosarcoma xenografts (27). In a
clinical trial, targeting GPNMB with the antibody-drug conjugate
glembatumumab vedotin demonstrated the potential utility of
targeting GPNMB for the treatment of osteosarcoma (28), however, its role in human
osteosarcoma remains unclear.
In the present study, the expression of GPNMB was
highly expressed in osteosarcoma tissue and osteosarcoma cells. To
elucidate the biological function of GPNMB in osteosarcoma cells,
we used GPNMB siRNA to knock down the expression of GPNMB. GPNMB
silencing inhibited the proliferation, migration and invasion of
the MG63 and U2OS cells. Collectively, these results indicated that
GPNMB plays a vital role in the tumorigenicity and progression of
osteosarcoma, which is consistent with the role of GPNMB in other
cancers (14,29,30).
The PI3K/Akt/mTOR signaling pathway plays a critical
role in many biological processes, such as the survival, migration
and progression of various types of cancer (31,32).
PI3K activates its downstream molecule Akt, the core component of
the PI3K/AKT signaling pathway, which in turn results in the
phosphorylation and activation of mTOR through a cascade of
regulators and ultimately increases the proliferation and
metastasis of the tumor cells (33). A previous study has revealed that
the aberrant activation of the PI3K/Akt/mTOR signaling pathway was
a pivotal event in the pathogenesis and progression of osteosarcoma
cells (34). To investigate the
mechanisms of GPNMB silencing in the progression of osteosarcoma
cells, we assessed the effect of GPNMB silencing on the
PI3K/Akt/mTOR signaling pathway-related proteins p-PI3K, PI3K,
p-AKT, AKT, p-mTOR and mTOR. Consistent with previous studies
(35,36), we revealed that the PI3K/Akt/mTOR
signaling pathway was activated in osteosarcoma cells, however the
GPNMB silencing inhibited the activation of the PI3K/Akt/mTOR
signaling pathway. The present study also revealed that the
inhibitory effect of GPNMB silencing on the proliferation,
migration and invasion of the MG63 and U2OS cells was partly
reversed by the PI3K/Akt agonist IGF-1. These results demonstrated
that the GPNMB silencing exerted an inhibitory effect on the
progression of osteosarcoma by suppressing the PI3K/Akt/mTOR
signaling pathway. However, IGF-1 did not completely reverse the
effect of GPNMB, indicating that other signaling pathways may be
involved in the effect of GPNMB on osteosarcoma. Future research is
warranted to further investigate these pathways.
In conclusion, the present study revealed that GPNMB
was highly expressed in osteosarcoma and that GPNMB silencing
inhibited the proliferation and metastasis of osteosarcoma cells by
suppressing the PI3K/Akt/mTOR signaling pathway in vitro.
Other possible effects of GPNMB should be investigated in
vivo, as GPNMB may be a potential therapeutic target for the
treatment of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The osteosarcoma and adjacent normal tissues were
received from patients in the Second Affiliated Hospital of Xi'an
Jiaotong University from January to March, 2017 (Xi'an, China).
Authors' contributions
RJ and YYJ conceived and designed the study. RJ,
YYJ, YLT and HJY performed the experiments. YLT and ZL analyzed the
data. RJ and XQZ wrote and reviewed the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All patients provided informed written consent and
the study was approved by the Institutional Research Ethics
Committee of Xi'an Jiaotong University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vasquez L, Tarrillo F, Oscanoa M, Maza I,
Geronimo J, Paredes G, Silva JM and Sialer L: Analysis of
prognostic factors in high-grade osteosarcoma of the extremities in
children: A 15-year single-institution experience. Front Oncol.
6:222016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon N and Kleinerman ES: Aerosol
therapy for the treatment of osteosarcoma lung metastases:
Targeting the Fas/FasL pathway and rationale for the use of
gemcitabine. J Aerosol Med Pulm Drug Deliv. 23:189–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Safadi FF, Xu J, Smock SL, Rico MC, Owen
TA and Popoff SN: Cloning and characterization of osteoactivin, a
novel cDNA expressed in osteoblasts. J Cell Biochem. 84:12–26.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maric G, Rose AA, Annis MG and Siegel PM:
Glycoprotein non-metastatic b (GPNMB): A metastatic mediator and
emerging therapeutic target in cancer. Onco Targets Ther.
