Introduction
Non-melanoma skin cancer (NMSC) includes squamous
cell carcinoma (SCC) and basal cell carcinoma (BCC) (1,2). BCC
represents ~80%, and SCC accounts for ~20% of all diagnosed NMSC
cases worldwide (3). According to
previous reports, SCC has a higher prevalence in comparison to BCC
(4). Apart from ultraviolet
radiation (UVR), other common risk factors of NMSC include
occupational and environmental exposures to polycyclic aromatic
hydrocarbons, arsenic, and ionizing radiation (5). Polycyclic aromatic hydrocarbons
originate during the incomplete combustion of organic materials,
including wood, petroleum and coal, and are well known for their
toxic abilities apart from being carcinogenic and mutagenic in
nature (6,7). 7,12-Dimethylbenz(a)anthracene
(DMBA) is the most common polycyclic aromatic hydrocarbon used as
an initiating agent in chemically triggered skin cancer models, and
12-O-tetradecanoylphorbol-13-acetate (TPA), as a tumor
promoter inducing two-stage skin cancer model, has been illustrated
to closely mimic human SCC (8–10). In
general, skin carcinogenesis is known as a multistep procedure,
which consists of initiation, acceleration, development and
progression (11). Identification
of effective chemoprevention agents seems to be one of the most
feasible strategies to reverse or impede carcinogenesis (12,13).
Salidroside (SR), 2-(4-hydroxyphenyl)ethyl
β-D-glucopyranoside, is a phenylpropanoid glycoside, which is
extracted from the root of Rhodiola rosea L. and has been
applied as a medicinal herb to protect erythrocytes against
oxidative stress and enhance resistance to fatigue (14,15).
In addition, SR was found to inhibit the inflammatory response by
regulating the nuclear factor (NF)-κB pathway, ameliorating gastric
damage (16). Furthermore, SR was
reported to modulate the apoptotic response via altering the
expression levels of apoptosis-related signals in various diseases
(17,18). In addition, the protective effects
of SR are considered to be related to its anti-inflammatory
properties in different diseases (15,19).
However, to date, the innate mechanism of skin cyto-protection is
consistently diminished or is not adequate to ameliorate cellular
transformation caused by radiation and various chemical carcinogens
(20). Considering the application
of SR in various diseases, it may be an effective candidate with
which to prevent skin carcinogenesis.
Inflammation is a key molecular mechanism which
induces disorders in organisms, including skin disease (21,22).
According to previous studies, a variety of pro-inflammatory
cytokines, such as interleukins (ILs), and tumor necrosis factor
(TNF), and the anti-inflammatory factor TGF-β1, are overexpressed
in the skin under different conditions (23). NF-κB plays an essential role in
different pathologies via regulation of chemokines, cytokines as
well as cell adhesion molecules (24,26).
Liberation from IκB promotes NF-κB to translocate into the nucleus.
Then, it induces gene transcription through combination with NF-κB
responsive gene promoter (26).
Furthermore, apoptosis is the most common, gene-directed form of
programmed cell death, contributing to different physiologic and
pathologic processes (27).
Apoptosis has been characterized as an important molecular
mechanism and various drugs have been explored for preventing
apoptosis in different types of tumors (28,29).
As previously reported, apoptosis is involved in skin cancer
development, which is dependent on the expression of anti-apoptotic
and pro-apoptotic signals (30,31).
Based on the effects of SR on inflammation and apoptosis, here, in
our study, we attempted to assess the preventive role of SR in
DMBA/TPA-induced two-stage skin cancer. Parameters of body weight,
tumor incidence, tumor size and the number of lesions were measured
to calculate the chemo-preventive value of SR. The inflammatory and
apoptotic response were also investigated to explain the molecular
mechanisms of SR during the regulation of skin carcinogenesis.
Materials and methods
Animals and treatments
Eighy female Institute of Cancer Research (ICR)
mice, 6–7 weeks of age, were purchased from the Shanghai
Experimental Animal Center (Shanghai, China) and kept in
climate-controlled quarters with a 12-h light and dark cycle with
food and water in cages under germ-free conditions. All
experimental procedures were carried out following the Guide for
the Care and Use of Laboratory Animals of Huai'an First People's
Hospital, Nanjing Medical University (Nanjing, China) and before
the animal experiments were performed, the procedures were approved
by the Research Ethics Committee of Huai'an First People's
Hospital, Nanjing Medical University (Nanjing, China).
ICR mice were randomly divided into four groups, 20
animals per group. The experimental design of the in vivo
study is exhibited in Fig. 1A. All
mice were shaved ahead of our study. In brief, the groups receiving
DMBA/TPA, and DMBA/TPA+SR were first administered 60 µg DMBA
dissolved in 0.2 ml to the naked backs. DMBA was administered to
mice for two weeks, from week 1 to week 3. The first two weeks
after skin tumor initiation with DMBA, animals in the DMBA/TPA and
DMBA/TPA+SR groups were further exposed to 4 µg TPA twice a week
for a total of 20 weeks ranging from week 3 to week 23. In
addition, the mice treated with SR (20 and 40 mg/kg) were topically
treated 30 min before each DMBA/TPA treatment five times a week
until the sacrifice of the animals at week 22 (32–35).
DMBA and TPA were purchased from Sigma-Aldrich (St. Louis, MO,
USA). SR (>98% purity, molecular formula:
C14H20O7, CAS 10338-51-9) was
purchased from Shanghai Ronghe Medicine Science and Technology
Development Co, Ltd. (Shanghai, China). Sizes of the skin tumors
>1 mm in diameter were measured every week using calipers. The
dorsal skin of mice derived from different experiments was excised.
After the fat was removed from the dorsal skin on ice, the skin
tissue samples were placed in liquid nitrogen immediately for
further research. The eye blood was collected for pro-inflammatory
cytokine determination.
Cells and culture
Normal human epidermal keratinocytes, HaCaT, were
purchased from Combioer Biosciences Co., Ltd. (Nanjing, China).
Human hypertrophic scar fibroblasts (HSFs) were purchased from
Bioleaf Corp. (Shanghai, China). Human normal liver cell line L02
was obtained from the Cell Bank of the Type Culture Collection of
the Chinese Academy of Science (Shanghai, China). All cells were
cultured and maintained in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in
a humidified 5% CO2 atmosphere at 37°C. SR used for the
treatment of skin cancer was dissolved in DMSO (KeyGen Biotech Co.,
Ltd., Nanjing, China) and stored at −20°C, and then it was diluted
in DMEM at the indicated concentrations for experimental treatment.
The final DMSO concentration was no more than 0.1% (v/v) in each
treatment.
MTT assay
To calculate the growth inhibitory role of SR in
different cell lines, ~1×103 cells/well were planted in
96-well plates (Corning Inc., Corning, NY, USA) with complete
growth medium. On the following day, the cells were treated with
different concentrations of SR ranging from 0 to 160 µM and
incubated at 37°C for 24 h. Then, the cell viability was determined
by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay at 570 nm.
Flow cytometric analysis
The Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit was purchased from KeyGen Biotech Co., Ltd., to
measure the apoptotic cell levels. All cells after the different
treatments as described were harvested and washed with ice-cold PBS
for twice, then incubated in a darkroom with Annexin V-FITC and PI
for 15 min. Subsequently, the cells were analyzed by flow cytometry
(BD Biosciences, San Jose, CA, USA). The percentage of cells
undergoing apoptosis was quantified.
ELISA methods
The eye blood was subjected to centrifugation at
12,000 × g for 10 min to carefully collect the supernatant. Then,
TNF-α, IL-1β, IL-18, IL-6, COX2 and TGF-β1 levels in serum were
determined using the Mouse TNF-α Quantikine ELISA kit (R&D
Systems, Inc., Minneapolis, MN, USA), Mouse IL-1β ELISA kit (IL-1β)
(Abcam, Cambridge, UK), Mouse IL-18 ELISA (R&D Systems, Inc.),
Mouse IL-6 Quantikine ELISA kit (R&D Systems, Inc.), Mouse COX2
ELISA kit (Abcam) and Mouse TGF-β1 ELISA kit (Abcam) following the
manufacturer's instructions.
Histochemical analysis
Fixed skin and tumor tissues obtained from mice were
embedded in paraffin blocks and 3-µm thick sections were cut. Skin
and tumor sections were then deparaffinized and stained with
hematoxylin and eosin (H&E) staining. The thickness of the skin
epidermis was measured using Magnus Analytics Magnuspro software.
Epidermal thickness of H&E staining sections was further
assessed by ImageJ software (National Institutes of Health,
Bethesda, MD, USA). For immunohistochemical images, the skin tissue
sections were then exposed to HCl (3.5 M) for 20 min at room
temperature and washed using PBS for 3 times. Subsequently, the
skin tissue sections were treated with peroxidase (0.3%) to
diminish endogenous peroxidase activity. Then, tissue sections were
incubated with normal goat serum (5%) for 30 min followed by
incubation with primary antibodies (anti-p53 antibody, ab131442;
Abcam) at 1:100 dilution for 2 h at room temperature. The section
was then incubated with HRP-conjugated compact polymer systems.
Diaminobenzidine (DAB; ChemService, West Chester, PA, USA) was used
as the chromogen according to the manufacturer's instructions.
Apoptosis assay of tissue samples was determined by TUNEL using an
In Situ Cell Death Detection kit, Fluorescein (Roche Applied
Science, USA) following the manufacturer's protocol. Tumor tissue
sections were counterstained with hematoxylin. Then, the number of
TUNEL-positive cells was evaluated under a microscope. The ratio of
apoptotic cells was determined by the ratio of the apoptotic cells
to total cells.
