Introduction
Breast cancer is a heterogeneous disease. Based on
comprehensive gene expression profiling, five types of breast
cancer have been determined including, luminal A and B, human
epidermal growth factor receptor-2 (HER-2)-overexpressing, basal-
and normal-like (1). Luminal A
breast cancers are characterized by the existence of estrogen
receptor (ER), progesterone receptor (PR) and lack of
overexpression of HER-2 and Ki-67 (2); triple-negative breast cancers (TNBCs)
are characterized by the absence of the ER and PR and lack of
overexpression of HER-2 (3). In
addition, luminal A breast cancers are associated with a good
prognosis and patients with TNBCs exhibit a poor prognosis.
Poly(ADP-ribose) polymerase (PARP) is a family of
proteins that have enzymatic features, scaffolding characteristics
and the ability to recruit other necessary DNA repair proteins
(4). Among these, PARP1 and 2 are
well known and they are critical for base excision repair (BER)
function. The function of BER is to repair the breaks that are
found in single-strand DNA and BER inhibition can result in cell
death. Thus, PARP proteins make ideal targets for anticancer
treatment. PARP inhibitors interfere with BER and DNA repair, and
PARP inhibitors can influence the death of tumor cells via this
pathway (5).
Paclitaxel targeting tubulin is used for the
treatment of various types of cancer; however, the use of cremophor
in the formulation restricts the utility of this drug. On the other
hand, nanoparticle albumin-bound paclitaxel [nab-paclitaxel
(Abraxane®); Celgene Corp., Summit, NJ, USA] is
solvent-free; it minimizes hypersensitivity reactions and has the
potential to inhibit other solvent-related toxicities such as
neutropenia (6–8).
The aim of the present study was to investigate the
effect of a PARP inhibitor alone and in combination with
nab-paclitaxel on MDA-MB-231 and MCF-7 cell lines using cell
kinetic parameters including cell index (CI), mitotic index (MI),
labelling index (LI) and apoptotic index (AI).
Materials and methods
Cell culture
MDA-MB-231 and MCF-7 cells were grown in Dulbecco's
modified Eagle's medium (DMEM, high glucose) (Gibco: Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 2 mM
L-glutamine and 10% fetal bovine serum (FBS; Gibco: Thermo Fisher
Scientific, Inc.) plus antibiotics in a humidified atmosphere with
5% CO2 in air. The pH of the medium was adjusted to 7.4
with NaHCO3.
Inhibitor and drug concentrations
PARP inhibitor and nab-paclitaxel concentrations
that were used in the present study were determined as 50, 75 and
100 µM for PARP inhibitor concentrations and 5.8, 11.72 and 17.59
µM for nab-paclitaxel concentrations. At first, 1 mM stock solution
was prepared by dilution of a stock solution for both inhibitor and
the drug.
Cell index
Experiments were carried out using the xCELLigence
Real-Time Cell Analysis (RTCA) DP instrument (Roche Diagnostics
GmbH, Mannheim, Germany) which was placed in a humidified incubator
at 37°C with 5% CO2. Cytotoxicity experiments were
performed using modified 16-well plates (E-Plate; Roche Diagnostics
GmbH). Microelectrodes were attached at the bottom of the wells for
impedance-based detection of attachment, spreading and
proliferation of the cells. The background impedance signal was
evaluated with 100 µl of cell culture medium/well. The final volume
in a single well was adjusted to 200 µl of cell culture medium by
adding an additional 100 µl of medium containing cells. Cell
numbers were 5,000 cells/well for MDA-MB-231 and 10,000 cells/well
for MCF-7. The impedance was recorded in 15-min intervals. Twenty
hours after seeding, the drug concentrations were added to the
culture. All incubations were performed at a volume of 200 µl.
Mitotic index
MDA-MB-231 and MCF-7 cells were plated on coverslips
and treated with the control or experimental agents for 0–72 h. The
cells were then fixed using Carnoy fixative (ethanol:acetic acid,
3:1) and stained using the Feulgen method. The number of cells in
the mitotic phases (n) per total cells (3,000-3,500; C) was
determined by the same individual. The MI (%) was scorred using the
following formula: MI = (n/C) ×100.
3H-thymidine LI
analysis
For 3H-thymidine LI analysis, which
detects cells in the S phase, MDA-MB-231 and MCF-7 cells were
seeded into round coverslips which were in 24-well plates at a
density of 2×104 cells/well and incubated for 24 h. Then
the experimental treatments were applied to cells. At the end of
the experimental period, cells were treated with medium containing
1 µCi/ml 3H-thymidine for 20 min for evaluation of the
LI.
Autoradiography
After exposure for 3 days at 4°C, autoradiograms
were developed with a D-19 developer solution and fixed with Fixaj
B (both from Kodak, Rochester, NY, USA). The coverslips were
evaluated after being stained with Giemsa for 3 min. The labeling
index (LI) was determined by counting at least 3,000
cells/coverslip. The index is expressed as the percentage of
labeled nuclei.
Apoptotic index
MDA-MB-231 and MCF-7 cells were collected and then
fixed with methanol:phosphate-buffered saline (PBS) (1:1) and
methanol. The cells were fixed and mounted on slides, stained with
0.5 mg/ml DAPI for 30 min and washed with PBS. Nuclear morphology
of the cells was visualized using an Olympus fluorescence
microscope (Olympus Corp., Tokyo, Japan). For evaluation of the AI,
at least 100 cells were counted for the control and each of the
experimental groups.
Statistical analysis
CI, MI, AI and LI values were evaluated relative to
the controls and to each other. For this reason, the values
obtained from all experimental groups were analyzed using the
one-way ANOVA test. The significance between the control and the
experimental groups was determined by the Dunnett's test and the
significance between the experimental groups was determined by the
Student's t-test. P<0.01 was considered to indicate a
statistically significant result.
Results
Cell index
CI values obtained from xCELLigence RTCA system
demonstrated that the PARP inhibitor at both concentrations alone
and in combination with nab-paclitaxel had significant
anti-proliferative effects on both MDA-MB-231 and MCF-7 cell lines.