6:839–852. 2013.PubMed/NCBI
|
|
7
|
Rose AA, Annis MG, Dong Z, Pepin F,
Hallett M, Park M and Siegel PM: ADAM10 releases a soluble form of
the GPNMB/Osteoactivin extracellular domain with angiogenic
properties. PLoS One. 5:e120932010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh M, Del Carpio-Cano F, Belcher JY,
Crawford K, Frara N, Owen TA, Popoff SN and Safadi FF: Functional
roles of osteoactivin in normal and disease processes. Crit Rev
Eukaryot Gene Expr. 20:341–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rose AA and Siegel PM: Osteoactivin/HGFIN:
Is it a tumor suppressor or mediator of metastasis in breast
cancer? Breast Cancer Res. 9:4032007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maric G, Annis MG, Dong Z, Rose AA, Ng S,
Perkins D, MacDonald PA, Ouellet V, Russo C and Siegel PM: GPNMB
cooperates with neuropilin-1 to promote mammary tumor growth and
engages integrin α5β1 for efficient breast cancer metastasis.
Oncogene. 34:5494–5504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuo H and Zhou L: Gpnmb/osteoactivin: An
indicator and therapeutic target in tumor and nontumorous lesions.
Pharmazie. 71:555–561. 2016.PubMed/NCBI
|
|
12
|
Williams MD, Esmaeli B, Soheili A,
Simantov R, Gombos DS, Bedikian AY and Hwu P: GPNMB expression in
uveal melanoma: A potential for targeted therapy. Melanoma Res.
20:184–190. 2010.PubMed/NCBI
|
|
13
|
Fiorentini C, Bodei S, Bedussi F, Fragni
M, Bonini SA, Simeone C, Zani D, Berruti A, Missale C, Memo M, et
al: GPNMB/OA protein increases the invasiveness of human metastatic
prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9
activity. Exp Cell Res. 323:100–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oyewumi MO, Manickavasagam D, Novak K,
Wehrung D, Paulic N, Moussa FM, Sondag GR and Safadi FF:
Osteoactivin (GPNMB) ectodomain protein promotes growth and
invasive behavior of human lung cancer cells. Oncotarget.
7:13932–13944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halim A, Bagley RG and Keler T: Abstract
5032: Glycoprotein NMB (gpNMB) overexpression is prevalent in human
cancers: Pancreatic cancer, non-small cell lung cancer, head and
neck cancer, and osteosarcoma. Cancer Res. 76:5032. 2016.
View Article : Google Scholar
|
|
16
|
Ludwig JA, Lamhamedi-Cherradi SE, Lee HY,
Naing A and Benjamin R: Dual targeting of the insulin-like growth
factor and collateral pathways in cancer: combating drug
resistance. Cancers. 3:3029–3054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke M, Mo L, Li W, Zhang X, Li F and Yu H:
Ubiquitin ligase SMURF1 functions as a prognostic marker and
promotes growth and metastasis of clear cell renal cell carcinoma.
FEBS Open Bio. 7:577–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Bi T, Dai W, Wang G, Qian L, Shen G
and Gao Q: Lupeol induces apoptosis and cell cycle arrest of human
osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer
Res Treat. 15:NP16–NP24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ono Y, Tsuruma K, Takata M, Shimazawa M
and Hara H: Glycoprotein nonmetastatic melanoma protein B
extracellular fragment shows neuroprotective effects and activates
the PI3K/Akt and MEK/ERK pathways via the
Na+/K+-ATPase. Sci Rep. 6:232412016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu H, Wu Y, Zheng W and Lu S: CO-029 is
overexpressed in gastric cancer and mediates the effects of EGF on
gastric cancer cell proliferation and invasion. Int J Mol Med.