As for the fluorescence assays, the cells were
carefully harvested after various treatments and then fixed in 4%
paraformaldehyde for 30 min. Then, the cells were incubated with
primary antibody (p-NF-κB; Abcam) at 4°C overnight.
Fluorophore-conjugated secondary antibodies were treated for 1 h at
25°C. The skin tissue sections were dried for 10 min at room
temperature, fixed with chilled acetone for 10 min at −20°C, and
washed with PBS for three times (5 min each). The pre-incubation
was conducted with 5% normal rabbit serum at room temperature for 1
h, and sections were incubated with specific antibody: polyclonal
rabbit anti-p-NF-κB (1:50; Abcam, Cambridge, MA, USA) at 4°C
overnight. The Alexa Fluor 488 and 594 labeled anti-rabbit
secondary antibodies (Invitrogen) were used in this part. Sections
were then subjected to immunofluorescence staining via
epifluorescence microscopy (Sunny Co.).
Real-time quantitative (qPCR) and
reverse transcription PCR assays
Total RNA was isolated from the skin tissue samples
and HaCaT cells after various treatments using TRIzol reagent
(KeyGen Biotech Co., Ltd.). Reverse transcription PCR was conducted
using the PrimeScript RT Reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China) following the manufacturer's instructions and
cDNA was then used as the template for the subsequent reactions.
qPCR was conducted with SYBR Premix Ex Taq II obtained from Takara.
The ABI-PRISM 7500 Sequence Detection System (Applied Biosystems
Life Technologies, Foster City, CA, USA) was used according to the
manufacturer's instructions. The primer sequences used in our study
were commercially synthesized and are as follows. The mRNA level of
GAPDH was used as the loading control. The 2−∆∆Cq
analyzing method was applied to evaluate the fold changes in mRNA
levels in each group. The primers were as follows: TNF-α forward,
5′-CGAAAGGGAGTAGAAGTGCG-3′ and reverse,
5′-AAACATACAGAGCCGGCTAGCC-3′; IL-1β forward,
5′-ACATAGAGAGGGAGTACAC-3′ and reverse, 5′-CAGCGTAGATTACTAGTTCG-3′;
IL-6 forward, 5′-GAGAGACGGAGTGGCCAC and reverse,
5′-CTCAAGTGAGAAGAGGCAACGGTAGT-3′; IL-18 forward,
5′-CTGATGAGCGGTCACAAGAAC-3′ and reverse,
5′-TTCTCTAACGCGTTAAGAGGAC-3′; TGF-β1 forward,
5′-TCGTGGAGCTCGAAGAACAC-3′ and reverse,
5′-TGGCTGACTTCACAACAGCGTA-3′; GAPDH forward,
5′-AACGGTGTCACAGACAGGCTCA-3′ and reverse,
5′-TCCACCTGACACGACACAACA-3′.
Western blot analysis
The western blot analysis was performed as
previously described (36).
Briefly, after treatments under different conditions, the skin
cells were colllected and the medium was removed. Then, cells were
washed with ice-cold PBS three times and lysed in ice-cold lysis
buffer (pH 7.4, 50 mM Tris-HCl, 150 mM NaCl, 1 mM NaF, 1 mM
ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 1% NP-40, 1
mM phenylmethane-sulfonyl fluoride, and 10 µg/ml leupeptin) in the
presence of fresh protease inhibitor cocktail. Frozen dorsal skins
and epidermal and tumor of mice were obtained from the experimental
mice treated under various conditions. Approximately 100 mg tissue
sample was lysed with 1 ml lysis buffer. The cell lysates were
centrifuged at 15,000 × g for 15 min at 4°C to collect the
supernatant. BSA protein assay kit (Thermo Fisher Scientific, Inc.)
was used to calculate the protein concentrations following the
manufacturer's instructions. Protein extracts (40 ng) were
separated by 10% SDS-PAGE and were then transferred to
polyvinylidene fluoride membrane (PVDF; Millipore, Billerica, MA,
USA). The PVDF membranes with proteins were blocked with 5% skim
fat dry milk in 0.1% Tween-20 in Tris-buffered saline (TBS) for 2 h
to block the non-specific sites on the blots. The primary
antibodies dissolved in blocking buffer were used to detect the
target protein blots at 4°C overnight for incubation. The bands on
the PVDF membranes were visualized using chemiluminescence with
Pierce ECL Western Blotting Substrate reagents (Thermo Fisher
Scientific, Inc.). All experiments were performed in triplicate and
carried out three times independently. The primary antibodies used
in our study included: anti-p21 (1:1,000, ab86696), anti-PUMA
(1:1,000, ab9643), anti-Bax (1:1,000, ab32503), anti-p53 (1:1,000,
ab131442), anti-caspase-3 (1:1,000, ab90437), anti-IκBα (1:1,000,
ab32518), anti-p-IκBα (1:1,000, ab133462), anti-NF-κB (1:1,000,
ab16502), anti-p-NF-κB (1:1,000, ab86299) and GAPDH (1:1,000,
ab8245) all from Abcam.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM). Statistical analyses were carried out by GraphPad
Prism (version 6.0; GraphPad software) by ANOVA with Dunnet's least
significant difference post hoc tests. P<0.05 was considered to
indicate a statistically significant result.
Results
SR shows inhibitory effects on
DMBA/TPA-induced mouse skin tumorigenesis
In order to explore the chemopreventive effect of
SR, DMBA-initiated and TPA-promoted mouse skin carcinogenesis in
mice was first established in vivo. Fisrt, the body weight
of mice was measured, and no significant difference was observed
among the different groups, although reduced body weight was
exerted in the DMBA/TPA-treated group (Fig. 1B). Compared to the Con group,
DMBA/TPA-induced mice showed a significantly high incidence of
papillomas, which was reduced by SR (Fig. 1C). In addition, DMBA/TPA exposure
triggered a higher multiplicity of skin papilloma formation that
was suppressed in the mice treated with SR, exhibiting a reduced
number of tumors per mouse (Fig.
1D). Furthermore, the suppressive effect of SR on tumorigenesis
was supported by the papilloma size distribution (Fig. 1E). Consistently, tumor area was
increased by DMBA/TPA exposure, which was reduced after SR
administration with an increase in time (Fig. 1F). Consistently, the tumor area was
elevated by DMBA/TPA treatment, which was reduced after SR
administration with the increase of time (Fig. 1F). In addition, the histological
analysis further revealed that SR markedly ameliorated the increase
in epidermal thickness (hyperplasia) in mice with DMBA/TPA
induction (Fig. 2). In conclusion,
the animal study above strongly indicated that SR efficiently
prevented skin carcinogenesis induced by DMBA/TPA and TPA in
mice.
SR suppresses the secretion of
pro-inflammatory cytokines in the serum of mice following DMBA/TPA
induction
Pro-inflammatory cytokines were also determined to
assess the role of SR in DMBA/TPA-induced mice with skin cancer. As
shown in Fig. 3A, serum TNF-α was
higher in the DMBA/TPA-treated mice, which was enhanced with
increasing time. SR also significantly suppressed TNF-α expression.
Next, cytokines IL-1β (Fig. 3B),
IL-18 (Fig. 3C), IL-6 (Fig. 3D), COX2 (Fig. 3E) and TGF-β1 (Fig. 3F) were all observed to have elevated
expression in the DMBA/TPA-treated mice, which were suppressed by
SR during the treatment procedure. Taken together, the findings
above indicated that SR has a potential role in blocking the
secretion of pro-inflammatory cytokines.
