Introduction
Melanoma is a highly malignant tumour originated
from neural crest-derived melanocytes. Melanoma can occur in the
skin, eye, digestive tract, reproductive systems and in other
organs, but it mostly occurs in cutaneous tissues. In general,
melanoma constitutes only 5% of all skin cancers. However, this
disease has the highest malignancy and mortality rate due to its
propensity for abnormal proliferation and early lymphatic and
haematogenous metastasis (1).
Currently, the incidence of melanoma is increasing at a rate of
4.1% per year, which is higher than that of many other
malignancies, and there are over 76,000 new cases of melanoma and
10,000 melanoma-related deaths in the United States each year
(2). Despite the advances and
breakthroughs in the field of melanoma treatment, an effective
diagnostic and therapeutic method is urgently needed. Thus, the
mechanisms of proliferation and metastasis of melanoma need to be
further studied.
Ubiquitin-like with PHD and ring finger domains 1
(UHRF1), which consists of 793 amino acids and binds to the
inverted CCAAT box (ICB2) in the promoter of topoisomerase Iiα
(3), acts as an epigenetic
integrator that possesses five functional domains, including
ubiquitin-like domain, tandem tudor domain, plant homeodomain, SET
and RING associated domain as well as an interesting new gene
domain (4). UHRF1 was reported to
cooperatively regulate DNA methylation and histone modification,
which epigenetically silenced tumour suppressor genes (TSGs)
(5) and played a positive role in
cell proliferation and tumour progression (6). A number of studies have demonstrated
that aberrations in the expression of UHRF1 were a reliable
biomarker for the diagnosis and prognostic prediction of lung
(7) breast (8) bladder (9) and hepatocellular carcinoma (10) and that the knockdown or knockout of
its expression led to weakened cell proliferation and increased
cell apoptosis, which made it a potential anticancer drug target
(11,12). Additionally, the elevated expression
of UHRF1was recently revealed to be associated with tumour cell
resistance to antitumour drugs, whereas the interference of UHRF1
sensitised cancer cells to chemotherapies or radiotherapy.
Obviously, UHRF1 is considered to be a powerful diagnostic and
prognostic biomarker to predict the therapeutic response and assess
the risk of tumour progression and recurrence. Therefore, further
investigation into the molecular mechanisms of the action of UHRF1
would help develop and improve cancer therapies.
At present, the expression and roles of UHRF1 in
melanoma remain largely unknown. In the present study, the
expression of UHRF1 in melanoma and the correlation between UHRF1
and the major pathological parameters of melanoma were
investigated. Additionally, the prognosis of the expression of
UHRF1 in melanoma was analysed.
Materials and methods
Bioinformatic analysis of the
expression of UHRF1 in melanoma
To determine the expression of UHRF1 in melanoma, we
used the Oncomine database (https://www.oncomine.org/resource/login.html) where we
inputted the keyword ‘UHRF1’ and chose ‘melanoma, please check
cancer vs. normal analysis’ as our target cancer and analysis type.
Subsequently, we selected the GSE4587 and GSE7553 datasets to
analyse the expression of UHRF1 mRNA between melanoma and benign
nevi, respectively. In order to understand the relationship between
UHRF1 and Ki-67, we searched for available data on mRNA expression
in the public datasets of TCGA (https://cancergenome.nih.gov/), which contains data of
471 melanoma patients. Subsequently, the data were visualised in a
heatmap using the RStudio software (RStudio, Inc., Boston, MA,
USA). For analysing the correlation between the prognosis of
patients with melanoma and the mRNA expression level of UHRF1 in
melanoma, we inputted the keyword of ‘UHRF1’ in OncoLnc (http://www.oncolnc.org/) and chose the survival curve
of skin cutaneous melanoma (SKCM) patients. Patients were sorted
based on the expression of UHRF1, and we compared the bottom third
vs. the top third as recommended. We obtained the survival curve
and detailed survival time of the low- and high-expression groups
(from TCGA).
Patients and follow-up
The present study enrolled a total of 56 melanoma
patients and 16 benign nevus patients. Tumor samples and matched
adjacent normal control tissues were obtained from 20 of those
melanoma patients. All patients were subjected to a complete
excision followed by tissue verification through pathological
examination at Zhongshan Hospital Affiliated to Fudan University
(Shanghai, China) from January 1, 2012 to December 31, 2016. Prior
to the surgery, none of the patients had received any form of
radiotherapy or chemotherapy and detailed clinicopathological and
follow-up data had been obtained from them. The clinical stage of
patients was evaluated by the TNM staging system of the American
Joint Committee on Cancer (AJCC) and IUCC (7th edition) (13) and it was evaluated at the time of
the formal pathology report. Ethical approval of the study was
obtained by The Ethics Committee of the Zhongshan Hospital
Biomedical Research and written informed consent was obtained from
each patient. The follow-up of patients ended in December 31, 2016,
and the average observation time was 25 months.
Cell culture and transfection
The melanoma cell lines, including A375, A2058,
A875, M14 and MV3, were purchased from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China) and they were grown based on
the recommended media and conditions. Knockdown of UHRF1 was
performed with the specific shRNAs delivered by a lentiviral system
purchased from Shanghai GeneChem Co. Ltd., (Shanghai, China)
according to the manufacturer's instructions and pLenti-shRNA-Mock
was used as a negative control. Subsequently, the cells with
suiTable fluorescent expression were screened with puromycin at a
concentration of 3 µg/ml. The transfection efficiency was verified
by western blotting and RT-qPCR. Each experiment was performed in
triplicate.
Tissue microarray (TMA) construction,
immunohistochemical and immunofluorescence staining
TMA was constructed according to a previous study
(14). Briefly, the above mentioned
specimens of melanoma and benign nevus were stained with
hematoxylin and eosin (H&E), and representative blocks were
sampled for the TMA blocks. Subsequently, they were punched by a
tissue cylinder with a 3-mm diameter and then were taken from the
paraffin blocks. Finally, sections of 4-µm thickness were placed on
the slides.
