Introduction
Gastric cancer is the third leading cause of
mortality throughout the world especially in developing countries
(1,2). Among them, colon cancer is one of the
most common cancers resulting in cancer-related deaths in China
(3–5). Over the past decade, surgery and
chemotherapy have been chosen as the main treatments for colon
cancer (6,7). Although major advances have been
developed in the treatment of colon cancer, the therapeutic
efficacy of anticancer agents remains very poor (8). Thus, it is urgent to develop novel,
safe and effective therapeutic agents for colon cancer.
miRNAs are a class of 22–25-nucleotide-long
non-coding RNA molecules which can exert their functions by
inhibiting mRNA translation or promoting mRNA degradation.
Increasing evidence has revealed that miRNAs have various
biological functions such as regulating cell viability, death and
migration in cultured cells (9,10).
Notably, miRNAs have been reported to be involved in cancer
development through their tumor-suppressing or -activating
functions (11–13). Recently, miR-218 was reported to
play a tumor-suppressive role in various types of human cancers,
such as lung, bladder and gastric cancer, hepatocellular carcinoma,
oral cancer and renal cell carcinoma (14–20),
indicating that miR-218 may be a potential therapeutic target for
the treatment of cancers.
c-FLIP, a death effector domain (DED)-containing
anti-apoptotic protein, can suppress cell apoptosis induced by
tumor necrosis factor-α (TNF-α), Fas-L, TNF-related
apoptosis-inducing ligand (TRAIL), and chemotherapy agents in
cancer cells by regulating the activation of caspase-8 and
caspase-10. New findings have provided evidence that c-FLIP can be
regarded as a critical target for therapeutic intervention due to
its inhibition in transcription and post-transcription (21,22).
Increased expression of c-FLIP has been found in cell lines from
various types of cancers, including colorectal (21,22),
head and neck (23), prostate
(24), cervical (25), breast (26), lung (27) and hepatocellular cancers (28), and c-FLIP has been demonstrated to
be an important indicator in these types of cancers. In our study,
we evaluated the roles and underlying mechanisms of miR-218 in
human colon cancer cells. It was demonstrated that miR-218 exerted
its anti-apoptotic functions in SW1417 colon cancer cells by
downregulating c-FLIP and may become a biomarker and therapeutic
target in colon cancer treatment.
Materials and methods
Patients and tissue sample
collection
A total of 20 patients diagnosed with colon cancer
were involved in this study. Tissue samples were obtained from the
tumoral area and adjacent area of patients who had not undergone
radiotherapy or chemotherapy prior to surgery at the Cancer
Hospital of China Medical University. All the patients that
participated in this study provided written informed consent. The
protocol of this study was approved by the local Ethics Committee
(Cancer Hospital of China Medical University, Liaoning Cancer
Hospital and Institute, Shenyang, China. Permit no. 122/17).
Cell culture
Human colon cancer cell line SW1417 was purchased
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
low-glucose Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL;
Thermo Fisher Scientific, Inc., Grand Island, NY, USA) supplemented
with 10% (v/v) fetal bovine serum (FBS), 100 IU/ml penicillin, and
10 mg/ml streptomycin. Cultures were incubated in 5% CO2
at 37°C.
Analysis of cell viability
Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck
KGaA; St. Louis, MO, USA) was used to determine cell proliferation.
Briefly, SW1417 cells at a density of 1.0×105 cells/ml
were seeded in 96-well plates in DMEM containing 10% FBS for 24 h.
Then, the cells were cultured in a serum-free medium. Following
incubation for 24 h, the cells were incubated with miR-218-mimic
control, miR-218 mimics, cFLIP-siRNA control, cFLIP siRNA and
miR-218 mimics plus cFLIP siRNA in fresh DMEM using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA). At 24 and 48 h, cell viability was evaluated according to a
previous study (7).
