Introduction
It has been widely reported that curcumin has
anti-inflammatory, antimicrobial, antioxidant, antitumor, and even
anti-neurodegenerative properties (1,2). The
underlying mechanism involved in the effect of curcumin is not
understood especially in liver cancer inhibition. However,
activation of the NF-κB signaling pathway has often been associated
with progression of liver cancer (3,4), and
HSP70 can activate NF-κB by binding to the TLR4 receptor (5). In addition, we revealed the
anti-inflammatory effect of curcumin by decrease of plasma HSP70
levels in a rat model of thrombosis (unpublished data) therefore,
we speculated that curcumin may have some interaction with
HSP70.
The ‘danger theory’ (6,7) of
immunology suggests that DAMPs are associated with inflammation,
autoimmune disease, cancer, and a variety of diseases associated
with aging (8–10). Extracellular HSP70 is a typical DAMP
(11), a pro-inflammatory molecule
that causes the immune system of body to produce an immune
response. Since many diseases associated with aging share a common
origin and characteristics, such as genomic and epigenetic
alterations, abnormal telomeres, inflammation and immune damage, a
focus on DAMPs may reveal a new direction for disease research
(8,12).
In the present study, we demonstrated that curcumin
inhibited proliferation, invasion, and metastasis of HepG2 cells,
caused cells to remain in the DNA S phase, promoted apoptosis, and
decreased the level of extracellular HSP70 and TLR4 of HepG2TT
cells. Heat stress increases extracellular HSP70, which is used to
study the change of the TLR4 pathway induced by it. Cell
destruction, death or apoptosis can lead to the release of HSP70
from the intracellular to the extracellular space (13). The target of curcumin inhibition in
liver cancer has not been clearly elucidated. This preliminary
study revealed that curcumin could reduce extracellular HSP70 (DAMP
molecule), and inhibit TLR4 signaling. Therefore, we deduced that
the inhibition of liver cancer by curcumin was related to the
inhibition of HSP70 and TLR4 signaling.
Materials and methods
Cell culture and heat stress cell
model establishment
HepG2 cells (a cell line originating from the liver
cancer tissue of a 15-year old white Caucasian male; is suitable
for use in liver cancer cell metabolism although its histological
type is derived from hepatoblastoma) (14) were obtained from the Central
Laboratory of Xiangya School, Central South University (Changsha,
China) and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS Premium; cat. no. P20-3302; PAN-Biotech GmbH, Aidenbach,
Germany), 100 U/ml penicillin/streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. Cells were grown in
sterilized culture flasks and were passaged every two days with
0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.). For heat
stress, the cells were heated in an incubator at 43°C for 80 min,
and named thermal tolerance HepG2 (HepG2TT) cells. Cells were
treated with curcumin (Sigma-Aldrich, St. Louis, MO, USA) in
complete medium, and the treatment concentrations of curcumin were
20 to 100 µM.
CCK-8 assay
HepG2 cells were seeded in 96-well plates (Corning,
Inc., Corning, NY, USA) at a density of 5×104 cells/ml
in RPMI-1640 medium with 10% FBS and 1% antibiotic-antimycotic
solution and then incubated for 24 h at 37°C with 5%
CO2. HepG2 cells were treated with curcumin at
concentrations of 20 to 100 µM. Following 24, 48 and 72 h of
incubation, 10 µl of CCK-8 (Dojindo Technologies, Inc., Kumamoto,
Japan) was added to each well, and the cells were further incubated
for 1 h. The absorbance of each well was measured using a
microplate reader (Addcare Bio-Tech Co., Ltd., Yantai, China) at a
wavelength of 450 nm. Cell proliferation was calculated according
to the following equation: Survival percentage (%) = (absorption
value of the treatment group)/(absorption value of the control
group) × 100%.
Wound-healing assay
Cells were plated in 6-well plates and grown
overnight to confluence. Monolayers of cells were wounded by manual
scraping with a 10-µl pipette tip. Cells were rinsed with PBS and
then treated with curcumin in serum-free medium at concentrations
of 50 and 80 µM. Images were obtained at 0, 24 and 48 h after
wounding under an inverted microscope.