TMA was stained with H&E for verifying the
presence of tumour tissue and stained with mouse anti-human
monoclonal UHRF1 (1:100; cat. no. 612264; BD Biosciences, Franklin
Lakes, NJ, USA) as well as rabbit anti-human monoclonal Ki-67
(1:100; cat. no. ab16667; Abcam, Cambridge, UK) to evaluate the two
protein expression levels by immunohistochemistry (IHC). Briefly,
the slides were deparaffinised and rehydrated following the
manufacturer's instructions. After incubation in 0.3%
H2O2 to abolish the activity of endogenous
peroxidase, antigen retrieval was conducted in citrate buffer.
Subsequently, the primary antibodies (UHRF1 and Ki-67) were
incubated overnight, and then the immunohistochemistry kits (cat.
no. GK500705; Gene Tech Co., Shanghai, China) were used for 1 h.
The sections were counterstained with H&E. The TMA was viewed
at ×40 and ×200 magnification and images were captured from each
area using a standard Olympus microscope (Olympus Corp., Tokyo,
Japan). For the IHC quantification, IPP (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA) was used for digital
photograph analysis of the antigen expression. The total area
stained with brown was assessed in pixels, and the integrated
optical density (IOD) values of each block were exported. The TMA
was then examined independently by two pathologists who were
unaware of the patient clinical information, and disagreements were
solved by reaching to a consensus. The score for nuclear UHRF1
staining was determined based on a combination of staining
percentage and intensity as previously described (15). The staining intensity was scored as
follows: 0 (negative), 1 (low), 2 (moderate), or 3 (high), and the
staining percentage was scored as follows: 1 (0–25%), 2
(>25–50%), 3 (>50–75%) or 4 (>75–100%). The sum was used
for evaluating the expression level of UHRF1 and it was classified
into two grades: low (0–3) or high (4–7).
For immunofluorescence staining, A375 and A2058
cells were seeded onto diagnostic glass slides and cultivated for
24 h with a suiTable degree of fusion. Subsequently, they were
fixed with 4% paraformaldehyde (cat. no. 30314ES76; Yeasen,
Shanghai, China), incubated with Triton X-100 (cat. no. 20107ES76;
Yeasen) and blocked with 5% bovine serum albumin (BSA) (cat. no.
36101ES25; Yeasen) orderly. Specific primary antibodies UHRF1
and/or Ki-67 were incubated in the same conditions as IHC. After
being washed with phosphate-buffered saline (PBS), they were
incubated with Alexa Fluor 488-labeled anti-rabbit IgG antibody
(1:100; cat. no. 33106ES60; Yeasen) and/or DyLight 594-labeled
anti-mouse antibody (cat. no. 33212ES60; Yeasen) for 60 min and
then, nuclear counterstaining was performed with DAPI for 15 min.
The fluorescence intensities were analysed by fluorescence
microscopy (Olympus Corp.).
Real-time reverse transcription-PCR
(RT-qPCR) and western blot analysis
Total RNA was extracted using TRIzol reagent and
reverse-transcribed using the cDNA Synthesis kit (Takara Bio, Inc.,
Otsu, Japan). The primer sequences used for PCR are as follows:
UHRF1: 5′-ACAACGTGTGCAAGGACTGC-3′ (forward) and
5′-GAGCTGGTTGAGGACGGTCT-3′ (reverse); GAPDH:
5′-CCTGCACCACCAACTGCTTA-3′ (forward and 5′-GGCCATCCACAGTCTTCTGG-3′
(reverse). The RNA quantity and density were verified using a
spectrophotometer (DeNovix Inc., Wilmington, DE, USA). RT-qPCR was
performed using the SYBR-Green Master Mix kit (Takara Bio, Inc.,
Otsu, Japan) and the conditions were as follows: 95°C for 1 min, 35
cycles of 95°C for 15 sec, 60°C for 15 sec, 72°C for 30 sec and a
final extension at 95, 65 and 30°C for 1 min. The assay was
performed in triplicate using the ABI PRISM 7900HT RT-PCR system,
and the relative gene expression level was determined using the
2−ΔΔCt method (16).
For total protein extraction, cell lysates were
obtained using the RIPA buffer supplemented with phosphatase
inhibitors. Total protein (15 µg) was injected into Bis-Tris
SDS/PAGE gel and transferred to polyvinylidene difluoride (PVDF)
membranes. After blocking with 5% BSA for 60 min, the membranes
were incubated with mouse anti-human monoclonal UHRF1 (1:500; cat.
no. 612264; BD Biosciences, Franklin Lakes, NJ, USA) and mouse
monoclonal GAPDH (1:10,000; cat. no. ab8245; Abcam, Cambridge, UK)
overnight at 4°C. The membranes were then exposed to the secondary
antibody (1:3,000; cat. no. A0216; Beyotime Institute of
Biotechnology, Shanghai, China) for 60 min. The bands were
incubated with a DAB kit and analysed with an imaging system.
Densitometric analysis of UHRF1 and GAPDH in every sample and cell
line was performed using Adobe Photoshop CS6 (Adobe, San Jose, CA,
USA).
Flow cytometric analysis, CCK-8 and
colony formation assays
Flow cytometric analysis was used for detecting the
cell cycle and evaluating cell proliferation. The cells were washed
with PBS and fixed in 70% ethanol overnight at 4°C. Subsequently,
propidium iodide (cat. no. 550825; BD Biosciences, Franklin Lakes,
NJ, USA) was used for staining the cells for 30 min. Stained cells
were detected by a flow cytometer and analysed using FlowJo
software version 10 (BD Biosciences, Franklin Lakes, NJ, USA).