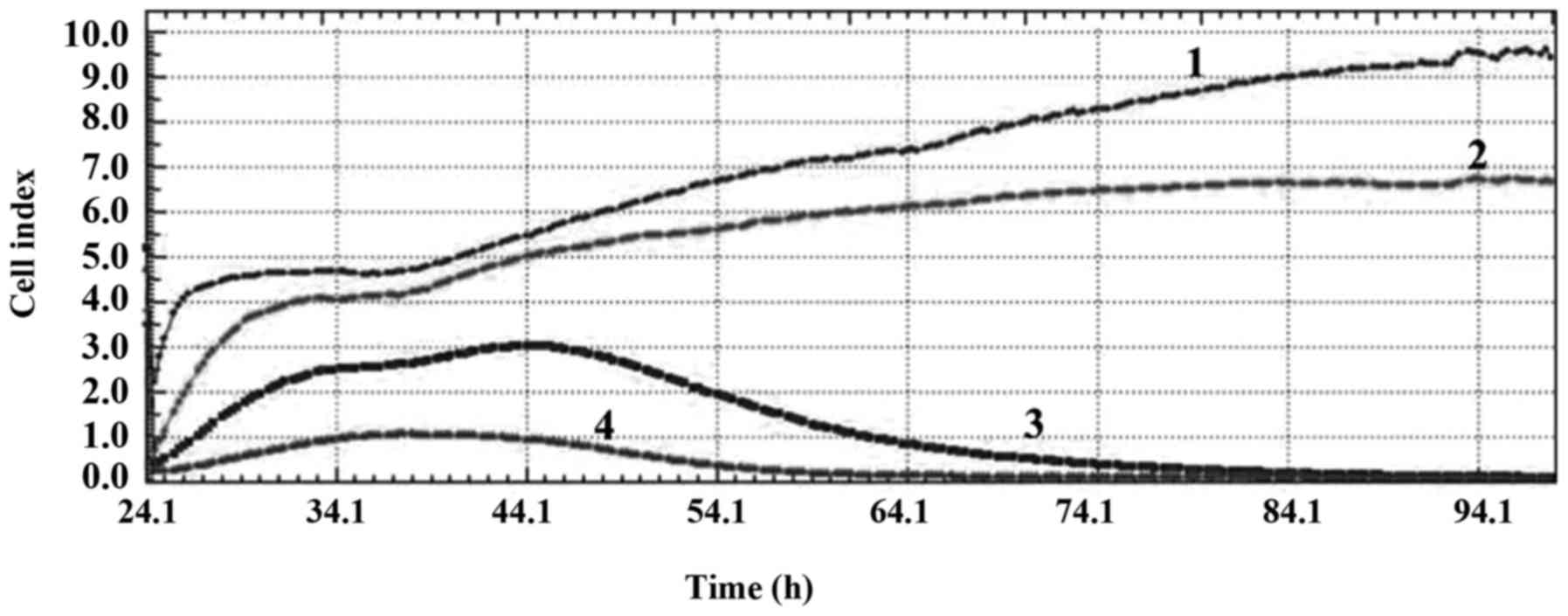
These values also showed that while the PARP inhibitor at a
concentration of 50 µM had a cytostatic effect, the PARP inhibitor
at concentrations 75 and 100 µM had DNA damaging effect on the
MDA-MB-231 cell line (Fig. 1). PARP
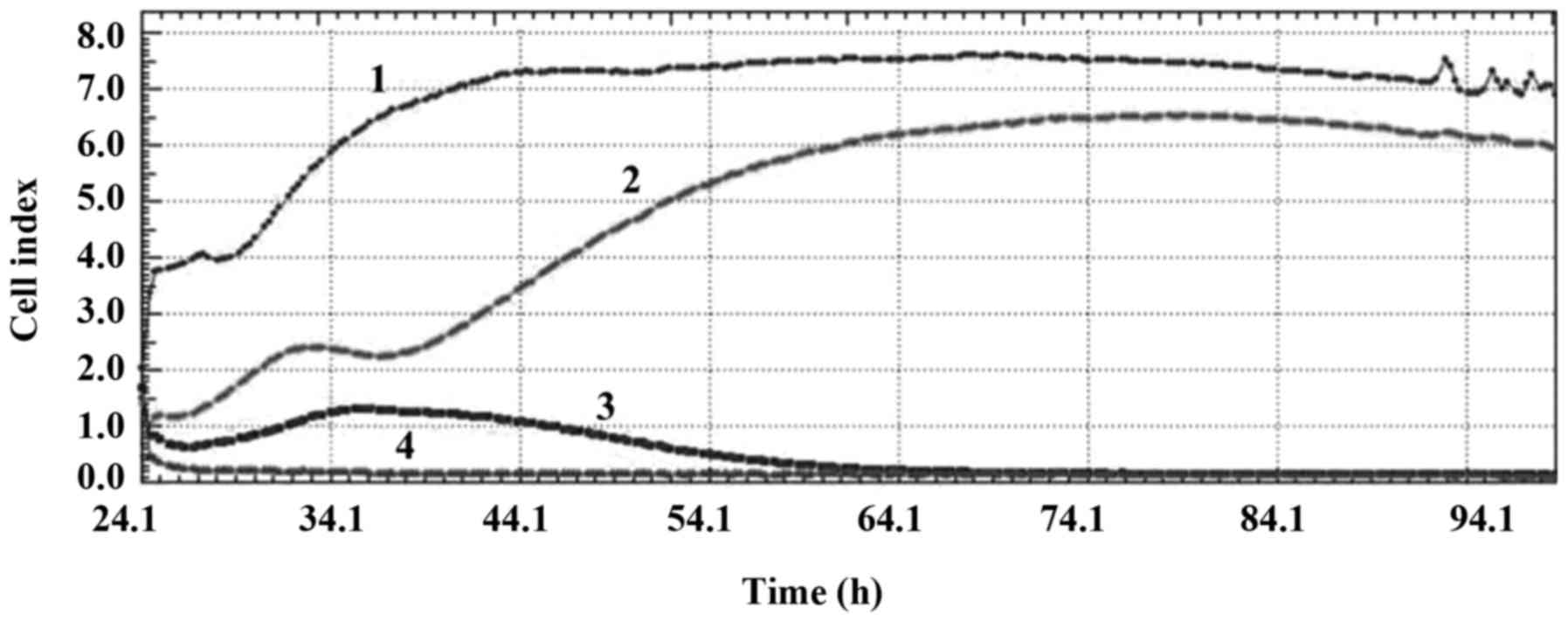
inhibitor at a concentration of 50 µM had an anti-mitotic effect,
the PARP inhibitor at a concentration of 75 µM had a DNA damaging
effect and the PARP inhibitor at a concentration of 100 µM had a
cytoskeletal effect on the MCF-7 cell line (Fig. 2).
