Introduction
Colorectal cancer (CRC) is the third leading cause
of cancer-related deaths, and more than 130,000 people are
diagnosed with CRC annually in the United States of America
(1). In Japan, CRC is the second
leading cause of cancer-related deaths, and out of 360,000
cancer-related deaths in Japan, more than 50,000 were caused by CRC
(2,3). The treatment of advanced CRC has
evolved from single modality treatment to multimodal treatment.
However, some patients still experience tumor recurrence and
metastasis, even after curative resection of the tumor and
administration of adjuvant chemotherapy. Therefore, it is necessary
to identify novel biomarkers for prediction of the risk of
recurrence or metastasis in high-risk patients.
Several studies have identified clinicopathological
and biological markers that predict the prognosis of patients with
CRC after administration of adjuvant chemotherapy. For example,
Shirota et al reported that thymidylate synthase mRNA levels
predicted survival in patients with CRC receiving a combination of
oxaliplatin and fluorouracil (5FU) adjuvant chemotherapy (4). Additionally, Crozier et al
demonstrated that the prognosis of patients with CRC after 5FU
adjuvant chemotherapy was poor in cases exhibiting high systemic
inflammatory responses (5). Ogata
et al revealed that the expression of vascular endothelial
growth factor (VEGF) was associated with the prognosis of patients
treated with adjuvant UFT and 5′-DFUR therapy (6). Furthermore, Ogawa et al
demonstrated that thymidine phosphorylase mRNA expression is an
effective marker predicting prognosis in patients who received S-1
adjuvant chemotherapy (7). These
biomarkers may become specific indicators for the corresponding
drugs. However, if the therapeutic regimen is combined with
multiple drugs, these biomarkers generally cannot predict patient
outcomes. Thus, it is necessary to develop biomarkers that can
clearly and broadly predict the outcome of various adjuvant
chemotherapies. Myc-induced nuclear antigen with a molecular weight
of 53 kDa (Mina53), also known as ribosomal oxygenase 2 (RIOX2), is
a novel Myc target gene located on chromosome 3q11.1. This gene was
initially identified by Tsuneoka et al (8). The Mina53 protein is not expressed in
cells of the normal colonic mucous membrane, but is induced
directly by c-Myc, an important oncogene (9). Moreover, the Mina53 protein is
localized in the nucleus and nucleolus. Following induction by
c-Myc, Mina53 in the nucleus and nucleolus contributes to cell
proliferation. Therefore, the localized expression of Mina53 in the
nucleus can be an important indicator of cancer cell proliferation.
Furthermore, Tsuneoka et al reported that specific
inhibition of Mina53 expression by RNA interference markedly
suppresses cell proliferation (8).
However, the correlation between the intracellular localization of
Mina53 and clinicopathological factors in CRC is still unknown.
Accordingly, in this study, we aimed to investigate
the cellular localization patterns of Mina53 in CRC tissues and to
assess the clinical significance of the nuclear expression of
Mina53 as a biomarker in patients with CRC after adjuvant
therapy.
Materials and methods
Patients
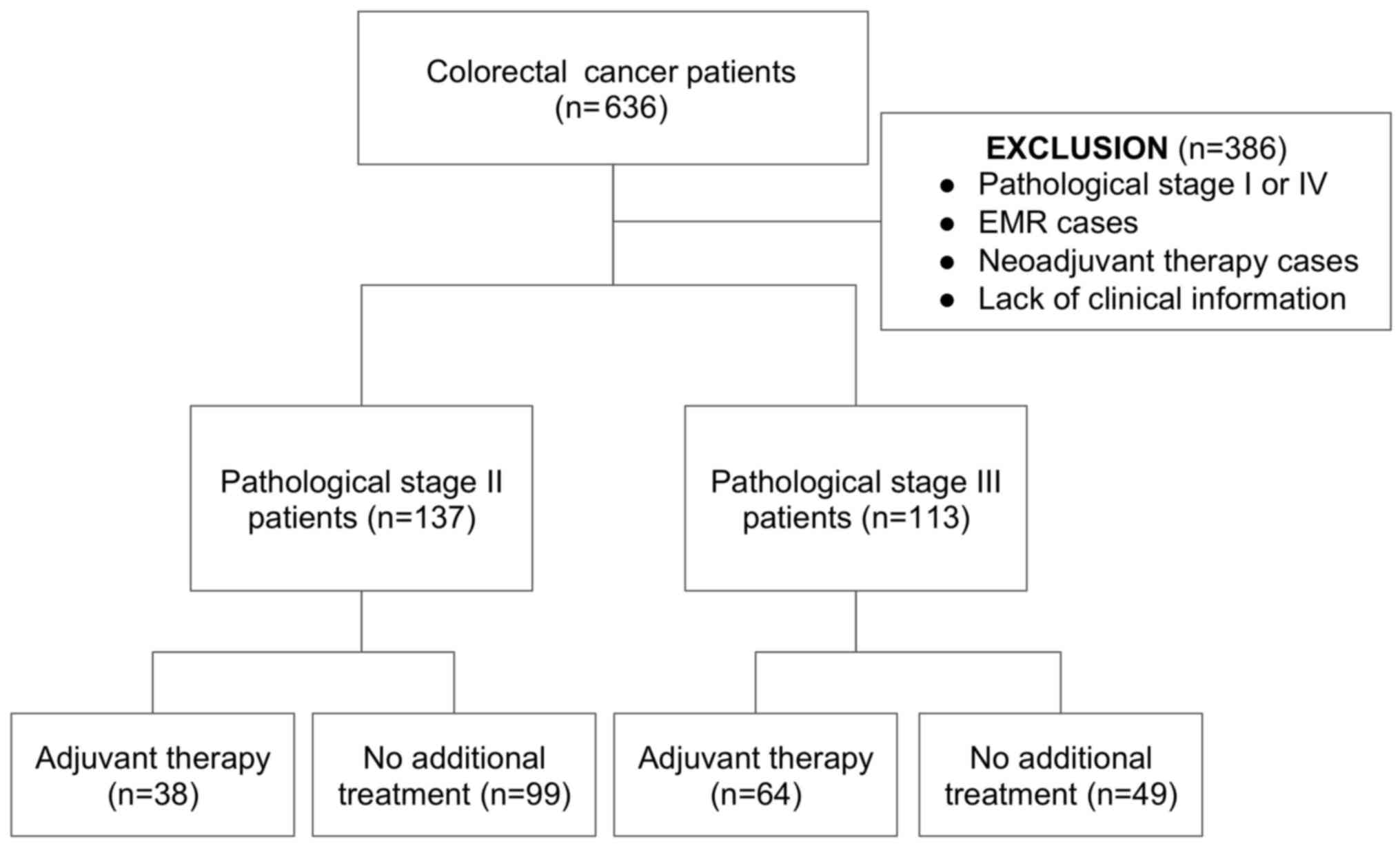
A total of 636 patients who were diagnosed with CRC