UHRF1 shRNA or negative control shRNA cells were
inoculated into 96-well plates (1,000 cells/well). At each
time-point (24, 48, 72 and 96 h), 10 µl of CCK-8 solution was added
into the sextuplicate wells. The wells were incubated for 3 h and
the absorbance of each individual well was determined at 490 nm.
The data obtained are presented as a line chart. Each experiment
was performed in triplicate.
The colony formation assay was also used to
investigate the cell proliferation capacity. The cells were
digested into a single cell suspension and seeded in a 6-well plate
(1,000 cells/well). The appropriate complete medium was added in
each well and then the wells were placed into an incubator with the
culture medium refreshed every three days for two weeks. Following
the two-week period, the cells were washed with PBS, fixed with 4%
paraformaldehyde and stained with 0.4% crystal violet for 15 min.
The number of colonies containing >10 cells was counted manually
and averaged from the duplicate wells.
Statistical analysis
The data were analysed using SPSS statistical
software package (v.20.0; IBM Corp., Armonk, NY, USA).
Qualitative variables of two groups were compared by the
independent or paired Student's t-test, multiple groups were
compared by the one-way ANOVA and post hoc Dunnett t-test and the
median UHRF1 expression values among three groups were compared by
the Kruskal-Wallis test followed by a Mann-Whitney U post hoc test
with Bonferroni's correction. Pearson's correlation test was
adopted to analyse the relationship between the expression of UHRF1
and Ki-67 by GraphPad version 6.0 (GraphPad Software, Inc., San
Diego, CA, USA). A Chi-squared test was used to analyse the
association between the expression of UHRF1 and the clinical
parameters of melanoma patients. Overall survival (OS) was
evaluated using the Kaplan-Meier method and analysed by the
log-rank test. For the univariate and multivariate analysis, a Cox
proportional hazard regression model was used. A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
The expression of UHRF1 is
significantly upregulated in melanoma
From the GSE4587 dataset, the expression of UHRF1
mRNA in benign nevus and cutaneous melanoma tissues was analysed
(Fig. 1A). We revealed that the
expression of UHRF1 in melanoma was significantly upregulated
compared with benign nevus tissues (Fig. 1A, P=0.0112), and the average
expression in the melanoma group was 5.2-fold higher than that in
the benign nevus tissue group. To confirm these results, the
GSE7553 dataset was further analysed (Fig. 1B) and the findings were consistent
with the above mentioned results. UHRF1 mRNA expression in
pre-metastatic and metastatic melanoma was obviously higher than
that in benign nevus tissues (Fig.
1B, P=0.05 and P=0.0032, respectively). Additionally, the
expression of UHRF1 in metastatic melanoma was found to be higher
than that in pre-metastatic melanoma tissues (Fig. 1B, P=0.0146).
Subsequently, we attempted to investigate the