Analysis of cell apoptosis
SW1417 cells at a concentration of
1.0×105 cells/ml were seeded onto a 96-well plate and
treated with miR-218 mimic control, miR-218 mimics, cFLIP-siRNA
control, cFLIP siRNA and miR-218 mimics plus cFLIP siRNA for 24 or
48 h, and the cell apoptotic rate was assessed using TUNEL
Apoptosis Detection kit (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturer's instructions (27). The cell apoptotic rate was assayed
using a FACScan flow cytometric apparatus (BD Biosciences, San
Jose, CA, USA) (1).
Western blot analysis
Total protein was extracted and the protein
expression levels of caspase-8, c-FLIP and GAPDH were performed
separately using western blot analysis according to a previous
study (29). Antibodies including
caspase-8 (1:2,500 dilution; cat. no. MAB704), c-FLIP (1:2,500
dilution; cat. no. MAB8430) and GAPDH (1:5,000 dilution; cat. no.
MAB5718) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The protein expression levels were obtained using
the chemiluminescence reader, ImageQuant™ LAS 4000 (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and the band density was
analyzed using ImageJ software (National Institutes of Health,
Bethesda, MD, USA) (1).
Gene expression analysis
RNA preparation and quantitative reverse
transcription polymerase chain reaction (RT-PCR) was used to detect
the expression levels of c-FLIP, caspase-8, miR-218 and GAPDH in
SW1417 cells at indicated time-points. Following the manufacturer's
instructions, total cellular RNA was isolated from SW1417 cells
cultured in 6-well plates using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). GAPDH expression was used as an internal
control. All primer sequences used in this study were synthesized
at BioSune Biotechnology Co., Ltd. (Shanghai, China), and the
sequences of the primers were as follows: c-FLIP forward,
5′-TAACATTTCAGCCGGTGGGT-3′ and reverse, 5′-ATCCTTTCCAGTGGGGGAGT-3′;
caspase-8 forward, 5′-CTGGTCTGAAGGCTGGTTGT-3′ and reverse,
5′-CAGGCTCAGGAACTTGAGGG-3′; GAPDH forward,
5′-AACGGATTTGGTCGTATTGGG-3′ and reverse,
5′-CCTGGAAGATGGTGATGGGAT-3′; miR-218 forward,
5′-GCGGCTTTGTGCTTGATCTAA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′.
Transfection
miR-218 mimics and their non-specific control miRNAs
(miR-218-NC, miR-218-NC-In), c-FLIP siRNA (AAGGAACAGCTTGGCGCTCAA)
and c-FLIP-control siRNA (AATTCTCCGAACGTGTCACGT) were all purchased
from RiboBio (Guangzhou, China). A total of 3 µg of miR-218-mimics,
non-specific control microRNAs, c-FLIP-specific siRNA or
non-targeting siRNA was transfected into SW1417 cells using
HiPerFect transfection reagent (Qiagen, Inc., Valencia, CA, USA)
according to the manufacturer's protocols. After SW1417 cells were
cultured in medium for 48 h, RT-PCR and western blot assays were
used to determine the efficiency of miR-218 mimics and c-FLIP
siRNA, respectively.
Immunohistochemistry
Immunohistochemical analysis of c-FLIP and
capspase-8 was performed using Image Analysis System (Leica
Microsystems, Wetzlar, Germany) according to the previous study
(2). Primary monoclonal antibodies
of c-FLIP (1:1,000 dilution; cat. no. MAB8430) and capspase-8
(1:1,000 dilution; cat. no. MAB704) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Biotinylated goat
anti-rabbit antibody (dilution, 1:1,000) was provided by Vector
Laboratories, Inc. (Burlingame, CA, USA). Positive immunoreactivity
was semi-quantitatively scored as 0 for none to trace, 1+ for
<10%, 2+ for 10–50%, and 3+ for >50% tumor cells showing
positive expression.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). One-way analysis of variance (ANOVA) with Duncan's new
multiple range test (MRT) in SPSS 15.0 software was used to compare
the statistical differences between the treatment and control
groups. P<0.05 indicated a statistically significant
difference.