and underwent curative resection of the tumor in our department
from 2005 to 2008 were enrolled in this study. Patients who
underwent additional colorectal resection after endoscopic mucosal
resection (EMR) of the primary tumor, patients lacking clinical
information, and patients who had received neoadjuvant chemotherapy
were excluded from the study. Clinical records and pathological
reports were closely reviewed retrospectively.
Among the included cases, 250 patients were
diagnosed with pathological stage II or III in accordance with the
seventh edition of the UICC TNM classification of malignant tumors.
All patients received curative resection of the tumor, including D3
regional lymphadenectomy. Thirty-eight of the 137 patients with
stage II disease and 64 of the 113 patients with stage III disease
received adjuvant therapy. The backgrounds and selection of
patients are summarized in Fig. 1
and Table I.
 | Table I.Clinicopathological backgrounds and
summary of recurrence of patients with stage II and stage III
disease enrolled in this study. |
Table I.
Clinicopathological backgrounds and
summary of recurrence of patients with stage II and stage III
disease enrolled in this study.
| A,
Clinicopathological backgrounds of patients enrolled in this
study |
|---|
|
|---|
| Clinicopathological
variables | Stage II n=137
(%) | Stage III n=113
(%) | P-value |
|---|
| Sex |
|
|
|
| Male | 94 (68.6) | 69 (61.1) | 0.21 |
|
Female | 43 (31.4) | 44 (38.9) |
|
| Age, average ±
SD | 67.83±11.27 | 67.68±11.97 | 0.91 |
| T grade |
|
|
|
| T1 | 0 (0) | 3 (2.65) |
<0.0001a |
| T2 | 0 (0) | 13 (11.5) |
|
| T3 | 60 (43.8) | 89 (78.76) |
|
| T4 | 77 (56.2) | 8 (7.08) |
|
| N grade |
|
|
|
| N0 | 137 (100) | 0 (0) |
<0.0001a |
| N1 | 0 (0) | 89 (78.76) |
|
| N2 | 0 (0) | 18 (15.93) |
|
| N3 | 0 (0) | 6 (5.31) |
|
| Tumor size, mm,
average ± SD | 51.21±23.0 | 48.93±19.87 | 0.41 |
| Location |
|
|
|
|
Colon | 85 (62.04) | 75 (66.37) | 0.47 |
|
Rectum | 52 (37.96) | 38 (33.63) |
|
| Histological
type |
|
|
|
|
Well-mod | 128 (93.43) | 100 (88.5) | 0.17 |
|
Others | 9 (6.57) | 13 (11.5) |
|
| Lymphatic
invasion |
|
|
|
|
Negative | 67 (48.91) | 28 (24.78) |
<0.0001a |
|
Positive | 70 (51.09) | 85 (75.22) |
|
| Vascular
invasion |
|
|
|
|
Negative | 31 (22.63) | 17 (15.04) | 0.12 |
|
Positive | 106 (77.37) | 96 (84.96) |
|
| Budding |
|
|
|
|
Negative | 52 (38.24) | 29 (25.66) | 0.034a |
|
Positive | 84 (61.76) | 84 (74.34) |
|
| Perineural
invasion |
|
|
|
|
Negative | 116 (84.67) | 80 (70.80) | 0.008a |
|
Positive | 21 (15.33) | 33 (29.20) |
|
| Adjuvant
chemotherapy |
|
|
|
|
Yes | 37 (27.0) | 64 (56.64) |
<0.0001a |
| No | 100 (73.0) | 49 (43.36) |
|
| CEA (ng/ml) |
|
|
|
|
<5 | 73 (53.28) | 63 (55.75) | 0.69 |
| ≥5 | 64 (46.72) | 50 (44.25) |
|
| Recurrence |
|
|
|
|
Negative | 114 (83.21) | 85 (75.22) | 0.11 |
|
Positive | 23 (16.79) | 28 (24.78) |
|
|
| B, Summary of
recurrence in patients with stage II and stage III disease |
|
| Patterns of
recurrence | Stage II n=23
(%) | Stage III n=28
(%) |
|
|
| Hematogenous
recurrence | 16 (69.6) | 19 (67.8) |
|
| Lymphogenous
recurrence | 3 (13.0) | 2 (7.2) |
|
| Disseminated
recurrence | 4 (17.4) | 7 (25) |
|
Institutional review board
statement
All of the protocols used in this study were in
compliance with the guidelines of the Ethics Committee of Kurume