expression of UHRF1 in our well-characterised melanoma TMA. H&E
staining was used to verify the presence of melanoma cells, and
then IHC was performed to detect the expression of the UHRF1
protein. As displayed in Fig. 1C,
representative images revealed that the UHRF1 protein was mainly
distributed in the nucleus. Through quantification using Image-Pro
Plus 6.0, it was observed that the expression of UHRF1 in the
melanoma sections was obviously upregulated compared with the
benign naevus sections (P=0.0243, Fig.
1D). Further analysis using western blotting confirmed that the
expression of UHRF1 protein in melanoma tissues was significantly
higher compared with that of the non-cancerous tissues (Fig. 1E).
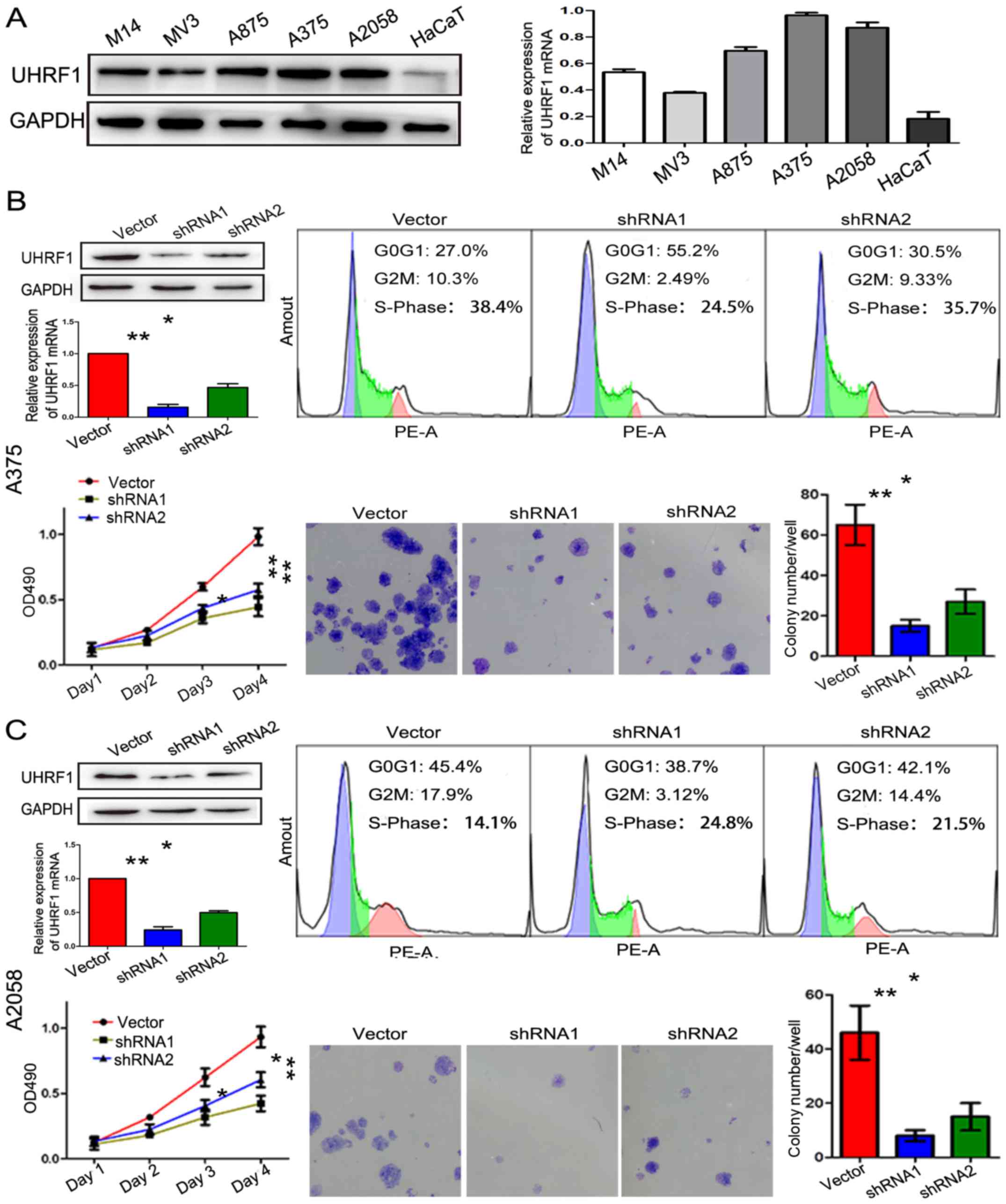
UHRF1 promotes cell proliferation and
the expression of UHRF1 is positively correlated to the expression
of Ki-67
As an epigenetic integrator, UHRF1 plays a crucial
role in cell proliferation in many types of cancer. As displayed in
Fig. 2A, the expression of UHRF1 in
five melanoma cells lines (A375, A2058, A875, M14 and MV3) and a
normal skin cell line (HaCaT) was detected by western blot analysis
and RT-qPCR. UHRF1 mRNA and protein were shown to be obviously
upregulated in the melanoma cell lines compared with the HaCaT cell
line, especially in the A375 and A2058 cell lines. In order to
reveal its role in melanoma, the expression of UHRF1 in A375 and
A2058 cells was downregulated by shRNAs and the downregulation was
verified by RT-qPCR and western blot analysis (Figs. 2B, C and 3D). The UHRF1-shRNA significantly
decreased the cell proliferation assessed by flow cytometric assay.
Furthermore, the colony formation and CCK-8 assay also confirmed
that the above mentioned result was statistically significant.
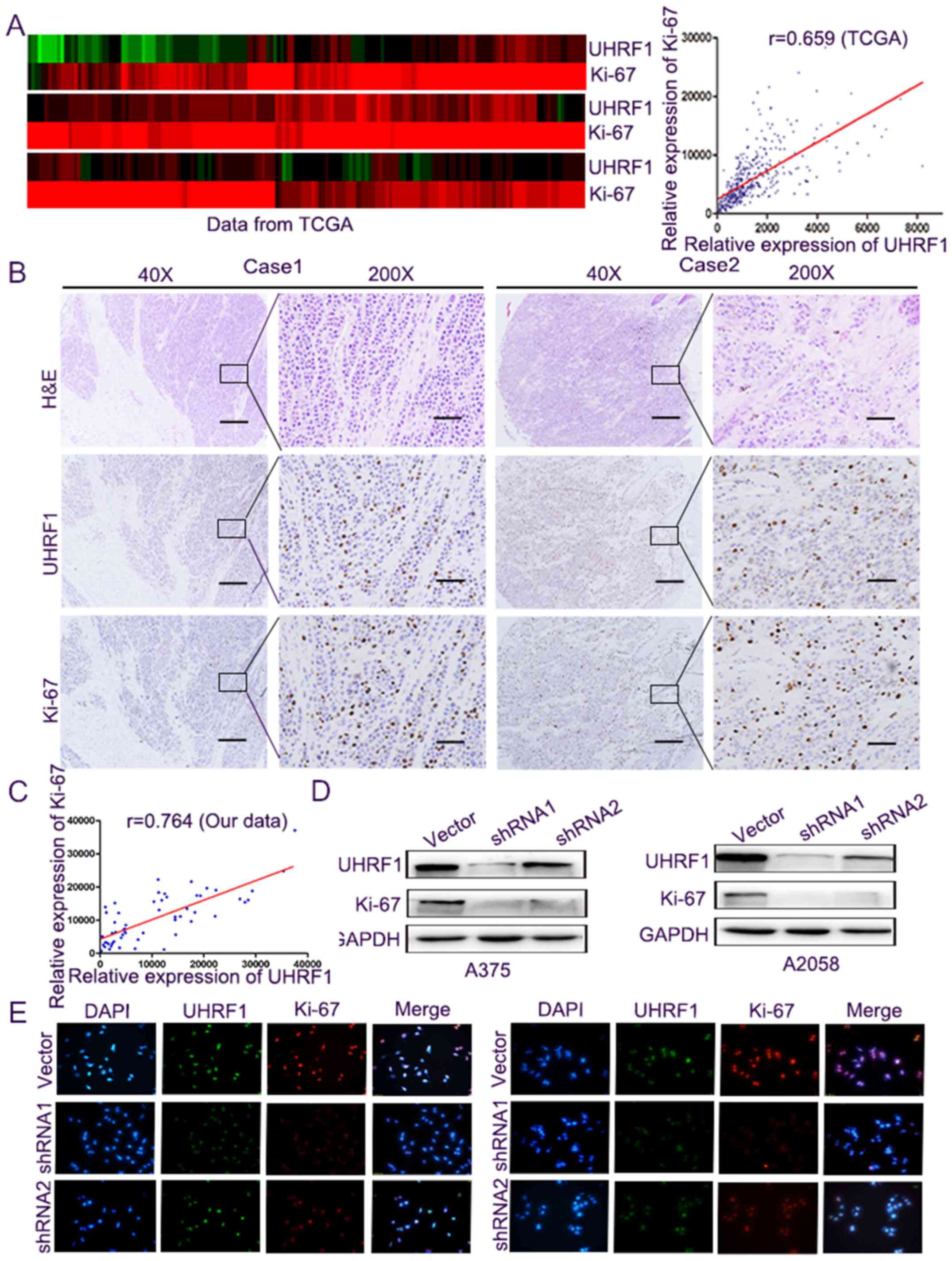
Subsequently, the relationship between the level of
UHRF1 and Ki-67, a well-known biomarker of cell proliferation
(17), was analysed to further
determine the role of UHRF1 in cell proliferation. Firstly, we
compared the mRNA expression through the public TCGA datasets, and
the expression levels were converted into a heatmap where they were
more easily presented. Subsequently, the correlation between them
was performed, and it demonstrated a markedly positive correlation
between the mRNA expression of UHRF1 and Ki-67 (Fig. 3A, r=0.659, P<0.001). Secondly, we
performed immunohistochemical staining (Fig. 3B) and we calculated the comparative
expression of UHRF1 and Ki-67 in TMA. The results revealed a
significantly positive correlation between the expression of UHRF1
and Ki-67 (Fig. 3C, r=0.764,
P<0.001). Western blot analysis and immunofluorescence staining
in the A375 and A2058 melanoma cells indicated that UHRF1 and Ki-67
proteins were co-expressed in the nucleus and the knockdown of the
expression of UHRF1 was accompanied by a decrease in Ki-67 protein
(Fig. 3D and E).
The expression of UHRF1 is clinically
relevant to melanoma
In the TMA, the blocks were divided into two groups