Results
Increased c-FLIP expression and
decreased miR-218 and caspase-8 expression is revealed in human
colon cancer tissues
In human colon cancer tissues and normal colon
tissues, the expression of c-FLIP, caspase-8 and miR-218 was
assessed and compared. The results revealed that compared with
normal colon tissues, miR-218 expression as determined by RT-PCR
was significantly reduced in human colon cancer tissues (Fig. 1A). As revealed in Fig. 1B, upregulation of c-FLIP expression
was observed, whereas downregulation of caspase-8 was found in
human colon cancer tissues in comparison with normal colon
tissues.
miR-218 overexpression suppresses the
proliferation while it promotes the apoptosis of SW1417 cells
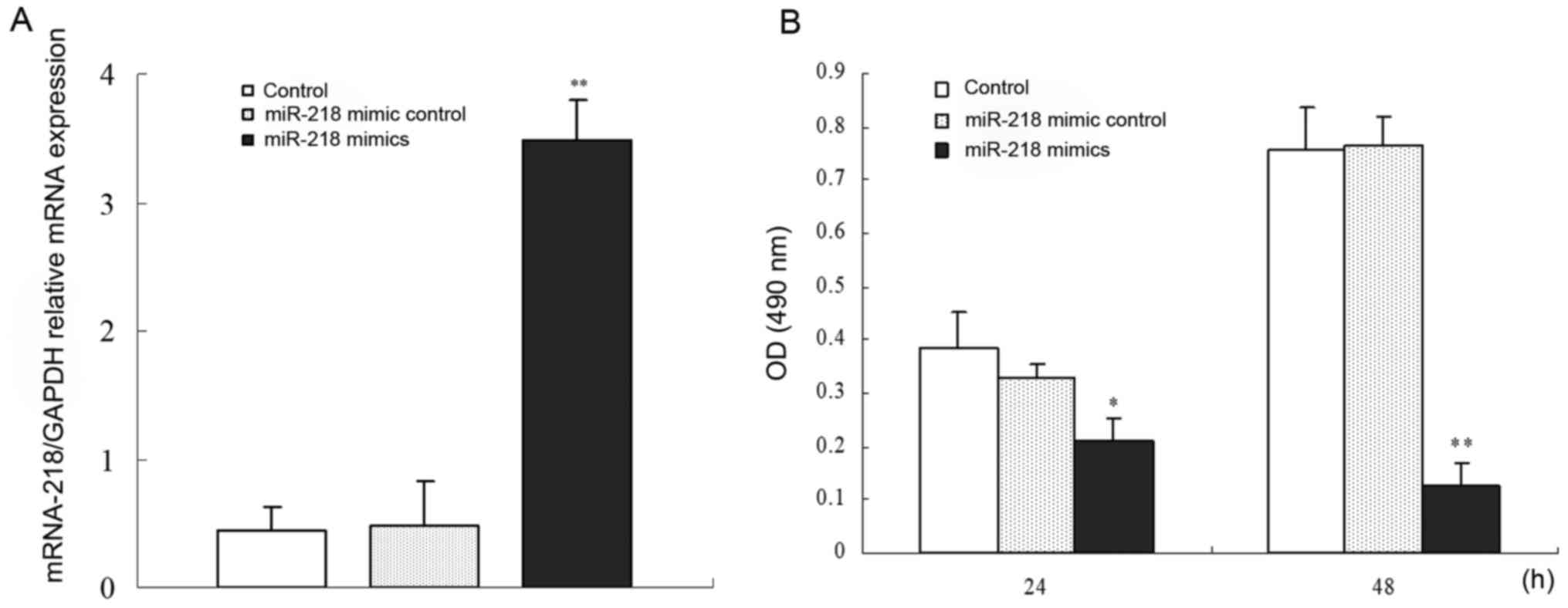
Firstly, miR-218 mimics were stably transfected into
SW1417 cells to increase the expression level of miR-218. A high
expression level of miR-218 was determined by RT-PCR, indicating
that miR-218 was successfully introduced into SW1417 cells
(Fig. 2A). Next, we ascertained the
effects of the transfected miR-218 mimics on the proliferation and
apoptosis of SW1417 cells. The results revealed that overexpression
of miR-218 inhibited the proliferation of SW1417 cells compared
with the mock-transfected cells, and the effects were
time-dependent (P<0.05) (Fig.
