Introduction
Although melanoma comprises 5% of all skin-related
tumors, it is responsible for 75% of the deaths caused by this type
of cancer. Although significant progress has been made in the last
decade and the number of cases has significantly decreased, the
overall mortality rate has remained steady. New treatment
strategies based on BRAF inhibitors or CTLA-4 blocking antibodies
have provided only slight benefit to patients with stage IV
melanoma and melanoma metastases. This moderate success provides
the rationale to continue research on expanding therapies focusing
on cancer biology and targeting molecular pathways crucial for
proliferation, metastasis and respond to treatment (1–3).
DNA synthesis and repair require coordinated
deoxyribonucleoside triphosphate (dNTP) supply as basic building
blocks. Impaired balance of the dNTP pool affects S phase duration
time, DNA synthesis fidelity, as well as the ability and
effectiveness of DNA repair. Loss of control over these processes
can also trigger genome instability and may initiate
cancerogenesis. The increased demand for deoxyribonucleotides is
serviced by upregulation of ribonucleotide reductase (RNR), which
reduces the 2′ carbon of a ribonucleoside diphosphate and has been
considered as the rate-limiting step in dNTP production. RNR as a
heterodimeric protein consists of three subunits – one
ribonucleotide reductase family member 1 (RRM1) and two molecules
of RRM2. While RRM1 expression is constant throughout the cell
cycle, the expression of RRM2 fluctuates and peaks at S phase, when
the need for nucleotide synthesis is the highest. The degradation
of RRM2 occurs in late G2 phase of the cell cycle in the nucleus
and is controlled by Skp, Cullin, F-box containing (SCF)cyclin
F ubiquitin ligase complex. The SCF complex is composed of
three proteins: Skp1 and Cul1, which provide a scaffold, and F-box
protein, which is responsible for target recognition (4).
Cyclin F, like other cyclins, has both cyclin and
F-box domains, but it does not bind or activate any known
cyclin-dependent kinase (CDK). The expression profile of cyclin F
is similar to cyclin A and fluctuates throughout the cell cycle. At
the protein level, cyclin F appears in the S phase, peaks before M
phase, and then its expression decreases dramatically. It is
clearly visible that changes in the expression of cyclin F
negatively correlates with the RRM2 level, which may suggest their
cooperation in the axis, important for genome stability and DNA
repair (5). As it has been
suggested, overexpression of RRM2 is associated with poorer patient
prognosis in melanoma and many other cancers. Furthermore, cells
with high content of RRM2 are characterized by much more effective
DNA repair systems which impair the effectiveness of therapy
(6–9).
The aim of our in silico analysis was to take
the first step in the elucidation of the precise mechanism of the
cyclin F (CCNF)-RRM2 axis in skin melanoma. The study aims to