according to the expression of UHRF1. Twenty-nine samples were
found to have high expression of UHRF1 and 27 cases had low
expression of UHRF1. The relationship between the expression of
UHRF1 and the clinical parameters of melanoma patients is displayed
in Table I. The results revealed
that the expression of UHRF1 was positively correlated with TNM
classification (P=0.017) and Breslow's thickness (P=0.034).
However, UHRF1 did not correlate with sex (P=0.186), age (P=0.189),
lymph node metastasis (P=0.299) or distant metastasis
(P=0.672).
 | Table I.Association of the expression of
UHRF1 with the clinical parameters of 56 patients with
melanoma. |
Table I.
Association of the expression of
UHRF1 with the clinical parameters of 56 patients with
melanoma.
| Clinical
characteristics | UHRF1Low
No. |
UHRF1High No. | P-value |
|---|
| Age (years) |
|
| 0.189 |
|
≥55 | 14 | 10 |
|
|
<55 | 13 | 19 |
|
| Sex |
|
| 0.186 |
|
Male | 12 | 18 |
|
|
Female | 15 | 11 |
|
| Lymph nodes
metastasis |
|
| 0.299 |
| No | 13 | 10 |
|
|
Yes | 14 | 19 |
|
| Distant
metastasis |
|
| 0.672 |
| No | 20 | 20 |
|
|
Yes | 7 | 9 |
|
| Breslow's depth
(mm) |
|
| 0.034a |
| ≤5 | 16 | 9 |
|
|
>5 | 11 | 20 |
|
| Clinical stage |
|
| 0.017a |
|
I–II | 16 | 8 |
|
|
III–IV | 11 | 21 |
|
The overall survival (OS) time of 302 melanoma
patients was analysed from the dataset on OncoLnc. Significantly
higher survival rates were found in the low UHRF1 expression group
compared with those in the high UHRF1 expression group
(representive figures shown in Fig.
4A), which are displayed in Fig.
4B (P=0.0044). In our melanoma cohort, the survival rate was
also found to be different between the high- and low-UHRF1
expression groups (P<0.001), and high UHRF1 expression predicted
poorer prognosis (Fig. 4C). One-and
two-year OS was 84.6 and 52.2% for the low UHRF1 expression group,
and only 37.1 and 15.9% for the high UHRF1 expression group,
respectively.
The univariate analysis is displayed in Table II. We identified that lymph node
metastasis, distant metastasis, Ki-67 expression, UHRF1 expression
and TNM stage were correlated with the OS of melanoma patients. In
particular, the expression of UHRF1 had a great impact on OS time,
and the average OS for the low- and high-UHRF1 expression patients
was 37 and 13.5 months, respectively.
 | Table II.Univariate and multivariate analyses
of factors associated with survival. |
Table II.
Univariate and multivariate analyses
of factors associated with survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables |
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | <55 vs. ≥55 | 1.409 | 0.658–3.017 | 0.378 | NA |
|
|
| Sex | Male vs.
female | 0.575 | 0.274–1.206 | 0.143 | NA |
|
|
| Lymph nodes
metastasis | No vs. yes | 0.296 | 0.129–0.680 | 0.004a | 0.253 | 0.088–0.728 | 0.011a |
| Distant
metastasis | No vs. yes | 2.707 | 1.296–5.657 | 0.008a | 2.616 | 1.034–6.619 | 0.04 a |
| Breslow's depth
(mm) | ≤5 vs. >5 | 2.105 | 0.979–4.529 | 0.057 | NA |
| Clinical stage | I–II vs.