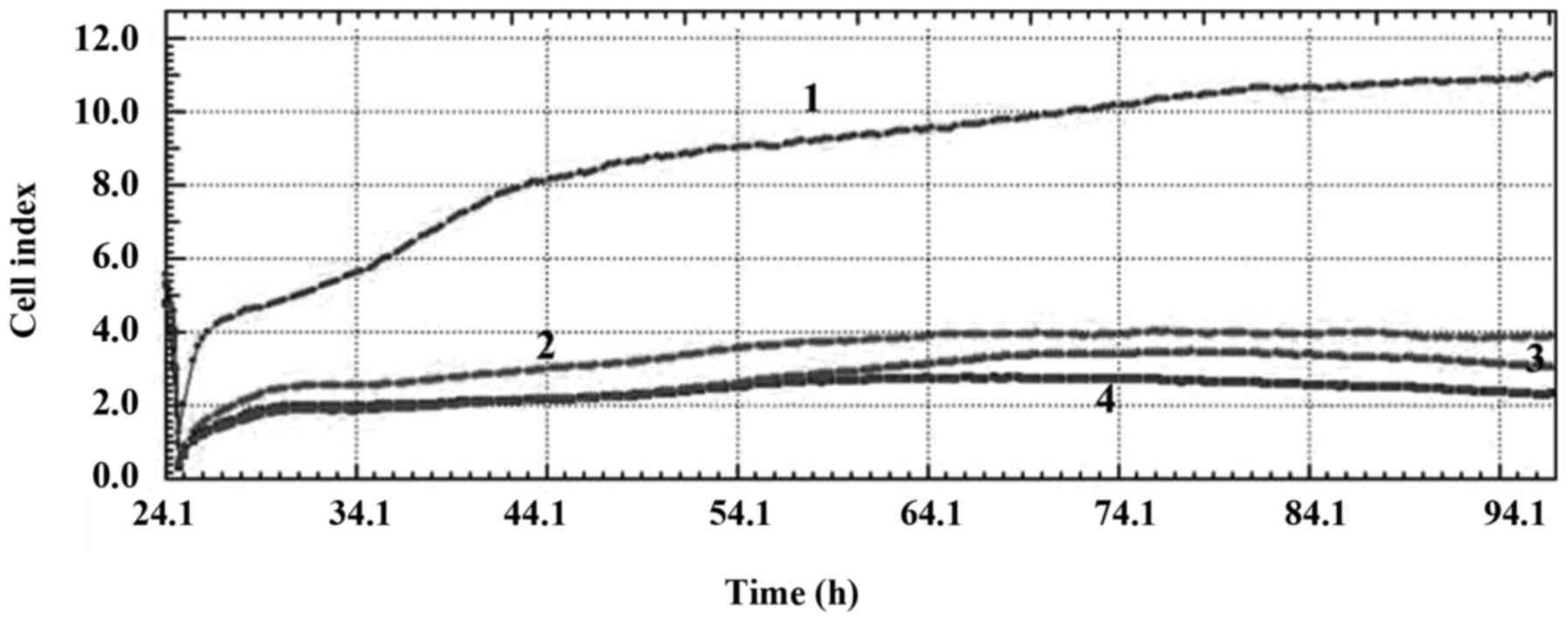
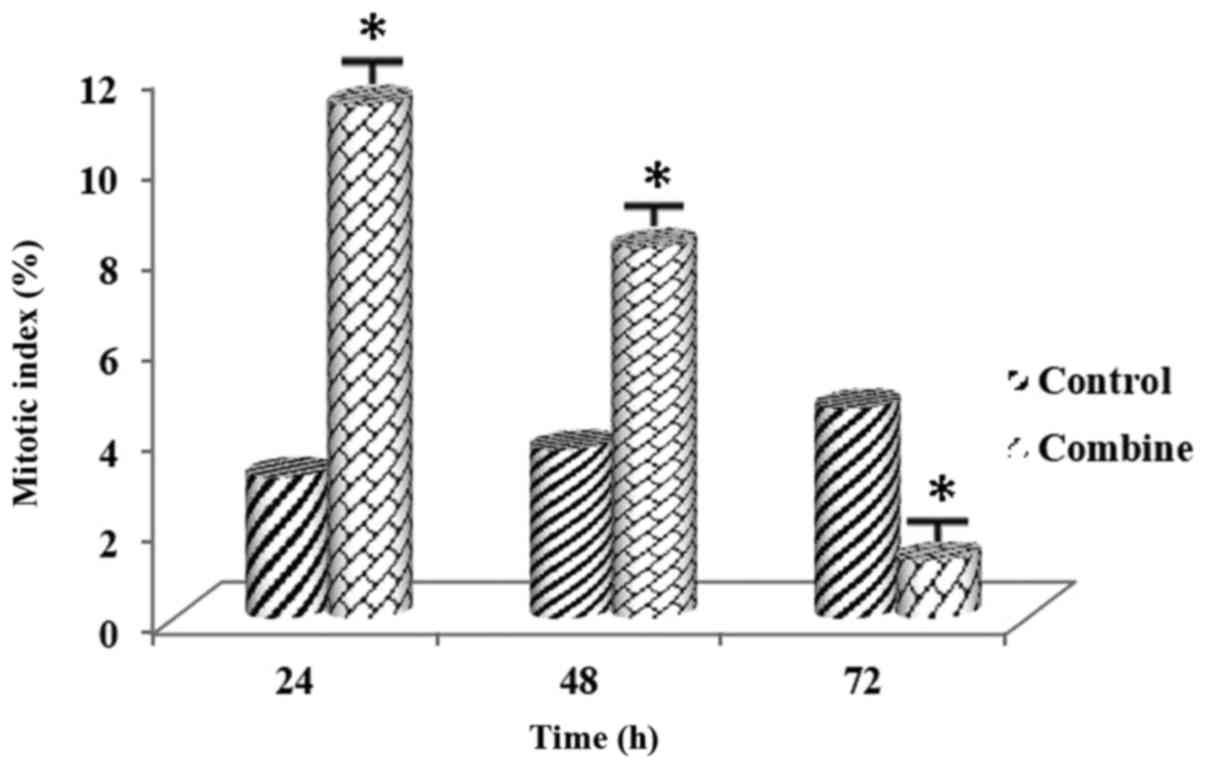
Combination treatment of the PARP inhibitor at
different concentrations with nab-paclitaxel had significant
anti-proliferative effects on the MDA-MB-231 and MCF-7 cell lines.