accelerate the development and to inspire other scientific teams to
conduct similar research in the field.
In the present study, using the data available in
the cBioPortal database, we showed for first time that high
expression of cyclin F mRNA is associated with poorer prognosis in
patients with skin cutaneous melanoma. Additionally, we present an
overview of the molecular pathways involved in the cell cycle, cell
death and DNA repair which are activated differentially in patients
who exhibit high and low expression of cyclin F and RRM2.
Materials and methods
Analysis of publicly available
data
To assess the expression profile of cyclin F and
RMM2 mRNA, we obtained data from The Cancer Genome Atlas via
www.cBioPortal.org (10). Patients were divided into groups:
with CCNF or RRM2 mRNA upregulated expression (z-score >0) and
with downregulated mRNA expression (z-score ≤0) and then, for each
mRNA, we conducted overall survival and disease-free survival
analysis. The same source was used for protein level comparison in
patients with upregulated and downregulated cyclin F and RRM2 mRNA.
In turn, we analyzed obtained information and used Reactome
(http://reactome.org) and ToppGene Suite
(http://toppgene.cchmc.org) to organize
data into biological processes and functional molecular
pathways.
Statistical analysis
In the life span study of the melanoma patients, the
data were analyzed with Kaplan-Meier survival analysis with
included log-rank test for trend tests. Comparisons between groups
expressing different levels of mRNA or proteins were conducted
using Mann-Whitney U-test. All statistical analyses were performed
using GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
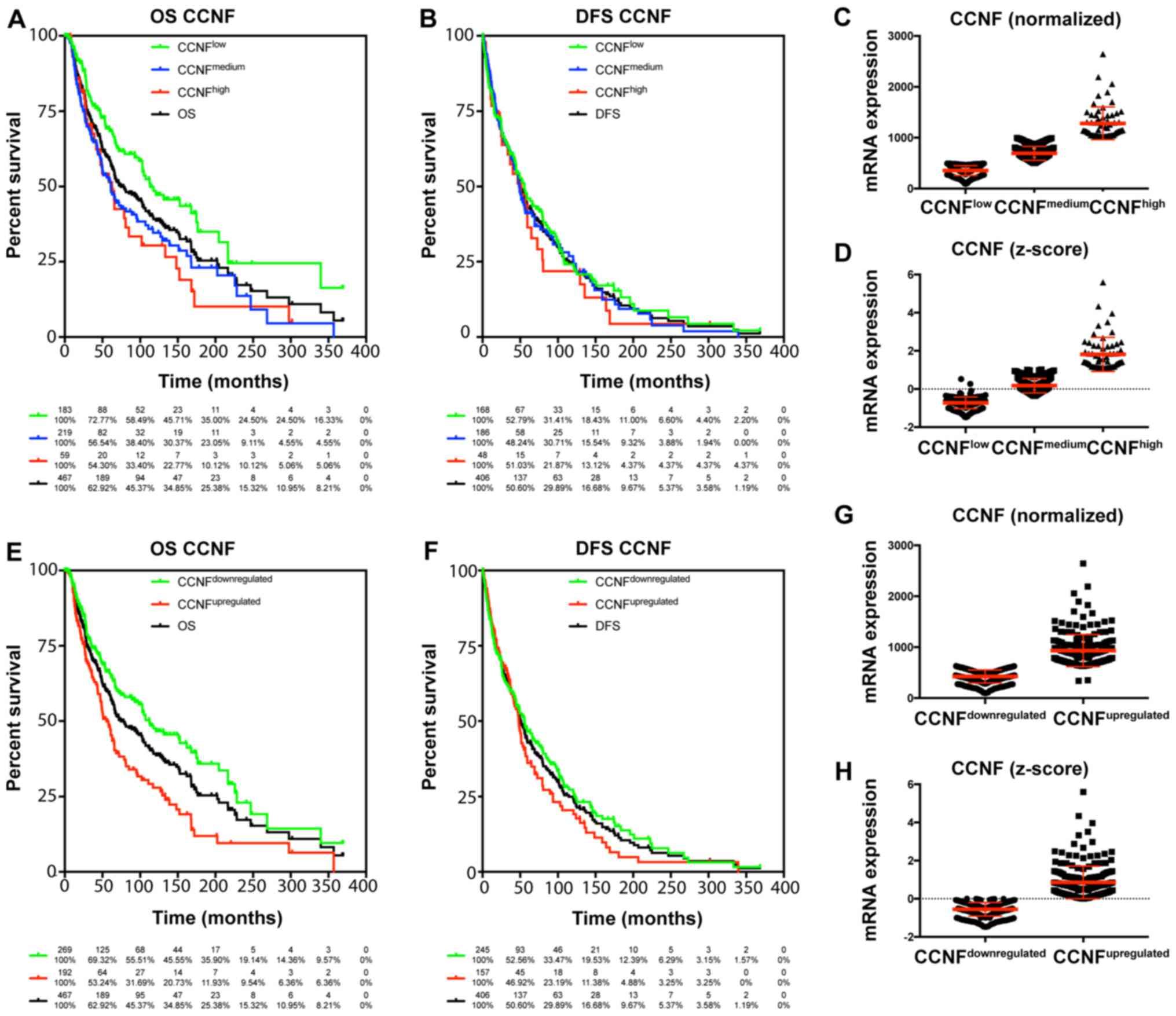
The TCGA data were used to characterize the
prognostic value of cyclin F and RRM2 mRNA in melanoma. The results
showed that increased expression of cyclin F mRNA is associated
with worse outcome in melanoma patients (Fig. 1; Tables
I and II). Median survival in
patients with upregulated cyclin F was significantly lower (112.48
vs. 55.55 months; P<0.0001). No significance in disease-free
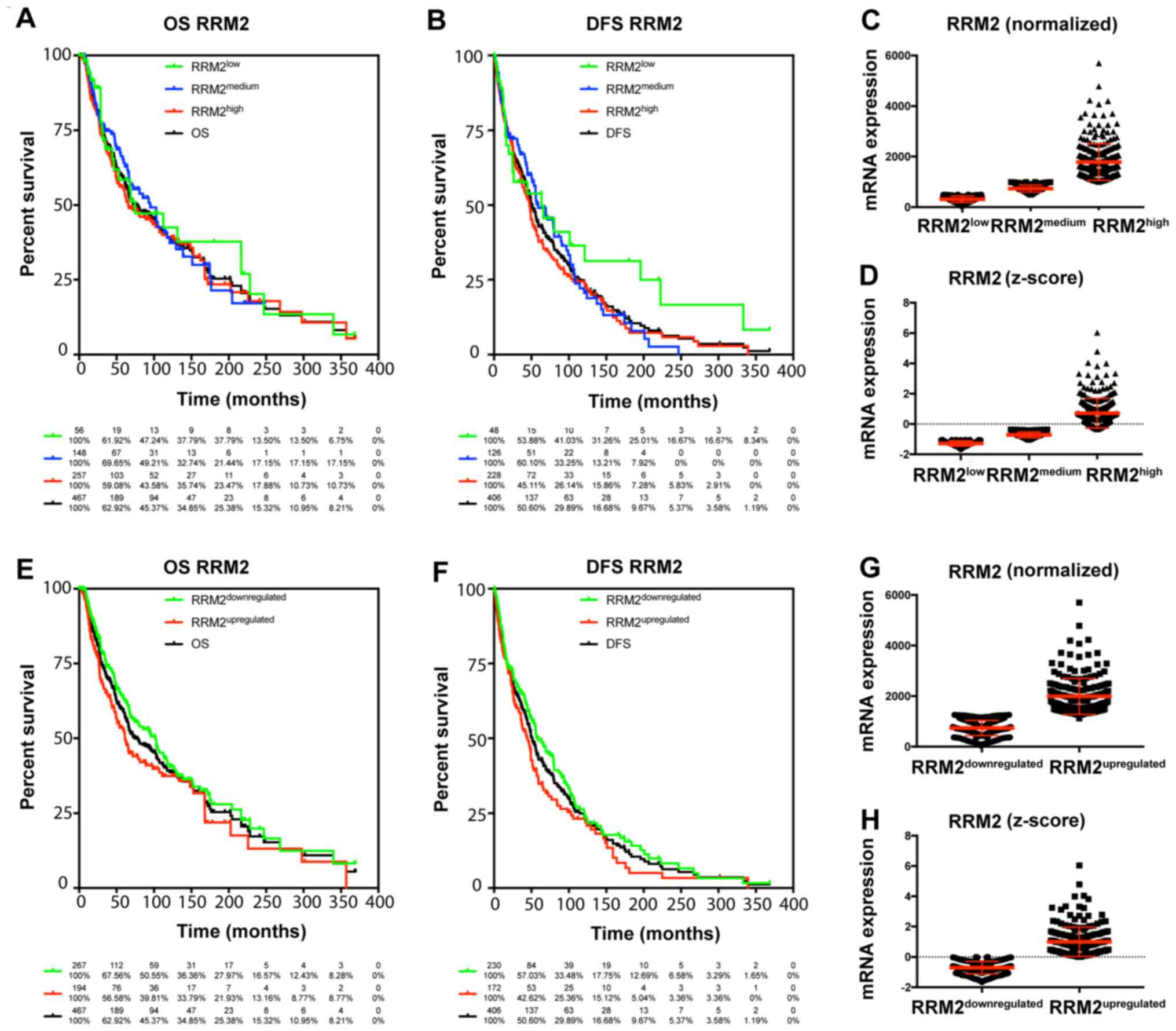
survival (DFS) was found. Furthermore, expression of RRM2 mRNA had
a significant influence on median survival (102.04 vs. 61.47;
P=0.034), but no effect on DSF was noted (Fig. 2; Tables
I and II). Cyclin F
significantly altered the expression of different cellular
proteins. The expression of proteins negatively and positively
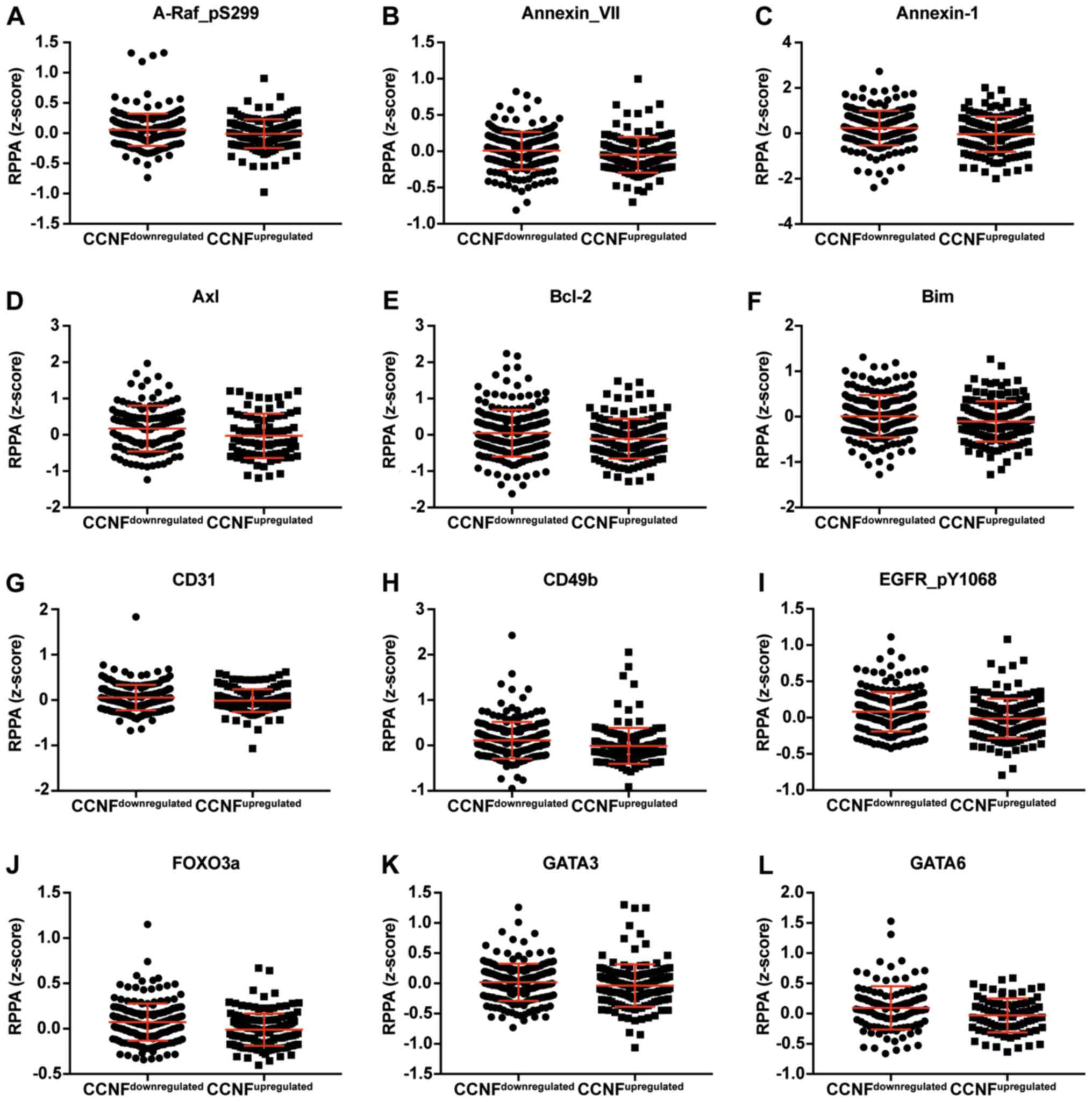
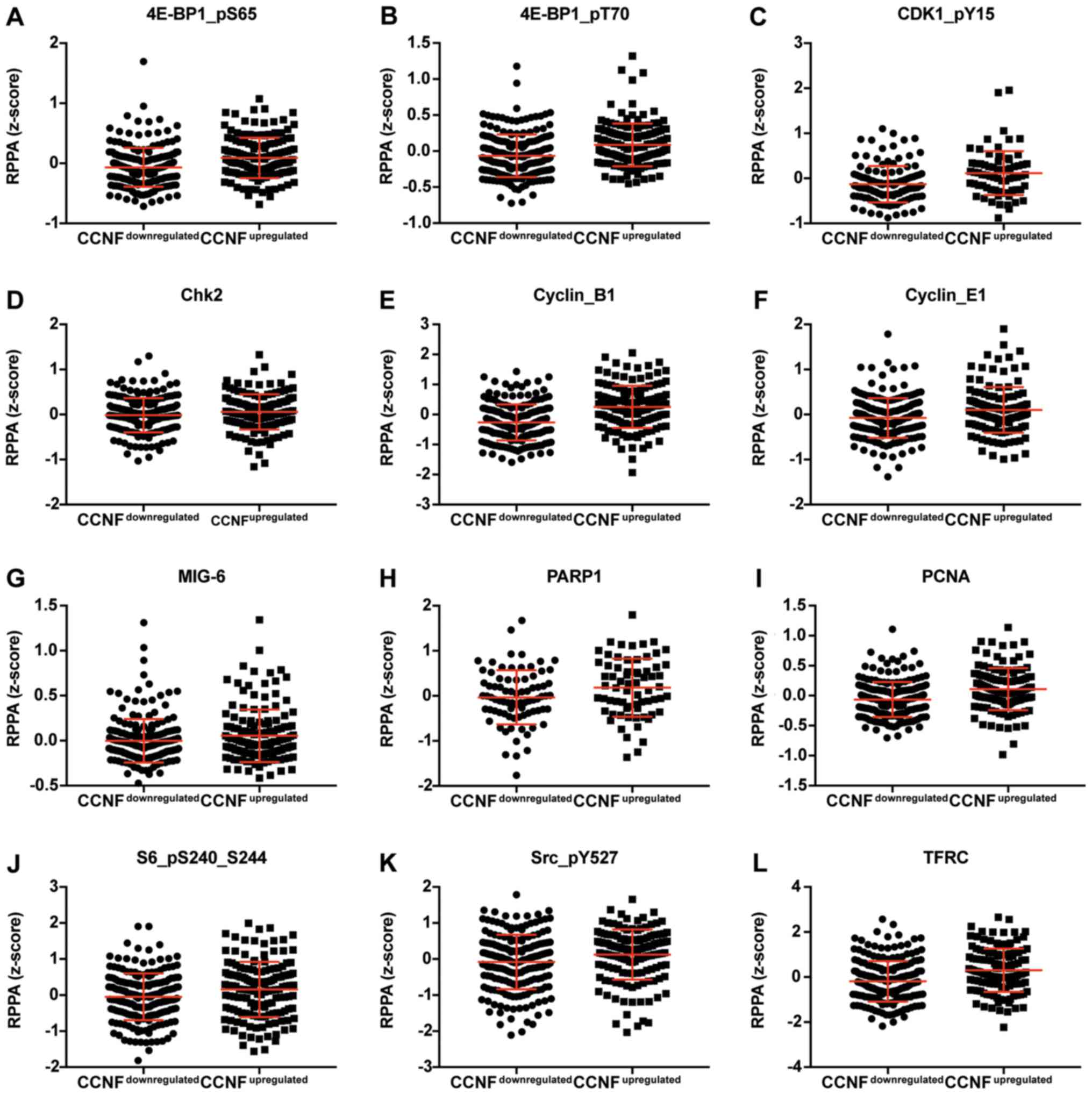
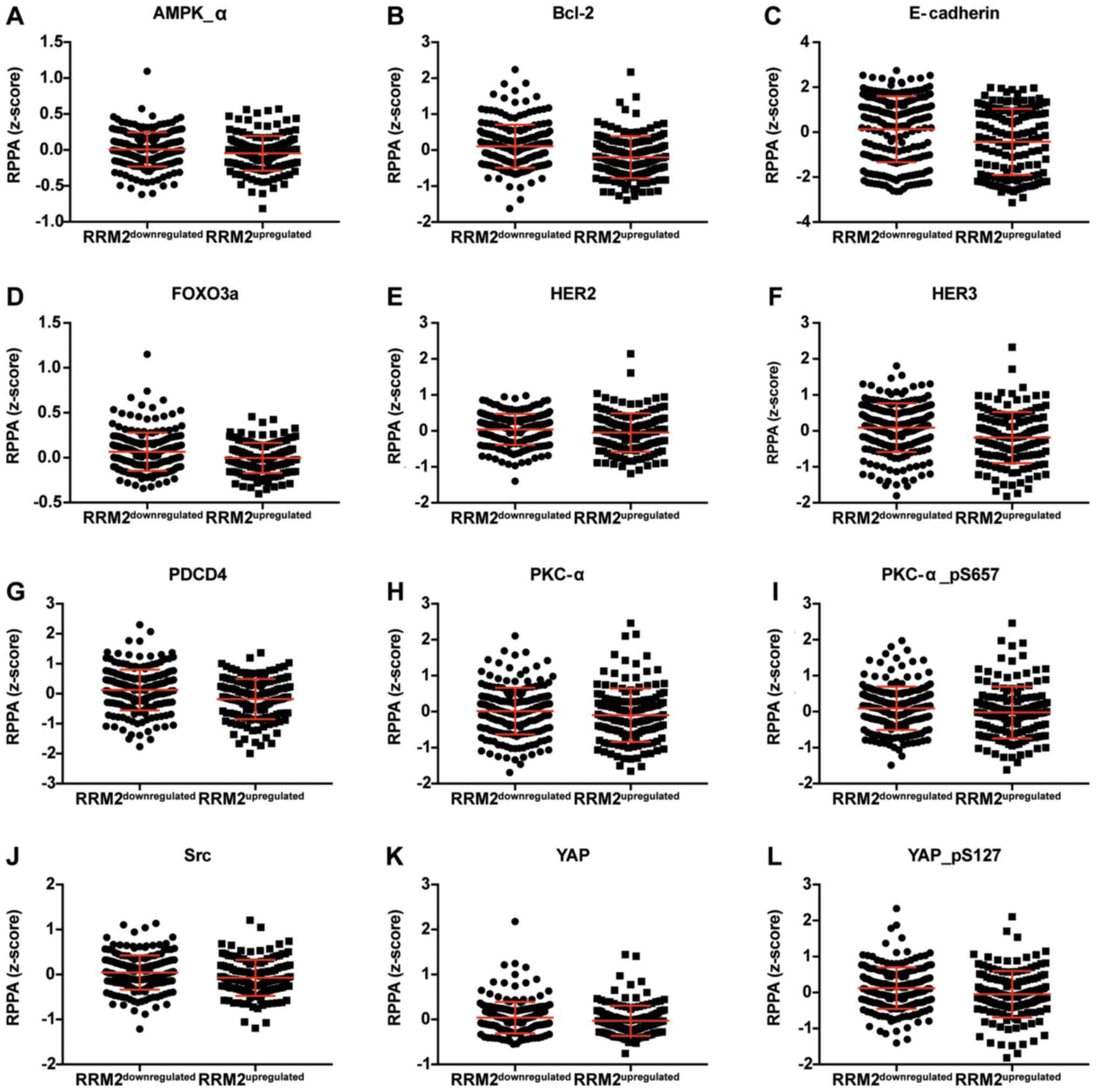
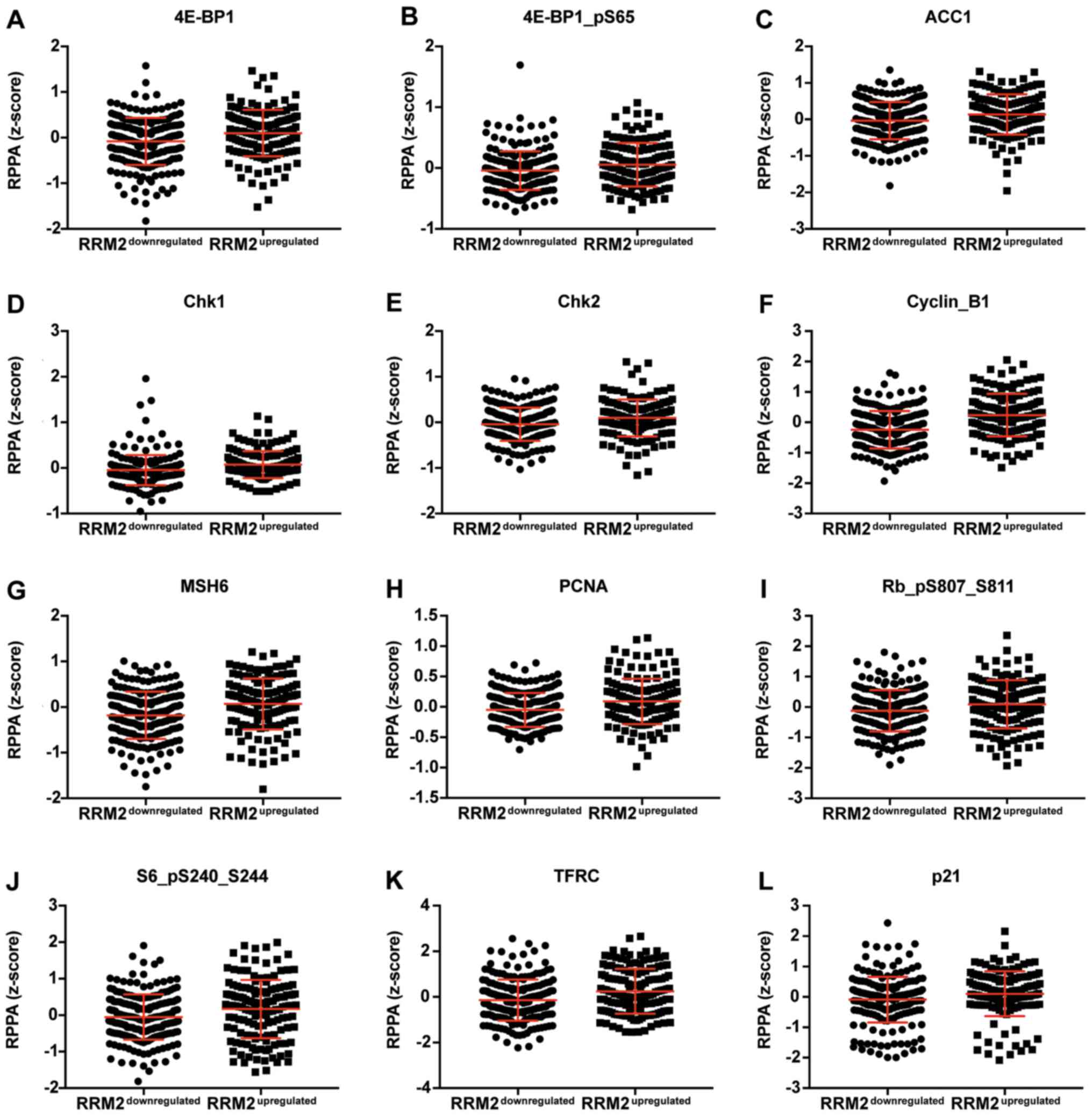
correlated with CCNF mRNA are listed in Tables III and V. Representative plots are shown in
Figs. 3 and 4. Analogous data for RRM2 mRNA expression
are shown in Tables VII and
IX, and representative plots are
presented in Figs. 5 and 6.
 | Table I.Association of CCNF and RRM2 mRNA
expression on the survival of melanoma patients. |
Table I.