III–IV | 0.234 | 0.099–0.551 | 0.001a | 1.239 | 0.365–4.200 | 0.731 |
| UHRF1 | Low vs. high | 3.889 | 1.718–8.801 | 0.001a | 3.520 | 1.312–9.442 | 0.012a |
| Ki-67 | Low vs. high | 3.023 | 1.378–6.631 | 0.006a | 1.239 | 0.754–4.157 | 0.189 |
Subsequently, we synthesised all the factors that
were statistically significant in a univariate analysis to verify
the role of the expression of UHRF1 in prognosis, and then a
multivariate analysis was performed. In conclusion, a high
expression of UHRF1 represented a promising and independent
prognostic variable for the prediction of melanoma pathogenesis
(P=0.012). Other factors, including lymph node metastasis and
distant metastasis, were also assessed and the results are
presented in Table II.
Discussion
Recently, ubiquitin-like with PHD and ring finger
domains 1 (UHRF1) has been regarded as a hub protein that
participates in various activities ranging from embryonic formation
to cell and tissue development. Generally, UHRF1 is highly
expressed in proliferative tissues, while it is hardly expressed in
low proliferative tissues (3). In
addition, the expression of UHRF1 changes through the cell cycle;
it peaks during late G1 and G2/M stages in normal cells and remains
at a high level in tumour cells (18). Indeed, the expression of UHRF1 is
markedly upregulated in multiple types of cancer, such as lung,
liver and breast cancer (19–21).
Overexpressed UHRF1 plays a crucial role in epigenetic changes (DNA
methylation and histone modification), and several tumour
suppressor genes (including p16INK4A, BRCA1, RB1, CDH13, SHP1,
SOCS3 and CDX2) were reported to be related to UHRF1 epigenetic
silencing, which allows cancer cells to escape apoptosis and
promote tumour progression (22).
Furthermore, UHRF1 plays a vital role in repairing damaged DNA and
in G1/S-phase transition through its five structural constituents
(6). At present, in vitro
and in vivo studies have demonstrated that UHRF1 plays a
vital role in tumour formation, indicating that UHRF1 is a
promising candidate for cancer therapy (23).
High levels of UHRF1 act as a promoter of cell
proliferation and tumour progression. In the present study, the
interference of the expression of UHRF1 in A375 and A2058 melanoma
cells markedly weakened the ability of cell proliferation, which is
in line with the role of UHRF1 in tumour growth (24). In addition, we demonstrated that the
expression of UHRF1 is positively related to the expression of
Ki-67, which has been widely accepted as a biomarker of the
actively proliferating cells and plays an important role in cell
proliferation. Furthermore, combined research from other studies
indicated that the upregulated expression of UHRF1 may be a better
index in predicting tumour growth, since it is maintained
throughout the cell cycle in cancer cells, but not in normal cells,
while Ki-67 is overexpressed in all kinds of proliferating cells.
Previous research has revealed several multi-markers that aid in
the diagnosis of melanoma. For example, the co-expression of ARPC2,
FN1, RGS1, SPP1 and WNT2 was found to correctly diagnose a high
percentage of melanomas arising in a nevus (25), and the increasing multi-marker,
NCOA3, SPP1 and RGS1, and positively immunostaining for c-Kit were
reported to be helpful in predicting disease-specific survival time
for melanomas. Allowing for the dynamic tumour heterogeneity of
melanoma, our findings were still important in predicting the
degree of melanoma malignancy.
Clinically, the information presented in our
survival curve revealed that melanoma patients with a high level of
UHRF1 had poorer prognosis than those with low levels of UHRF1 and
that the expression of UHRF1 could be an independent prognostic
factor for melanoma patients through both univariate and
multivariate analyses. From the above mentioned results, it can be
hypothesized that UHRF1 may be an ideal target for cancer
treatment. Indeed, several studies confirmed that downregulating
the expression of UHRF1 may have promising therapeutic effects.
Firstly, it has been reported that many natural anticancer drugs
involve an overexpression of tumour suppressor genes, accompanied
with downregulation of the expression of UHRF1 (26,27).
For instance, epigallocatechin-3-gallate (EGCG) has been
demonstrated to decrease the proliferation of cancer cells by
downregulating the expression of UHRF1 (26). Secondly, UHRF1 can inhibit tumour
cell apoptosis by silencing the tumour suppressor genes (27). A relevant study demonstrated that
inhibiting the expression of UHRF1 can induce apoptosis and enhance
chemosensitivity in breast cancer, however it had no effect on
normal cells (28). The above
mentioned studies strongly support the view that UHRF1 targeting
has several advantages, including specific targeting of cancer
cells and enhancing chemosensitivity of cancer cells.
Currently, differential diagnosis of melanoma and
benign nevi is mainly based on the H&E-stained sections and
researchers have attempted to find specific markers that may
distinguish melanoma from benign nevi and the progression stages
(29,30). There is a number of studies on the
potential biomarkers for diagnosis and prognosis of melanoma, but
their sensitivity and specificity remain insufficient. C-Kit has
been proposed to discriminate metastatic from non-metastatic
melanoma in patients, but it cannot differentiate Spitz nevus from
malignant melanoma (31).
Overexpressed WT1 has been reported as an indicator of melanoma
cells, however as a single immunohistochemical marker, WT1 is not
able to distinguish melanoma from benign nevi (32). Other indicators, such as S100A6
(33), Melan-A (34) and HMB-45 (35), have been indicated to contribute to
the differential diagnosis of melanocytic lesions. However, these
markers are unlikely to be useful diagnostic tools for
distinguishing malignant from benign cells because they have no
differential expression between nevi and melanoma (36). Recently, a study reported a panel of
five overexpressed markers (WNT2, ARPC2, RGS1, SPP1 and FN1)
obtained by transcriptome analysis, and this five marker assay
achieved 95% specificity and 97% sensitivity in the diagnosis of
melanoma (25). However, due to the
obvious heterogeneity of melanoma and the significant differences
between patients, not every patient expresses common markers
through transcriptome analysis in a particular population.