University School of Medicine. The protocol of the study was
approved by the hospital Ethics Review Board (no. 203), and
informed consent was obtained from all of the enrolled patients
pre-operatively.
Immunohistochemical analysis of tissue
microarray (TMA)
Tissue samples were obtained from the selected
patients after surgery, and formalin-fixed, paraffin-embedded
blocks were created. One cylindrical core biopsy with a diameter of
3.0 mm was punched out from the center of every tumor tissue sample
using a tissue microarray instrument. These samples were assembled
and embedded in a recipient paraffin block to make a TMA
sample.
A 4-µm section was cut from this TMA block and used
for immunohistochemical staining. Paraffin sections were
deparaffinized in xylene and rehydrated in graded ethanol.
Microwave-mediated antigen retrieval was performed in 0.01 M
citrate buffer, pH 6.0. Endogenous peroxidase activity was blocked
with 0.3% hydrogen peroxide in methanol for 15 min. Sections were
incubated at room temperature with anti-Mina53 antibody (dilution
1:100; cat. no. ab126282; Abcam, Cambridge, MA).
Immunohistochemical staining was performed using the Dako Chem Mate
EnVision system (Dako, Glostrup, Denmark) and a Peroxidase/DAB Kit
(Dako). Sections were counterstained with hematoxylin, the slides
were dehydrated, coverslipped and observed in a ×100 magnifying
field with a microscope (Olympus BX51; Olympus Optical, Co., Ltd.,
Tokyo, Japan). Images were thoroughly evaluated by two independent
pathologists.
Statistical analysis
Patients were divided into Mina53 nuclear
expression-positive and -negative groups. The Chi-squared test was
used to evaluate the significance of correlations between groups.
Survival curves were computed by the Kaplan-Meier method and
statistical significance was assessed by the log-rank test.
Univariate and multivariate analyses were performed using the Cox
proportional-hazard model, and are summarized using false-discovery
rate comparisons. Findings with P-values <0.05 were considered
to indicate a statistically significant difference. All statistical
analyses were performed using the computational statistical
software JMP version 11.0 (SAS Institute, Cary, NC, USA).
Results
Patient backgrounds and evaluation of
Mina53
A flowchart of the selection process and a summary
of the enrolled patients are presented in Fig. 1 and Table IA. There were significant
differences in tumor depth and lymph node metastasis between
patients with stage II and stage III disease. Furthermore,
lymphatic invasion, vascular invasion, tumor budding and perineural
invasion (PNI) were more common in patients with stage III disease
than in patients with stage II disease. However, there were no
significant differences in tumor size, tumor location, and tumor
marker expression between patients with stage II and stage III
disease. Adjuvant chemotherapy was performed in 27% of patients
with stage II disease and 56.64% of patients with stage III
disease; this difference was statistically significant. The
percentages of recurrence were 16.8% in patients with stage II
disease and 24.8% in patients with stage III disease. The patterns
of recurrence are summarized in Table
IB. There were no significant differences in recurrence form
between patients with stage II and III disease.