The CI values demonstrated that the combination treatment with all
concentrations had a cytostatic effect on the MDA-MB-231 cell line
and a cytoskeletal effect on the MCF-7 cell line (Figs. 3 and 4).
Mitotic index
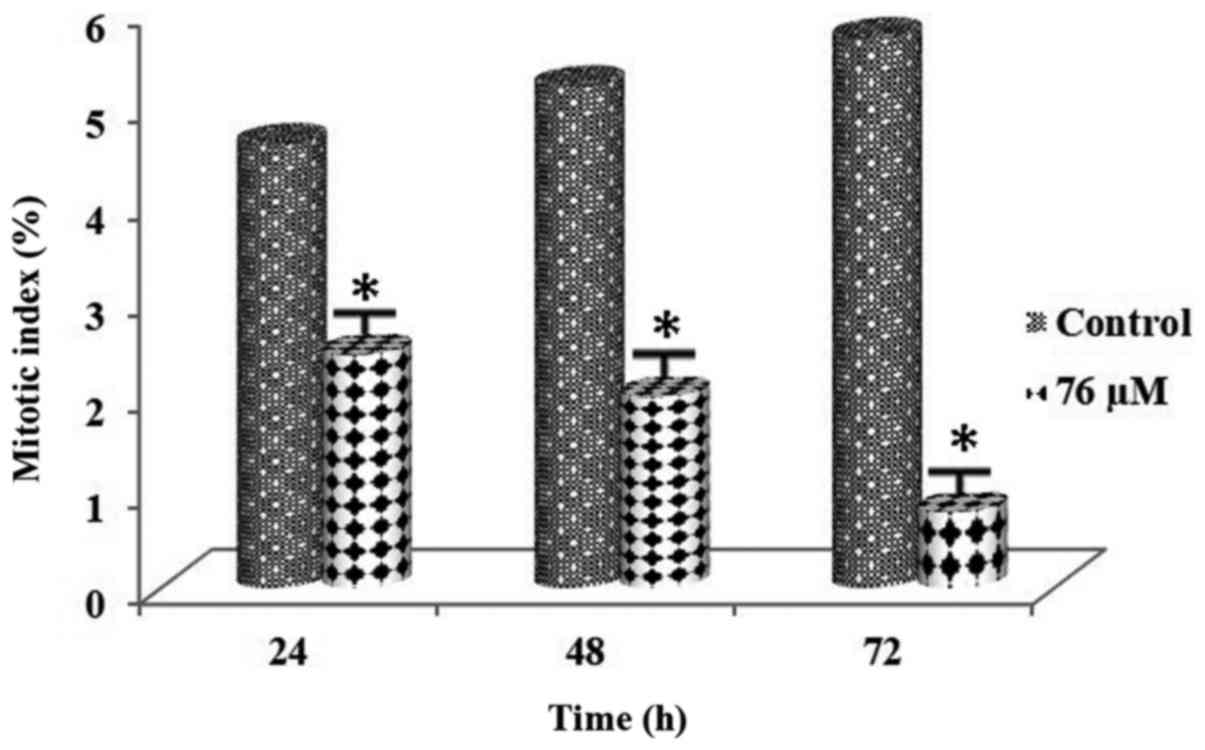
Following administration of the PARP inhibitor at a
concentration of 58 µM in MDA-MB-231 cells and administration of
the PARP inhibitor at a concentration of 76 µM in MCF-7 cells for
0–72 h, 3,000 cells were counted for both the control and the
experimental groups. MI values of these concentrations are shown in
Tables I and II. In addition, MI values for both cell
lines showed that there was a significant decrease in the mitosis
rates in the cell lines (Figs. 5
and 6). The difference was found to
be significant between the control and the experimental groups
(P<0.01). In addition, a statistically significant difference
was noted among all the experimental groups (P<0.01).
 | Table I.MI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h. |
Table I.
MI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h.
|
| MI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | 58 µM |
|---|
| 24 | 3.1±0.03 |
1.78±0.02a |
| 48 | 3.7±0.03 |
1.18±0.03a |
| 72 | 4.6±0.04 |
0.68±0.01a |
 | Table II.MI (%) values of MCF-7 cells treated
with 76 µM dose of the PARP inhibitor for 0–72 h. |
Table II.
MI (%) values of MCF-7 cells treated
with 76 µM dose of the PARP inhibitor for 0–72 h.
|
| MI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | 76 µM |
|---|
| 24 | 4.6±0.02 |
2.24±0.04a |
| 48 | 5.2±0.04 |
1.98±0.02a |
| 72 | 5.7±0.06 |
0.79±0.01a |
Following the combined administration of the PARP
inhibitor at a concentration of 58 µM and nab-paclitaxel at a
concentration of 5.8 µM in the MDA-MB-231 cells and administration
of the PARP inhibitor at a concentration of 76 µM and
nab-paclitaxel at a concentration of 5.8 µM in MCF-7 for 0–72 h,
3,000 cells were counted both for the control and the experimental
groups. MI values of these concentrations are shown in Tables III and IV. MI values for both cell lines
demonstrated that there was a significant increase at 24 h and a
significant decrease at 72 h (Figs.