Association of CCNF and RRM2 mRNA
expression on the survival of melanoma patients.
|
|
|
| Overall survival
(%) | Disease-free
survival (%) |
|---|
|
|
|
|
|
|
|---|
| Factor | Median survival
(months) | Disease-free median
survival (months) | 5 years | 10 years | 15 years | 5 years | 10 years | 15 years |
|---|
| Total | 74.67 | 51.08 | 58.79 | 39.20 | 25.38 | 42.85 | 24.93 | 12.09 |
| CCNF expression
(normalized) |
|
|
|
|
|
|
|
|
|
CCNFlow | 113.44 | 55.49 | 68.40 | 48.55 | 35.00 | 46.05 | 24.17 | 15.40 |
|
CCNFmedium | 61.10 | 48.59 | 52.24 | 34.64 | 23.05 | 41.18 | 26.87 | 10.88 |
|
CCNFhigh | 62.75 | 51.08 | 51.44 | 30.36 | 10.12 | 36.45 | 21.87 | 4.37 |
| CCNF expression
(z-score) |
|
|
|
|
|
|
|
|
|
CCNFdownregulated | 112.48 | 55.85 | 65.83 | 47.57 | 35.90 | 46.50 | 27.27 | 15.15 |
|
CCNFupregulated | 55.55 | 48.00 | 48.06 | 27.93 | 11.93 | 36.14 | 20.46 | 6.50 |
| RRM2 expression
(normalized) |
|
|
|
|
|
|
|
|
|
RRM2low | 74.67 | 63.40 | 58.48 | 42.51 | 37.79 | 53.88 | 36.47 | 31.26 |
|
RRM2medium | 94.91 | 58.97 | 64.07 | 39.11 | 21.44 | 49.07 | 22.17 | 10.57 |
|
RRM2high | 65.83 | 47.60 | 55.55 | 39.11 | 23.47 | 37.74 | 24.46 | 8.74 |
| RRM2 expression
(z-score) |
|
|
|
|
|
|
|
|
|
RRM2downregulated | 102.04 | 58.97 | 63.15 | 41.44 | 27.97 | 49.34 | 26.30 | 15.51 |
|
RRM2upregulated | 61.47 | 44.15 | 52.73 | 37.40 | 21.93 | 34.77 | 23.16 | 6.72 |
 | Table II.Changes in overall survival and
disease-free survival as associated with CCNF and RRM2 mRNA
expression in melanoma patients. |
Table II.
Changes in overall survival and
disease-free survival as associated with CCNF and RRM2 mRNA
expression in melanoma patients.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | Significance | HR | 95% CI | P-value | Significance |
|---|
| CCNF expression
(normalized) |
|
|
|
|
|
|
|
|
|
CCNFlow vs.
total | 0.73 | 0.57–0.93 | 0.0119 | * | 0.96 | 0.77–1.19 | 0.6915 | NS |
|
CCNFmedium vs.
total | 1.21 | 0.96–1.54 | 0.1070 | NS | 1.01 | 0.81–1.27 | 0.9072 | NS |
|
CCNFhigh vs.
total | 1.33 | 0.90–1.97 | 0.1576 | NS | 1.11 | 0.75–1.62 | 0.6087 | NS |
|
CCNFlow vs.
CCNFmedium | 0.60 | 0.45–0.80 | 0.0005 | *** | 0.95 | 0.73–1.27 | 0.6733 | NS |
|
CCNFlow vs.
CCNFhigh | 0.48 | 0.30–0.77 | 0.0022 | ** | 0.86 | 0.57–1.30 | 0.4748 | NS |
|
CCNFmedium vs.
CCNFhigh | 0.94 | 0.64–1.39 | 0.7717 | NS | 0.92 | 0.61–1.37 | 0.6784 | NS |
| CCNF expression
(z-score) |
|
|
|
|
|
|
|
|
|
CCNFdownregulated
vs. total | 0.79 | 0.63–0.98 | 0.0317 | * | 0.94 | 0.78–1.15 | 0.5671 | NS |
|
CCNFupregulated vs.
total | 1.42 | 1.11–1.82 | 0.0053 | ** | 1.11 | 0.87–1.40 | 0.3980 | NS |
|
CCNFdownregulated
vs. CCNFupregulated | 0.54 | 0.43–0.75 | <0.0001 | **** | 0.85 | 0.66–1.10 | 0.2211 | NS |
| RRM2 expression
(normalized) |
|
|
|
|
|
|
|
|
|
RRM2low vs.
total | 0.87 | 0.59–1.30 | 0.5052 | NS | 0.77 | 0.53–1.12 | 0.1693 | NS |
|
RRM2medium vs.
total | 0.93 | 0.71–1.22 | 0.5970 | NS | 0.96 | 0.75–1.24 | 0.7756 | NS |
|
RRM2high vs.
total | 0.95 | 0.76–1.17 | 0.6165 | NS | 1.08 | 0.88–1.32 | 0.4596 | NS |
|
RRM2low vs.
RRM2medium | 1.10 | 0.68–1.76 | 0.7059 | NS | 1.31 | 0.85–2.03 | 0.2260 | NS |
|
RRM2low vs.
RRM2high | 0.84 | 0.56–1.27 | 0.4156 | NS | 0.73 | 0.50–1.08 | 0.1134 | NS |
|
RRM2medium vs.
RRM2high | 0.88 | 0.66–1.18 | 0.3845 | NS | 0.90 | 0.69–1.17 | 0.4161 | NS |
| RRM2 expression
(z-score) |
|
|
|
|
|
|
|
|
|
RRM2downregulated
vs. total | 0.88 | 0.71–1.09 | 0.2507 | NS | 1.11 | 0.91–1.36 | 0.3133 | NS |
|
RRM2upregulated vs.
total | 1.17 | 0.92–1.50 | 0.1960 | NS | 1.15 | 0.92–1.44 | 0.2233 | NS |
|
RRM2downregulated
vs. RRM2upregulated | 0.75 | 0.57–0.98 | 0.0344 | * | 0.78 | 0.61–1.00 | 0.0529 | NS |
 | Table III.Expression of proteins which are
negatively correlated with CCNF. |
Table III.
Expression of proteins which are
negatively correlated with CCNF.
|
|
|
CCNFdownregulated |
CCNFupregulated |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| RPPA (z-score) |
|
|
|---|
| Protein | Gene | upregulated | downregulated | P-value | Significance |
|---|
| A-Raf_pS299 | ARAF | 0.0567 | −0.0105 | 0.0279 | * |
| Annexin_VII | ANXA7 | 0.0085 | −0.0491 | 0.0055 | ** |
| Annexin-1 | ANXA1 | 0.2359 | −0.0402 | 0.0006 | *** |
| AR | AR | 0.0662 | −0.0072 | 0.0380 | * |
| Axl | AXL | 0.1741 | −0.0276 | 0.0283 | * |
| Bak | BAK1 | 0.0059 | −0.0199 | 0.5374 | NS |
| Bcl-2 | BCL2 | 0.0461 | −0.1069 | 0.0190 | * |
| Bcl-xL | BCL2L1 | 0.0578 | −0.0127 | 0.0609 | NS |
| Bim | BCL2L11 | 0.0081 | −0.1046 | 0.0200 | * |
| Caveolin-1 | CAV1 | 0.2809 | −0.0344 | 0.0013 | ** |
| CD31 | PECAM1 | 0.0548 | −0.0108 | 0.0260 | * |
| CD49b | ITGA2 | 0.1129 | −0.0100 | <0.0001 | **** |
| Chk1_pS345 | CHEK1 | 0.0011 | −0.0009 | 0.6241 | NS |
| DJ-1 | PARK7 | 0.0503 | −0.0112 | 0.0743 | NS |
| EGFR_pY1068 | EGFR | 0.0817 | −0.0107 | 0.0015 | ** |
| ER-α | ESR1 | 0.0900 | −0.0314 | 0.0002 | *** |
| FOXO3a | FOXO3 | 0.0724 | −0.0102 | <0.0001 | **** |
| GATA3 | GATA3 | 0.0186 | −0.0356 | 0.0287 | * |
| GATA6 | GATA6 | 0.0949 | −0.0295 | 0.0132 | * |
| HER2 | ERBB2 | 0.0678 | −0.0827 | 0.0036 | ** |
| HER3 | ERBB3 | 0.0023 | −0.0620 | 0.2038 | NS |
| HER3_pY1289 | ERBB3 | 0.0086 | −0.0137 | 0.1953 | NS |
| INPP4B | INPP4B | 0.0761 | −0.0258 | 0.0008 | *** |
| JAB1 | COPS5 | 0.0558 | −0.1180 | <0.0001 | **** |
| JNK2 | MAPK9 | 0.0404 | −0.0589 | 0.0083 | ** |
| Myosin-IIa | MYH9 | 0.0003 | −0.0030 | 0.9509 | NS |
| p27 | CDKN1B | 0.0582 | −0.1027 | <0.0001 | **** |
| p38_pT180_Y182 | MAPK14 | 0.0115 | −0.0346 | 0.3252 | NS |
| p53 | TP53 | 0.0557 | −0.0223 | 0.0021 | ** |
| PARP_cleaved | PARP1 | 0.0227 | −0.0241 | 0.0773 | NS |
| PDCD4 | PDCD4 | 0.0854 | −0.1255 | 0.0025 | ** |
| PEA15 | PEA15 | 0.0238 | −0.0052 | 0.4346 | NS |
| PI3K-p110-α | PIK3CA | 0.0097 | −0.0625 | 0.0315 | * |
| PKC-α | PRKCA | 0.1358 | −0.2574 | <0.0001 | **** |
| PKC-α_pS657 | PRKCA | 0.1951 | −0.1638 | <0.0001 | **** |
| PKC-δ_pS664 | PRKCD | 0.0194 | −0.0539 | 0.1518 | NS |
| PRDX1 | PRDX1 | 0.0266 | −0.0393 | 0.2556 | NS |
| Rab25 | RAB25 | 0.0432 | −0.0767 | 0.0011 | ** |
| Rad50 | RAD50 | 0.0579 | −0.0170 | 0.1381 | NS |
| Shc_pY317 | SHC1 | 0.0064 | −0.0834 | 0.0026 | ** |
| Src_pY416 | SRC | 0.0296 | −0.0103 | 0.4421 | NS |
| VEGFR2 | KDR | 0.0142 | −0.0164 | 0.3048 | NS |
 | Table V.Expression of proteins which
positively correlate with CCNF. |
Table V.