To evaluate the clinical impact of Mina53 nuclear
staining in patients with stage II and III CRC, immunohistochemical
analysis using a TMA was performed, and clinicopathological factors
were investigated. Representative cases of immunostaining of Mina53
are shown in Fig. 2. Primary tumor
cells displayed several Mina53 staining patterns. As shown in
Fig. 2A, Mina53 was clearly stained
in the nucleus of tumor cells. Fig.
2B shows Mina53 staining in both the cytoplasm and nucleus of
tumor cells. In Fig. 2C, Mina53 was
present in the cytoplasm but not in the nucleus of tumor cells.
Finally, Fig. 2D shows negative
results for Mina53 staining in tumor cells. We defined Mina53
positivity as shown in Fig. 2A and
B.
Status of Mina53 in stage II and III
CRC and clinicopathological variables
Patients were classified into Mina53-positive and
-negative groups according to the immunohistochemical results. As
shown in Table IIA, 148 out of 250
cases (59.6%) were assigned into the positive group. Neither T
factor nor N factor were significantly correlated with Mina53
positivity. The percentage of Mina53-positive cases was almost
identical in patients with stage II and III disease. There were no
significant differences in the majority of clinicopathological
factors, other than patient age and tumor recurrence, between the
Mina53-positive and -negative groups. Vascular invasion was
relatively higher in the Mina53-negative group than in the
Mina53-positive group; however, this difference was not
significant. Patient age was significantly higher in the
Mina53-positive group. Tumor recurrence was also significantly
higher in the Mina53-positive group than in the Mina53-negative
group. Metastatic lesions did not differ significantly between the
two groups. However, the Mina53-positive group exhibited
significantly poorer RFS than the Mina53-negative group (Fig. 3).
 | Table II.Mina53 nuclear localization and
clinicopathological variables and univariate/multivariate analyses
of patients with stage II/III colorectal cancer. |
Table II.
Mina53 nuclear localization and
clinicopathological variables and univariate/multivariate analyses
of patients with stage II/III colorectal cancer.
| A, Mina53 nuclear
localization and clinicopathological variables |
|---|
|
|---|
| Mina53 nuclear
localization |
| Negative (%)
n=102 |
|
| Positive (%)
n=148 | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male |
| 62 (60.7) |
|
| 101 (68.2) | 0.224 |
|
Female |
| 40 (39.3) |
|
| 47 (31.8) |
|
| Age, average ±
SD |
| 65.79±1.13 |
|
| 69.12±0.94 | 0.0252a |
| T grade |
|
|
|
|
|
|
| T1 |
| 2 (1.9) |
|
| 1 (0.7) | 0.24 |
| T2 |
| 8 (7.6) |
|
| 5 (3.5) |
|
| T3 |
| 62 (58.9) |
|
| 87 (60.9) |
|
| T4 |
| 30 (31.6) |
|
| 55 (34.9) |
|
| N grade |
|
|
|
|
|
|
| N0 |
| 51 (50.0) |
|
| 86 (58.1) | 0.139 |
| N1 |
| 44 (43.14) |
|
| 45 (30.4) |
|
| N2 |
| 6 (5.88) |
|
| 12 (8.1) |
|
| N3 |
| 1 (0.98) |
|
| 5 (3.4) |
|
| Tumor stage |
|
|
|
|
|
|
| II |
| 51 (50.0) |
|
| 86 (58.1) | 0.205 |
|
III |
| 51 (50.0) |
|
| 62 (41.2) |
|
| Tumor size (mm),
average ± SD |
| 51.08±2.14 |
|
| 49.56±1.78 | 0.584 |
| Location |
|
|
|
|
|
|
|
Colon |
| 70 (68.6) |
|
| 90 (60.8) | 0.204 |
|
Rectum |
| 32 (31.4) |
|
| 58 (39.2) |
|
| Histological
type |
|
|
|
|
|
|
|
Well-mod |
| 94 (92.2) |
|
| 134 (90.5) | 0.656 |
|
Poorly |
| 8 (7.8) |
|
| 14 (9.5) |
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
Negative |
| 33 (32.4) |
|
| 62 (41.9) | 0.125 |
|
Positive |
| 69 (67.6) |
|
| 86 (58.1) |
|
| Vascular
invasion |
|
|
|
|
|
|
|
Negative |
| 25 (24.5) |
|
| 23 (15.5) | 0.076 |
|
Positive |
| 77 (75.5) |
|
| 125 (54.5) |
|