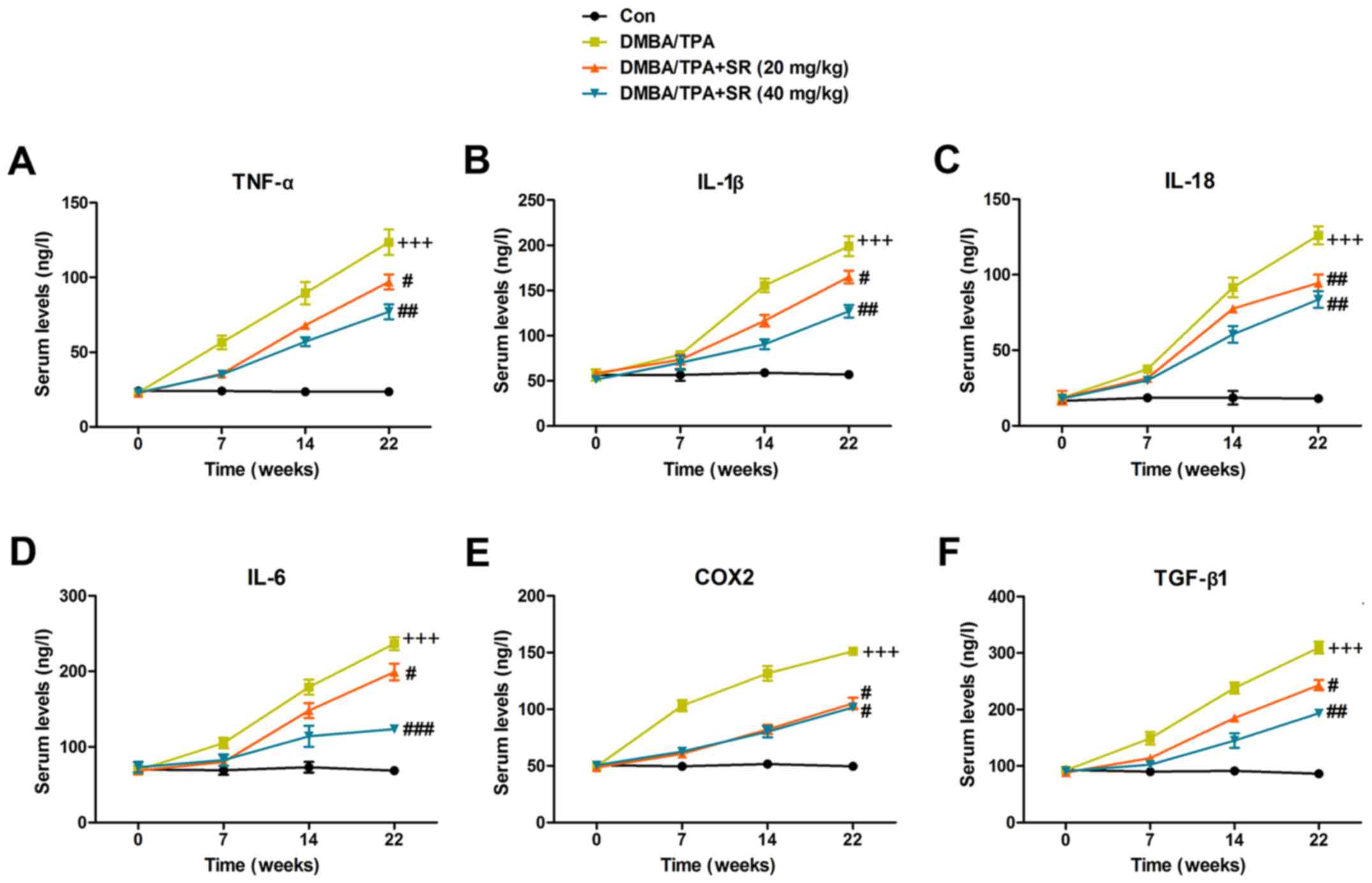 | Figure 3.SR suppresses pro-inflammatory
cytokine release in the serum of mice with DMBA/TPA induction. The
levels of circulating pro-inflammatory cytokines of (A) TNF-α, (B)
IL-1β, (C) IL-18, (D) IL-6, (E) COX2 and (F) TGF-β1 were evaluated
by ELISA methods. Data are presented as mean ± SEM (n=20).
+P<0.05, ++P<0.01 and
+++P<0.001 vs. the Con group; #P<0.05,
##P<0.01 and ###P<0.001 <0.001 vs.
the DMBA/TPA group. SR, salidroside. DMBA,
7,12-dimethylbenz(a)anthracene. TPA,
12-O-tetradecanoylphor-bol-13-acetate. |
SR reduces DMBA/TPA-induced
inflammation in the skin of mice by inactivating NF-κB in vivo
Next, we attempted to ascertain whether the NF-κB
signaling pathway is also involved in SR-ameliorated skin cancer
induced by DMBA/TPA. As shown in Fig.
4A, RT-qPCR analysis further indicated that pro-inflammatory
cytokines, including TNF-α, IL-1β, IL-18, IL-6, and COX2, as well
as anti-inflammatory factor TGF-β1, were increased in the tissue
samples of DMBA/TPA-induced mice, which were all reduced by SR
administration in a dose-dependent manner. The IκB/NF-κB signaling
pathway was also explored. The data indicated that IκBα
phosphorylation was upregulated in the DMBA/TPA-treated mice, while
IκBα was downregulated. Phosphorylated NF-κB levels were also
elevated due to DMBA/TPA induction in mice (Fig. 4B). Of note, SR exerted an inhibitory
effect on IκBα and NF-κB phosphorylation, indicating its
anti-inflammatory property. Furthermore, the immunofluorescence
analysis revealed that enhanced NF-κB phosphorylated levels by
DMBA/TPA were reduced after SR administration (Fig. 4C). Together, the results above
demonstrated that SR inhibited skin carcinogenesis by impeding
inflammation, linked to the suppression of the IκBα/NF-κB signaling
pathway.
 | Figure 4.SR reduces DMBA/TPA-induced
inflammation in the skin of mice through inactivation of NF-κB
in vivo. The skin was removed from mice treated under
different conditions. Then, (A) RT-qPCR analysis was used to
determine TNF-α, IL-1β, IL-18, IL-6, COX2 and TGF-β1 mRNA levels.
(B) Western blot analysis was carried out to evaluate
phosphorylated IκBα, IκBα and phosphorylated NF-κB in the skin
tissue samples obtained from mice. (C) Representative images of
skin after exposure to DMBA/TPA in the absence or presence of SR.
The quantification of phosphorylated NF-κB was measured using
immunofluorescence analysis. Data are presented as mean ± SEM
(n=20). +P<0.05, ++P<0.01 and
+++P<0.001 vs. the Con group; #P<0.05,
##P<0.01 and ###P<0.001 <0.001 vs.
the DMBA/TPA group. SR, salidroside. DMBA,
7,12-dimethylbenz(a)anthracene. TPA,
12-O-tetradecanoylphor-bol-13-acetate. |
SR suppresses the inflammation
response in DMBA-induced cells in vitro
As it was described above, we found that
inflammation blockage by SR might be a possible molecular mechanism
by which to prevent skin carcinogenesis progression in
DMBA/TPA-induced mice in vivo. In order to further confirm
our findings above, the in vitro study was conducted. First
MTT assays were used to calculate the safety of SR to normal cells.
The results indicated that compared to the group in the absence of
any treatment, no significant difference was observed among the
various groups of treated cells as indicated (Fig. 5A). Thus, we supposed that SR might
be safe for application with little cytotoxicity to normal cells.
Normal human epidermal keratinocytes, HaCaT, were treated with 20
µg/ml DMBA for 48 h in the absence or presence of SR at 40, 80 and
160 µM. As shown in Fig. 5B, we
found that cells exposed to DMBA displayed accelerated IκBα
phosphorylation and reduced IκBα expression, which were reversed by
SR administration. NF-κB was also activated by DMBA exposure.
Significantly, SR treatment dose-dependently reduced NF-κB activity
in the cells. RT-qPCR analysis indicated that SR suppressed high
levels of pro-inflammatory cytokines, including TNF-α, IL-1β,
IL-18, IL-6, and COX2, and TGF-β1 was also found to be reduced,
indicating the attenuated inflammatory response, which were in line
with the in vivo results (Fig.
5C). Moreover, the immunofluorescence assays also showed that
SR reduced NF-κB phosphorylation caused by DMBA in normal human
epidermal keratinocytes (Fig. 5D).
Next, to further illustrate the role of SR in suppressing
inflammation, the cells were pre-treated with SR (80 µM) for
different times, ranging from 0 to 48 h. Then, they were exposed to
50 ng/ml TNF-α for another 1 h to induce inflammation. The western
blot analysis indicated that IκBα and NF-κB were activated, which
were markedly reduced by SR administration in a time-dependent
manner (Fig. 6A, B and D).
Oppositely, the downregulated level of IκBα due to TNF-α exposure
was reversed by SR treatment (Fig. 6A
and C). In conclusion, the data above indicate that SR, indeed,
suppressed the inflammatory response in DMBA-treated cells or skin
tissue samples, exhibiting its preventive effects on skin
carcinogenesis.
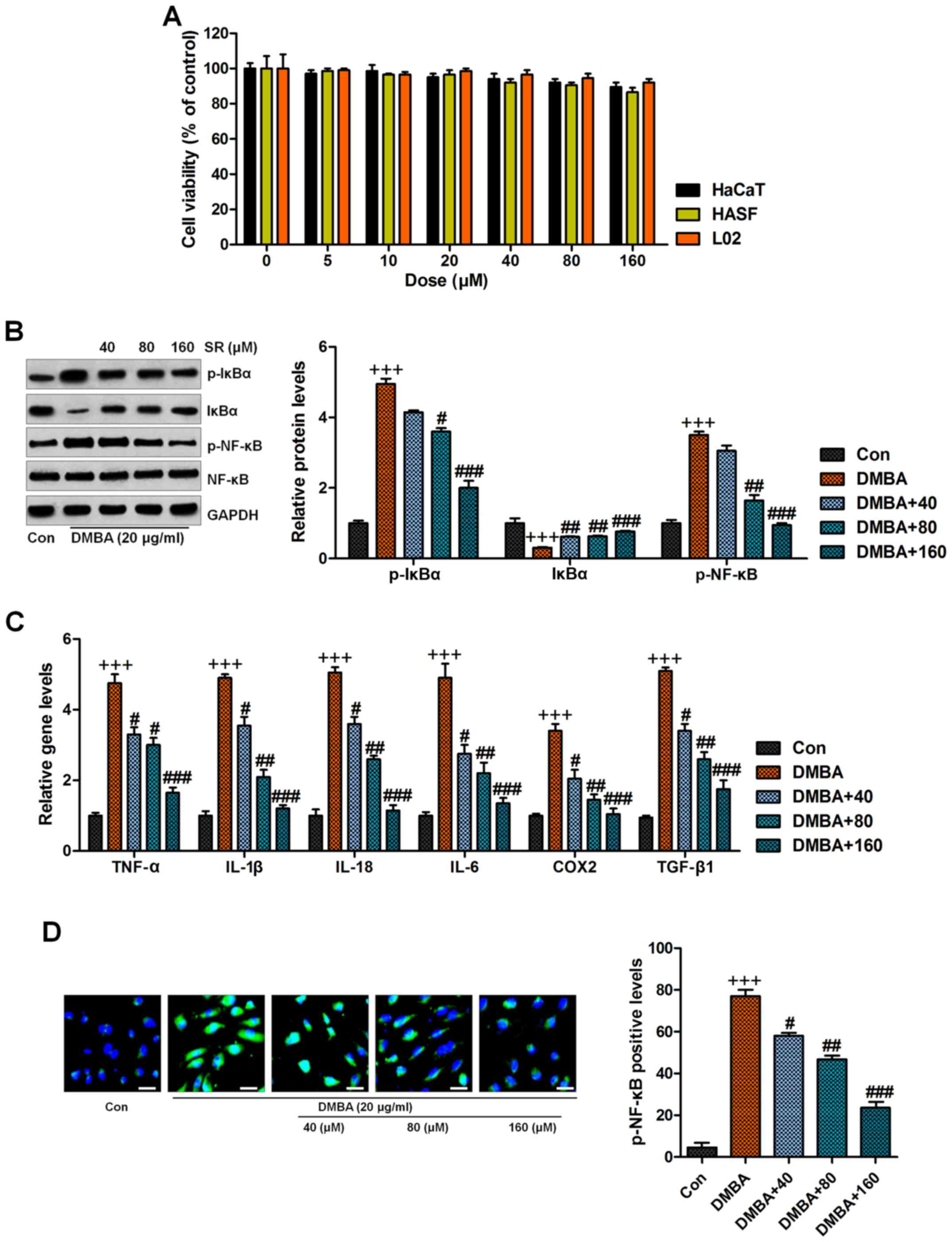 | Figure 5.SR suppresses the inflammation
response in DMBA-induced cells in vitro. (A) Normal human
epidermal keratinocytes, HaCaT, human hypertrophic scar fibroblasts
(HSFs) and human normal liver cell line L02 were treated with
different concnetrations of SR (0, 5, 10, 20, 40, 80 and 160 µM)
for 48 h. Then, all cells were harvested for MTT assay. Normal
human epidermal keratinocytes, HaCaT, were treated with 20 µg/ml
DMBA for 48 h with or without SR treatment at 40, 80 and 160 µM.