Expression of proteins which
positively correlate with CCNF.
|
|
|
CCNFdownregulated |
CCNFupregulated |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| RPPA (z-score) |
|
|
|---|
| Protein | Gene | Downregulated | Upregulated | P-value | Significance |
|---|
| 4E-BP1 | EIF4EBP1 | −0.0327 | 0.0285 | 0.3383 | NS |
| 4E-BP1_pS65 | EIF4EBP1 | −0.0677 | 0.0891 | <0.0001 | **** |
| 4E-BP1_pT70 | EIF4EBP1 | −0.0655 | 0.0860 | <0.0001 | **** |
| ACC_pS79 | ACACA | −0.0054 | 0.0362 | 0.3200 | NS |
| C-Raf | RAF1 | −0.0110 | 0.0031 | 0.3485 | NS |
| CDK1_pY15 | CDK1 | −0.1318 | 0.1145 | <0.0001 | **** |
| Chk1 | CHEK1 | −0.0156 | 0.0301 | 0.0333 | * |
| Chk2 | CHEK2 | −0.0137 | 0.0578 | 0.0421 | * |
| Cyclin_B1 | CCNB1 | −0.2619 | 0.2546 | <0.0001 | **** |
| Cyclin_E1 | CCNE1 | −0.0766 | 0.1020 | 0.0009 | *** |
| eEF2 | EEF2 | −0.0851 | 0.0434 | 0.0449 | * |
| FoxM1 | FOXM1 | −0.0423 | 0.1525 | <0.0001 | **** |
| GAPDH | GAPDH | −0.0492 | 0.0336 | 0.5952 | NS |
| MIG-6 | ERRFI1 | −0.0025 | 0.0536 | 0.1141 | NS |
| MSH2 | MSH2 | −0.0296 | 0.0032 | 0.3485 | NS |
| MSH6 | MSH6 | −0.1614 | 0.0359 | 0.0003 | *** |
|
NF-kB-p65_pS536 | NFKB1 | −0.0494 | 0.0028 | 0.6642 | NS |
| NF2 | NF2 | −0.0131 | 0.0148 | 0.7265 | NS |
| p21 | CDKN1A | −0.0769 | 0.0860 | 0.0186 | * |
| p38_MAPK | MAPK14 | −0.0104 | 0.0141 | 0.7464 | NS |
| p62-LCK-ligand | SQSTM1 | −0.0667 | 0.0042 | 0.1143 | NS |
| PARP1 | PARP1 | −0.0340 | 0.1803 | 0.0425 | * |
| PCNA | PCNA | −0.0654 | 0.1086 | <0.0001 | **** |
| PRAS40_pT246 | AKT1S1 | −0.0248 | 0.0148 | 0.1293 | NS |
| Rb_pS807_S811 | RB1 | −0.1385 | 0.1091 | 0.0014 | ** |
| S6_pS240_S244 | RPS6KB1 | −0.0474 | 0.1577 | 0.0086 | ** |
| SLC1A5 | SLC1A5 | −0.0542 | 0.0092 | 0.2129 | NS |
| Src | SRC | −0.0137 | 0.0101 | 0.4254 | NS |
| Src_pY527 | SRC | −0.0814 | 0.1241 | 0.0022 | ** |
| TFRC | TFRC | −0.1894 | 0.3049 | <0.0001 | **** |
| Tuberin_pT1462 | TSC2 | −0.0626 | 0.0410 | 0.0488 | * |
| XRCC1 | XRCC1 | −0.0784 | 0.0078 | 0.0065 | ** |
 | Table VII.Expression of proteins which are
negatively correlated with RRM2. |
Table VII.
Expression of proteins which are
negatively correlated with RRM2.
|
|
|
RRM2downregulated |
RRM2upregulated |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| RPPA (z-score) |
|
|
|---|
| Protein | Gene | Upregulated | Downregulated | P-value | Significance |
|---|
| 14-3-3_ζ | YWHAZ | 0.0436 | −0.0176 | 0.1293 | NS |
| α-catenin | CTNNB1 | 0.0676 | −0.0037 | 0.0957 | NS |
| AMPK_α | PRKAA1 | 0.0076 | −0.0467 | 0.0311 | * |
| Bcl-2 | BCL2 | 0.1080 | −0.1967 | <0.0001 | **** |
| cIAP | BIRC2 | 0.0042 | −0.0600 | 0.0043 | ** |
| E-cadherin | CDH1 | 0.1282 | −0.4331 | 0.0003 | *** |
| ER-α | ESR1 | 0.0796 | −0.0194 | 0.0013 | ** |
| FOXO3a | FOXO3 | 0.0664 | −0.0035 | 0.0041 | ** |
| GATA3 | GATA3 | 0.0059 | −0.0189 | 0.0886 | NS |
| HER2 | ERBB2 | 0.0414 | −0.0487 | 0.0298 | * |
| HER3 | ERBB3 | 0.0900 | −0.1863 | <0.0001 | **** |
| INPP4B | INPP4B | 0.0749 | −0.0261 | 0.0007 | *** |
| JAB1 | COPS5 | 0.0083 | −0.0772 | 0.0957 | NS |
| JNK2 | MAPK9 | 0.0047 | −0.0109 | 0.6772 | NS |
| p27_pT198 | CDKN1B | 0.0017 | −0.0069 | 0.7788 | NS |
| p38_MAPK | MAPK14 | 0.0349 | −0.0488 | 0.0165 | * |
| p38_pT180_Y182 | MAPK14 | 0.0086 | −0.0314 | 0.4440 | NS |
| PARP_cleaved | PARP1 | 0.0073 | −0.0035 | 0.4065 | NS |
| PDCD4 | PDCD4 | 0.1225 | −0.1818 | 0.0001 | *** |
| PDK1 | PDPK1 | 0.0294 | −0.0014 | 0.1348 | NS |
| PDK1_pS241 | PDPK1 | 0.0071 | −0.0442 | 0.2341 | NS |
| PI3K-p85 | PIK3R1 | 0.0116 | −0.0613 | 0.0605 | NS |
| PKC-α | PRKCA | 0.0155 | −0.0969 | 0.0414 | * |
| PKC-α_pS657 | PRKCA | 0.0906 | −0.0247 | 0.0307 | * |
| PRDX1 | PRDX1 | 0.0146 | −0.0238 | 0.5525 | NS |
| PREX1 | PREX1 | 0.0619 | −0.0082 | 0.2623 | NS |
| Rab25 | RAB25 | 0.0707 | −0.1177 | <0.0001 | **** |
| Rad50 | RAD50 | 0.0570 | −0.0174 | 0.0624 | NS |
| Src | SRC | 0.0459 | −0.0729 | 0.0033 | ** |
| Src_pY527 | SRC | 0.0315 | −0.0300 | 0.3020 | NS |
| VEGFR2 | KDR | 0.0191 | −0.0239 | 0.4077 | NS |
| YAP | YAP1 | 0.0412 | −0.0283 | 0.0215 | * |
| YAP_pS127 | YAP1 | 0.1242 | −0.0491 | 0.0106 | * |
 | Table IX.Expression of proteins which are
positively correlated with RRM2. |
Table IX.
Expression of proteins which are
positively correlated with RRM2.
|
|
|
RRM2downregulated |
RRM2upregulated |
|
|
|---|
|
|
|
|
|
|
|---|
|
|
| RPPA (z-score) |
|
|
|---|
| Protein | Gene | Downregulated | Upregulated | P-value | Significance |
|---|
| 4E-BP1 | EIF4EBP1 | −0.0824 | 0.0995 | 0.0010 | *** |
| 4E-BP1_pS65 | EIF4EBP1 | −0.0413 | 0.0554 | 0.0126 | * |
| 4E-BP1_pT70 | EIF4EBP1 | −0.0152 | 0.0185 | 0.2779 | NS |
| ACC_pS79 | ACACA | −0.0209 | 0.0587 | 0.0782 | NS |
| ACC1 | ACACA | −0.0340 | 0.1352 | 0.0024 | ** |
| Bax | BAX | −0.0251 | 0.0043 | 0.8262 | NS |
| C-Raf | RAF1 | −0.0244 | 0.0222 | 0.0089 | ** |
| CDK1_pY15 | CDK1 | −0.0711 | 0.0147 | 0.1638 | NS |
| Chk1 | CHEK1 | −0.0460 | 0.0737 | <0.0001 | **** |
| Chk1_pS345 | CHEK1 | −0.0142 | 0.0205 | 0.0618 | NS |
| Chk2 | CHEK2 | −0.0412 | 0.0977 | 0.0002 | *** |
| Cyclin_B1 | CCNB1 | −0.2434 | 0.2391 | <0.0001 | **** |
| Cyclin_E1 | CCNE1 | −0.0330 | 0.0445 | 0.1473 | NS |
| eEF2 | EEF2 | −0.0765 | 0.0339 | 0.1272 | NS |
| EGFR_pY1173 | EGFR | −0.0088 | 0.0283 | 0.1524 | NS |
| eIF4E | EIF4E | −0.0487 | 0.0033 | 0.1951 | NS |
| FoxM1 | FOXM1 | −0.0608 | 0.1823 | <0.0001 | **** |
| GAPDH | GAPDH | −0.0538 | 0.0416 | 0.0996 | NS |
| HER3_pY1289 | ERBB3 | −0.0062 | 0.0066 | 0.3676 | NS |
| MSH2 | MSH2 | −0.0493 | 0.0314 | 0.0703 | NS |
| MSH6 | MSH6 | −0.1812 | 0.0677 | <0.0001 | **** |
| Myosin-IIa | MYH9 | −0.0317 | 0.0371 | 0.4099 | NS |
| NF2 | NF2 | −0.0205 | 0.0258 | 0.2552 | NS |
| p21 | CDKN1A | −0.0880 | 0.1049 | 0.0025 | ** |
| p62-LCK-ligand | SQSTM1 | −0.0850 | 0.0313 | 0.1070 | NS |
| p90RSK | RPS6KA1 | −0.0133 | 0.0750 | 0.0363 | * |
| PCNA | PCNA | −0.0499 | 0.0905 | 0.0001 | *** |
| PRAS40_pT246 | AKT1S1 | −0.0225 | 0.0124 | 0.3327 | NS |
| Rb_pS807_S811 | RB1 | −0.1222 | 0.0913 | 0.0035 | ** |
| S6_pS235_S236 | RPS6KB1 | −0.0110 | 0.2044 | 0.0053 | ** |
| S6_pS240_S244 | RPS6KB1 | −0.0516 | 0.1676 | 0.0024 | ** |
| SLC1A5 | SLC1A5 | −0.0858 | 0.0421 | 0.0743 | NS |
| Src_pY416 | SRC | −0.0307 | 0.0735 | 0.0630 | NS |
| TFRC | TFRC | −0.1404 | 0.2463 | 0.0007 | *** |
|
Transglutaminase | TGM1 | −0.0275 | 0.0094 | 0.5674 | NS |
| TSC1 | TSC1 | −0.0611 | 0.0051 | 0.1200 | NS |
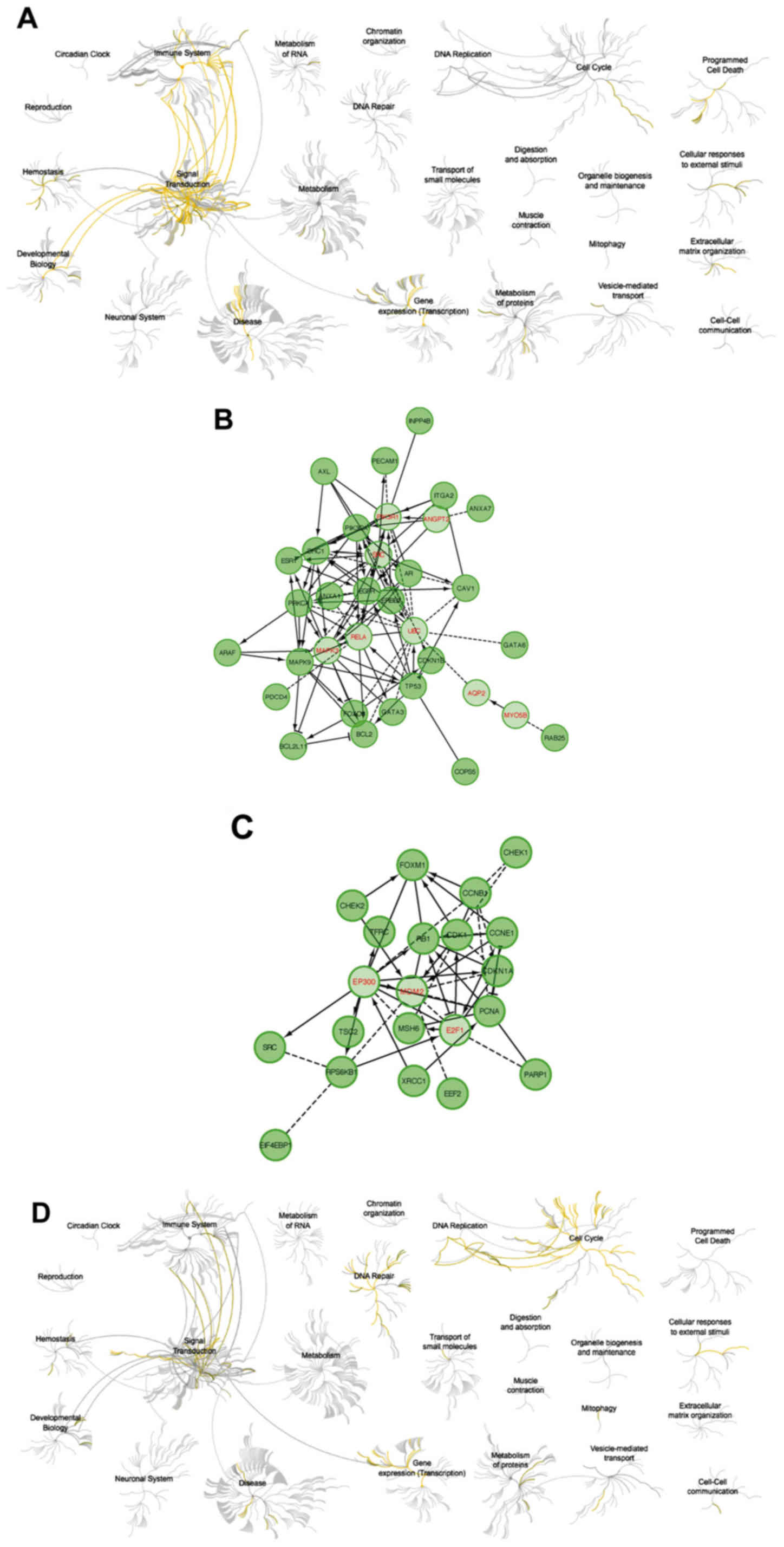
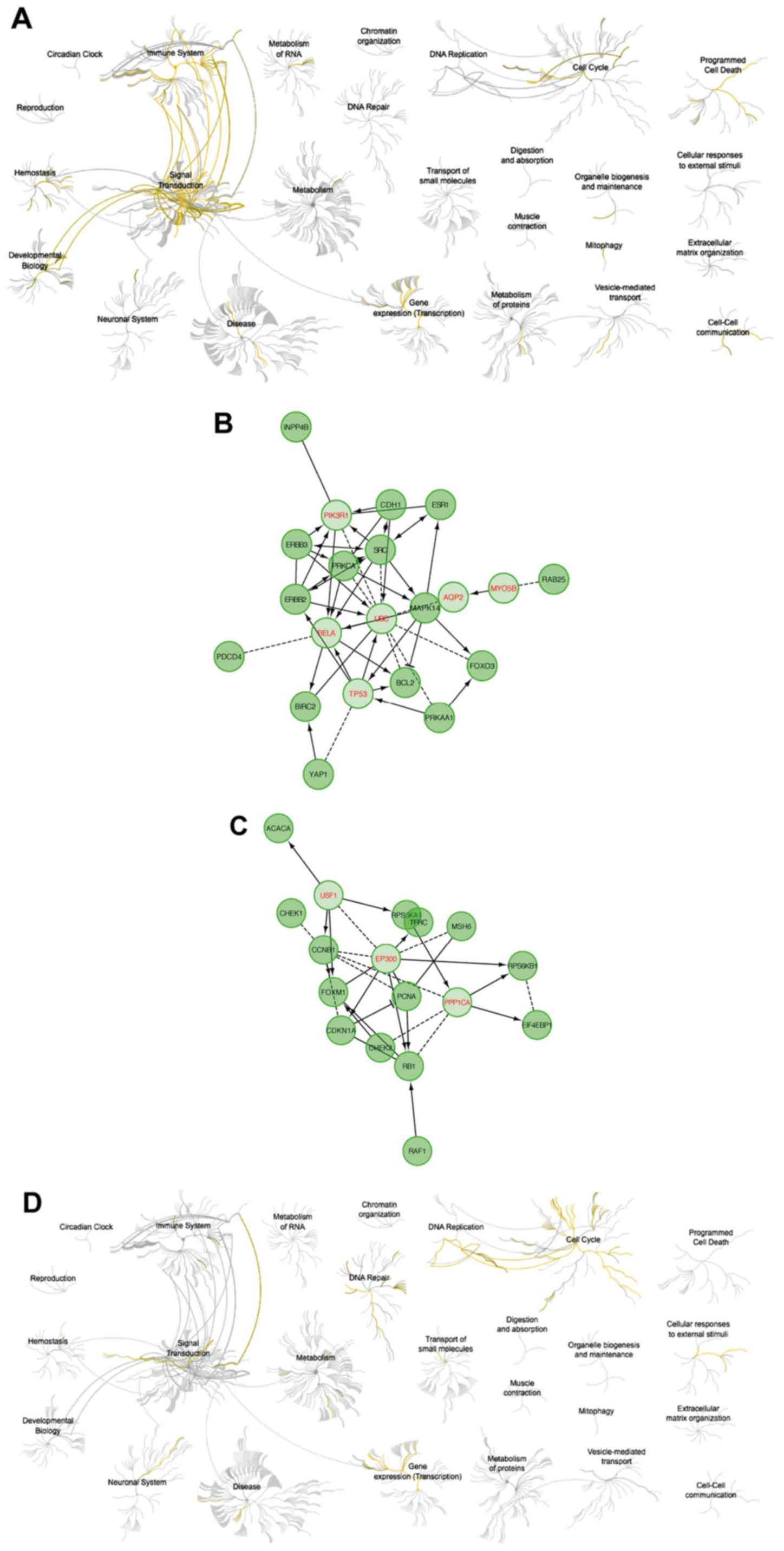
The analysis using Reactome showed that upregulation
of cyclin F resulted in downregulation of pathways responsible for
signal transduction and activation of cell cycle-related and DNA
repair (Fig. 7). High expression of
RRM2 mRNA also resulted in downregulation of cell signaling
pathways. Activation of the cell cycle and DNA pathways was also
visible but less univocal (Fig. 8).