| Tumor budding |
|
|
|
|
|
|
|
Negative |
| 32 (31.4) |
|
| 49 (33.3) | 0.745 |
|
Positive |
| 70 (68.6) |
|
| 98 (66.7) |
|
| Perineural invasion
(PNI) |
|
|
|
|
|
|
|
Negative |
| 83 (81.4) |
|
| 113 (76.4) | 0.34 |
|
Positive |
| 19 (18.6) |
|
| 35 (23.6) |
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
Yes |
| 44 (43.1) |
|
| 58 (39.2) | 0.532 |
| No |
| 58 (56.9) |
|
| 90 (60.8) |
|
| CEA (ng/ml) |
|
|
|
|
|
|
|
<5 |
| 55 (53.9) |
|
| 81 (54.7) | 0.899 |
| ≥5 |
| 47 (46.1) |
|
| 67 (45.3) |
|
| Recurrence |
|
|
|
|
|
|
|
Negative |
| 90 (85.3) |
|
| 109 (73.6) | 0.003a |
|
Positive |
| 12 (11.7) |
|
| 39 (26.4) |
|
|
| B, Univariate
and multivariate analyses for relapse-free survival in patients
with stage II/III colorectal cancer |
|
|
| Odds
ratio | 95% CI | P-value | Odds
ratio | 95% CI | P-value |
|
| Factors |
|
|
|
|
|
|
| Age |
|
|
|
|
|
|
|
≥67/<67 | 0.77 | 0.44–1.34 | 0.35 | – | – | – |
| Sex |
|
|
|
|
|
|
|
Male/female | 1.05 | 0.6–1.94 | 0.84 | – | – | – |
| Primary tumor |
|
|
|
|
|
|
|
T3-4/T2-1 | 1.86 | 0.57–11.42 | 0.33 | – | – | – |
| Tumor size |
|
|
|
|
|
|
|
≥50/<50 | 0.91 | 0.52–1.59 | 0.76 | – | – | – |
| Location |
|
|
|
|
|
|
|
Colon/rectum | 0.67 | 0.37–1.17 | 0.16 | – | – | – |
| Histological
type |
|
|
|
|
|
|
|
Poor/well-mod | 6.83 | 2.36–15.72 | 0.0014a | 7.62 | 2.6–17.9 | 0.0009a |
| Lymphatic
invasion |
|
|
|
|
|
|
|
ly+/ly− | 1.66 | 0.92–3.18 | 0.09 | – | – | – |
| Vascular
invasion |
|
|
|
|
|
|
|
v+/v− | 0.98 | 0.51–2.07 | 0.95 | – | – | – |
| Tumor budding |
|
|
|
|
|
|
|
b+/b− | 1.19 | 0.66–2.28 | 0.56 | – | – | – |
| PNI |
|
|
|
|
|
|
|
PNI+/PNI− | 1.44 | 0.75–2.6 | 0.25 | – | – | – |
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
Yes/no | 1.97 | 1.14–3.48 | 0.0152a | 2.01 | 1.16–3.55 | 0.0128a |
| CEA |
|
|
|
|
|
|
|
>5.0/≤5.0 | 1.95 | 1.12–3.48 | 0.0175a | 2.2 | 1.25–3.97 | 0.0058a |
| Mina53 status |
|
|
|
|
|
|
|
Positive/negative | 2.58 | 1.39–5.16 | 0.002a | 2.92 | 1.56–5.87 | 0.0005a |
To evaluate the influence of Mina53 nuclear
expression on RFS, univariate and multivariate analyses were
performed. In the univariate analysis, histological type, adjuvant
chemotherapy status, CEA status, and Mina53 status were prognostic
factors for RFS in patients with stage II and stage III disease. In
multivariate analysis, each of the variables selected in the
univariate analysis became an independent prognostic factor for RFS
(Table IIB). In false-discovery
rate (FDR) analysis for RFS, Mina53 status and histological type
were the most significantly contributing variables, followed by CEA
level and adjuvant chemotherapy status (Fig. 4A).
Mina53 nuclear expression did not
correlate with prognosis in patients who did not receive adjuvant
chemotherapy
Next, we performed an analysis of patients who had
or had not undergone adjuvant chemotherapy (Fig. 1). There were no significant
differences in RFS in the Mina53-positive and -negative groups in
patients who did not receive adjuvant chemotherapy (data not
shown). These results were also confirmed separately for patients
with stage II and stage III disease (data not shown).
Mina53 nuclear expression was an
indicator of poor prognosis in patients who had received adjuvant
chemotherapy
Nuclear positivity of Mina53 was significantly
associated with shorter RFS compared with that in the
Mina53-negative group (Fig. 5A).
Furthermore, in subgroup analysis, there were no significant
differences in RFS in patients with stage II disease who received
adjuvant chemotherapy (Fig. 5B).
However, patients treated with adjuvant chemotherapy in stage III
exhibited a significantly shorter RFS (Fig. 5C).