Then, all cells were harvested for the following research. (B)
Western blot analysis was used to determine levels in
phosphorylated (p)-IκBα, IκBα and p-NF-κB in cells treated under
various conditions. (C) Cytokines of TNF-α, IL-1β, IL-18, IL-6,
COX2 and TGF-β1 were evaluated by RT-qPCR analysis. (D) The
immunofluorescence analysis was used to assess NF-κB
phosphorylation. Data are presented as mean ± SEM (n=10).
+P<0.05, ++P<0.01 and
+++P<0.001 vs. the Con group; #P<0.05,
##P<0.01 and ###P<0.001 <0.001 vs.
the DMBA group. SR, salidroside. DMBA,
7,12-dimethylbenz(a)anthracene. TPA,
12-O-tetradecanoylphorbol-13-acetate. |
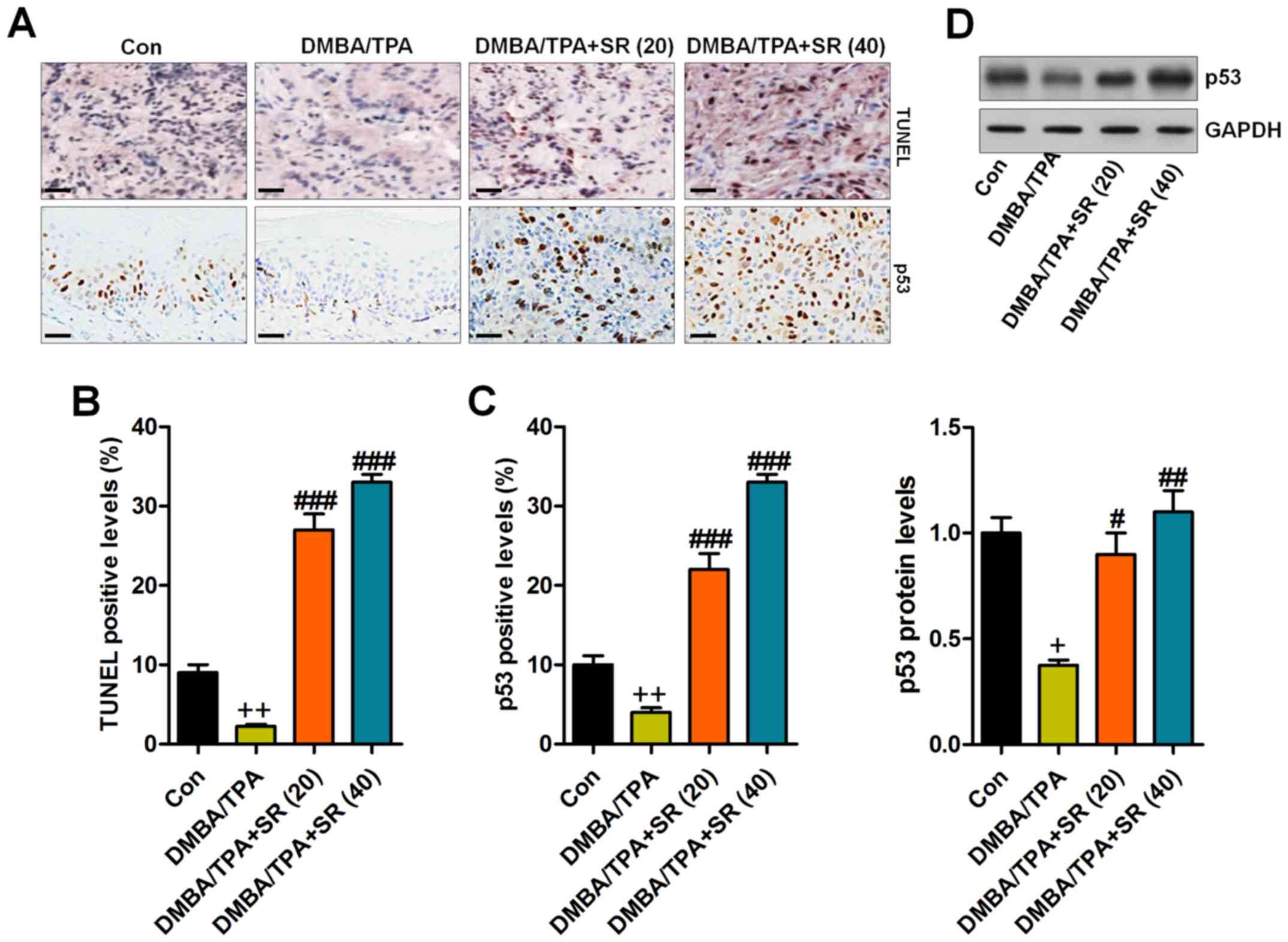
SR prevents skin carcinogenesis
through apoptosis induction in mice in vivo
Immunohistochemical analysis indicated that in the
skin tissue samples of the DMBA/TPA-induced mice reduced TUNEL
levels were noted when compared to the control group, revealing
that the apoptotic response might be disrupted following DMBA/TPA
treatment. In contrast, SR treatment significantly enhanced the
percentage of TUNEL-positive cells, suggesting cell death during
skin carcinogenesis (Fig. 7A and
B). p53, an essential tumor suppressor, was found to be
downregulated by DMBA/TPA, and in agreement with TUNEL alterations,
SR administration reversed the p53 reduction (Fig. 7A and C). Additionally, western blot
analysis illustrated that DMBA/TPA reduced p53 expression, while SR
augmented p53 protein expression levels (Fig. 7D). In summary, the data above
indicated that apoptosis might be involved in SR-regulated skin
carcinogenesis caused by DMBA/TPA.