Upregulation of cyclin F coincides with altered expression of
factors that were associated with worse patient outcome.
Furthermore, patients with worse outcome had increased levels of
proliferative proteins, such as cyclin E, cyclin B, PCNA,
pro-survival factors such as p27 or FOXM1 and connected with AKT
pathway activation (INPP4B). The list of biological processes
altered by cyclin F dysregulation are presented in Tables IV and VI. Furthermore, data presenting
biological processes influenced by changes in RRM2 expression are
presented in Tables VIII and
X.
 | Table IV.Biological process and pathway
analysis of genes whose products are negatively correlated with
CCNF expression. |
Table IV.
Biological process and pathway
analysis of genes whose products are negatively correlated with
CCNF expression.
| Factor | P-value | Number of
genes | Gene list |
|---|
| Biological
process |
|
|
|
|
Regulation of apoptotic
process | 1.26E-12 | 19 | GATA3, GATA6,
CDKN1B, FOXO3, PRKCA, ERBB2, BCL2, CAV1, MAPK9, BCL2L11, EGFR,
PIK3CA, ANXA1, AXL, AR, ARAF, PDCD4, ESR1, TP53 |
|
Regulation of intracellular
signal transduction | 7.99E-12 | 19 | SHC1, GATA3, PRKCA,
ERBB2, BCL2, CAV1, MAPK9, BCL2L11, EGFR, PIK3CA, COPS5, AXL, AR,
ARAF, PDCD4, ESR1, INPP4B, TP53, PECAM1 |
|
Apoptotic process | 2.63E-11 | 19 | GATA3, GATA6,
CDKN1B, FOXO3, PRKCA, ERBB2, BCL2, CAV1, MAPK9, BCL2L11, EGFR,
PIK3CA, ANXA1, AXL, AR, ARAF, PDCD4, ESR1, TP53 |
|
Negative regulation of
apoptotic process | 3.01E-11 | 15 | GATA3, GATA6,
CDKN1B, PRKCA, ERBB2, BCL2, CAV1, EGFR, PIK3CA, ANXA1, AXL, AR,
ARAF, PDCD4, TP53 |
|
Positive regulation of
cellular protein metabolic process | 1.08E-10 | 17 | SHC1, GATA3,
CDKN1B, PRKCA, ERBB2, BCL2, ITGA2, CAV1, MAPK9, BCL2L11, EGFR,
PIK3CA, AR, ARAF, ESR1, TP53, PECAM1 |
|
Regulation of protein
modification process | 1.30E-10 | 18 | SHC1, GATA3,
CDKN1B, PRKCA, ERBB2, BCL2, ITGA2, CAV1, MAPK9, EGFR, PIK3CA,
COPS5, AR, ARAF, PDCD4, ESR1, TP53, PECAM1 |
|
Positive regulation of
signaling | 3.37E-10 | 17 | SHC1, GATA3, GATA6,
PRKCA, ERBB2, BCL2, ITGA2, CAV1, MAPK9, BCL2L11, EGFR, AXL, AR,
ARAF, ESR1, TP53, PECAM1 |
|
Positive regulation of cell
communication | 3.61E-10 | 17 | SHC1, GATA3, GATA6,
PRKCA, ERBB2, BCL2, ITGA2, CAV1, MAPK9, BCL2L11, EGFR, AXL, AR,
ARAF, ESR1, TP53, PECAM1 |
|
Regulation of
phosphorylation | 7.58E-10 | 16 | SHC1, CDKN1B,
PRKCA, ERBB2, BCL2, CAV1, MAPK9, EGFR, PIK3CA, COPS5, AR, ARAF,
PDCD4, ESR1, TP53, PECAM1 |
|
Positive regulation of
phosphorylation | 7.76E-10 | 14 | SHC1, CDKN1B,
PRKCA, ERBB2, BCL2, CAV1, MAPK9, EGFR, PIK3CA, AR, ARAF, ESR1,
TP53, PECAM1 |
|
Regulation of cell
proliferation | 2.67E-09 | 16 | SHC1, GATA3, GATA6,
CDKN1B, FOXO3, PRKCA, ERBB2, BCL2, RAB25, ITGA2, CAV1, EGFR, ANXA1,
AR, ESR1, TP53 |
|
Positive regulation of cell
proliferation | 4.30E-09 | 13 | SHC1, GATA6,
CDKN1B, PRKCA, ERBB2, BCL2, RAB25, ITGA2, CAV1, EGFR, ANXA1, AR,
ESR1 |
| Cell
adhesion | 1.11E-07 | 14 | SHC1, GATA3, PRKCA,
ERBB2, BCL2, ITGA2, CAV1, BCL2L11, EGFR, PIK3CA, ANXA1, AXL, TP53,
PECAM1 |
|
Positive regulation of
apoptotic process | 3.01E-07 | 10 | GATA6, CDKN1B,
FOXO3, BCL2, CAV1, MAPK9, BCL2L11, ANXA1, PDCD4, TP53 |
| Pathway |
|
|
|
| EGFR
tyrosine kinase inhibitor resistance | 2.50E-13 | 10 | SHC1, FOXO3, PRKCA,
ERBB2, BCL2, BCL2L11, EGFR, PIK3CA, AXL, ARAF |
|
Endocrine resistance | 9.60E-13 | 10 | SHC1, CDKN1B,
ERBB2, BCL2, MAPK9, EGFR, PIK3CA, ARAF, ESR1, TP53 |
|
Proteoglycans in cancer | 1.01E-09 | 10 | PRKCA, ERBB2,
ITGA2, CAV1, EGFR, PIK3CA, ARAF, PDCD4, ESR1, TP53 |
| ErbB
signaling pathway | 1.01E-09 | 8 | SHC1, CDKN1B,
PRKCA, ERBB2, MAPK9, EGFR, PIK3CA, ARAF |
| Focal
adhesion | 1.57E-08 | 9 | SHC1, PRKCA, ERBB2,
BCL2, ITGA2, CAV1, MAPK9, EGFR, PIK3CA |
|
Pathways in cancer | 1.57E-08 | 11 | CDKN1B, PRKCA,
ERBB2, BCL2, ITGA2, MAPK9, EGFR, PIK3CA, AR, ARAF, TP53 |
|
MicroRNAs in cancer | 2.01E-08 | 10 | SHC1, CDKN1B,
PRKCA, ERBB2, BCL2, BCL2L11, EGFR, PIK3CA, PDCD4, TP53 |
| FoxO
signaling pathway | 4.15E-07 | 7 | CDKN1B, FOXO3,
MAPK9, BCL2L11, EGFR, PIK3CA, ARAF |
|
Signaling by SCF-KIT | 7.61E-07 | 9 | SHC1, CDKN1B,
FOXO3, PRKCA, ERBB2, EGFR, PIK3CA, ARAF, TP53 |
|
PI3K-Akt signaling
pathway | 7.61E-07 | 9 | CDKN1B, FOXO3,
PRKCA, BCL2, ITGA2, BCL2L11, EGFR, PIK3CA, TP53 |
|
Signaling by NGF | 9.31E-07 | 10 | SHC1, CDKN1B,
FOXO3, PRKCA, ERBB2, BCL2L11, EGFR, PIK3CA, ARAF, TP53 |
| HIF-1
signaling pathway | 1.43E-06 | 6 | CDKN1B, PRKCA,
ERBB2, BCL2, EGFR, PIK3CA |
|
Apoptosis signaling
pathway | 1.43E-06 | 6 | PRKCA, BCL2, MAPK9,
BCL2L11, PIK3CA, TP53 |
|
Signaling by ERBB2 | 1.54E-06 | 5 | SHC1, PRKCA, ERBB2,
EGFR, PIK3CA |
 | Table VI.Biological process and pathway
analysis of genes whose products are positively correlated with
CCNF expression. |
Table VI.