7 and 8). The difference was
significant between the control and the experimental groups
(P<0.01). In addition, a statistically significant difference
was noted among all the experimental groups (P<0.01).
 | Table III.MI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h. |
Table III.
MI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h.
|
| MI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | Combination |
|---|
| 24 | 3.1±0.03 |
11.27±0.03a |
| 48 | 3.7±0.03 |
8.13±0.02a |
| 72 | 4.6±0.04 |
1.27±0.01a |
 | Table IV.MI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h. |
Table IV.
MI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h.
|
| MI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | Combination |
|---|
| 24 | 4.6±0.02 |
12.32±0.03a |
| 48 | 5.2±0.04 |
9.96±0.02a |
| 72 | 5.7±0.06 |
1.23±0.01a |
Labelling index
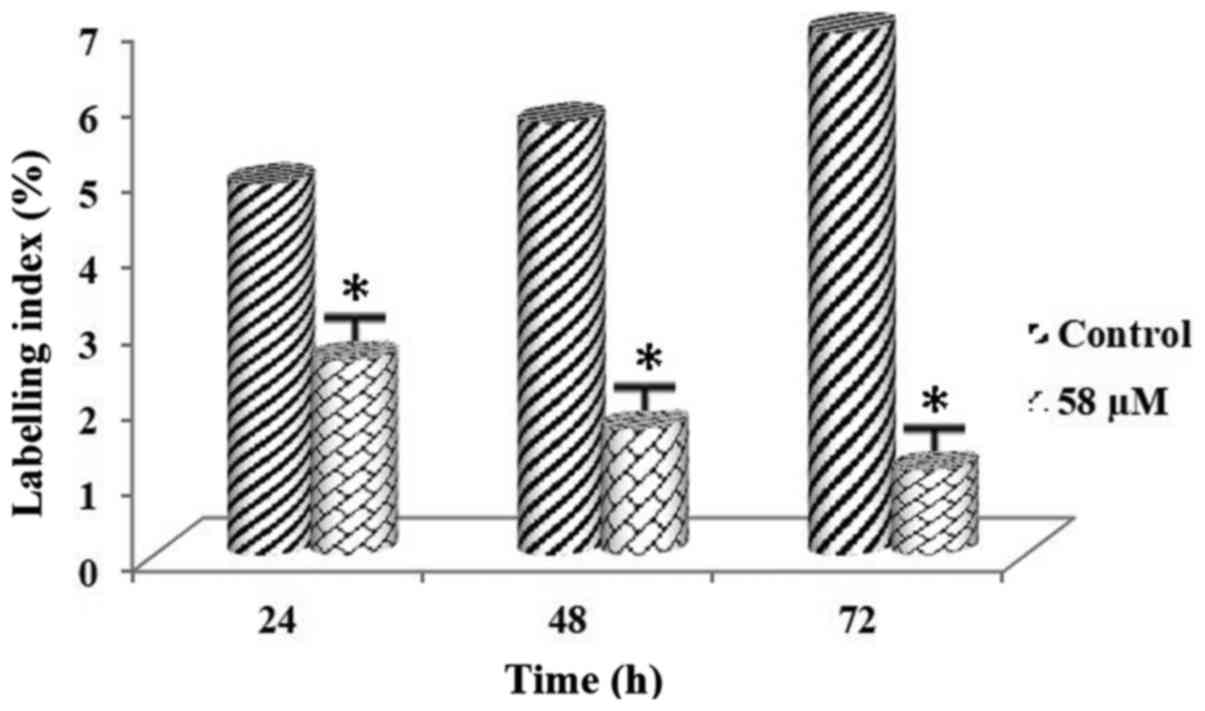
Following administration of the PARP inhibitor at a
concentration of 58 µM in MDA-MB-231 cells and administration of
the PARP inhibitor at a concentration of 76 µM in MCF-7 cells for
0–72 h, 3,000 cells were counted both for the control and the
experimental groups. LI values of these concentrations are shown in
Tables V and VI. LI values belonging to both cell lines
demonstrated that there was a significant decrease in DNA synthesis
rates of the cell lines (Figs. 9
and 10). The difference was
significant between the control and the experimental groups
(P<0.01). In addition, a statistically significant difference
was noted among all the experimental groups (P<0.01).
 | Table V.LI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h. |
Table V.
LI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h.
|
| LI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | 58 µM |
|---|
| 24 | 4.9±0.04 |
2.57±0.03a |
| 48 | 5.7±0.02 |
1.67±0.05a |
| 72 | 6.9±0.03 |
1.12±0.03a |
 | Table VI.LI (%) values of MCF-7 cells treated
with 76 µM dose of the PARP inhibitor for 0–72 h. |
Table VI.
LI (%) values of MCF-7 cells treated
with 76 µM dose of the PARP inhibitor for 0–72 h.