Transwell migration assay
Experiments were performed using a 24-well Τranswell
chamber (Corning Inc.). Briefly, 600 µl of RMPI-1640 complete
medium containing 10% FBS was placed in the lower chamber. A total
of 1×105 HepG2 cells in 100 µl medium were seeded into the upper
chamber (pore size, 8 µm). Then, HepG2 cells and medium were
transferred into the chamber with curcumin (final concentrations of
curcumin were 50 or 80 µM); untreated HepG2 cells were used as
controls. The chamber was then incubated for 24 h at 37°C in a
humidified atmosphere with 5% CO2. The membrane was
removed and its upper surface was wiped with a cotton swab to
remove cells that had not migrated through the membrane. The
membrane was then fixed in methyl alcohol for 20 min at room
temperature and then stained with 0.1% crystal violet for 15 min.
The number of HepG2 cells that had migrated to the lower surface of
the membrane were counted in 5 random high-power fields (HPFs)
under an Olympus CKX41 light microscope.
Flow cytometric analysis of the cell
cycle
The effect of curcumin on the cell cycle
distribution was determined by flow cytometry. Briefly, HepG2 cells
were seeded in culture bottles and treated with curcumin at 50 and
80 µM. Cells were harvested after 24 h and fixed at least 1 h with
70% ice-cold ethanol at 4°C. Cells were washed with PBS and
resuspended in 1 ml of PBS, and then stained with propidium iodide
PI). Cells were analyzed by flow cytometry using a FACSCalibur flow
cytometer (Becton-Dickinson; BD Biosciences, CA, USA). The fraction
of cells in the G0/G1, S and G2/M phases were analyzed using ModFit
software, which was provided by the Third Hospital of Central South
University.
Flow cytometric analysis of cell
apoptosis
HepG2 cells were seeded in culture bottles and
treated with curcumin at 50 and 80 µM. Cells were trypsinized
(Gibco; Thermo Fisher Scientific, Inc.) and washed with PBS. The
samples were centrifuged for 5 min at 2,000 × g and the cells were
harvested. The cells were resusupended in 500 µl of binding buffer,
then stained with Annexin V-FITC and PI. The number of apoptotic
cells was detected and analyzed using flow cytometry.
Western blot analysis
Total proteins were extracted in lysis buffer and
quantified using the BCA method. Proteins (50 µg) were separated by
SDS-PAGE (8 and 10%). Proteins were then transferred to
polyvinylidene fluoride (PVDF) membranes, and the membranes were
incubated overnight at 4°C with the following antibodies: SPAG9
(1:2,000; cat. no. ab12331; Abcam, Cambridge, MA, USA), HSP70
(1:2,000; cat. no. 10995-1-AP; Proteintech Group, Inc., Chicago,
IL, USA), TLR4 (1:1,000; cat. no. 66350-1-Ig; Proteintech Group)
and β-actin (1:4,000; cat. no. 60008-1-Ig; Proteintech Group).
After incubation with goat anti-mouse/anti-rabbit IgG (1:4,000;
cat. no. SA00001-1/SA00001-15; Proteintech Group) at 37°C for 2 h,
bound proteins were visualized using superECL plus western blotting
substrate (Pierce; Thermo Fisher Scientific, Inc.). Blots were
exposed to a radiographic film.
ELISA assay
Levels of HSP70 were detected in the supernatant of
HepG2 cells using the Human Heat Shock Protein 70 ELISA kit
(Cusabio Technology, LLC, Wuhan, China) according to the
manufacturer's instructions.
Statistical analyses
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the means ±
standard deviations. Statistical analyses were performed with
two-way repeated measures ANOVA and two-way ANOVA. An LSD t-test
was used in multiple comparison tests, and the statistical
significance level was set at P< 0.05 (two-sided).
Results
Curcumin inhibits proliferation,
migration, and invasion of HepG2 cells
In order to understand the effect of curcumin on
cell proliferation, we performed a CCK-8 assay, which is a
sensitive colorimetric assay using WST-8 dye to stain viable cells.