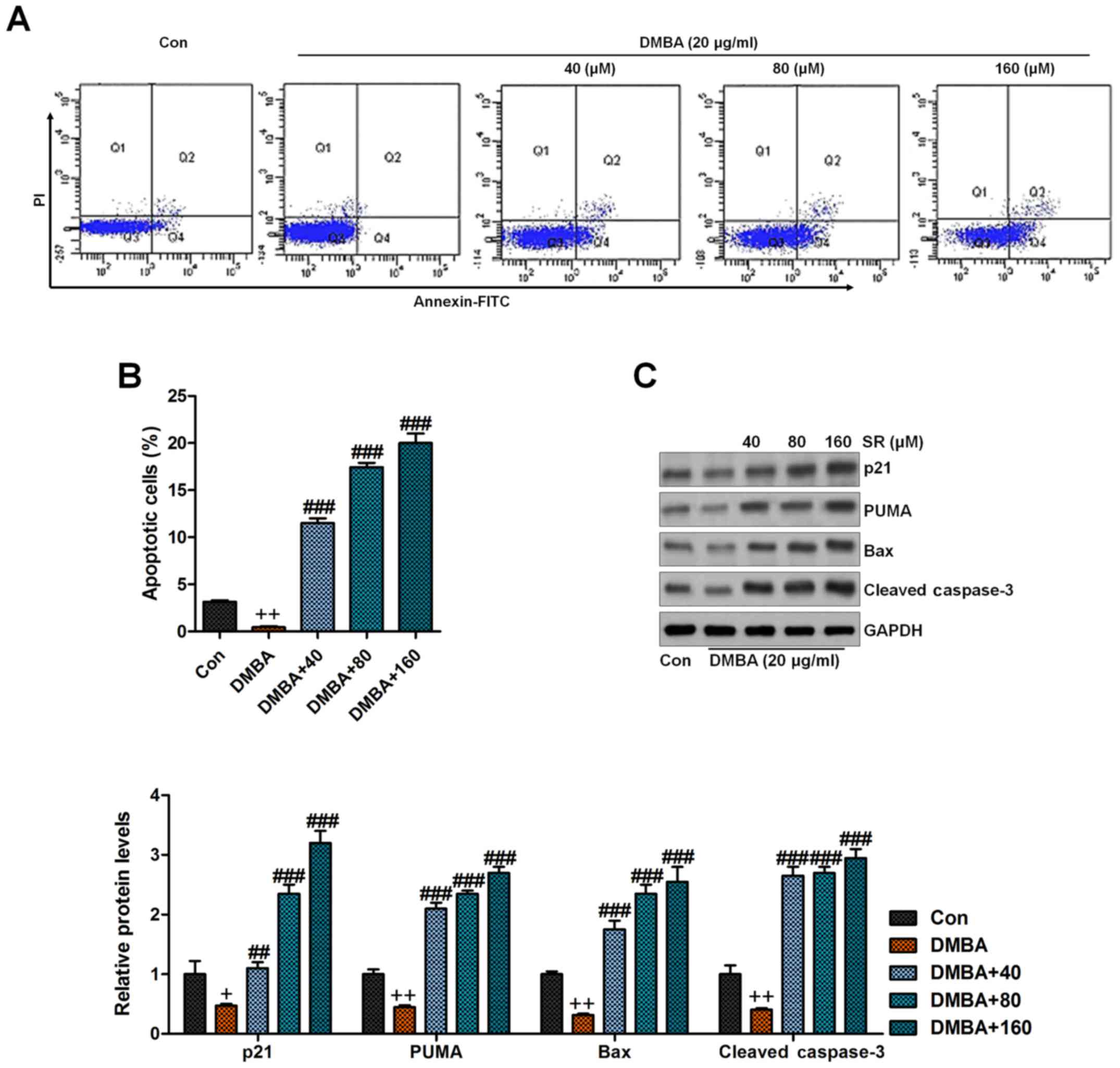
SR induces apoptosis in DMBA-induced
cells in vitro
p53 can induce apoptosis, which is linked to Bax or
other pro-apoptotic molecules (37). Therefore, we analyzed the expression
of these proteins in the skin tissues of mice after various
treatments. The immunoblot analysis showed a decrease in p21, PUMA,
Bax, and cleaved caspase-3 in the DMBA/TPA-treated mice, which were
significantly reversed by SR (Fig.
8). Next, the in vitro study was conducted to further
confirm our data above. Human normal epidermal keratinocytes,
HaCaT, exposed to DMBA were treated with or without SR at the
indicated concentrations. Then, flow cytometric analysis indicated
that DMBA caused reduced percentages of apoptotic cells, which was
in line with the TUNEL assays in vivo. Notably, SR treatment
enhanced apoptosis in the DMBA-treated cells (Fig. 9A and B). Finally, pro-apoptotic
signals of p21, PUMA, Bax and cleaved caspase-3 protein levels were
also reduced by DMBA in vitro, which were elevated after SR
administration, indicating the role of SR in inducing apoptosis to
avoid skin carcinogenesis in vitro (Fig. 9C).
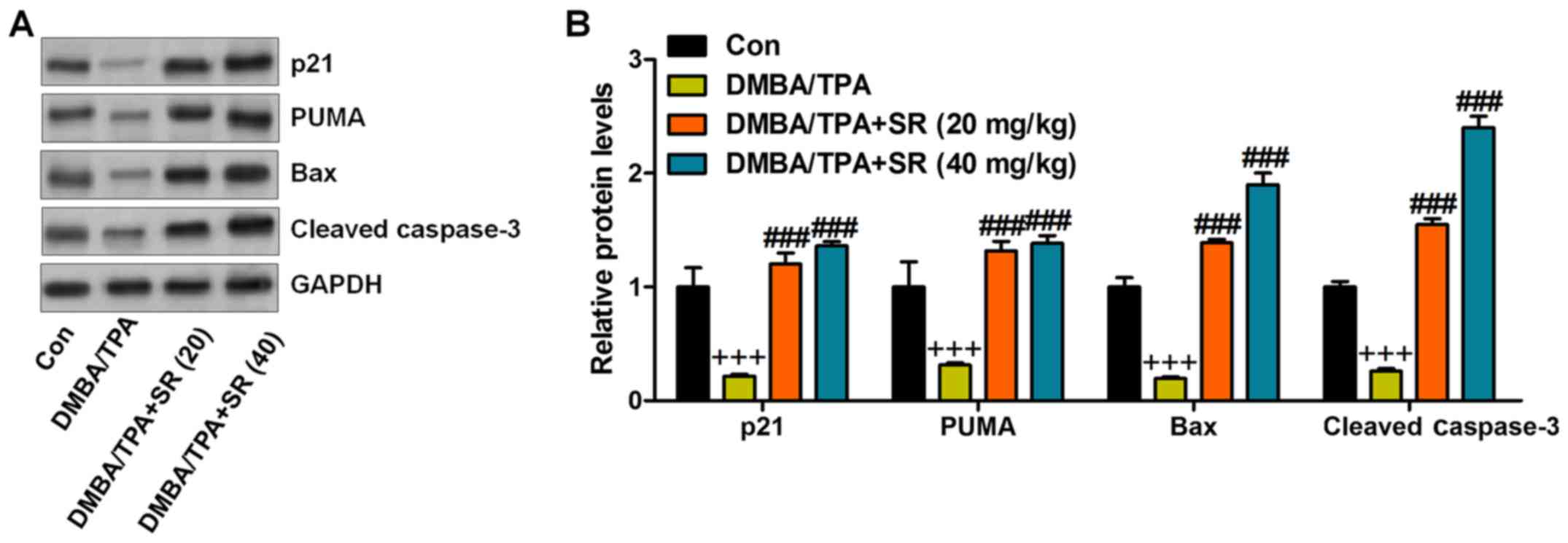 | Figure 8.SR promotes apoptosis following
DMBA/TPA treatment in mice. (A) The representative images of p21,
PUMA, Bax and cleaved caspase-3 protein bands. (B) The
quantification of p21, PUMA, Bax and cleaved caspase-3 is shown.
Data are presented as mean ± SEM (n=20). +P<0.05,
++P<0.01 and +++P<0.001 vs. the Con
group; #P<0.05, ##P<0.01 and
###P<0.001 <0.001 vs. the DMBA/TPA group. SR,
salidroside. DMBA, 7,12-dimethylbenz(a)anthracene. TPA,
12-O-tetradecanoylphorbol-13-acetate. |
Discussion
Non-melanoma skin cancers (NMSCs) are reported as
one of the most commonly diagnosed cancers in the world (1–3,38). The
chronic exposure to solar UR is a major etiological factor for skin
disease. Due to various changes in human life style, the incidence
of NMSCs is increasing due to oxidative stress, inflammatory and
immunosuppressive factors induced by solar UR exposure (3,4,39).
Furthermore, patients with organ transplants are at greater risk to
develop skin cancer in comparison to healthy individuals. Because
of the rising risk of skin cancer, more effective, safe, potent,
and affordable anticancer therapeutic strategies are required to
prevent this disease (40,41). In addition, one limitation is that
it is difficult to predict the location of the initiation of human
skin tumorigenesis. In the present study, we attempted to evaluate
the anti-skin cancer effect of SR using DMBA/TPA-induced skin
tumors as an in vivo model and human skin epidermal HaCaT
cells, as an in vitro model. Following previous studies,
DMBA-initiated and TPA-enhanced mouse skin tumorigenesis is
essential for the investigation of cancer prevention. SR is the
main effective component of Rhodiola rosea L. with a variety
of pharmacologic properties, such as anti-oxidative, anti-aging,
anti-inflammatory, anticancer, anti-fatigue and anti-depressant
activities, to protect tissues or cells from being injured under
various stresses (42,43). Here, in the present study in
vivo, we found that SR treatment reduced tumor incidence and
the total number of tumors in each mouse. In addition, SR-mediated
suppression of skin tumors in mice was related to the inactivation
of the IκBα/NF-κB pathway, thus decreasing the secretion of
pro-inflammatory cytokines. In addition, p53 and caspase-3
signaling pathways were enhanced by SR, leading to apoptosis
against skin carcinogenesis. Furthermore, the MTT assay showed
little cytotoxicity of SR to normal cells, suggesting its safety
for application. Therefore, the results above indicate that SR may
be a promising candidate for inhibiting skin cancer.
Pro-inflammatory cytokines, such as IL-1β, TNF-α,
IL-18, IL-6 and COX2, are suggested to play crucial roles in the
progression of diseases by inflammation response (23,44,45).
NF-κB has been reported to be involved in skin damage, and whose
sustained activation has been elucidated in numerous tumors and
involved in various stages of carcinogenesis (24,26,46).
NF-κB phosphorylation is crucial for the release of
pro-inflammatory cytokines (47).
Its activation leads to the subsequent induction of
pro-inflammatory cytokines (i.e., TNF-α, IL-1β and IL-6),
contributing to inflammation response and disease progression
(48). Excessive release of TNF-α,
IL-1β, IL-18, IL-6 and COX2 is associated with the enhanced risk of
various diseases, including skin carcinogenesis (42,45).
TNF-α was reported to be an inflammatory response primarily, which
is related to skin injury (49).
IL-1β is generated by macrophages activated as proteins and is also
known as catabolin. This cytokine is known as a crucial regulator
of the inflammatory response and various cellular functions,
including cell differentiation and apoptosis (50,51).
UVB, as previously reported, can lead to a high release of COX2.
COX2 participates in the inflammatory response, cell survival and
proliferation (52). In skin damage
induced under various situations, hyper-proliferation of
keratinocytes is induced, which has a close relationship with
pro-inflammatory cytokine secretion (52,53).