Biological process and pathway
analysis of genes whose products are positively correlated with
CCNF expression.
| Factor | P-value | Number of
genes | Gene list |
|---|
| Biological
process |
|
|
|
|
Regulation of cell cycle | 1.88E-10 | 13 | CHEK2, FOXM1,
CCNE1, CDKN1A, RB1, TSC2, RPS6KB1, CDK1, CHEK1, PCNA, EIF4EBP1,
SRC, CCNB1 |
| Cell
cycle phase transition | 1.94E-10 | 11 | CHEK2, FOXM1,
CCNE1, CDKN1A, RB1, RPS6KB1, CDK1, CHEK1, PCNA, EIF4EBP1,
CCNB1 |
| Cell
cycle G1/S phase transition | 2.22E-10 | 9 | CHEK2, CCNE1,
CDKN1A, RB1, RPS6KB1, CDK1, PCNA, EIF4EBP1, CCNB1 |
|
Positive regulation of cell
cycle | 1.82E-09 | 9 | CHEK2, CDKN1A, RB1,
RPS6KB1, CDK1, PCNA, EIF4EBP1, SRC, CCNB1 |
|
Regulation of DNA metabolic
process | 2.32E-09 | 9 | CHEK2, FOXM1,
CDKN1A, MSH6, PARP1, CDK1, CHEK1, PCNA, SRC |
| Cell
cycle arrest | 4.10E-09 | 8 | CHEK2, FOXM1,
CDKN1A, RB1, TSC2, CDK1, PCNA, CCNB1 |
|
Negative regulation of mitotic
cell cycle phase transition | 8.06E-09 | 7 | CHEK2, CDKN1A, RB1,
CDK1, CHEK1, PCNA, CCNB1 |
|
Negative regulation of G1/S
transition of mitotic cell cycle | 2.07E-08 | 6 | CHEK2, CDKN1A, RB1,
CDK1, PCNA, CCNB1 |
| DNA
damage checkpoint | 1.70E-07 | 6 | CHEK2, CDKN1A,
CDK1, CHEK1, PCNA, CCNB1 |
|
Positive regulation of
macromolecule biosynthetic process | 1.94E-07 | 12 | CHEK2, FOXM1,
CCNE1, RB1, PARP1, TSC2, EEF2, RPS6KB1, CDK1, CHEK1, PCNA, SRC |
| DNA
repair | 3.08E-07 | 8 | CHEK2, FOXM1, MSH6,
PARP1, CDK1, CHEK1, PCNA, XRCC1 |
|
Positive regulation of gene
expression | 3.21E-07 | 12 | CHEK2, FOXM1,
CCNE1, RB1, PARP1, TSC2, EEF2, RPS6KB1, CDK1, CHEK1, SRC,
CCNB1 |
|
Positive regulation of cell
cycle arrest | 3.74E-07 | 5 | CHEK2, CDKN1A,
CDK1, PCNA, CCNB1 |
|
Positive regulation of
cellular biosynthetic process | 3.74E-07 | 12 | CHEK2, FOXM1,
CCNE1, RB1, PARP1, TSC2, EEF2, RPS6KB1, CDK1, CHEK1, PCNA, SRC |
| Pathway |
|
|
|
| Cell
cycle | 1.06E-09 | 8 | CHEK2, CCNE1,
CDKN1A, RB1, CDK1, CHEK1, PCNA, CCNB1 |
| p53
signaling pathway | 1.06E-09 | 7 | CHEK2, CCNE1,
CDKN1A, TSC2, CDK1, CHEK1, CCNB1 |
| FOXM1
transcription factor network | 1.61E-09 | 6 | CHEK2, FOXM1, RB1,
CDK1, CCNB1, XRCC1 |
| E2F
mediated regulation of DNA replication | 9.24E-08 | 5 | CCNE1, RB1, CDK1,
PCNA, CCNB1 |
| mTOR
signaling pathway | 9.46E-07 | 5 | CCNE1, TSC2, EEF2,
RPS6KB1, EIF4EBP1 |
| ATM
signaling pathway | 4.84E-05 | 3 | CHEK2, CDKN1A,
CHEK1 |
| DNA
double-strand break repair | 5.32E-05 | 5 | CHEK2, PARP1,
CHEK1, PCNA, XRCC1 |
| ErbB
signaling pathway | 8.14E-05 | 4 | CDKN1A, RPS6KB1,
EIF4EBP1, SRC |
|
Endocrine resistance | 1.16E-04 | 4 | CDKN1A, RB1,
RPS6KB1, SRC |
| HIF-1
signaling pathway | 1.39E-04 | 4 | CDKN1A, RPS6KB1,
EIF4EBP1, TFRC |
| Base
excision repair | 1.57E-04 | 3 | PARP1, PCNA,
XRCC1 |
| AMPK
signaling pathway | 2.39E-04 | 4 | TSC2, EEF2,
RPS6KB1, EIF4EBP1 |
|
PI3K-Akt signaling
pathway | 7.78E-04 | 5 | CCNE1, CDKN1A,
TSC2, RPS6KB1, EIF4EBP1 |
|
Mismatch repair | 1.42E-03 | 2 | MSH6, PCNA |
 | Table VIII.Biological process and pathway
analysis of genes whose products are negatively correlated with
RRM2 expression. |
Table VIII.
Biological process and pathway
analysis of genes whose products are negatively correlated with
RRM2 expression.
| Factor | P-value | Number of
genes | Gene list |
|---|
| Biological
process |
|
|
|
|
Regulation of cell
proliferation | 4.03E-09 | 13 | FOXO3, CDH1, BIRC2,
PRKCA, YAP1, ERBB2, ERBB3, ESR1, BCL2, RAB25, MAPK14, PRKAA1,
SRC |
|
Apoptotic process | 1.03E-08 | 13 | FOXO3, CDH1, BIRC2,
PRKCA, YAP1, ERBB2, ERBB3, PDCD4, ESR1, BCL2, MAPK14, PRKAA1,
SRC |
|
Regulation of apoptotic
process | 1.39E-08 | 12 | FOXO3, CDH1, BIRC2,
PRKCA, YAP1, ERBB2, ERBB3, PDCD4, ESR1, BCL2, PRKAA1, SRC |
|
Negative regulation of signal
transduction | 2.30E-08 | 11 | FOXO3, CDH1, PRKCA,
YAP1, ERBB3, PDCD4, ESR1, BCL2, MAPK14, PRKAA1, SRC |
|
Negative regulation of
apoptotic process | 8.07E-07 | 9 | BIRC2, PRKCA, YAP1,
ERBB2, ERBB3, PDCD4, BCL2, PRKAA1, SRC |
|
Regulation of intracellular
signal transduction | 8.07E-07 | 11 | BIRC2, PRKCA,
ERBB2, ERBB3, PDCD4, ESR1, BCL2, INPP4B, MAPK14, PRKAA1, SRC |
|
Positive regulation of
intracellular signal transduction | 1.09E-06 | 9 | BIRC2, PRKCA,
ERBB2, ERBB3, ESR1, BCL2, MAPK14, PRKAA1, SRC |
|
Regulation of cell
motility | 4.00E-06 | 8 | CDH1, PRKCA, ERBB2,
ERBB3, BCL2, RAB25, MAPK14, SRC |
|
Positive regulation of protein
modification process | 5.45E-06 | 9 | BIRC2, PRKCA,
ERBB2, ERBB3, ESR1, BCL2, MAPK14, PRKAA1, SRC |
|
Regulation of cellular
component movement | 6.29E-06 | 8 | CDH1, PRKCA, ERBB2,
ERBB3, BCL2, RAB25, MAPK14, SRC |
| MAPK
cascade | 8.78E-06 | 8 | PRKCA, ERBB2,
ERBB3, PDCD4, ESR1, MAPK14, PRKAA1, SRC |
|
Positive regulation of protein
phosphorylation | 1.03E-05 | 8 | PRKCA, ERBB2,
ERBB3, ESR1, BCL2, MAPK14, PRKAA1, SRC |
| Signal
transduction by protein phosphorylation | 1.03E-05 | 8 | PRKCA, ERBB2,
ERBB3, PDCD4, ESR1, MAPK14, PRKAA1, SRC |
|
Regulation of canonical Wnt
signaling pathway | 3.05E-05 | 5 | FOXO3, CDH1, YAP1,
MAPK14, SRC |
| Pathway |
|
|
|
| EGFR
tyrosine kinase inhibitor resistance | 1.74E-07 | 6 | FOXO3, PRKCA,
ERBB2, ERBB3, BCL2, SRC |
|
Proteoglycans in cancer | 5.39E-07 | 7 | PRKCA, ERBB2,
ERBB3, PDCD4, ESR1, MAPK14, SRC |
| a6b1
and a6b4 Integrin signaling | 9.50E-06 | 4 | CDH1, PRKCA, ERBB2,
ERBB3 |
|
Endocrine resistance | 1.17E-05 | 5 | ERBB2, ESR1, BCL2,
MAPK14, SRC |
|
Signaling by ERBB2 | 4.47E-05 | 4 | PRKCA, ERBB2,
ERBB3, SRC |
| Focal
adhesion | 2.06E-04 | 5 | BIRC2, PRKCA,
ERBB2, BCL2, SRC |
| ErbB
signaling pathway | 2.06E-04 | 4 | PRKCA, ERBB2,
ERBB3, SRC |
| NGF
signalling via TRKA from the plasma membrane | 2.42E-04 | 6 | FOXO3, PRKCA,
ERBB2, ERBB3, MAPK14, SRC |
| FAS
(CD95) signaling pathway | 4.38E-04 | 3 | BIRC2, MAPK14,
SRC |
|
Signalling by NGF | 4.82E-04 | 6 | FOXO3, PRKCA,
ERBB2, ERBB3, MAPK14, SRC |
|
PI3K/AKT activation | 4.82E-04 | 4 | FOXO3, ERBB2,
ERBB3, SRC |
|
Cadherin signaling
pathway | 6.77E-04 | 4 | CDH1, ERBB2, ERBB3,
SRC |
|
Pathways in cancer | 1.04E-03 | 5 | CDH1, BIRC2, PRKCA,
ERBB2, BCL2 |
|
Signaling by SCF-KIT | 6.93E-04 | 5 | FOXO3, PRKCA,
ERBB2, ERBB3, SRC |
 | Table X.Biological process and pathway
analysis of genes whose products are positively correlated with
RRM2 expression. |
Table X.
Biological process and pathway
analysis of genes whose products are positively correlated with
RRM2 expression.
| Factor | P-value | Number of
genes | Gene list |
|---|
| Biological
process |
|
|
|
| Cell
cycle phase transition | 1.99E-08 | 9 | CHEK2, FOXM1,
CDKN1A, RB1, RPS6KB1, CHEK1, PCNA, EIF4EBP1, CCNB1 |
| Cell
cycle G1/S phase transition | 9.08E-08 | 7 | CHEK2, CDKN1A, RB1,
RPS6KB1, PCNA, EIF4EBP1, CCNB1 |
|
Negative regulation of cell
cycle phase transition | 1.97E-07 | 6 | CHEK2, CDKN1A, RB1,
CHEK1, PCNA, CCNB1 |
| Cell
cycle | 1.97E-07 | 11 | CHEK2, FOXM1,
CDKN1A, RB1, MSH6, RPS6KA1, RPS6KB1, CHEK1, PCNA, EIF4EBP1,
CCNB1 |
|
Positive regulation of cell
cycle | 2.96E-07 | 7 | CHEK2, CDKN1A, RB1,
RPS6KB1, PCNA, EIF4EBP1, CCNB1 |
| Cell
cycle process | 3.83E-07 | 10 | CHEK2, FOXM1,
CDKN1A, RB1, MSH6, RPS6KB1, CHEK1, PCNA, EIF4EBP1, CCNB1 |
|
Regulation of cell cycle | 5.63E-07 | 9 | CHEK2, FOXM1,
CDKN1A, RB1, RPS6KB1, CHEK1, PCNA, EIF4EBP1, CCNB1 |
|
Negative regulation of cell
cycle G1/S phase transition | 5.94E-07 | 5 | CHEK2, CDKN1A, RB1,
PCNA, CCNB1 |
|
Regulation of cell cycle
arrest | 7.05E-07 | 5 | CHEK2, FOXM1,
CDKN1A, PCNA, CCNB1 |
| Signal
transduction by p53 class mediator | 1.11E-06 | 6 | CHEK2, FOXM1,
CDKN1A, CHEK1, PCNA, CCNB1 |
| Signal
transduction in response to DNA damage | 1.11E-06 | 5 | CHEK2, FOXM1,
CDKN1A, PCNA, CCNB1 |
| DNA
integrity checkpoint | 3.58E-06 | 5 | CHEK2, CDKN1A,
CHEK1, PCNA, CCNB1 |
|
Regulation of cell
proliferation | 1.36E-04 | 8 | FOXM1, CDKN1A, RB1,
RAF1, RPS6KB1, CHEK1, CCNB1, TFRC |
|
Regulation of cell growth | 2.26E-04 | 5 | FOXM1, CDKN1A, RB1,
RPS6KA1, TFRC |
| Pathway |
|
|
|
| Cell
cycle | 6.87E-07 | 6 | CHEK2, CDKN1A, RB1,
CHEK1, PCNA, CCNB1 |
| Insulin
signalling | 9.14E-06 | 4 | RAF1, RPS6KA1,
RPS6KB1, EIF4EBP1 |
| FOXM1
transcription factor network | 9.14E-06 | 4 | CHEK2, FOXM1, RB1,
CCNB1 |
| mTOR
signaling pathway | 5.69E-05 | 4 | RAF1, RPS6KA1,
RPS6KB1, EIF4EBP1 |
| p53
signaling pathway | 6.70E-05 | 4 | CHEK2, CDKN1A,
CHEK1, CCNB1 |
| ATM
signaling pathway | 9.01E-05 | 3 | CHEK2, CDKN1A,
CHEK1 |
| ErbB
signaling pathway | 1.04E-04 | 4 | CDKN1A, RAF1,
RPS6KB1, EIF4EBP1 |
| HIF-1
signaling pathway | 1.53E-04 | 4 | CDKN1A, RPS6KB1,
EIF4EBP1, TFRC |
| E2F
mediated regulation of DNA replication | 2.21E-04 | 3 | RB1, PCNA,
CCNB1 |
| G2/M
DNA damage checkpoint | 2.21E-04 | 2 | CHEK1, CCNB1 |
| G1/S
Transition | 2.34E-04 | 4 | CDKN1A, RB1, PCNA,
CCNB1 |
| EGFR
tyrosine kinase inhibitor resistance | 1.20E-03 | 3 | RAF1, RPS6KB1,
EIF4EBP1 |
| RB
tumor suppressor/checkpoint signaling in response to DNA
damage | 1.25E-03 | 2 | RB1, CHEK1 |
|
MAPKinase signaling
pathway | 1.49E-03 | 3 | RAF1, RPS6KA1,
RPS6KB1 |
Discussion
There is only limited data describing cyclin F and
its possible role in human cancer. D'Angiolella et al
characterized the functional axis which is responsible for DNA
repair following genotoxic stress (5). It is possible that interaction between
cyclin F and RRM2 is significantly responsible for treatment
response, thus detailed recognition of its nature may be useful for
cancer clinical outcome prediction. Nuclear accumulation of RRM2,
which allows efficient DNA repair, is preceded by downregulation of
cyclin F. As it has been shown by D'Angiolella et al the
insertion of wild-type cyclin F into hTERT RPE-1 cells prevents
transposition of RRM2 from the cytoplasm to the nucleus (5). It has also been shown that
overexpression of RRM2 may affect the proliferation of melanoma
cells, their response to treatment in vivo, and is
associated with worse overall survival in melanoma patients bearing
mutations in the BRAF oncogene (8,11,12).