|
| LI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | 76 µM |
|---|
| 24 | 5.8±0.05 |
2.27±0.02a |
| 48 | 6.3±0.05 |
1.76±0.04a |
| 72 | 8.9±0.03 |
1.53±0.03a |
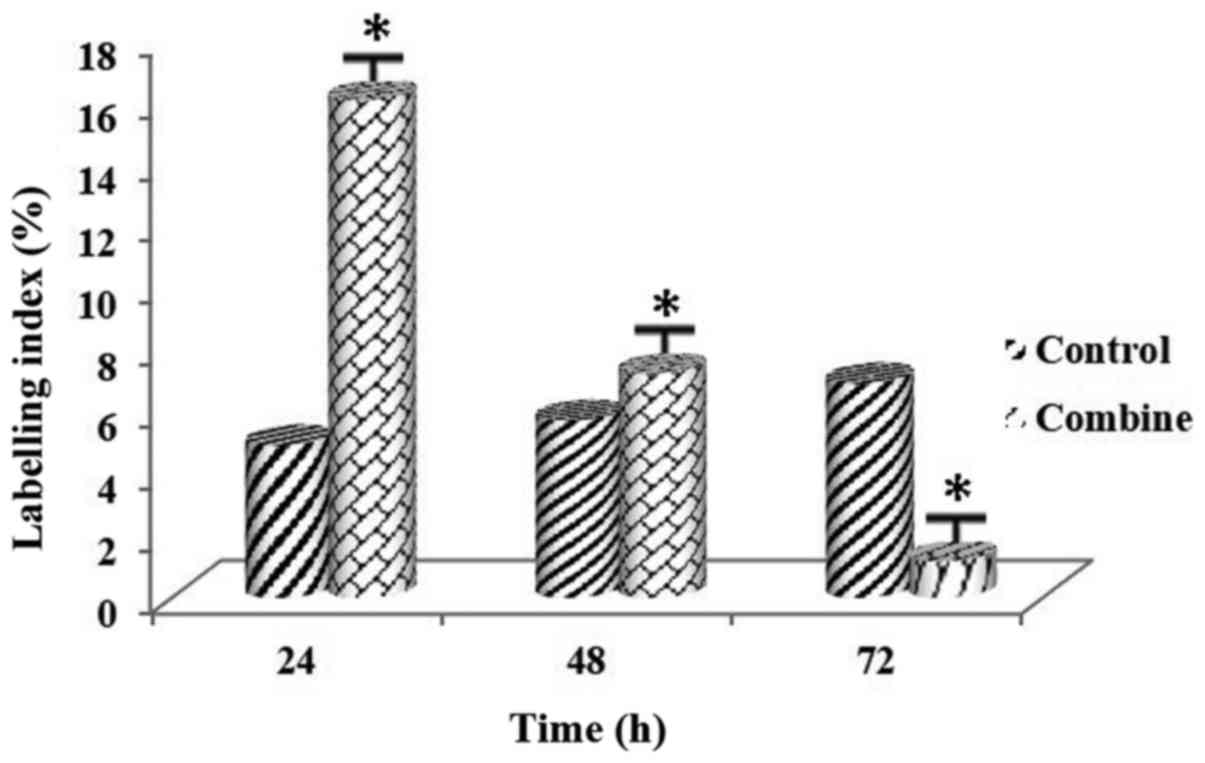
Following the combined administration of the PARP
inhibitor at a concentration of 58 µM and nab-paclitaxel at a
concentration of 5.8 µM in MDA-MB-231 and the combined
administration of the PARP inhibitor at a concentration of 76 µM
and nab-paclitaxel at a concentration of 5.8 µM in MCF-7 cells for
0–72 h, 3,000 cells were counted both for the control and the
experimental groups. LI values of these concentrations are shown in
Tables VII and VIII. LI values for both cell lines
indicated that there was an increase at 24 h, and a significant
decrease at 72 h (Figs. 11 and
12). The difference was
significant between the control and the experimental groups
(P<0.01). In addition, a statistically significant difference
was noted among all the experimental groups (P<0.01).
 | Table VII.LI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h. |
Table VII.
LI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h.
|
| LI (%) |
|---|
|
|
|
|---|
| Time (h)
Combination | Control |
|
|---|
| 24 | 4.9±0.04 |
16.03±0.05a |
| 48 | 5.7±0.02 |
7.22±0.03a |
| 72 | 6.9±0.03 |
1.18±0.03a |
 | Table VIII.LI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h. |
Table VIII.
LI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h.
|
| LI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | Combination |
|---|
| 24 | 5.8±0.05 |
15.13±0.03a |
| 48 | 6.3±0.05 |
10.98±0.05a |
| 72 | 8.9±0.03 |
2.13±0.04a |
Apototic index
Following administration of the PARP inhibitor at a
concentration of 58 µM in MDA-MB-231 cells and the PARP inhibitor
at a concentration of 76 µM in MCF-7 cells for 0–72 h, 250 cells
were counted both for the control and the experimental groups. AI
values of these concentrations are shown in Table IX and X. Also AI values for both
cell lines demonstrated that there was a significant increase in
apoptosis rates of the cell lines (Figs. 13 and 14). The difference was significant
between the control and the experimental groups (P<0.01). In
addition, statistically significant difference was noted among all
the experimental groups (P<0.01).
 | Table IX.AI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h. |
Table IX.
AI (%) values of MDA-MB-231 cells
treated with 58 µM dose of the PARP inhibitor for 0–72 h.
|
| AI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | 58 µM |
|---|
| 24 | 1.17±0.01 |
4.87±0.06a |
| 48 | 1.89±0.01 |
10.17±0,09a |
| 72 | 2.17±0.03 |
17.83±0.11a |
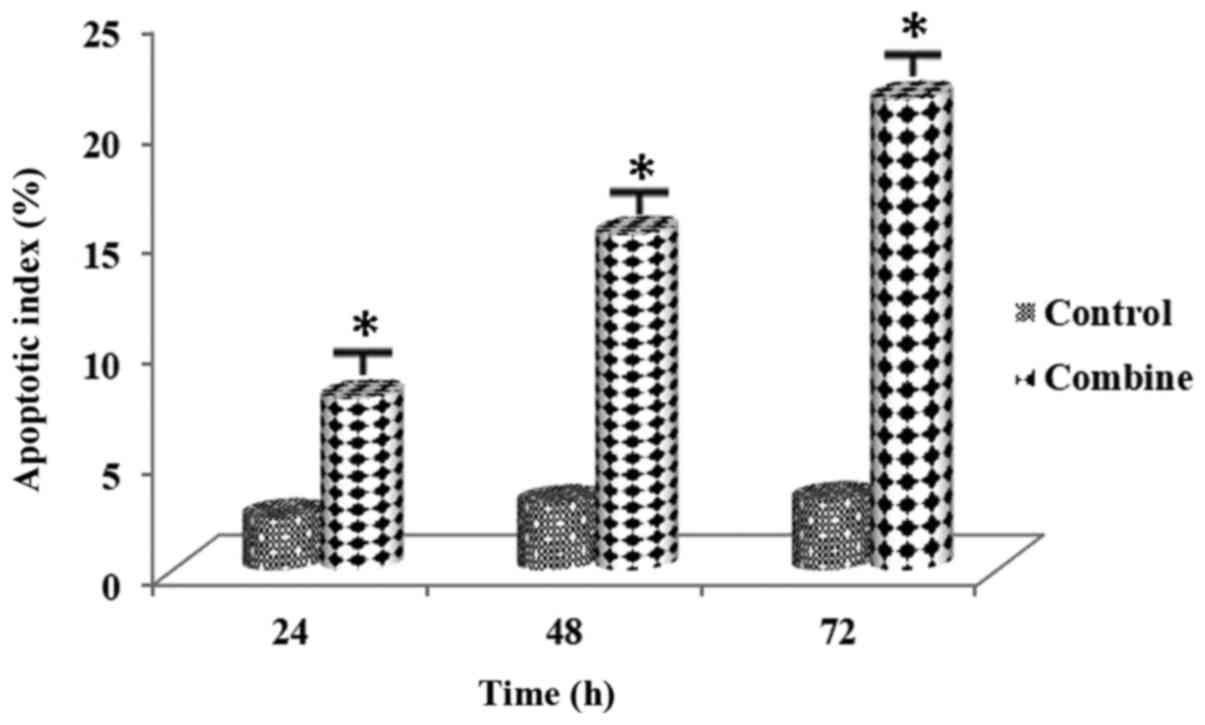
Following the combined administration of the PARP
inhibitor at a concentration of 58 µM and nab-paclitaxel at a
concentration of 5.8 µM in MDA-MB-231 and the combined
administration of the PARP inhibitor at a concentration of 76 µM
and nab-paclitaxel at a concentration of 5.8 µM in MCF-7 cells for
0–72 h, 250 cells were counted both for the control and the
experimental groups. AI values of these concentrations are shown in
Tables XI and XII. AI values for both cell lines
demonstrated that there was a significant increase in apoptosis
rates of the cell lines (Figs. 15
and 16). The difference was
significant between the control and the experimental groups
(P<0.01). In addition, statistically significant difference was
noted among all the experimental groups (P<0.01).