Univariate and multivariate analyses were performed
to determine which clinicopathological variables contributed to RFS
(Table III). Histological type
and Mina53 status were chosen as factors significantly contributing
to RFS in both univariate and multivariate analyses. Furthermore,
Mina53 was superior to histological status in the FDR analysis
(Fig. 4B).
 | Table III.Univariate and multivariate analyses
for relapse-free survival in patients with stage III disease
treated with adjuvant chemotherapy. |
Table III.
Univariate and multivariate analyses
for relapse-free survival in patients with stage III disease
treated with adjuvant chemotherapy.
|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Factors |
|
|
|
|
|
|
| Age |
|
|
|
|
|
|
|
≥67/<67 | 0.91 | 0.42–1.9 | 0.81 | – | – | – |
| Sex |
|
|
|
|
|
|
|
Male/female | 0.79 | 0.37–1.73 | 0.54 | – | – | – |
| Primary tumor |
|
|
|
|
|
|
|
T3-4/T2-1 | 3.59 | 0.76–64.12 | 0.12 | – | – | – |
| Tumor size |
|
|
|
|
|
|
|
≥50/<50 | 0.89 | 0.43–1.93 | 0.78 | – | – | – |
| Location |
|
|
|
|
|
|
|
Colon/rectum | 1 | 0.46–2.09 | 0.98 | – | – | – |
| Histological
type |
|
|
|
|
|
|
|
Poor/well-mod | 2.56 | 1.17–6.15 | 0.0167a | 3.11 | 1.42–7.51 | 0.0038a |
| Lymphatic
invasion |
|
|
|
|
|
|
|
ly+/ly− | 1.55 | 0.69–3.93 | 0.29 | – | – | – |
| Vascular
invasion |
|
|
|
|
|
|
|
v+/v− | 0.77 | 0.32–2.29 | 0.61 | – | – | – |
| Tumor budding |
|
|
|
|
|
|
|
b+/b− | 1.8 | 0.78–4.88 | 0.17 | – | – | – |
| PNI |
|
|
|
|
|
|
|
PNI+/PNI− | 0.93 | 0.37–2.08 | 0.87 | – | – | – |
| CEA |
|
|
|
|
|
|
|
>5.0/≤5.0 | 1.55 | 0.74–3.28 | 0.23 | – | – | – |
| Mina53 status |
|
|
|
|
|
|
|
Positive/negative | 4.29 | 1.77–12.77 | 0.0007a | 5.02 | 2.06–15 | 0.0002a |
Discussion
In the present study, we focused on Mina53 nuclear
expression in primary lesions in patients with stage II/III CRC
treated with radical resection of the tumor. Of the 250 patients,
113 (45.2%) had stage III disease, and of these 113, 49 patients
(43.33%) did not receive adjuvant chemotherapy. Currently, almost
all patients with stage III disease receive adjuvant chemotherapy.
However, the patients in this study were treated from 2005 to 2008,
during which time the choice of therapy was at the discretion of
the doctor, without an obvious consensus. Thus, many patients with
stage III disease did not receive adjuvant chemotherapy.
There were no significant differences in RFS between
Mina53-positive and -negative groups in patients with stage II
disease; however, a difference was observed in patients with stage
III disease. In Japan, the surgical procedure performed in patients
with stage II/III disease allows for a sufficient surgical margin
and lymphadenectomy around the main tumor feeder in the upstreaming
artery (10). In patients with
stage II disease, the lymphadenectomy procedure is thought to be
sufficient for complete cancer resection. In contrast, in patients
with stage III disease, there is a chance that some cancer cells
are not fully resected. The residual cancer cells could cause
recurrence and metastasis, thereby affecting RFS.
As reported previously, Mina53 is a direct target of
the c-Myc proto-oncogene, which regulates cell proliferation, cell
growth, differentiation, and apoptosis. c-Myc is a well-studied
oncogene that is overexpressed in many cancers (11,12).
Tuneoka et al demonstrated that Mina53 expression is
controlled by c-Myc (8). Thus,
localization of Mina53 expression in the nucleus may reflect the
activation of c-Myc. In this study, there were no significant
differences in RFS between Mina53-negative and -positive groups in
patients who did not undergo adjuvant chemotherapy. However,
significantly poorer RFS was observed in the Mina53-positive group
in patients who received adjuvant chemotherapy compared with those
in patients who did not receive adjuvant chemotherapy. The
correlations between Mina53 nuclear expression and
clinicopathological variables were also analyzed, and recurrence
was revealed to be significantly correlated with Mina53 positivity.
Thus, these findings indicated that nuclear expression of Mina53
was associated with the acquisition of chemotherapy resistance and
tumorigenic ability often observed in cancer stem cells. Mina53 is
induced by Myc (8), which functions
as a transcriptioN factor and reprogramming regulator in embryonic
stem cells and cancer cells (13).
Thus, Myc is also involved in the regulation of cancer stem cells.
Consistent with our findings, several studies have demonstrated
that cancer stem cells are related to chemotherapy resistance in
many types of cancer (14–17).