Based on these data, we hypothesized that low expression of cyclin
F in melanoma patients can be related to a poorer prognosis. This
hypothesis was strengthened by the fact that the relationship
between low cyclin F expression and poorer prognosis was
demonstrated by Fu et al in patients with hepatocellular
carcinoma. They showed that downregulation of cyclin F in
hepatocellular carcinoma tissue samples was related to larger tumor
size and poor tumor differentiation (13). Interestingly our analysis revealed
that high expression of cyclin F mRNA is associated with poorer
prognosis in skin cutaneous melanoma. Much as the result differs
from what was expected, it is not surprising as overexpression of
cyclin proteins is more common in cancer rather than their
downregulation. Sun et al showed that overexpression of
cyclin B1 is associated with poorer prognosis and reduced overall
survival in breast cancer (14). Li
et al revealed an association between high expression of
cyclin B1 and claudin-1 with worse outcome in patients with
hypopharyngeal squamous cell carcinoma (15). On the other hand, high cyclin B1
expression was found to reduce lymph node metastasis and distant
metastasis stage, and was also associated with higher survival
rates in colorectal cancer (16).
High expression of cyclin D1 is a poor prognostic factor in
gastric, oropharyngeal and breast cancer (17–19).
Additionally, the overexpression of cyclin E correlates with worse
outcome in patients with breast cancer, rectal cancer and
gastrointestinal cancer (20–22).
Some evidence has shown that low expression of cyclin F may be
tumorigenic. It has been proposed that the downregulation of cyclin
F promotes centrosomal and mitotic abnormalities associated with
impaired degradation of CP110, an important centriolar protein
(23). Moreover, cyclin F-mediated
degradation of CDC-6 suppresses genome instability and prevents
re-replication, limiting the number of cells with DNA content
greater than 4N (24). Pan et
al showed that different levels of cyclin F, cyclin D and RBL1
between 2D and 3D cultured cells may be associated with
radioresistance of cells in 3-dimensional culture. They noted that
A549 cells cultured in 3D exhibited lower levels of cyclin F and
were less susceptible to G2/M cell cycle arrest after X-ray
irradiation (25). However, the
potential role of cyclin F as a tumor-promoting factor and the
underlying mechanism remain elusive. The Oct4/NIPP1-CCNF/PP1 axis
is responsible for maintenance of retinoblastoma protein 1 (Rb1) in
the hyperphosphorylated state providing stem cell self-renewal and
increased proliferation. Inactivation of Rb1 via CCNF/PP1 is also
associated with enhanced ovarian cancer aggressiveness (26,27).
In our pathway analysis, we observed a decrease in the cell
signaling-related pathway activity and increase in the cell
cycle-related pathways in patients with upregulated levels of
cyclin F. A recent report showed that cyclin F is a bridge between
AKT kinase and cell cycle machinery. Choudhury et al
hypothesized a model where growth signaling initiates a positive
loop where AKT phosphorylates and stabilizes cyclin F in the SCF
complex. This stabilization inhibits degradation of cyclin F via
APC/C (Cdh1) complex and promotes SCF-dependent degradation of
Cdh1. Degradation of Cdh1 is essential for S phase entry and loss
of cyclin F impairs cell cycle progression (28,29).
Activation of the PI3K/AKT pathway is a common event in a variety
of cancer diseases and it is believed to contribute to drug
resistance. Although, we did not observe clear symptoms of PI3K/AKT
activation, our analysis revealed downregulated INPP4B, tumor
suppressor antagonizing PI3K/AKT pathway. Loss of INPP4B was found
to increase AKT activation and drive higher proliferation rate and
metastasis (30). It has been also
reported that a decreased level of INPP4B is releted to higher
proliferative, invasive and metastatic potential of melanocytic
neoplasms (31). In contradiction
to these reports is a study by Chi et al where upregulation
of INPP4B in a melanoma subset was observed. Furthermore, INPP4B
driven proliferation was Akt-independent and was mediated by serum-
and glucocorticoid-regulated kinase 3 (SGK3). Additionally, they
observed no significant differences between primary and metastatic
melanoma suggesting the involvement of INPP4B in developing cancer
from the early stages (32).
In the present study, the upregulation of cyclin F
mRNA was found to coincide with the downregulation of p27 protein,
important cell cycle regulator involved in G1 arrest. Akman et
al found that patients with melanoma are characterized by lower
p27 expression in comparison to patients with benign nevi and
dysplastic nevi (33). Furthermore,
Florenes et al reported that decreased expression of p27 is
associated with increasing Breslow thickness and lower disease-free
survival rates in primary nodular melanoma (34). Additionally, the low expression of
p27 in melanocytic lesions may be responsible for its high
proliferation rate (35). The lack
of proper control in regards to cell cycle events is typical for
cancer cells. As was mentioned in the introduction, the
overexpression of cyclins is very common in various malignancies.
In our analysis, elevated levels of cyclin F mRNA were also
associated with upregulation of cyclin E1 and B1 proteins. Elevated
levels of cyclin E1 were observed in melanoma and enhanced
expression of cyclin E was noted in both primary and metastatic
melanomas. In contrast, its overexpression was not observed in
non-malignant nevi (36). Bales
et al reported that cyclin E is overexpressed in melanoma
and present in the low-molecular form. Noteworthy, transfection of
a primary cutaneous melanoma cell line with low tumorigenic and
metastatic potential with low-molecular cyclin E forms resulted in
the development of angiogenic tumors with prominent perineural
invasion. Additionally, truncated forms of cyclin E triggered a
dramatic increase in a number of metastasis events (37). In turn, cyclin B1 is involved in
proliferation and metastatic potential of melanoma cells (38). Silencing of cyclin B exerts an
antitumor effect on melanoma cells and lung metastases, both in
vitro and in vivo (39).
Kruiswijk et al reported that patients with
elevated levels of cyclin B1, Pin1 and FOXM1 display a worse
outcome and exhibit increased mortality (40). FOXM1 is a pro-proliferative and
pro-survival transcription factor participating in DNA repair.
Moreover, these data are in agreement with our analysis, where a
significant increase in FOXM1 protein in patients with upregulated
cyclin F mRNA was noted. It suggests possible activation of cyclin
F expression by FOXM1, but further research is needed to clarify
this. Moreover, the upregulation of FOXM1 coincides with
downregulation of FOXO3a. The abrogation of FOXO3a function was
found to lead to increased tumor aggressiveness in melanoma and
renal carcinoma (41,42). Another important observation made in
this study is that 4E-BP1 (4E binding protein 1) was
hyperphosphorylated in patients with upregulated cyclin expression.
Phosphorylation of 4E-BP1 results in dissociation from translation
factor eIF4E and allows cap-dependent translation. Phospho-4E-BP1
may also be useful as a marker of mTOR pathway activity and
integrates signals obtained from PI3K/AKT and RAS/RAF/MEK/ERK
pathways (43). Additionally,
concomitant hyperphosphorylation of 4E-BP1 and activation of the
PI3K/AKT pathway results in resistance to mTOR inhibitors.
Moreover, in hypoxic conditions, 4E-BP1 initiates translation of
proteins responsible for angiogenesis (VEGF-A), hypoxia response
(HIF1α) and apoptosis resistance (Bcl-2) in advanced cancer
(44,45). Increased levels of phosphorylated
4E-BP1 are also associated with poor overall survival and
significant difference in post-recurrence survival (46). It is possible that cyclin F is a
part of the specific cellular environment, promoting cell
proliferation and survival.
The ability of cancer cells to efficiently repair
DNA is a significant barrier to successful treatment. RRM2 is a
part of the RNR and has been reported to be partially responsible
for chemoresistance of cancer cells, including melanoma. However,
our analysis did not reveal significant changes in overall survival
or disease-free survival between patients with differential RRM2
mRNA expression. Aird et al showed that high RRM2 expression
is correlated with worse outcome in melanoma patients (8). Silencing of RRM2 inhibited melanoma
growth which suggests the involvement of RRM2 in melanoma
progression. Silencing of RRM2 and treatment with mutant BRAF
inhibitor PLX4720 simultaneously and synergistically inhibited
melanoma growth (11). It is
possible that the negative effect of RRM2 overexpression is limited
to patients bearing BRAFV600E mutation, but we cannot
confirm this using TCGA data due to an insufficient number of
patients with the BRAF mutation in the cohort.
Beyond controlling RRM2 levels, cyclin F is a
limiting factor in histone H2.AX signalization. In the G2 phase
cyclin F mediates degradation of SLBP protein which promotes
synthesis of H2AFX mRNA. Presence of SLBP in the G2 phase increases
H2.AX levels and makes the cell more susceptible to apoptosis under
genotoxic stress. It is another piece of evidence showing how
cyclin F promotes cancer progression (47). Moreover, we observed an alteration
in expression of other DNA-repair related proteins: XRCC1, PARP1,
PCNA, and MSH6. All proteins were upregulated which is a hallmark
of efficient DNA repair systems and a potential obstacle to
successful treatment. However, the prognostic status of XRCC1 is
ambiguous. Its overexpression is associated with less favorable
prognosis in head and neck squamous carcinoma. Decreased levels of
XRCC1 are responsible for acute side-effects after radiotherapy in
breast cancer patients. Loss of XRCC1 confers a more aggressive
phenotype in melanoma (48–50). It suggests an indirect effect of
cyclin F overexpression on the DNA damage repair system.
Additionally, PCNA in patients with upregulated cyclin F is very
significantly increased, what confirms the higher proliferation
potential of cells overexpressing cyclin F. These findings confirm
a study by Wang et al in which treatment of cells with
stimulatory polysaccharides from abalone, significantly increased
the expression of cyclin B1, CDK1 and cyclin F (51).
Another interesting observation was increased
expression of TFRC (transferrin receptor 1) gene in patients with
high expression of cyclin F and RRM2 mRNA. It has been reported
that melanoma cells are able to upregulate transferrin receptor 1
through the hyaluronan/CD44 pathway. It is possible that this
pathway promotes proliferation providing alternative iron supply
for melanoma cells. High expression of TFRC is associated with
unfavorable prognosis in breast and pancreatic cancer (52–54).
This newly discovered relationship between mRNA
expression of CCNF and RRM2 provide and attractive point for
further investigations in the field of dermato-oncology. Our
analysis was performed using independent data obtained from TCGA
and provide many key results that can be used in further
explanation of the precise mechanisms. Moreover, we expect that the
present results will be useful to other researchers and induce
further investigations, essential for better diagnosis, prediction,
therapy response, but also for better selection of patients for
optimal therapy against skin melanoma. A high number of clones
contributes to an exceptional level of intratumor heterogeneity of
melanoma, but also refers to metastases which may originate from
different subclones of the primary tumor. This creates an obstacle
to proper diagnosis and successful treatment (55). Increased research on the topic is
needed for understanding the limitation or failure of contemporary
therapies and the precise mechanism must and will be elucidated by
our team in vitro in the immediate future using melanoma
cancer cell panels. We suggest here to investigate the precise
mechanism indicated in the study using all following cell lines:
SK-MEL-1, A375, G-361, SK-MEL-3, SH-4, SK-MEL-24, RPMI-7951.
However, we hope that the publication of in silico analyses
accelerates the development and inspires other scientific teams to
conduct similar research in the field.
In conclusion, the present study is a first attempt
to elucidate the influence of cyclin F mRNA expression on the
outcome of melanoma patients. High expression of cyclin F mRNA is
associated with worse overall survival. Moreover, in silico
analysis revealed that upregulated cyclin F mRNA expression is
associated with activation of molecular pathways responsible for
melanoma proliferation, metastatic potential and survival. These
findings are a good starting point to address new cyclin F targets
and interactions which drive the increased aggressiveness of the
tumor.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the
National Science Centre, Poland (grant no. 2016/21/B/NZ7/01121 to
AG).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MG and AG designed the study. MG and AK performed
the analyses, interpreted the data and wrote the study. DG and AG
revised manuscript critically for important intellectual content.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Bioethics
Committee of the Nicolaus Copernicus University in Toruń
functioning at Collegium Medicum in Bydgoszcz (KB 554/2016).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niezgoda A, Niezgoda P and Czajkowski R:
Novel approaches to treatment of advanced melanoma: A review on
targeted therapy and immunotherapy. BioMed Res Int.
2015:8513872015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DB and Sosman JA: Therapeutic
advances and treatment options in metastatic melanoma. JAMA Oncol.