35:798–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Wang Q, Wang GD, Wang HS, Huang Y,
Liu XM and Cai XH: miR-16 inhibits cell proliferation by targeting
IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS
Lett. 587:1366–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boniface K, Bernard FX, Garcia M, Gurney
AL, Lecron JC and Morel F: IL-22 inhibits epidermal differentiation
and induces proinflammatory gene expression and migration of human
keratinocytes. J Immunol. 174:3695–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Sun Y, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci
Rep: BSR20160165. 2016. View Article : Google Scholar
|
|
25
|
Hu K, Dai HB and Qiu ZL: mTOR signaling in
osteosarcoma: Oncogenesis and therapeutic aspects (Review). Oncol
Rep. 36:1219–1225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang XJ, Yu HY, Cai YJ and Ke M: Lycium
barbarum polysaccharides inhibit proliferation and migration of
bladder cancer cell lines BIU87 by suppressing Pi3K/AKT pathway.
Oncotarget. 8:5936–5942. 2017.PubMed/NCBI
|
|
27
|
Kolb EA, Gorlick R, Billups CA, Hawthorne
T, Kurmasheva RT, Houghton PJ and Smith MA: Initial testing (stage
1) of glembatumumab vedotin (CDX-011) by the pediatric preclinical
testing program. Pediatr Blood Cancer. 61:1816–1821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roth M, Barris DM, Piperdi S, Kuo V,
Everts S, Geller D, Houghton P, Kolb EA, Hawthorne T, Gill J and
Gorlick R: Targeting glycoprotein NMB with antibody-drug conjugate,
glembatumumab vedotin, for the treatment of osteosarcoma. Pediatr
Blood Cancer. 63:32–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rose AA, Grosset AA, Dong Z, Russo C,
Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park
M, et al: Glycoprotein nonmetastatic B is an independent prognostic
indicator of recurrence and a novel therapeutic target in breast
cancer. Clin Cancer Res. 16:2147–2156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torres C, Linares A, Alejandre MJ,
Palomino-Morales R, Martin M, Delgado JR, Martinez J and Perales S:
The potential role of the glycoprotein osteoactivin/glycoprotein
nonmetastatic melanoma protein B in pancreatic cancer. Pancreas.
44:302–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le B, Powers GL, Tam YT, Schumacher N,
Malinowski RL, Steinke L, Kwon G and Marker PC: Multi-drug loaded
micelles delivering chemotherapy and targeted therapies directed
against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer.
PloS One. 12:e01746582017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M, Bamodu OA, Huang WC, Zucha MA, Lin
YK, Wu ATH, Huang CC, Lee WH, Yuan CC, Hsiao M, et al:
4-Acetylantroquinonol B suppresses autophagic flux and improves
cisplatin sensitivity in highly aggressive epithelial cancer
through the PI3K/Akt/mTOR/p70S6K signaling pathway. Toxicol Appl
Pharmacol. 325:48–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodon J, Dienstmann R, Serra V and
Tabernero J: Development of PI3K inhibitors: Lessons learned from
early clinical trials. Nat Rev Clin Oncol. 10:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Sun X, Wu J and Li Z: MicroRNA-613
suppresses proliferation, migration and invasion of osteosarcoma by
targeting c-MET. Am J Cancer Res. 6:2869–2879. 2016.PubMed/NCBI
|
|
35
|
Song R, Tian K, Wang W and Wang L: P53
suppresses cell proliferation, metastasis, and angiogenesis of
osteosarcoma through inhibition of the PI3K/AKT/mTOR pathway. Int J
Surg. 20:80–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keremu A, Maimaiti X, Aimaiti A, Yushan M,
Alike Y, Yilihamu Y and Yusufu A: NRSN2 promotes osteosarcoma cell
proliferation and growth through PI3K/Akt/MTOR and Wnt/β-catenin
signaling. Am J Cancer Res. 7:565–573. 2017.PubMed/NCBI
|