Therefore, suppression of the inflammation response is a key to
preventing skin tumor, which is also a molecular mechanism for drug
exploration (54). Consistently, in
our study, we first found that pro-inflammatory cytokines were
highly induced by DMBA/TPA in vivo and in vitro,
which were significantly reduced by SR administration in a
dose-dependent manner. The activated IKK kinase leads to the
phosphorylation and degradation of IκB in the proteasome, and
thereby the release of NF-κB from the NF-IKB-κB complex, enabling
the translocation of NF-κB to the nucleus, where the expression of
genes encoding pro-inflammatory cytokines is induced (55). Furthermore, in our study, we found
that the IκB/NF-κB signaling pathway was markedly activated by
DMBA/TPA treatment, while being inactivated by SR. The data here
indicated that the function of SR to prevent skin carcinogenesis
might be attributed to suppression of inflammation.
Apoptosis is considered as a key molecular mechanism
by which various cancer cells are induced to death (27,40,41,56).
Apoptosis is tightly controlled by the balance between pro- and
anti-apoptotic members of the Bcl-2 protein family. Changes in the
relative expression levels of such molecules will ultimately decide
the cell fate (57). Additionally,
cysteinyl aspartate specific proteinases (caspases) play important
roles during apoptosis (58).
Increase in pro-apoptotic molecules, including Bax, helps to induce
apoptosis by enhancing caspase-3 cleavage, which has been well
known to play an important role in inducing apoptosis (59). Therefore, we subsequently evaluated
the protein levels of pro-apoptotic Bax and caspase-3 molecules
which define the cell propensity to apoptosis (60). p53, an important tumor suppressor,
provides powerful intrinsic defense against various cancers through
its diverse function as a major modulator of apoptosis, the cell
cycle and senescence (61,62). Abnormalities of p53 have been
observed in patients suffering from different cancers (63). Furthermore, p53 shows
transcriptional activities to regulate the expression of
pro-apoptotic gene: PUMA (64).
PUMA is known as the activator for Bax, and is involved in
mitochondrial-mediated apoptosis (65,66).
p21 is a downstream signal of p53, which participates in apoptosis
induction (67). SR has been
confirmed to modulate apoptosis in numerous types of injuries,
including brain, vascular disease and colon cancer (68–70).
SR was observed to promote an apoptotic response to induce cell
death, contributing to colon cancer and leukemia prevention. In the
present study, western blot analysis indicated that SR upregulated
p53, p21, PUMA, Bax and caspase-3 cleavage, contributing to
apoptosis development both in in vivo and in vitro
models induced by DMBA/TPA and DMBA, respectively. Furthermore,
TUNEL assay of tissue samples and flow cytometry analysis of cells
also confirmed the role of SR in apoptosis induction during skin
carcinogenesis. Together, our study revealed that triggering
apoptosis might be a possible molecular mechanism by which SR
showed preventive effects against skin cancer.
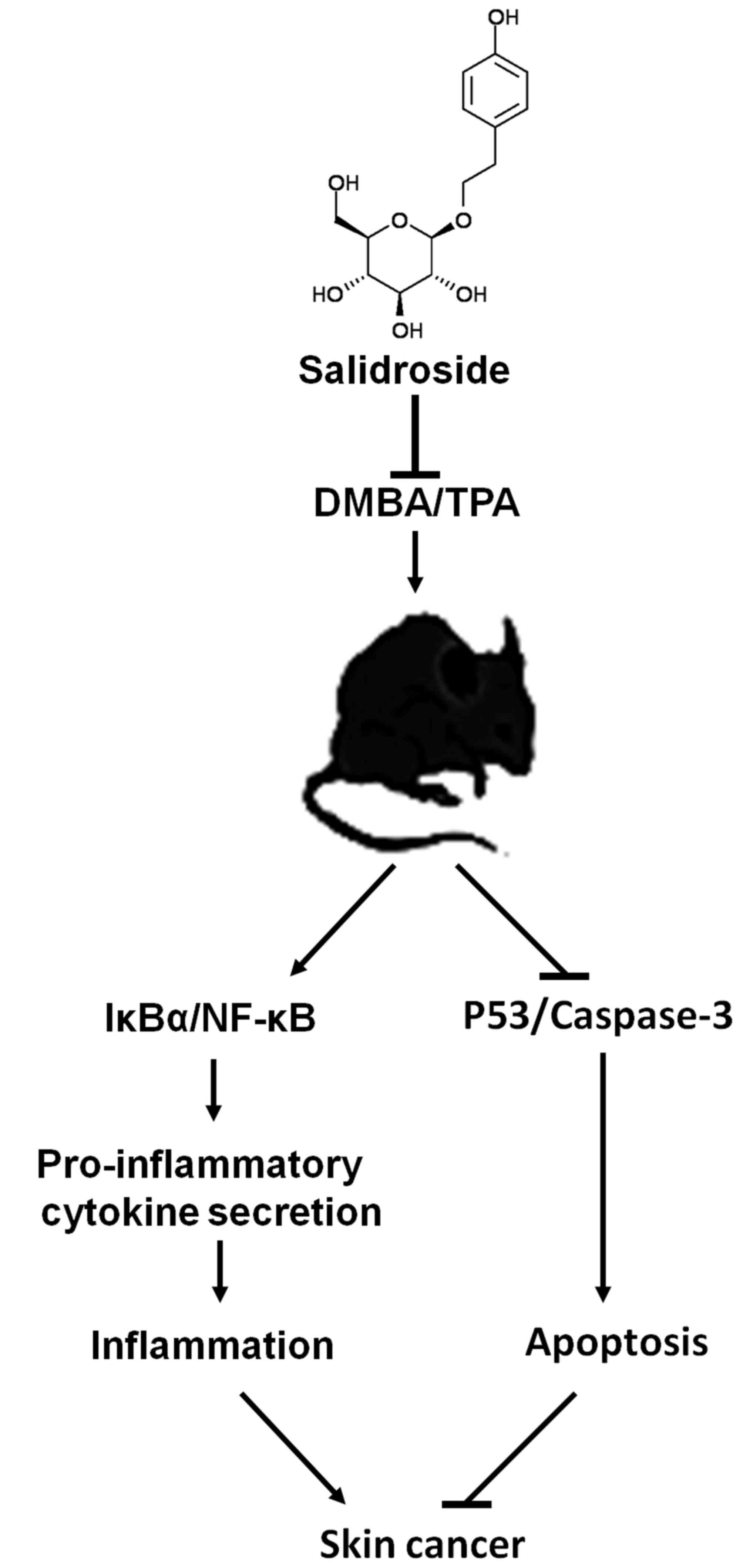
In conclusion, we found that SR prevents the
carcinogenesis of mouse skin tissue initiated by DMBA/TPA. SR acts
as a drug to suppress skin tumors in mice by inactivating the
IκBα/NF-κB pathway, thus reducing the secretion of pro-inflammatory
cytokines. In addition, p53 and caspase-3 signaling pathways were
enhanced by SR, resulting in apoptosis to prevent skin
carcinogenesis (Fig. 10). The
finding supports the proposal that SR has skin tumor-suppressive
activity, which may be a therapeutic strategy for human skin cancer
treatment. However, further study is required to confirm its
function in patients with skin tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YHK did experiments, and SPX did the calculation and
wrote the manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were carried out
following the Guide for the Care and Use of Laboratory Animals of
Huai'an First People's Hospital, Nanjing Medical University
(Nanjing, China) and before the animal experiments were performed,
the procedures were approved by the Research Ethics Committee of
Huai'an First People's Hospital, Nanjing Medical University
(Nanjing, China).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Eisemann N, Waldmann A, Geller AC,
Weinstock MA, Volkmer B, Greinert R, Breitbart EW and Katalinic A:
Non-melanoma skin cancer incidence and impact of skin cancer
screening on incidence. J Invest Dermatol. 134:43–50. 2014.
View Article : Google Scholar
|
|
2
|
Katalinic A, Eisemann N and Waldmann A:
Skin cancer screening in Germany. Dtsch Arztebl Int. 112:629–634.
2015.
|
|
3
|
Fusi C, Materazzi S, Minocci D, Maio V,
Oranges T, Massi D and Nassini R: Transient receptor potential
vanilloid 4 (TRPV4) is downregulated in keratinocytes in human
non-melanoma skin cancer. J Invest Dermatol. 134:2408–2417. 2014.
View Article : Google Scholar
|
|
4
|
Brantsch KD, Meisner C, Schönfisch B,
Trilling B, Wehner-Caroli J, Röcken M and Breuninger H: Analysis of
risk factors determining prognosis of cutaneous squamous-cell
carcinoma: a prospective study. Lancet Oncol. 9:713–720. 2008.
View Article : Google Scholar
|
|
5
|
Surdu S, Fitzgerald EF, Bloom MS, Boscoe
FP, Carpenter DO, Haase RF, Gurzau E, Rudnai P, Koppova K, Vahter
M, et al: Polymorphisms in DNA repair genes XRCC1 and XRCC3,
occupational exposure to arsenic and sunlight, and the risk of
non-melanoma skin cancer in a European case-control study. Environ
Res. 134:382–389. 2014. View Article : Google Scholar
|
|
6
|
Ju Q and Zouboulis CC:
Endocrine-disrupting chemicals and skin manifestations. Rev Endocr
Metab Disord. 17:449–457. 2016. View Article : Google Scholar
|
|
7
|
Goldenberg A, Ortiz A, Kim SS and Jiang
SB: Squamous cell carcinoma with aggressive subclinical extension:
5-year retrospective review of diagnostic predictors. J Am Acad
Dermatol. 73:120–126. 2015. View Article : Google Scholar
|
|
8
|
Goldstein J, Roth E, Roberts N, Zwick R,
Lin S, Fletcher S, Tadeu A, Wu C, Beck A, Zeiss C, et al: Loss of
endogenous Nfatc1 reduces the rate of DMBA/TPA-induced skin
tumorigenesis. Mol Biol Cell. 26:3606–3614. 2015. View Article : Google Scholar
|
|
9
|
Bai Y, Edamatsu H, Maeda S, Saito H,
Suzuki N, Satoh T and Kataoka T: Crucial role of phospholipase
Cepsilon in chemical carcinogen-induced skin tumor development.