2B). Furthermore, a higher apoptosis rate was found in the
miR-218-mimic treated SW1417 cells when compared to cells treated
with negative control mimic (P<0.05) (Fig. 3). Therefore, miR-218 is an important
molecule in the promotion of the apoptosis of human colon cancer
cells.
miR-218 overexpression inhibits the
expression level of c-FLIP in SW1417 cells
To determine the molecular mechanism in apoptosis
signaling involved in the inhibition of cell apoptosis induced by
miR-218, we further assessed the expression of c-FLIP and caspase-8
by western blotting and RT-PCR. In vector-transfected SW1417 cells,
c-FLIP mRNA expression was detectable and significantly decreased
with miR-218 stimulation. In contrast, mRNA expression of caspase-8
was evidently elevated in miR-218-overexpressing SW1417 cells
(P<0.05) (Fig. 4A). Accordingly,
compared to the control, the protein expression of c-FLIP was
decreased while caspase-8 was increased by miR-218 (Fig. 4B). Thus, miR-218 overexpression
inhibited the expression level of c-FLIP and promoted the
expression level of caspase-8 in human colon cancer cells.
miR-218-triggered cell apoptosis in
SW1417 cells is c-FLIP-dependent
The molecular mechanism of miR-218 on cell apoptosis
in human colon cancer cells was further investigated.
c-FLIP-targeted siRNA was used to transfect SW1417 cells and
western blot analyses indicated that c-FLIP-targeted siRNA
efficiently inhibited c-FLIP expression (Fig. 5A). Notably, cell viability assays
revealed that transfection with miR-218 mimics or c-FLIP-targeted
siRNA decreased the number of SW1417 cells, however the effect of
miR-218 mimics was more significant (P<0.05). Moreover, there
was no difference in cell viability between thr c-FLIP-targeted
siRNA group and c-FLIP-targeted siRNA+miR-218 mimic group
(P>0.05) (Fig. 5B). Furthermore,
flow cytometric experiments indicated that miR-218 overexpression
resulted in a marked increase in cell apoptosis, and c-FLIP
knockdown also led to an upregulation of the apoptotic rate in
SW1417 cells. A similar effect was observed in c-FLIP-knockdown
SW1417 cells and c-FLIP knockdown plus miR-218-mimic SW1417 cells
(Fig. 5C). These results revealed
that miR-218 induced the onset of apoptosis through the suppression
of c-FLIP in human colon cancer cells.
Discussion
Recently, miR-218 has been reported to be involved
in the proliferation, apoptosis and migration of different types of
tumor cells (9,10). The present study demonstrated that
the miR-218 expression level was significantly downregulated in
colon cancer tissues. Additionally, the results revealed that
miR-218 may play a role in the inhibition of colon cancer cell
apoptosis through downregulation of c-FLIP expression.
As demonstrated in a previous research, changes in
miR-218 expression were associated with cancer development. For
instance, compared to normal tissues without cancer, the expression
levels of miR-218 were significantly inhibited in lung cancer
tissues (15). In cultured gastric
cancer cell lines SGC7901 and BGC823, it was revealed that miR-218
mRNA and protein expression were significantly decreased compared
to those in normal gastric epithelial cell line GES-1 (30). Furthermore, compared with gastric
cancer cell lines MGC80-3 and HGC-27, decreased miR-218 expression
levels were also found in the more aggressive gastric cancer cell
line NCI-87 (17). Notably, the
present study revealed a significant decrease in miR-218 expression
in human colon cancer tissues and SW1417 cells.