The expression of UHRF1 was significantly
upregulated, which made it easy to distinguish it from benign nevi,
and this process can be completely realized by
immunohistochemistry. Furthermore, UHRF1 plays an important role in
epigenetic code, and is altered during the course of tumour
formation. Therefore, it is no surprising that UHRF1 may be a
potential biomarker for cancer. A study has reported that UHRF1 may
be an effective biomarker for differential diagnosis of pancreatic
adenocarcinoma (37), and its
expression is closely related to patient clinicopathological
parameters (38). Similarly, it has
been proposed that UHRF1 is a promising biomarker for the diagnosis
and prognosis of bladder cancer, and it has been demonstrated that
the expression of UHRF1 was significantly upregulated and
correlated with the malignancy of bladder cancer (39).
In conclusion, the present study demonstrated that
the expression of UHRF1 was markedly upregulated in melanoma, and
that the downregulated expression of UHRF1 significantly decreased
cell proliferation. Clinically, overexpressed UHRF1 was related to
high TNM classification and Breslow's thickness. Furthermore, high
UHRF1 was positively associated with shorter overall survival of
melanoma patients and the expression of UHRF1 was an independent
prognostic factor for the overall survival of melanoma
patients.
Acknowledgements
The authors would like to thank the Pathology
Department of Zhongshan Hospital for providing tumour samples.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JG and FQ conceived and designed the study. CW, NL,
LW and YZ performed the experiments. CW and ZF wrote the
manuscript. CW, YY and NL reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accounTable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval of the study was obtained by The
Ethics Committee of the Zhongshan Hospital Biomedical Research and
written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UHRF1
|
ubiquitin-like with PHD and ring
finger domains 1
|
|
TSGs
|
tumour suppressor genes
|
|
GEO
|
Gene Expression Omnibus
|
|
CCK-8
|
Cell Counting Kit-8
|
|
AJCC
|
American Joint Committee on Cancer
|
|
IUCC
|
International Union of Cancer
Control
|
|
IHC
|
immunohistochemistry
|
|
BSA
|
bovine serum albumin
|
|
TCGA
|
The Cancer Genome Atlas
|
|
H&E
|
hematoxylin and eosin
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
real-time quantitative PCR
|
|
TMA
|
tissue microarray
|
References
|
1
|
Rajabi P, Bagheri M and Hani M: Expression
of estrogen receptor alpha in malignant melanoma. Adv Biomed Res.
6:142017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Girotti MR, Gremel G, Lee R, Galvani E,
Rothwell D, Viros A, Mandal AK, Lim KH, Saturno G, Furney SJ, et
al: Application of sequencing, liquid biopsies, and patient-derived
xenografts for personalized medicine in melanoma. Cancer Discov.
6:286–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hopfner R, Mousli M, Jeltsch JM, Voulgaris
A, Lutz Y, Marin C, Bellocq JP, Oudet P and Bronner C: ICBP90, a
novel human CCAAT binding protein, involved in the regulation of
topoisomerase IIalpha expression. Cancer Res. 60:121–128.
2000.PubMed/NCBI
|
|
4
|
Bronner C, Krifa M and Mousli M:
Increasing role of UHRF1 in the reading and inheritance of the
epigenetic code as well as in tumorogenesis. Biochem Pharmacol.
86:1643–1649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JK, Estève PO, Jacobsen SE and Pradhan
S: UHRF1 binds G9a and participates in p21 transcriptional
regulation in mammalian cells. Nucleic Acids Res. 37:493–505. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bronner C, Fuhrmann G, Chédin FL, Macaluso
M and Dhe-Paganon S: UHRF1 links the histone code and DNA
methylation to ensure faithful epigenetic memory inheritance. Genet
Epigenet. 2009:29–36. 2010.PubMed/NCBI
|
|
7
|
Unoki M, Daigo Y, Koinuma J, Tsuchiya E,
Hamamoto R and Nakamura Y: UHRF1 is a novel diagnostic marker of
lung cancer. Br J Cancer. 103:217–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Unoki M, Nishidate T and Nakamura Y:
ICBP90, an E2F-1 target, recruits HDAC1 and binds to methyl-CpG
through its SRA domain. Oncogene. 23:7601–7610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang GL, Zhang LH, Bo JJ, Chen HG, Cao M,
Liu DM and Huang YR: UHRF1 is associated with tumor recurrence in
non-muscle-invasive bladder cancer. Med Oncol. 29:842–847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang D, Xue H, Yu Y, Lv F, You W and
Zhang B: Elevated expression of UHRF1 predicts unfavorable
prognosis for patients with hepatocellular carcinoma. Int J Clin
Exp Pathol. 8:9416–9421. 2015.PubMed/NCBI
|
|
11
|
Yan F, Tan XY, Geng Y, Ju HX, Gao YF and
Zhu MC: Inhibition effect of siRNA-downregulated UHRF1 on breast
cancer growth. Cancer Biother Radiopharm. 26:183–189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan F, Wang X, Shao L, Ge M and Hu X:
Analysis of UHRF1 expression in human ovarian cancer tissues and
its regulation in cancer cell growth. Tumour Biol. 36:8887–8893.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kallioniemi OP, Wagner U, Kononen J and
Sauter G: Tissue microarray technology for high-throughput
molecular profiling of cancer. Hum Mol Genet. 10:657–662. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang JH, Gao Q, Ke AW, Yu Y, Shi GM, Fan
J, Zhou J and Huang XW: Prognostic significance of nuclear RNA
export factor 3 in hepatocellular carcinoma. Oncol Lett. 7:641–646.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(Review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mousli M, Hopfner R, Abbady AQ, Monté D,
Jeanblanc M, Oudet P, Louis B and Bronner C: ICBP90 belongs to a
new family of proteins with an expression that is deregulated in
cancer cells. Br J Cancer. 89:120–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang P, Cheng CL, Chang YH, Liu CH, Hsu
YC, Chen JS, Chang GC, Ho BC, Su KY, Chen HY and Yu SL: Molecular
gene signature and prognosis of non-small cell lung cancer.