Cancer Res. 64:8808–8810. 2004. View Article : Google Scholar
|
|
10
|
Wang Z, Pedersen E, Basse A, Lefever T,
Peyrollier K, Kapoor S, Mei Q, Karlsson R, Chrostek-Grashoff A and
Brakebusch C: Rac1 is crucial for Ras-dependent skin tumor
formation by controlling Pak1-Mek-Erk hyperactivation and
hyperproliferation in vivo. Oncogene. 29:3362–3373. 2010.
View Article : Google Scholar
|
|
11
|
Drexler SK, Bonsignore L, Masin M,
Tardivel A, Jackstadt R, Hermeking H, Schneider P, Gross O, Tschopp
J and Yazdi AS: Tissue-specific opposing functions of the
inflammasome adaptor ASC in the regulation of epithelial skin
carcinogenesis. Proc Natl Acad Sci USA. 109:18384–18389. 2012.
View Article : Google Scholar
|
|
12
|
Kang NJ, Jung SK, Lee KW and Lee HJ:
Myricetin is a potent chemopreventive phytochemical in skin
carcinogenesis. Ann NY Acad Sci. 1229:124–132. 2011. View Article : Google Scholar
|
|
13
|
Manoharan S and Selvan MV: Chemopreventive
potential of geraniol in 7,12-dimethylbenz(a) anthracene (DMBA)
induced skin carcinogenesis in Swiss albino mice. J Environ Biol.
33:255–260. 2012.
|
|
14
|
Qian EW, Ge DT and Kong SK: Salidroside
protects human erythrocytes against hydrogen peroxide-induced
apoptosis. J Nat Prod. 75:531–537. 2012. View Article : Google Scholar
|
|
15
|
Wang S, He H, Chen L, Zhang W, Zhang X and
Chen J: Protective effects of salidroside in the
MPTP/MPP(+)-induced model of Parkinson's disease through
ROS-NO-related mitochondrion pathway. Mol Neurobiol. 51:718–728.
2015. View Article : Google Scholar
|
|
16
|
Zhu L, Chen T, Chang X, Zhou R, Luo F, Liu
J, Zhang K, Wang Y, Yang Y, Long H, et al: Salidroside ameliorates
arthritis-induced brain cognition deficits by regulating
Rho/ROCK/NF-κB pathway. Neuropharmacology. 103:134–142. 2016.
View Article : Google Scholar
|
|
17
|
Zhang S, Chen X, Yang Y, Zhou X, Liu J and
Ding F: Neuroprotection against cobalt chloride-induced cell
apoptosis of primary cultured cortical neurons by salidroside. Mol
Cell Biochem. 354:161–170. 2011. View Article : Google Scholar
|
|
18
|
Li X, Ye X, Li X, Sun X, Liang Q, Tao L,
Kang X and Chen J: Salidroside protects against MPP(+)-induced
apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res.
1382:9–18. 2011. View Article : Google Scholar
|
|
19
|
Yang DW, Kang OH, Lee YS, Han SH, Lee SW,
Cha SW, Seo YS, Mun SH, Gong R, Shin DW, et al: Anti-inflammatory
effect of salidroside on phorbol-12-myristate-13-acetate plus
A23187-mediated inflammation in HMC-1 cells. Int J Mol Med.
38:1864–1870. 2016. View Article : Google Scholar
|
|
20
|
Shan Y, Wei Z, Tao L, Wang S, Zhang F,
Shen C, Wu H, Liu Z, Zhu P, Wang A, et al: Prophylaxis of diallyl
disulfide on skin carcinogenic model via p21-dependent Nrf2
stabilization. Sci Rep. 6:356762016. View Article : Google Scholar
|
|
21
|
Saw CL, Huang MT, Liu Y, Khor TO, Conney
AH and Kong AN: Impact of Nrf2 on UVB-induced skin
inflammation/photoprotection and photoprotective effect of
sulforaphane. Mol Carcinog. 50:479–486. 2011. View Article : Google Scholar
|
|
22
|
Kim EJ, Park H, Kim J and Park JH:
3,3′-diindolylmethane suppresses
12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor
promotion in mouse skin via the downregulation of inflammatory
mediators. Mol Carcinog. 49:672–683. 2010. View Article : Google Scholar
|
|
23
|
Delavary Mahdavian B, van der Veer WM, van
Egmond M, Niessen FB and Beelen RH: Macrophages in skin injury and
repair. Immunobiology. 216:753–762. 2011. View Article : Google Scholar
|
|
24
|
Sellami H, Said-Sadier N, Znazen A, Gdoura
R, Ojcius DM and Hammami A: Chlamydia trachomatis infection
increases the expression of inflammatory tumorigenic cytokines and
chemokines as well as components of the Toll-like receptor and
NF-κB pathways in human prostate epithelial cells. Mol Cell Probes.
28:147–154. 2014. View Article : Google Scholar
|
|
25
|
Xu X, Yang H, Wang X and Tu Y: The
significance of nuclear factor-kappa B signaling pathway in glioma:
A review. Cancer Transl Med. 3:181–184. 2017. View Article : Google Scholar
|
|
26
|
Liu Y, Liu Y, Xu D and Li J:
Latanoprost-induced cytokine and chemokine release rrom human
Tenon's capsule fibroblasts: Role of MAPK and NF-κB signaling
pathways. J Glaucoma. 24:635–641. 2015. View Article : Google Scholar
|
|
27
|
Wang HC, Yang JH, Hsieh SC and Sheen LY:
Allyl sulfides inhibit cell growth of skin cancer cells through
induction of DNA damage mediated G2/M arrest and apoptosis. J Agric
Food Chem. 58:7096–7103. 2010. View Article : Google Scholar
|
|
28
|
Wang T, Yang S, Mei LA, Parmar CK,
Gillespie JW, Praveen KP, Petrenko VA and Torchilin VP:
Paclitaxel-loaded PEG-PE-based micellar nanopreparations targeted
with tumor-specific landscape phage fusion protein enhance
apoptosis and efficiently reduce tumors. Mol Cancer Ther.
13:2864–2875. 2014. View Article : Google Scholar
|
|
29
|
Zhao Y, Guo Q, Chen J, Hu J, Wang S and
Sun Y: Role of long non-coding RNA HULC in cell proliferation,
apoptosis and tumor metastasis of gastric cancer: A clinical and in
vitro investigation. Oncol Rep. 31:358–364. 2014. View Article : Google Scholar
|
|
30
|
Claerhout S, Verschooten L, Van Kelst S,
De Vos R, Proby C, Agostinis P and Garmyn M: Concomitant inhibition
of AKT and autophagy is required for efficient cisplatin-induced
apoptosis of metastatic skin carcinoma. Int J Cancer.
127:2790–2803. 2010. View Article : Google Scholar
|
|
31
|
Strozyk E and Kulms D: The role of
AKT/mTOR pathway in stress response to UV-irradiation: Implication
in skin carcinogenesis by regulation of apoptosis, autophagy and
senescence. Int J Mol Sci. 14:15260–15285. 2013. View Article : Google Scholar
|
|
32
|
Li D, Fu Y, Zhang W, Su G, Liu B, Guo M,
Li F, Liang D, Liu Z, Zhang X, et al: Salidroside attenuates
inflammatory responses by suppressing nuclear factor-κB and mitogen
activated protein kinases activation in lipopolysaccharide-induced
mastitis in mice. Inflamm Res. 62:9–15. 2013. View Article : Google Scholar
|
|
33
|
Zhu L, Wei T, Gao J, Chang X, He H, Luo F,
Zhou R, Ma C, Liu Y and Yan T: The cardioprotective effect of
salidroside against myocardial ischemia reperfusion injury in rats
by inhibiting apoptosis and inflammation. Apoptosis. 20:1433–1443.
2015. View Article : Google Scholar
|
|
34
|
Fu W, McCormick T, Qi X, Luo L, Zhou L, Li
X, Wang BC, Gibbons HE, Abdul-Karim FW and Gorodeski GI: Activation
of P2X(7)-mediated apoptosis inhibits DMBA/TPA-induced formation of
skin papillomas and cancer in mice. BMC Cancer. 9:1142009.
View Article : Google Scholar
|
|
35
|
Zhang XR, Fu XJ, Zhu DS, Zhang CZ, Hou S,
Li M and Yang XH: Salidroside-regulated lipid metabolism with
down-regulation of miR-370 in type 2 diabetic mice. Eur J
Pharmacol. 779:46–52. 2016. View Article : Google Scholar
|
|
36
|
Xu MX, Zhao L, Deng C, Yang L, Wang Y, Guo
T, Li L, Lin J and Zhang L: Curcumin suppresses proliferation and
induces apoptosis of human hepatocellular carcinoma cells via the
wnt signaling pathway. Int J Oncol. 43:1951–1959. 2013. View Article : Google Scholar
|
|
37
|
Wang X, Simpson ER and Brown KA: p53:
protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015. View Article : Google Scholar
|
|
38
|
Madan V, Lear JT and Szeimies RM:
Non-melanoma skin cancer. Lancet. 375:673–685. 2010. View Article : Google Scholar
|
|
39
|
Saw CLL, Yang AY, Huang MT, Liu Y, Lee JH,
Khor TO, Su ZY, Shu L, Lu Y, Conney AH, et al: Nrf2 null enhances
UVB-induced skin inflammation and extracellular matrix damages.