miR-218 is also regarded as a tumor suppressor due
to its function in the inhibition of invasion and growth of
nasopharyngeal (31), oral
(19), lung (15,32),
and bladder (33) cancer cells. By
regulating the expression of Ang-2 in gastric cancer cells NCI-87
and HGC-27, miR-218 overexpression suppressed cell proliferation
and angiogenesis (17). An MTT
assay revealed that miR-218 overexpression markedly suppressed the
proliferation of gastric cancer cells and wound scratch assays
indicated that miR-218 inhibited cell migration and EMT by
targeting WASF3 (30). Another
study found that miR-218 could promote cell apoptosis and inhibit
cell growth in colorectal cancer cells (34). Notably, Tie et al provided
the evidence that upregulation of miR-218 could inhibit tumor cell
invasion and proliferation in gastric cancer cells by altering
miR-218-targeted genes (18). In
LoVo colon cancer cells, miR-218 overexpression suppressed the
invasion, proliferation migration of cells and the signaling
pathways including PI3K/Akt/mTOR and MMP9 were involved in these
functions (35). Furthermore,
miR-218 played an important role in colon cancer development
through inhibition of cell proliferation and promotion of cell
apoptosis by targeting BIRC5 (36),
BMI-1 (37) and MACC1 (38). In the present study, we also
revealed that miR-218 overexpression inhibited cell viability and
promoted cell apoptosis in human SW1417 colon cancer cells.
FU is a chemotherapy drug commonly used for the
treatment of colon cancer by targeting rpL3 to downregulate p53
(39). miR-218 enhanced
5-FU-induced apoptosis while the suppression of miR-218 expression
was associated to the resistance to 5-FU (40). These findings revealed that miR-218
could exert its functions by targeting some molecules. c-FLIP
belongs to a family of apoptosis inhibitors, and plays a crucial
role in tumor development and progression (41). Studies have revealed that c-FLIP
could inhibit apoptosis mediated by death receptors trough binding
to Fas-associated death domain and inhibit caspase-8 activation
(42). Overexpression of c-FLIP has
been revealed in different types of cancers (26,43).
c-FLIP upregulation has also been observed in gastric cancer
tissues and cells (44). Using
RT-PCR and flow cytometric analyses, the high expression level of
c-FLIP in colon carcinoma cell line HT-29 was detected.
Furthermore, silencing of c-FLIP with the specific siRNA promoted
Fas-mediated apoptosis (45).
Knockdown of c-FLIP by siRNA was found to sensitize colon cancer
cells to TRAIL-induced apoptosis (46). In the present study, the increased
expression of c-FLIP was also observed and knockdown of c-FLIP
could enhance cell apoptosis of human colon cancer cell line
SW1417. Furthermore, it was demonstrated that miR-218-induced cell
apoptosis in this type of cell line was c-FLIP dependent.
In summary, the present study revealed that miR-218
could inhibit the apoptosis of human colon cancer cell line SW1417
by suppressing c-FLIP expression, indicating that the
miR-218/c-FLIP axis could be an important target for gastric cancer
therapy. However, due to the limited support of funding, we only
used colon cancer cell line SW1417 in this study, and only provided
evidence that miR-218 inhibited c-FLIP expression using western
blotting and RT-PCR methods without a luciferase assay with a 3′
UTR region of c-FLIP. Thus, further investigations are warranted to
demonstrate the functions of miR-218/c-FLIP in other colon cancer
cell lines.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used in the present study are available
from the corresponding author YHM upon reasonable request.
Authors' contributions
QM designed the study and wrote the paper. YC, BL
and YS performed the experiments and analyzed data. HY reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the local Ethics Committee (Cancer Hospital of China Medical
University, Liaoning Cancer Hospital and Institute, Shenyang,
China. Permit no. 122/17).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Laks S, Meyers MO and Kim HJ: Surveillance
for gastric cancer. Surg Clin North Am. 97:317–331. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill S, Thomas RR and Goldberg RM: Review
article: Colorectal cancer chemotherapy. Aliment Pharmacol Ther.