The results revealed that curcumin inhibited the proliferation of
HepG2 cells in a dose- and time-dependent manner (F=8.383;
P<0.001) (Fig. 1; Table I).
 | Table I.Curcumin inhibits proliferation of
HepG2 cells in a concentration-dependent manner. |
Table I.
Curcumin inhibits proliferation of
HepG2 cells in a concentration-dependent manner.
|
| Cell proliferation
rate (%) |
|---|
|
|
|
|---|
| Concentration of
curcumin |
| 24 h | 48 h | 72 h |
|---|
| 20 µM |
| 93.43±3.77 | 82.44±6.58 | 84.17±5.93 |
| 50 µM |
| 53.26±2.57 | 48.24±3.14 | 28.34±1.80 |
| 80 µM |
| 45.86±1.76 | 40.95±2.85 | 24.62±6.86 |
| 100 µM |
| 39.61±0.37 | 37.10±2.39 | 8.730±0.55 |
| Two-way analysis of
variance with repeated measures |
| Mauchly's
test of sphericity | P | 0.868 |
|
|
| Main
effect of curcumin | F, P | 444.843,
<0.001 |
|
|
| Main
effect of time | F, P | 97.455,
<0.001 |
|
|
|
Interaction | F, P | 8.383, <0.001 |
|
|
| Multiple comparisons
between groups, LSD t-test |
| 20 µM and
50 µM | P | <0.001 | <0.001 | <0.001 |
| 20 µM and
80 µM | P | <0.001 | <0.001 | <0.001 |
| 20 µM and
100 µM | P | <0.001 | <0.001 | <0.001 |
| 50 µM
and 80 µM | P |
0.006 |
0.061 |
0.354 |
| 50 µM
and 100 µM | P | <0.001 |
0.010 |
0.001 |
| 80 µM
and 100 µM | P |
0.014 |
0.283 |
0.003 |
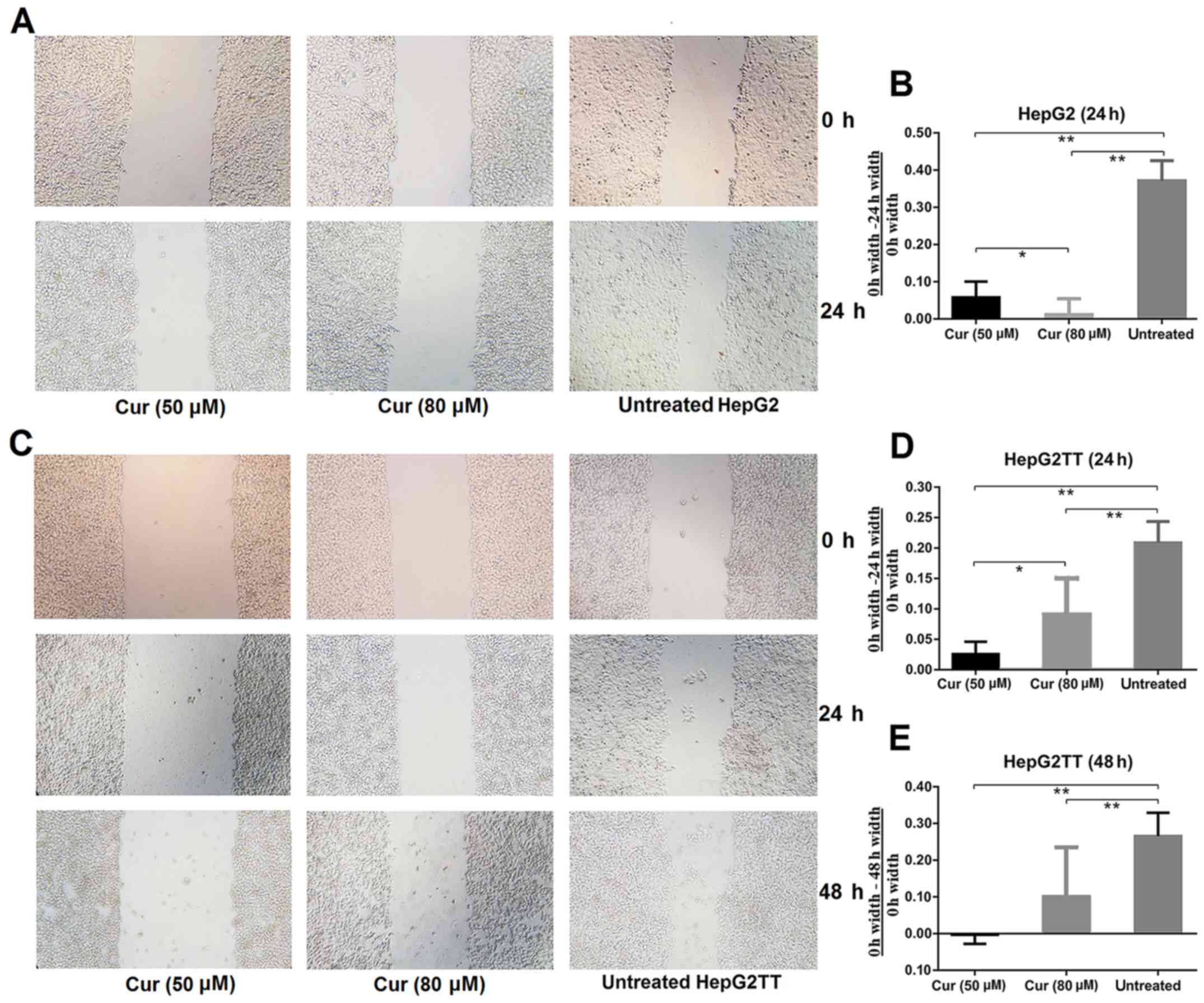
To investigate the effect of curcumin on cell
migration and invasion, we performed an in vitro wound
healing assay. As revealed in Fig. 2A
and B, significantly fewer cells had migrated into the
scratched area in the curcumin-treated cultures than in the
untreated samples after 24 h of culture. To investigate whether
heat stress, which increases extracellular HSP70 concentration,
would affect the invasive abilities of HepG2 cells, we performed
the wound healing assay with HepG2TT cells. As shown in Fig. 2C-E, the migration of cells that had
been subjected to heat stress was significantly slower in the
curcumin treated group than in the untreated group after 24 and 48