In the present study, Mina53 nuclear expression was
significantly associated with recurrence and was a biomarker of RFS
in patients with stage II/III CRC treated with adjuvant
chemotherapy. Several other biomarkers have been revealed to
indicate prognosis after adjuvant chemotherapy. For example, Lin
et al reported that pre-operative CEA levels, emergent
operation for obstruction/perforation, lymphovascular invasion, and
tumor depth were poor predictors of RFS in patients with stage II
disease (18). Additionally, the
presence of KRAS and BRAF mutations affects
recurrence or metastasis after surgical treatment in patients with
CRC (19). Mutations in epidermal
growth factor receptor (EGFR) and p53 (20) or microsatellite instability (MSI)
(21) have also been reported to be
biomarkers of CRC. However, some markers have not been fully
evaluated due to the long time required for analysis, as well as
the cost and complexity of the assays. Notably, Mina53 nuclear
expression could be detected using a simple IHC procedure. With
this assay, it was relatively easy to determine whether Mina53 was
expressed in the nucleus. Moreover, the assay is inexpensive when
compared with other potential methods.
Targeting of c-Myc in clinical trials is quite
difficult because c-Myc is involved in not only cancer cell
proliferation but also normal cell development (22). Furthermore, c-Myc is involved in a
number of molecular pathways associated with cell growth, cell
proliferation, and gene regulation. Myc was identified more than 30
years ago (23–27). Teye et al reported that
Mina53 is expressed in all pathological grades of colon cancer, but
that it is not or is only weakly expressed in non-neoplastic
colonic cells (9). They also
demonstrated that suppression of Mina53 expression in vitro
by Mina53-specific small interfering RNA suppressed the
proliferation of CRC cell lines. This suppressive effect was
thought to be a result of inhibition of Mina53, suggesting that
Mina53 may be a direct target of c-Myc as well as an attractive
target for controlling the c-Myc pathway in tumor cells without
harming normal cells. Further studies are required to elucidate
these mechanisms. Mina53 may not only be a crucial marker to
predict the prognosis of colorectal cancer patients who received
adjuvant chemotherapy, but may also be an important biological
target to treat those patients who failed to respond to
conventional chemotherapeutic treatment.
Mina53 nuclear expression in CRC cells was found to
be associated with tumor recurrence and could be used as an
effective marker for predicting the prognosis of patients with CRC
who received adjuvant chemotherapy. By investigating Mina53 nuclear
expression, patients who may experience tumor recurrence or
metastasis quickly after adjuvant chemotherapy can be selected.
Patients who received adjuvant chemotherapy should be carefully
screened and followed after treatment if their primary tumor
exhibited nuclear Mina53 expression.
Acknowledgements
The authors thank Nagako Shigenaga, Hiroko Takayama
and Michiko Nagamatsu for their technical support.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SF and TK conceived and designed the study. SF and
TK performed the experiments. SF, TK and TS wrote the paper. SF,
TK, TS, TM, TY, NY, TO, KT, KY, SN, MK and YA reviewed and edited
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All of the protocols used in this study were in
compliance with the guidelines of the Ethics Committee of Kurume
University School of Medicine. The protocol of the study was
approved by the hospital Ethics Review Board (no. 203), and
informed consent was obtained from all of the enrolled patients
pre-operatively.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Mina53
|
Myc-induced nuclear antigen with a
molecular weight of 53 kDa
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotake K, Honjo S, Sugihara K, Kato T,
Kodaira S, Takahashi T, Yasutomi M, Muto T and Koyama Y: Changes in
colorectal cancer during a 20-year period: An extended report from
the multi-institutional registry of large bowel cancer, Japan. Dis
Colon Rectum. 46 10 Suppl:S32–S43. 2003.PubMed/NCBI
|
|
3
|
Tamakoshi A, Nakamura K, Ukawa S, Okada E,
Hirata M, Nagai A, Matsuda K, Kamatani Y, Muto K, Kiyohara Y, et
al: Characteristics and prognosis of Japanese colorectal cancer
patients: The BioBank Japan Project. J Epidemiol. 27:S36–S42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels
predict survival for colorectal cancer patients receiving
combination oxaliplatin and fluorouracil chemotherapy. J Clin
Oncol. 19:4298–4304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crozier JEM, McKee RF, McArdle CS,
Angerson WJ, Anderson JH, Horgan PG and McMillan DC: The presence
of a systemic inflammatory response predicts poorer survival in
patients receiving adjuvant 5-FU chemotherapy following potentially
curative resection for colorectal cancer. Br J Cancer.
94:1833–1836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogata Y, Matono K, Mizobe T, Ishibashi N,
Mori S, Akagi Y, Ikeda S, Ozasa H, Murakami H and Shirouzu K: The
expression of vascular endothelial growth factor determines the
efficacy of post-operative adjuvant chemotherapy using oral
fluoropyrimidines in stage II or III colorectal cancer. Oncol Rep.