 | Table XI.AI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h. |
Table XI.
AI (%) values of MDA-MB-231 cells
treated with the combination of 58 µM dose of the PARP inhibitor
and 5.8 µM nab-paclitaxel for 0–72 h.
|
| AI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | Combination |
|---|
| 24 | 1.17±0.01 |
5.75±0.07a |
| 48 | 1.89±0.01 |
18.17±0.12a |
| 72 | 2.17±0.03 |
24.93±0.16a |
 | Table XII.AI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h. |
Table XII.
AI (%) values of MCF-7 cells treated
with the combination of 76 µM dose of the PARP inhibitor and 5.8 µM
nab-paclitaxel for 0–72 h.
|
| AI (%) |
|---|
|
|
|
|---|
| Time (h) | Control | Combination |
|---|
| 24 | 2.36±0.02 |
7.76±0.09a |
| 48 | 2.98±0.01 |
15.16±0.12a |
| 72 | 3.13±0.05 |
21.28±0.18a |
Discussion
Breast cancer is a heterogeneous disease. It
consists of several distinct entities having significantly
different biological characteristics and clinical behaviors
(9). TNBC is also a disease with
different characteristics and the standard treatment is cytotoxic
chemotherapy. Recent advances in the field of medicine have
demonstrated that multiple TNBC subtypes exist as well (10).
Today several limitations related to cancer
treatment exist. One of these limitations is the use of
conventional agents that not only target cancer cells, but also
result in high toxicity, thus precluding effective treatment.
Elucidation of cancer-causing mechanisms in recent studies has led
to the discovery of novel key molecules and pathways which have the
potential to become successful targets for the elimination of
cancer cells only (11). Although
TNBCs are reported to respond to neoadjuvant chemotherapy, the
survival of patients with such tumors is poor and their management
may therefore require a more aggressive alternative intervention
(12,13).
Upregulation of PARP1 is expressed by TNBCs
(14). This finding indicates that
TNBCs are sensitive to PARP inhibitors (5). The use of PARP inhibitors in
combination with systemic therapies for targeting DNA repair
deficiencies continues to present a significant clinical interest
(15). PARP1 inhibitors may
demonstrate synergism with various anti-metabolites and
anti-mitotic agents, as well. PARP1 inhibitors are also promising
agents based on the fact that they appear to possess few
side-effects, and also protect normal tissues. Indeed, reports from
clinical trials performed with PARP1 inhibitors indicate that phase
I studies have successfully been completed, and phase II studies
have been initiated for various ischemic disorders (16). Preclinical studies have demonstrated
that breast cancer cell lines having a TN phenotype appear to be
more sensitive to PARP inhibitors in comparison to non-TNBC cells
(17).
Preclinical studies indicate that a combination of
PARP inhibitor and various anti-proliferative agents yielded a more
sustainable recession of cell proliferation; it also enhanced the
retention of DNA damage and ultimately led to an increase in
apoptosis for pancreatic and TNBC cell lines (17,18).
The primary mechanism of the action of paclitaxel
has been intensively investigated. It leads to the promotion of
polymerization of tubulin heterodimers to microtubules. When
paclitaxel is administered at high concentrations, it both
stabilizes microtubules and also increases the total polymer mass
(19). However, these
concentrations are far higher than what is needed for the
inhibition of microtubule functions (20). Paclitaxel suppresses dynamic changes
in microtubules at clinically relevant concentrations, therefore,
it leads to mitotic arrest (21).
Paclitaxel has several accompanying toxic effects (22). Nab-paclitaxel is a novel formulation
of paclitaxel, which consists of a colloidal suspension formed by
albumin-bound paclitaxel nanoparticles (23). It has been shown in phase III trials
that nab-paclitaxel is a next-generation taxane having a superior
therapeutic index compared to paclitaxel (8,24).
The combination of paclitaxel with PARP inhibitor
olaparib has also been investigated in a phase I/II trial in TNBC.
Nineteen patients, most of whom were previously administered taxane
therapy were treated with daily 200 mg of olaparib (oral) in
combination with paclitaxel 90 mg/m2 (i.v. drip) weekly
for 3–4 weeks. When we consider the previous taxane exposure,
olaparib usage may be successful in overcoming taxane resistance
(25).