h, but a concentration dependence was not evident. These results
revealed that curcumin is an important inhibitor of migration and
invasion in HepG2 and HepG2TT cells.
A Transwell migration assay also revealed that
curcumin significantly inhibited the migration of HepG2 cells.
There were significantly fewer cells per view in the samples
treated with 80 µM curcumin than in cells treated with 50 µM
curcumin (Fig. 3).
Curcumin halts the cell cycle in the S
phase and promotes apoptosis of HepG2 cells
Flow cytometry was used to analyze the effects of
curcumin on the cell cycle and apoptosis. Compared with untreated
HepG2 cells, the percentage of cells in the S phase of HepG2 cells
treated with 80 µM curcumin was significantly higher than in the
untreated cells (Fig. 4A and B).
The percentage of apoptosis in cells treated with both 50 and 80 µM
of curcumin was significantly higher than that in the untreated
HepG2 cells (Fig. 5A and B).
Curcumin reduces the expression of
HSP70 and TLR4
Western blot analyses revealed that HepG2 cells
express both HSP70 and TLR4 and that curcumin treatment decreased
the expression of HSP70 and TLR4. Heat stress also significantly
reduced the expression of HSP70 and TLR4. Curcumin downregulates
the expression of intracellular HSP70 in HepG2 cells, but has the
opposite effect in HepG2TT cells. Curcumin downregulated the
expression of TLR4 in HepG2 cells, but had no effect in HepG2TT
cells (Fig. 6A and B).
Effects of curcumin on eHSP70 in
HepG2TT cells
Compared with HepG2 cells, the concentration of
eHSP70 was significantly increased in HepG2 cells subjected to heat
stress. Curcumin treatment significantly decreased eHSP70 of
HepG2TT cells; the changes of intracellular HSP70 were opposite to
that of eHSP70, and the change of TLR4 expression was consistent
with the change of eHSP70. After removing the effect of curcumin on
HepG2TT cells, eHSP70 was recovered again (Fig. 7).