Oncotarget. 7:51898–51907. 2016.PubMed/NCBI
|
|
20
|
Liu X, Ou H, Xiang L, Li X, Huang Y and
Yang D: Elevated UHRF1 expression contributes to poor prognosis by
promoting cell proliferation and metastasis in hepatocellular
carcinoma. Oncotarget. 8:10510–10522. 2017.PubMed/NCBI
|
|
21
|
Geng Y, Gao Y, Ju H and Yan F: Diagnostic
and prognostic value of plasma and tissue ubiquitin-like,
containing PHD and RING finger domains 1 in breast cancer patients.
Cancer Sci. 104:194–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashraf W, Ibrahim A, Alhosin M, Zaayter L,
Ouararhni K, Papin C, Ahmad T, Hamiche A, Mély Y, Bronner C and
Mousli M: The epigenetic integrator UHRF1: On the road to become a
universal biomarker for cancer. Oncotarget. 8:51946–51962. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bronner C, Achour M, Arima Y, Chataigneau
T, Saya H and Schini-Kerth VB: The UHRF family: Oncogenes that are
drugable targets for cancer therapy in the near future? Pharmacol
Ther. 115:419–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Yang YZ, Shi CZ, Zhang P, Moyer
MP, Zhang HZ, Zou Y and Qin HL: UHRF1 promotes cell growth and
metastasis through repression of p16ink4a in colorectal
cancer. Ann Surg Oncol. 19:2753–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kashani-Sabet M, Rangel J, Torabian S,
Nosrati M, Simko J, Jablons DM, Moore DH, Haqq C, Miller JR III and
Sagebiel RW: A multi-marker assay to distinguish malignant
melanomas from benign nevi. Proc Natl Acad Sci USA. 106:6268–6272.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Achour M, Mousli M, Alhosin M, Ibrahim A,
Peluso J, Muller CD, Schini-Kerth VB, Hamiche A, Dhe-Paganon S and
Bronner C: Epigallocatechin-3-gallate up-regulates tumor suppressor
gene expression via a reactive oxygen species-dependent
down-regulation of UHRF1. Biochem Biophys Res Commun. 430:208–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alhosin M, Sharif T, Mousli M,
Etienne-Selloum N, Fuhrmann G, Schini-Kerth VB and Bronner C:
Down-regulation of UHRF1, associated with re-expression of tumor
suppressor genes, is a common feature of natural compounds
exhibiting anti-cancer properties. J Exp Clin Cancer Res.
30:412011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang L, Shanqu L, Ping G, Ting H, Xi W, Ke
D, Min L, Junxia W and Huizhong Z: Gene therapy with RNAi targeting
UHRF1 driven by tumor-specific promoter inhibits tumor growth and
enhances the sensitivity of chemotherapeutic drug in breast cancer
in vitro and in vivo. Cancer Chemother Pharmacol. 69:1079–1087.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerriere-Kovach PM, Hunt EL, Patterson
JW, Glembocki DJ, English JC III and Wick MR: Primary melanoma of
the skin and cutaneous melanomatous metastases: Comparative
histologic features and immunophenotypes. Am J Clin Pathol.
122:70–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Kempen LC, van den Oord JJ, van Muijen
GN, Weidle UH, Bloemers HP and Swart GW: Activated leukocyte cell
adhesion molecule/CD166, a marker of tumor progression in primary
malignant melanoma of the skin. Am J Pathol. 156:769–774. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Isabel Y and Fitzpatrick JE:
Expression of c-kit (CD117) in Spitz nevus and malignant melanoma.
J Cutan Pathol. 33:33–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosner K, Mehregan DR, Moussai D, Abrams
J, Tromp G and Mehregan DA: WT1 marker is not sufficient for
distinguishing between melanoma and melanocytic nevi. J Cutan
Pathol. 36:1077–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fullen DR, Reed JA, Finnerty B and McNutt
NS: S100A6 preferentially labels type C nevus cells and nevic
corpuscles: Additional support for Schwannian differentiation of
intradermal nevi. J Cutan Pathol. 28:393–399. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busam KJ, Chen YT, Old LJ, Stockert E,
Iversen K, Coplan KA, Rosai J, Barnhill RL and Jungbluth AA:
Expression of melan-A (MART1) in benign melanocytic nevi and
primary cutaneous malignant melanoma. Am J Surg Pathol. 22:976–982.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kucher C, Zhang PJ, Pasha T, Elenitsas R,
Wu H, Ming ME, Elder DE and Xu X: Expression of Melan-A and Ki-67
in desmoplastic melanoma and desmoplastic nevi. Am J Dermatopathol.
26:452–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haqq C, Nosrati M, Sudilovsky D, Crothers
J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR III, Allen RE,
Singer MI, et al: The gene expression signatures of melanoma
progression. Proc Natl Acad Sci USA. 102:6092–6097. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crnogorac-Jurcevic T, Gangeswaran R,
Bhakta V, Capurso G, Lattimore S, Akada M, Sunamura M, Prime W,
Campbell F, Brentnall TA, et al: Proteomic analysis of chronic
pancreatitis and pancreatic adenocarcinoma. Gastroenterology.
129:1454–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sabatino L, Fucci A, Pancione M, Carafa V,
Nebbioso A, Pistore C, Babbio F, Votino C, Laudanna C, Ceccarelli
M, et al: UHRF1 coordinates peroxisome proliferator activated
receptor gamma (PPARG) epigenetic silencing and mediates colorectal
cancer progression. Oncogene. 31:5061–5072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Unoki M, Kelly JD, Neal DE, Ponder BA,
Nakamura Y and Hamamoto R: UHRF1 is a novel molecular marker for
diagnosis and the prognosis of bladder cancer. Br J Cancer.
101:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|