Cell Biosci. 4:392014. View Article : Google Scholar
|
|
40
|
Chinembiri TN, du Plessis LH, Gerber M,
Hamman JH and du Plessis J: Review of natural compounds for
potential skin cancer treatment. Molecules. 19:11679–11721. 2014.
View Article : Google Scholar
|
|
41
|
Simões MCF, Sousa JJS and Pais AA: Skin
cancer and new treatment perspectives: A review. Cancer Lett.
357:8–42. 2015. View Article : Google Scholar
|
|
42
|
Ma C, Hu L, Tao G, Lv W and Wang H: An
UPLC-MS-based metabolomics investigation on the anti-fatigue effect
of salidroside in mice. J Pharm Biomed Anal. 105:84–90. 2015.
View Article : Google Scholar
|
|
43
|
Zhong X, Lin R, Li Z, Mao J and Chen L:
Effects of Salidroside on cobalt chloride-induced hypoxia damage
and mTOR signaling repression in PC12 cells. Biol Pharm Bull.
37:1199–1206. 2014. View Article : Google Scholar
|
|
44
|
Tsimikas S, Duff GW, Berger PB, Rogus J,
Huttner K, Clopton P, Brilakis E, Kornman KS and Witztum JL:
Pro-inflammatory interleukin-1 genotypes potentiate the risk of
coronary artery disease and cardiovascular events mediated by
oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol.
63:1724–1734. 2014. View Article : Google Scholar
|
|
45
|
Lofrumento DD, Nicolardi G, Cianciulli A,
De Nuccio F, La Pesa V, Carofiglio V, Dragone T, Calvello R and
Panaro MA: Neuroprotective effects of resveratrol in an MPTP mouse
model of Parkinson's-like disease: Possible role of SOCS-1 in
reducing pro-inflammatory responses. Innate Immun. 20:249–260.
2014. View Article : Google Scholar
|
|
46
|
Kim BH, Choi MS, Lee HG, Lee SH, Noh KH,
Kwon S, Jeong AJ, Lee H, Yi EH, Park JY, et al: Photoprotective
potential of penta-O-galloyl-β-Dglucose by targeting NF-κB and MAPK
signaling in UVB radiation-induced human dermal fibroblasts and
mouse skin. Mol Cells. 38:982–990. 2015. View Article : Google Scholar
|
|
47
|
Yuan L, Wu Y, Ren X, Liu Q, Wang J and Liu
X: Isoorientin attenuates lipopolysaccharide-induced
pro-inflammatory responses through down-regulation of ROS-related
MAPK/NF-κB signaling pathway in BV-2 microglia. Mol Cell Biochem.
386:153–165. 2014. View Article : Google Scholar
|
|
48
|
Lin TH, Yao Z, Sato T, Keeney M, Li C,
Pajarinen J, Yang F, Egashira K and Goodman SB: Suppression of
wear-particle-induced pro-inflammatory cytokine and chemokine
production in macrophages via NF-κB decoy oligodeoxynucleotide: A
preliminary report. Acta Biomater. 10:3747–3755. 2014. View Article : Google Scholar
|
|
49
|
Choi H, Nguyen HN and Lamb FS: Inhibition
of endocytosis exacerbates TNF-α-induced endothelial dysfunction
via enhanced JNK and p38 activation. Am J Physiol Heart Circ
Physiol. 306:H1154–H1163. 2014. View Article : Google Scholar
|
|
50
|
Schett G, Dayer JM and Manger B:
Interleukin-1 function and role in rheumatic disease. Nat Rev
Rheumatol. 12:14–24. 2016. View Article : Google Scholar
|
|
51
|
Wu L, Zhou Y, Zhou Z, Liu Y, Bai Y, Xing X
and Wang X: Nicotine induces the production of IL-1β and IL-8 via
the α7 nAChR/NF-κB pathway in human periodontal ligament cells: An
in vitro study. Cell Physiol Biochem. 34:423–431. 2014. View Article : Google Scholar
|
|
52
|
Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo
C, Qin J, Wang H, Wang J and Yu Y: Vitamin D inhibits COX-2
expression and inflammatory response by targeting thioesterase
superfamily member 4. J Biol Chem. 289:11681–11694. 2014.
View Article : Google Scholar
|
|
53
|
Kumar D, Tewari-Singh N, Agarwal C, Jain
AK, Inturi S, Kant R, White CW and Agarwal R: Nitrogen mustard
exposure of murine skin induces DNA damage, oxidative stress and
activation of MAPK/Akt-AP1 pathway leading to induction of
inflammatory and proteolytic mediators. Toxicol Lett. 235:161–171.
2015. View Article : Google Scholar
|
|
54
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar
|
|
55
|
Song J, Feng L, Zhong R, Xia Z, Zhang L,
Cui L, Yan H, Jia X and Zhang Z: Icariside II inhibits the EMT of
NSCLC cells in inflammatory microenvironment via down-regulation of
Akt/NF-κB signaling pathway. Mol Carcinog. 56:36–48. 2017.
View Article : Google Scholar
|
|
56
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH, et al: Long noncoding RNA ANRIL
promotes non-small cell lung cancer cell proliferation and inhibits
apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther.
14:268–277. 2015. View Article : Google Scholar
|
|
57
|
Dai H, Meng XW and Kaufmann SH: BCL2
family, mitochondrial apoptosis, and beyond. Cancer Transl Med.
2:7–20. 2016. View Article : Google Scholar
|
|
58
|
White MJ, McArthur K, Metcalf D, Lane RM,
Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie
ME, et al: Apoptotic caspases suppress mtDNA-induced STING-mediated
type I IFN production. Cell. 159:1549–1562. 2014. View Article : Google Scholar
|
|
59
|
Samarghandian S, Nezhad Azimi M and
Mohammadi G: Role of caspases, Bax and Bcl-2 in chrysin-induced
apoptosis in the A549 human lung adenocarcinoma epithelial cells.
Anticancer Agents Med Chem. 14:901–909. 2014. View Article : Google Scholar
|
|
60
|
Jiang X, Jiang H, Shen Z and Wang X:
Activation of mitochondrial protease OMA1 by Bax and Bak promotes
cytochrome c release during apoptosis. Proc Natl Acad Sci USA.
111:14782–14787. 2014. View Article : Google Scholar
|
|
61
|
Seth R, Corniola RS, Gower-Winter SD,
Morgan TJ Jr, Bishop B and Levenson CW: Zinc deficiency induces
apoptosis via mitochondrial p53- and caspase-dependent pathways in
human neuronal precursor cells. J Trace Elem Med Biol. 30:59–65.
2015. View Article : Google Scholar
|
|
62
|
Al-Fatlawi AA, Al-Fatlawi AA, Irshad M,
Zafaryab M, Rizvi MM and Ahmad A: Rice bran phytic acid induced
apoptosis through regulation of Bcl-2/Bax and p53 genes in HepG2
human hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
15:3731–3736. 2014. View Article : Google Scholar
|
|
63
|
Gu JJ, Zhang Q, Mavis C, Czuczman MS and
Hernandez-Ilizaliturri FJ: Metformin induces p53-dependent
mitochondrial stress in therapy-sensitive and-resistant lymphoma
pre-clinical model and primary patients sample with B-cell
non-Hodgkin lymphoma (NHL). Blood. 126:4008. 2015.
|
|
64
|
Song H, Wei M, Liu W, Shen S, Li J and
Wang L: Cisplatin induced apoptosis of ovarian cancer A2780s cells
by activation of ERK/p53/PUMA signals. Histol Histopathol. Mar
13–2017.(Epub ahead of print). doi: 10.14670/HH-11-889.
|
|
65
|
Renault TT, Floros KV, Elkholi R, Corrigan
KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla
JJ, Buettner C, et al: Mitochondrial shape governs BAX-induced
membrane permeabilization and apoptosis. Mol Cell. 57:69–82. 2015.
View Article : Google Scholar
|
|
66
|
Singh N, Sarkar J, Sashidhara KV, Ali S
and Sinha S: Anti-tumour activity of a novel coumarin-chalcone
hybrid is mediated through intrinsic apoptotic pathway by inducing
PUMA and altering Bax/Bcl-2 ratio. Apoptosis. 19:1017–1028. 2014.
View Article : Google Scholar
|
|
67
|
Shamanna RA, Hoque M, Pe'ery T and Mathews
MB: Induction of p53, p21 and apoptosis by silencing the NF90/NF45
complex in human papilloma virus-transformed cervical carcinoma
cells. Oncogene. 32:5176–5185. 2013. View Article : Google Scholar
|
|
68
|
Dong X, Zhang X, Li D, Li B, Wang J, Meng
S, Luo W and Zhang W: Protective effect of salidroside against high
altitude hypoxia-induced brain injury in rats. Xi Bao Yu Fen Zi
Mian Yi Xue Za Zhi. 31:1327–1331. 2015.(In Chinese).
|
|
69
|
Teng L, Gao JF, Zhou L, Xian QY, Li JK and
Yang SJ: Influence of salidroside on expression level of
endothelin-1 and its receptors under hypoxic conditions in chicken
embryonic pulmonary artery smooth muscle cells. Pak Vet J.
36:214–218. 2016.
|
|
70
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar
|