Discussion
Curcumin is a polyphenol isolated from the
traditional Chinese medicine turmeric rhizome which has a wide
range of antitumor, anti-inflammatory, antioxidant,
anti-atherosclerosis, and anti-depression activity (1,2),
however, the underlying mechanism of curcumin action is
controversial (15,16).
We used a CCK-8 assay, an in vitro wound
healing assay and a Transwell migration assay and confirmed that
treatment of HepG2 cells with curcumin significantly inhibited
proliferation, invasion, and migration in a concentration-dependent
manner. Flow cytometric results revealed that the cell cycle was
blocked in the S phase in cells treated with curcumin, and
apoptosis was increased, although there was no statistical
significance. Further study is warranted to confirm the
results.
According to research, tumor cells can release HSP70
into the extracellular environment, and high levels of HSP70 are
detected in the peripheral blood of patients with various types of
tumors (17,18). High levels of HSP70 have also been
detected in the sera of patients with cancer including those with
liver cancer (18). We also
detected a high expression of HSP70 and TLR4 in cancer tissues of
patients with liver cancer. Our previous study revealed that the
serum HSP70 levels in patients with hepatitis cirrhosis, as well as
in patients with liver cancer, were higher than normal (19). These findings revealed that DAMP
HSP70 is associated with the pathological process both of
inflammation and cancer.
In this study, we found that HSP70 was excreted from
HepG2 cells, TLR4 was expressed on the surface of HepG2 cells, and
that heat stress increased the secretion of HSP70 into the HepG2
cell culture medium. As revealed in Fig. 6, in heat-stressed cells, the levels
of intracellular HSP70 were decreased compared with non-stressed
HepG2 cells, indicating that heat stress can release HSP70 from
cells and significantly increase eHSP70 (as shown in Fig. 7). Co-culture with curcumin in HepG2
cells significantly decreased eHSP70, however eHSP70 increased
again after the removal of the effect of curcumin. We also observed
that the expression of TLR4 in the cells, exhibited a
concentration-dependent relationship, indicating that the increased
expression of TLR4 in HepG2 cells was associated with eHSP70.
Extracellular HSP70 is a typical DAMP (20), and, like cytokines, it stimulates
the expression of TLR4 receptors on the surface of immune and tumor
cells (21). When HSP70 engages
TLR4, NF-κB is activated promoting the transcription of
inflammatory genes including cytokines, chemokines, and growth
factors. It has been previously reported that curcumin inhibits the
transcription of NF-κB (22), and
the NF-κB pathway plays important roles in the occurrence and
development of tumors (23,24). TLR4 is the receptor of eHSP70,
therefore, inhibition of this interaction can indirectly inhibit
NF-κB activation.
In summary, our results demonstrated that the
HSP70-mediated activation of TLR4 signaling could be inhibited by
curcumin, and the antitumor effect of curcumin was related to the
inhibition of HSP70-TLR4 signaling, thus we speculated that
curcumin inhibits the expression of TLR4 on the tumor cell by
decreasing the levels of eHSP70, thereby inhibiting the NF-κB
pathway initiated by TLR4 signaling. Our data provides a new
research direction to reveal the anticancer liver mechanism of
curcumin, supporting its further evaluation in liver cancer
therapy, and the HSP70-TLR4 pathway may be a potential target for
liver cancer therapy.
Acknowledgements
We would like to thank Min Xu and Weimin Wu for the
collection of materials.
Funding
The present study was supported in part by the Hunan
Administration of Traditional Chinese Medicine Key Fund (no.