18:683–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu T, Li LF, Shen J, Zhang L and Cho CH:
Chronic inflammation and colorectal cancer: The role of vascular
endothelial growth factor. Curr Pharm Des. 21:2960–2967. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu
WK, Wang L, Hu T, Li MX and Cho CH: Cigarette smoking and
gastrointestinal diseases: The causal relationship and underlying
molecular mechanisms (Review). Int J Mol Med. 34:372–380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein A, Atanackovic D and Bokemeyer C:
Current standards and new trends in the primary treatment of
colorectal cancer. Eur J Cancer. 47 (Suppl 3):S312–S314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dallas NA, Xia L, Fan F, Gray MJ, Gaur P,
van Buren G II, Samuel S, Kim MP, Lim SJ and Ellis LM:
Chemoresistant colorectal cancer cells, the cancer stem cell
phenotype, and increased sensitivity to insulin-like growth
factor-I receptor inhibition. Cancer Res. 69:1951–1957. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma L and Weinberg RA: Micromanagers of
malignancy: Role of microRNAs in regulating metastasis. Trends
Genet. 24:448–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson KM and Weiss GJ: MicroRNAs cancer:
Past, present, and potential future. Mol Cancer Ther. 7:3655–3660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs-the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng Y, Yang X, Deng X, Zhang X, Li P,
Tao J and Lu Q: MicroRNA-218 inhibits bladder cancer cell
proliferation, migration, and invasion by targeting BMI-1. Tumor
Biol. 36:8015–8023. 2015. View Article : Google Scholar
|
|
15
|
Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li
DM, Wen YY, Sun HR, Pan MH, Li W, et al: Downregulation of miR-218
contributes to epithelial-mesenchymal transition and tumor
metastasis in lung cancer by targeting Slug/ZEB2 signaling.
Oncogene. 36:2577–2588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sui C, Xu F, Shen W, Geng L, Xie F, Dai B,
Lu J, Zhang M and Yang J: Overexpression of miR-218 inhibits
hepatocellular carcinoma cell growth through RET. Tumor Biol.
36:1511–1518. 2015. View Article : Google Scholar
|
|
17
|
Tang S, Wang D, Zhang Q and Li L: miR-218
suppresses gastric cancer cell proliferation and invasion via
regulation of angiopoietin-2. Exp Ther Med. 12:3837–3842. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uesugi A, Kozaki K, Tsuruta T, Furuta M,
Morita K, Imoto I, Omura K and Inazawa J: The tumor suppressive
MicroRNA miR-218 targets the mTOR component rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res. 71:5765–5778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamasaki T, Seki N, Yoshino H, Itesako T,
Hidaka H, Yamada Y, Tatarano S, Yonezawa T, Kinoshita T, Nakagawa M
and Enokida H: MicroRNA-218 inhibits cell migration and invasion in
renal cell carcinoma through targeting caveolin-2 involved in focal
adhesion pathway. J Urol. 190:1059–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (c-FLIP): A novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Safa AR and Pollok KE: Targeting the
anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel).
3:1639–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seo SU, Cho HK, Min KJ, Woo SM, Kim S,
Park JW, Kim SH, Choi YH, Keum YS, Hyun JW, et al: Thioridazine
enhances sensitivity to carboplatin in human head and neck cancer
cells through downregulation of c-FLIP and Mcl-1 expression. Cell
Death Dis. 8:e25992017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yun H, Xie J, Olumi AF, Ghosh R and Kumar
AP: Activation of AKR1C1/ERβ induces apoptosis by downregulation of
c-FLIP in prostate cancer cells: A prospective therapeutic
opportunity. Oncotarget. 6:11600–11613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Wang S, Song X, Sima N, Xu X, Luo
A, Chen G, Deng D, Xu Q, Meng L, et al: The relationship between
c-FLIP expression and human papillomavirus E2 gene disruption in
cervical carcinogenesis. Gynecol Oncol. 105:571–577. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Yang G, Bai H, Zhang M and Mou D:
NCTD promotes Birinapant-mediated anticancer activity in breast
cancer cells by downregulation of c-FLIP. Oncotarget.