Paclitaxel is commonly being used in several
subtypes of cancer and a PARP inhibitor/PXL combination was
suggested as an alternative therapeutic approach in various patient
populations (26,27).
In a study conducted by Cetin and Topcul, the
effectiveness of nab-paclitaxel and liposomal cisplatin combination
was investigated in MDA-MB-231 and MCF-7 cell lines, and as a
result a significant decrease in cell viability and cell index
values for both cell lines was observed. MI and LI values of both
cell lines were seen to increase at 24 h, and then decreased
significantly at 72 h. There was a significant increase in AI
values, as well (28).
In the present study, we demonstrated that there was
a significant decrease in cell, mitotic and labelling indices and a
significant increase in the AI values specifically at 72 h. The
results of the present study seem to be concordant with the above
mentioned studies and addition of nab-paclitaxel to the treatment
combination showed similar profiles with the above mentioned
previous findings.
Acknowledgements
The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University (project
no. 41832).
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MT and İÇ conceived and designed the study. MT and
İÇ performed xCelligence experiments. MT performed labelling index
experiments. İÇ performed mitotic index experiments. Apoptotic
index experiments were performed by SÖT. Statistical analyses were
performed by MÖKO. All authors reviewed and edited the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sørlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cetin I and Topcul M: Triple negative
breast cancer. Asian Pac J Cancer Prev. 15:2427–2431. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouleau M, Patel A, Hendzel MJ, Kaufmann
SH and Poirier GG: PARP inhibition: PARP1 and beyond. Nat Rev
Cancer. 10:293–301. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weil MK and Chen AP: PARP inhibitor
treatment in ovarian and breast cancer. Curr Probl Cancer. 35:7–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibrahim NK, Desai N, Legha S, Soon-Shiong
P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A,
Hortobagyi GN, et al: Phase I and pharmacokinetic study of ABI-007,
a Cremophor-free, protein-stabilized, nanoparticle formulation of
paclitaxel. Clin Cancer Res. 8:1038–1044. 2002.PubMed/NCBI
|
|
7
|
ten Tije AJ, Verweij J, Loos WJ and
Sparreboom A: Pharmacological effects of formulation vehicles:
Implications for cancer chemotherapy. Clin Pharmacokinet.
42:665–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reis-Filho JS and Tutt ANJ: Triple
negative tumours: A critical review. Histopathology. 52:108–118.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saha P and Nanda R: Concepts and targets
in triple-negative breast cancer: Recent results and clinical
implications. Ther Adv Med Oncol. 8:351–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topcul M and Cetin I: Endpoint of cancer
treatment: Targeted therapies. Asian Pac J Cancer Prev.
15:4395–4403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Shaughnessy J, Yoffe M, Osborne C, Blum
J, Rocha C, Ossovskaya V, Sherman B and Bradley C: Triple negative
breast cancer: A phase 2, multi-center, open-label, randomized
trial of gemcitabine/carboplatin (G/C), with or without BSI-201, a
PARP inhibitor. Cancer Res. 69 Suppl:21202009. View Article : Google Scholar
|
|
15
|
Dent RA, Lindeman GJ, Clemons M, Wildiers
H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Watkins CL and
Carmichael J: Phase I trial of the oral PARP inhibitor olaparib in
combination with paclitaxel for first- or second-line treatment of
patients with metastatic triple-negative breast cancer. Breast
Cancer Res. 15:R882013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Graziani G and Szabó C: Clinical
perspectives of PARP inhibitors. Pharmacol Res. 52:109–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hastak K, Alli E and Ford JM: Synergistic
chemosensitivity of triple-negative breast cancer cell lines to
poly(ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin.
Cancer Res. 70:7970–7980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacob DA, Bahra M, Langrehr JM, Boas-Knoop
S, Stefaniak R, Davis J, Schumacher G, Lippert S and Neumann UP:
Combination therapy of poly (ADP-ribose) polymerase inhibitor
3-aminobenzamide and gemcitabine shows strong antitumor activity in
pancreatic cancer cells. J Gastroenterol Hepatol. 22:738–748.
2007.PubMed/NCBI
|
|
19
|
Schiff PB and Horwitz SB: Taxol stabilizes
microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA.
77:1561–1565. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jordan MA and Wilson L: Microtubules and
actin filaments: Dynamic targets for cancer chemotherapy. Curr Opin
Cell Biol. 10:123–130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crown J and O'Leary M: The taxanes: An
update. Lancet. 355:1176–1178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kratz F: Albumin as a drug carrier: Design
of prodrugs, drug conjugates and nanoparticles. J Control Release.
132:171–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Untch M, Jackisch C, Schneeweiss A, Conrad
B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kümmel S,
Hilfrich J, et al: German Breast Group (GBG); Arbeitsgemeinschaft
Gynäkologische Onkologie-Breast (AGO-B) Investigators:
Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant
chemotherapy for early breast cancer (GeparSepto-GBG 69): A
randomised, phase 3 trial. Lancet Oncol. 17:345–356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giaccone G, Rajan A, Kelly RJ, Gutierrez
M, Kummar S, Yancey M, Ji JJ, Zhang Y, Parchment RE and Doroshow
JH: A phase I combination study of olaparib (AZD2281; KU-0059436)
and cisplatin (C) plus gemcitabine (G) in adults with solid tumors.
J Clin Oncol. 28 suppl 15:30272010. View Article : Google Scholar
|
|
26
|
Haince JF, Kozlov S, Dawson VL, Dawson TM,
Hendzel MJ, Lavin MF and Poirier GG: Ataxia telangiectasia mutated
(ATM) signaling network is modulated by a novel
poly(ADP-ribose)-dependent pathway in the early response to
DNA-damaging agents. J Biol Chem. 282:16441–16453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang B, Guo RF, Tan XH, Zhao M, Tang ZB
and Lu YY: Expression status of ataxia-telangiectasia-mutated gene
correlated with prognosis in advanced gastric cancer. Mutat Res.
638:17–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cetin I and Topcul MR: In vitro
antiproliferative effects of nab-paclitaxel with liposomal
cisplatin on MDA-MB-231 and MCF-7 breast cancer cell lines. J BUON.
22:347–354. 2017.PubMed/NCBI
|