1:380–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maverakis E, Cornelius LA, Bowen GM, Phan
T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, et
al: Metastatic melanoma - a review of current and future treatment
options. Acta Derm Venereol. 95:516–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galper J, Rayner SL, Hogan AL, Fifita JA,
Lee A, Chung RS, Blair IP and Yang S: Cyclin F: A component of an
E3 ubiquitin ligase complex with roles in neurodegeneration and
cancer. Int J Biochem Cell Biol. 89:216–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Angiolella V, Donato V, Forrester FM,
Jeong YT, Pellacani C, Kudo Y, Saraf A, Florens L, Washburn MP and
Pagano M: Cyclin F-mediated degradation of ribonucleotide reductase
M2 controls genome integrity and DNA repair. Cell. 149:1023–1034.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grolmusz VK, Karászi K, Micsik T, Tóth EA,
Mészáros K, Karvaly G, Barna G, Szabó PM, Baghy K, Matkó J, et al:
Cell cycle dependent RRM2 may serve as proliferation marker and
pharmaceutical target in adrenocortical cancer. Am J Cancer Res.
6:2041–2053. 2016.PubMed/NCBI
|
|
7
|
Han P, Lin Z-R, Xu L-H, Zhong Q, Zhu XF,
Liang FY, Cai Q, Huang XM and Zeng MS: Ribonucleotide reductase M2
subunit expression and prognostic value in nasopharyngeal
carcinoma. Mol Med Rep. 12:401–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aird KM, Zhang G, Li H, Tu Z, Bitler BG,
Garipov A, Wu H, Wei Z, Wagner SN, Herlyn M, et al: Suppression of
nucleotide metabolism underlies the establishment and maintenance
of oncogene-induced senescence. Cell Reports. 3:1252–1265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mah V, Alavi M, Márquez-Garbán DC, Maresh
EL, Kim SR, Horvath S, Bagryanova L, Huerta-Yepez S, Chia D,
Pietras R, et al: Ribonucleotide reductase subunit M2 predicts
survival in subgroups of patients with non-small cell lung
carcinoma: Effects of gender and smoking status. PLoS One.
10:e01276002015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fatkhutdinov N, Sproesser K, Krepler C,
Liu Q, Brafford PA, Herlyn M, Aird KM and Zhang R: Targeting RRM2
and mutant BRAF is a novel combinatorial strategy for melanoma. Mol
Cancer Res. 14:767–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zuckerman JE, Hsueh T, Koya RC, Davis ME
and Ribas A: siRNA knockdown of ribonucleotide reductase inhibits
melanoma cell line proliferation alone or synergistically with
temozolomide. J Invest Dermatol. 131:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu J, Qiu H, Cai M, Pan Y, Cao Y, Liu L,
Yun J and Zhang CZ: Low cyclin F expression in hepatocellular
carcinoma associates with poor differentiation and unfavorable
prognosis. Cancer Sci. 104:508–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Zhangyuan G, Shi L, Wang Y, Sun B
and Ding Q: Prognostic and clinicopathological significance of
cyclin B expression in patients with breast cancer: A
meta-analysis. Medicine (Baltimore). 96:e68602017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Dong Q, Li L, Zhang Z, Cai X and Pan
X: Prognostic significance of claudin-1 and cyclin B1 protein
expression in patients with hypopharyngeal squamous cell carcinoma.
Oncol Lett. 11:2995–3002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Y, Liang X, Jiang W, Li J, Xu J and
Cai X: Cyclin b1 suppresses colorectal cancer invasion and
metastasis by regulating e-cadherin. PLoS One. 10:e01268752015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan YS, Hsu HP, Lai MD, Hung YH, Wang CY,
Yen MC and Chen YL: Cyclin D1 overexpression correlates with poor
tumor differentiation and prognosis in gastric cancer. Oncol Lett.
14:4517–4526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin RJ, Lubpairee T, Liu KY, Anderson DW,
Durham S and Poh CF: Cyclin D1 overexpression is associated with
poor prognosis in oropharyngeal cancer. J Otolaryngol Head Neck
Surg. 42:232013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahlin C, Lundgren C, Embretsén-Varro E,
Jirström K, Blomqvist C and Fjällskog ML: High expression of cyclin
D1 is associated to high proliferation rate and increased risk of
mortality in women with ER-positive but not in ER-negative breast
cancers. Breast Cancer Res Treat. 164:667–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luhtala S, Staff S, Tanner M and Isola J:
Cyclin E amplification, over-expression, and relapse-free survival
in HER-2-positive primary breast cancer. Tumour Biol. 37:9813–9823.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou YJ, Xie YT, Gu J, Yan L, Guan GX and
Liu X: Overexpression of cyclin E isoforms correlates with poor
prognosis in rectal cancer. Eur J Surg Oncol. 37:1078–1084. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang L, Ren F, Tang R, Feng Z and Chen G:
Prognostic value of expression of cyclin E in gastrointestinal
cancer: A systematic review and meta-analysis. Technol Cancer Res
Treat. 15:12–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Angiolella V, Donato V, Vijayakumar S,
Saraf A, Florens L, Washburn MP, Dynlacht B and Pagano M:
SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity
through CP110 degradation. Nature. 466:138–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walter D, Hoffmann S, Komseli E-S,
Rappsilber J, Gorgoulis V and Sørensen CS: SCF(Cyclin F)-dependent
degradation of CDC6 suppresses DNA re-replication. Nat Commun.
7:105302016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan D, Chen Y, Du Y, Ren Z, Li X and Hu B:
Methylation of promoter of RBL1 enhances the radioresistance of
three dimensional cultured carcinoma cells. Oncotarget.
8:4422–4435. 2017.PubMed/NCBI
|
|
26
|
Schoeftner S, Scarola M, Comisso E,
Schneider C and Benetti R: An Oct4-pRb axis, controlled by MiR-335,
integrates stem cell self-renewal and cell cycle control. Stem
Cells. 31:717–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Comisso E, Scarola M, Rosso M, Piazza S,
Marzinotto S, Ciani Y, Orsaria M, Mariuzzi L, Schneider C,
Schoeftner S, et al: OCT4 controls mitotic stability and
inactivates the RB tumor suppressor pathway to enhance ovarian
cancer aggressiveness. Oncogene. 36:4253–4266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choudhury R, Bonacci T, Wang X, Truong A,
Arceci A, Zhang Y, Mills CA, Kernan JL, Liu P and Emanuele MJ: The
E3 ubiquitin ligase SCF(cyclin F) transmits AKT signaling to the
cell-cycle machinery. Cell Reports. 20:3212–3222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choudhury R, Bonacci T, Arceci A, Lahiri
D, Mills CA, Kernan JL, Branigan TB, DeCaprio JA, Burke DJ and
Emanuele MJ: APC/C and SCF(cyclin F) constitute a reciprocal
feedback circuit controlling S-phase entry. Cell Reports.
16:3359–3372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Chew C, Lunardi A, Gulluni F, Ruan DT,
Chen M, Salmena L, Nishino M, Papa A, Ng C, Fung J, et al: In vivo
role of INPP4B in tumor and metastasis suppression through
regulation of PI3K-AKT signaling at endosomes. Cancer Discov.
5:740–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perez-Lorenzo R, Gill KZ, Shen C-H, Zhao
FX, Zheng B, Schulze HJ, Silvers DN, Brunner G and Horst BA: A
tumor suppressor function for the lipid phosphatase INPP4B in
melanocytic neoplasms. J Invest Dermatol. 134:1359–1368. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi MN, Guo ST, Wilmott JS, Guo XY, Yan
XG, Wang CY, Liu XY, Jin L, Tseng HY, Liu T, et al: INPP4B is
upregulated and functions as an oncogenic driver through SGK3 in a
subset of melanomas. Oncotarget. 6:39891–39907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akman A, Ciftcioglu MA, Ozbey C and Alpsoy
E: Expression of cell cycle inhibitor p27Kip1 in nevi and
melanomas. Indian J Dermatol Venereol Leprol. 74:5512008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flørenes VA, Maelandsmo GM, Kerbel RS,
Slingerland JM, Nesland JM and Holm R: Protein expression of the
cell-cycle inhibitor p27Kip1 in malignant melanoma: Inverse
correlation with disease-free survival. Am J Pathol. 153:305–312.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ivan D, Diwan AH, Esteva FJ and Prieto VG:
Expression of cell cycle inhibitor p27Kip1 and its inactivator Jab1
in melanocytic lesions. Mod Pathol. 17:811–818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Georgieva J, Sinha P and Schadendorf D:
Expression of cyclins and cyclin dependent kinases in human benign
and malignant melanocytic lesions. J Clin Pathol. 54:229–235. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bales E, Mills L, Milam N, McGahren-Murray
M, Bandyopadhyay D, Chen D, Reed JA, Timchenko N, van den Oord JJ,
Bar-Eli M, et al: The low molecular weight cyclin E isoforms
augment angiogenesis and metastasis of human melanoma cells in
vivo. Cancer Res. 65:692–697. 2005.PubMed/NCBI
|
|
38
|
Lu M, Breyssens H, Salter V, Zhong S, Hu
Y, Baer C, Ratnayaka I, Sullivan A, Brown NR, Endicott J, et al:
Restoring p53 function in human melanoma cells by inhibiting MDM2
and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell.
23:618–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kedinger V, Meulle A, Zounib O, Bonnet ME,
Gossart JB, Benoit E, Messmer M, Shankaranarayanan P, Behr JP,
Erbacher P, et al: Sticky siRNAs targeting survivin and cyclin B1
exert an antitumoral effect on melanoma subcutaneous xenografts and
lung metastases. BMC Cancer. 13:3382013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kruiswijk F, Hasenfuss SC, Sivapatham R,
Baar MP, Putavet D, Naipal KA, van den Broek NJ, Kruit W, van der
Spek PJ, van Gent DC, et al: Targeted inhibition of metastatic
melanoma through interference with Pin1-FOXM1 signaling. Oncogene.
35:2166–2177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ni D, Ma X, Li H-Z, Gao Y, Li XT, Zhang Y,
Ai Q, Zhang P, Song EL, Huang QB, et al: Downregulation of FOXO3a
promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin
Cancer Res. 20:1779–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zanella F, Renner O, García B, Callejas S,
Dopazo A, Peregrina S, Carnero A and Link W: Human TRIB2 is a
repressor of FOXO that contributes to the malignant phenotype of
melanoma cells. Oncogene. 29:2973–2982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ayuso MI, Hernández-Jiménez M, Martín ME,
Salinas M and Alcázar A: New hierarchical phosphorylation pathway
of the translational repressor eIF4E-binding protein 1 (4E-BP1) in
ischemia-reperfusion stress. J Biol Chem. 285:34355–34363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sherrill KW, Byrd MP, Van Eden ME and
Lloyd RE: BCL-2 translation is mediated via internal ribosome entry
during cell stress. J Biol Chem. 279:29066–29074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qin X, Jiang B and Zhang Y: 4E-BP1, a
multifactor regulated multifunctional protein. Cell Cycle.
15:781–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
O'Reilly KE, Warycha M, Davies MA, Rodrik
V, Zhou XK, Yee H, Polsky D, Pavlick AC, Rosen N, Bhardwaj N, et
al: Phosphorylated 4E-BP1 is associated with poor survival in
melanoma. Clin Cancer Res. 15:2872–2878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dankert JF, Rona G, Clijsters L, Geter P,
Skaar JR, Bermudez-Hernandez K, Sassani E, Fenyö D, Ueberheide B,
Schneider R, et al: Cyclin F-mediated degradation of SLBP limits
H2A.X accumulation and apoptosis upon genotoxic stress in G2. Mol
Cell. 64:507–519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ang M-K, Patel MR, Yin X-Y, Sundaram S,
Fritchie K, Zhao N, Liu Y, Freemerman AJ, Wilkerson MD, Walter V,
et al: High XRCC1 protein expression is associated with poorer
survival in patients with head and neck squamous cell carcinoma.
Clin Cancer Res. 17:6542–6552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Batar B, Guven G, Eroz S, Bese NS and
Guven M: Decreased DNA repair gene XRCC1 expression is associated
with radiotherapy-induced acute side effects in breast cancer
patients. Gene. 582:33–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhandaru M, Martinka M, Li G and Rotte A:
Loss of XRCC1 confers a metastatic phenotype to melanoma cells and
is associated with poor survival in patients with melanoma. Pigment
Cell Melanoma Res. 27:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang YM, Wu FJ, Du L, Li GY, Takahashi K,
Xue Y and Xue CH: Effects of polysaccharides from abalone (Haliotis
discus hannai Ino) on HepG2 cell proliferation. Int J Biol
Macromol. 66:354–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miller LD, Coffman LG, Chou JW, Black MA,
Bergh J, D'Agostino R Jr, Torti SV and Torti FM: An iron regulatory
gene signature predicts outcome in breast cancer. Cancer Res.
71:6728–6737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Laube F and Glanz D: Modulation of
Melanotransferrin and Transferrin Receptor 1 (TFRC)- and CD44-based
Signaling for TFRC Up-regulation in Human Melanoma Cells.
Anticancer Res. 37:3001–3007. 2017.PubMed/NCBI
|
|
54
|
Ryschich E, Huszty G, Knaebel HP, Hartel
M, Büchler MW and Schmidt J: Transferrin receptor is a marker of
malignant phenotype in human pancreatic cancer and in
neuroendocrine carcinoma of the pancreas. Eur J Cancer.
40:1418–1422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grzywa TM, Paskal W and Włodarski PK:
Intratumor and Intertumor Heterogeneity in Melanoma. Transl Oncol.
10:956–975. 2017. View Article : Google Scholar : PubMed/NCBI
|