8:26886–26895. 2017.PubMed/NCBI
|
|
27
|
Wright C, Iyer AKV, Yakisich JS and Azad
N: Anti-tumorigenic effects of resveratrol in lung cancer cells
through modulation of c-FLIP. Curr Cancer Drug Targets. 17:669–680.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun J, Luo H, Nie W, Xu X, Miao X, Huang
F, Wu H and Jin X: Protective effect of RIP and c-FLIP in
preventing liver cancer cell apoptosis induced by TRAIL. Int J Clin
Exp Pathol. 8:6519–6525. 2015.PubMed/NCBI
|
|
29
|
Scaffidi C, Schmitz I, Krammer PH and
Peter ME: The role of c-FLIP in modulation of CD95-induced
apoptosis. J Biol Chem. 274:1541–1548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang G, Fu Y, Liu G, Ye Y and Zhang X:
miR-218 Inhibits proliferation, migration, and EMT of gastric
cancer cells by targeting WASF3. Oncol Res. 25:355–364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopharyngeal cancer progression through downregulation
of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 71:2381–2391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu K, Ding H, Wang W, Liao Z, Fu Z, Hong
Y, Zhou Y, Zhang CY and Chen X: Tumor-suppressive miR-218-5p
inhibits cancer cell proliferation and migration via EGFR in
non-small cell lung cancer. Oncotarget. 7:28075–28085.
2016.PubMed/NCBI
|
|
33
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama
K, Seki N and Nakagawa M: miR-218 on the genomic loss region of
chromosome 4p15.31 functions as a tumor suppressor in bladder
cancer. Int J Oncol. 39:13–21. 2011.PubMed/NCBI
|
|
34
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Shi H, Tang H, Fang Z, Wang J and
Cui S: miR-218 inhibits the invasion and migration of colon cancer
cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol
Med. 35:1301–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li PL, Zhang X, Wang LL, Du LT, Yang YM,
Li J and Wang CX: MicroRNA-218 is a prognostic indicator in
colorectal cancer and enhances 5-fluorouracil-induced apoptosis by
targeting BIRC5. Carcinogenesis. 36:1484–1493. 2015.PubMed/NCBI
|
|
37
|
He X, Dong Y, Wu CW, Zhao Z, Ng SS, Chan
FK, Sung JJ and Yu J: MicroRNA-218 inhibits cell cycle progression
and promotes apoptosis in colon cancer by downregulating BMI1
polycomb ring finger oncogene. Mol Med. 18:1491–1498.
2013.PubMed/NCBI
|
|
38
|
Ilm K, Fuchs S, Mudduluru G and Stein U:
MACC1 is post-transcriptionally regulated by miR-218 in colorectal
cancer. Oncotarget. 7:53443–53458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pagliara V, Saide A, Mitidieri E,
d'Emmanuele di Villa Bianca R, Sorrentino R, Russo G and Russo A:
5-FU targets rpL3 to induce mitochondrial apoptosis via
cystathionine-β-synthase in colon cancer cells lacking p53.
Oncotarget. 7:50333–50348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shirley S and Micheau O: Targeting c-FLIP
in cancer. Cancer Lett. 332:141–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Safa AR: c-FLIP, a master anti-apoptotic
regulator. Exp Oncol. 34:176–184. 2012.PubMed/NCBI
|
|
43
|
Logan IR, McClurg UL, Jones DL, O'Neill
DJ, Shaheen FS, Lunec J, Gaughan L and Robson CN: Nutlin-3 inhibits
androgen receptor-driven c-FLIP expression, resulting in apoptosis
of prostate cancer cells. Oncotarget. 7:74724–74733. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryang DY, Joo YE, Chung KM, Lim SR, Jeong
HK, Kim HI, Lee WS, Park CH, Kim HS, Choi SK, et al: Expression of
c-FLIP in gastric cancer and its relation to tumor cell
proliferation and apoptosis. Korean J Intern Med. 22:263–269. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zang F, Sun B, Zhao X, Niu R, Zhang S, Yu
M, Wei X and Zhang L: Critical role for c-FLIP(L) on Fas resistance
in colon carcinoma cell line HT-29. Cell Biol Int. 32:329–336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: The roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|