15:1111–1116. 2006.PubMed/NCBI
|
|
7
|
Ogawa M, Watanabe M, Mitsuyama Y, Anan T,
Ohkuma M, Kobayashi T, Eto K and Yanaga K: Thymidine phosphorylase
mRNA expression may be a predictor of response to post-operative
adjuvant chemotherapy with S-1 in patients with stage III
colorectal cancer. Oncol Lett. 8:2463–2468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teye K, Tsuneoka M, Arima N, Koda Y,
Nakamura Y, Ueta Y, Shirouzu K and Kimura H: Increased expression
of a Myc target gene Mina53 in human colon cancer. Am J Pathol.
164:205–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
West NP, Kobayashi H, Takahashi K,
Perrakis A, Weber K, Hohenberger W, Sugihara K and Quirke P:
Understanding optimal colonic cancer surgery: Comparison of
Japanese D3 resection and European complete mesocolic excision with
central vascular ligation. J Clin Oncol. 30:1763–1769. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stewart J, Evan G, Watson J and Sikora K:
Detection of the c-myc oncogene product in colonic polyps and
carcinomas. Br J Cancer. 53:1–6. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sikora K, Chan S, Evan G, Gabra H, Markham
N, Stewart J and Watson J: c-myc oncogene expression in colorectal
cancer. Cancer. 59:1289–1295. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y,
Kim CG, Cantor AB and Orkin SH: A Myc network accounts for
similarities between embryonic stem and cancer cell transcription
programs. Cell. 143:313–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133+ cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salcido CD, Larochelle A, Taylor BJ,
Dunbar CE and Varticovski L: Molecular characterisation of side
population cells with cancer stem cell-like characteristics in
small-cell lung cancer. Br J Cancer. 102:1636–1644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bora-Singhal N, Perumal D, Nguyen J and
Chellappan S: Gli1-mediated regulation of Sox2 facilitates
self-renewal of stem-like cells and confers resistance to EGFR
inhibitors in non-small cell lung cancer. Neoplasia. 17:538–551.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Xu W, Guo H, Zhang Y, He Y, Lee
SH, Song X, Li X, Guo Y, Zhao Y, et al: NOTCH1 signaling regulates
self-renewal and platinum chemoresistance of cancer stem-like cells
in human non-small cell lung cancer. Cancer Res. 77:3082–3091.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HH, Chang YY, Lin JK, Jiang JK, Lin
CC, Lan YT, Yang SH, Wang HS, Chen WS, Lin TC and Chang SC: The
role of adjuvant chemotherapy in stage II colorectal cancer
patients. Int J Colorectal Dis. 29:1237–1243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roth AD, Tejpar S, Delorenzi M, Yan P,
Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C,
et al: Prognostic role of KRAS and BRAF in stage II
and III resected colon cancer: Results of the translational study
on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol.
28:466–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Resnick MB, Routhier J, Konkin T, Sabo E
and Pricolo VE: Epidermal growth factor receptor, c-MET,
beta-catenin, and p53 expression as prognostic indicators in stage
II colon cancer: A tissue microarray study. Clin Cancer.
10:3069–3075. 2004. View Article : Google Scholar
|
|
21
|
Ribic CM, Sargent DJ, Moore MJ, Thibodeau
SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R,
Shepherd LE, et al: Tumor microsatellite-instability status as a
predictor of benefit from fluorouracil-based adjuvant chemotherapy
for colon cancer. New Engl J Med. 349:247–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davis AC, Wims M, Spotts GD, Hann SR and
Bradley A: A null c-myc mutation causes lethality before 10.5 days
of gestation in homozygotes and reduced fertility in heterozygous
female mice. Genes Dev. 7:671–682. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duesberg PH and Vogt PK: Avian acute
leukemia viruses MC29 and MH2 share specific RNA sequences:
Evidence for a second class of transforming genes. Proc Natl Acad
Sci USA. 76:1633–1637. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheiness D and Bishop JM: DNA and RNA from
uninfected vertebrate cells contain nucleotide sequences related to
the putative transforming gene of avian myelocytomatosis virus. J
Virol. 31:514–521. 1979.PubMed/NCBI
|
|
25
|
McKeown MR and Bradner JE: Therapeutic
strategies to inhibit MYC. Cold Spring Harb Perspect Med.
4:a0142662014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Whitfield JR, Beaulieu M-E and Soucek L:
Strategies to inhibit Myc and their clinical applicability. Front
Cell Dev Biol. 5:102017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen BJ, Wu YL, Tanaka Y and Zhang W:
Small molecules targeting c-Myc oncogene: Promising anti-cancer
therapeutics. Int J Biol Sci. 10:1084–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|