201720).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BR contributed to the conception and design. SL, XT
and ZJ drafted and revised the manuscript. SL, GZ and FX acquired,
analyzed and interpreted the data. TY, YH and JL performed all the
experiments. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DAMP
|
damage-associated molecular
pattern
|
|
eHSP70
|
extracellular heat shock protein
70
|
|
TLR4
|
toll-like receptors 4
|
|
CCK-8
|
Cell Counting Kit-8
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IHC
|
immunohistochemistry
|
|
HepG2TT
|
thermal tolerance HepG2
|
References
|
1
|
Egan ME, Pearson M, Weiner SA, Rajendran
V, Rubin D, Glöckner-Pagel J, Canny S, Du K, Lukacs GL and Caplan
MJ: Curcumin, a major constituent of turmeric, corrects cystic
fibrosis defects. Science. 304:600–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeitlin P: Can curcumin cure cystic
fibrosis? N Engl J Med. 351:606–608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wheeler DS, Chase MA, Senft AP, Poynter
SE, Wong HR and Page K: Extracellular Hsp72, an endogenous DAMP, is
released by virally infected airway epithelial cells and activates
neutrophils via Toll-like receptor (TLR)-4. Respir Res. 10:312009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polly Matzinger P: Tolerance, danger, and
the extended family. Annu Rev lmmunol. 12:991–1045. 1994.
View Article : Google Scholar
|
|
7
|
Huang J, Xie Y, Sun X, Zeh HJ III, Kang R,
Lotze MT and Tang D: DAMPs, ageing, and cancer: The ‘DAMP
Hypothesis’. Ageing Res Rev. 24A:3–16. 2015. View Article : Google Scholar
|
|
8
|
Nefla M, Holzinger D, Berenbaum F and
Jacques C: The danger from within: Alarmins in arthritis. Nat Rev
Rheumatol. 12:669–683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pécheur EI: Curcumin against hepatitis C
virus infection: Spicing up antiviral therapies with
‘nutraceuticals’? Gut. 63:1035–1037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adewoye AH and McMahon L: Chaperones and
disease. N Engl J Med. 353:2821–2822; author reply 2821–2822. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Land WG: Role of heat shock protein 70 in
innate alloimmunity. Front Immunol. 2:892012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Tang D and Lotze MT: Ménage à Trois
in stress: DAMPs, redox and autophagy. Semin Cancer Biol.
23:380–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brusa D, Migliore E, Garetto S, Simone M
and Matera L: Immunogenicity of 56°C and UVC-treated prostate
cancer is associated with release of HSP70 and HMGB1 from necrotic
cells. Prostate. 69:1343–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
15
|
Baker M: Deceptive curcumin offers
cautionary tale for chemists. Nature. 541:144–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heger M: Drug screening: Don't discount
all curcumin trial data. Nature. 543:402017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozenberg P, Kocsis J, Saar M, Prohászka
Z, Füst G and Fishelson Z: Elevated levels of mitochondrial
mortalin and cytosolic HSP70 in blood as risk factors in patients
with colorectal cancer. Int J Cancer. 133:514–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gehrmann M, Cervello M, Montalto G,
Cappello F, Gulino A, Knape C, Specht HM and Multhoff G: Heat shock
protein 70 serum levels differ significantly in patients with
chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma.
Front Immunol. 5:3072014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren B, Luo S, Xu F, Zou G, Xu G, He J,
Huang Y, Zhu H and Li Y: The expression of DAMP proteins HSP70 and
cancer-testis antigen SPAG9 in peripheral blood of patients with
HCC and lung cancer. Cell Stress Chaperones. 22:237–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Timmermans K, Kox M, Vaneker M, van den
Berg M, John A, van Laarhoven A, van der Hoeven H, Scheffer GJ and
Pickkers P: Plasma levels of danger-associated molecular patterns
are associated with immune suppression in trauma patients.
Intensive Care Med. 42:551–561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camp SM, Ceco E, Evenoski CL, Danilov SM,
Zhou T, Chiang ET, Moreno-Vinasco L, Mapes B, Zhao J, Gursoy G, et
al: Unique toll-like receptor 4 activation by NAMPT/PBEF induces
NFκB signaling and inflammatory lung injury. Sci Rep. 5:131352015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marquardt JU, Gomez-Quiroz L, Arreguin
Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D,
Breuhahn K, Conner EA, et al: Curcumin effectively inhibits
oncogenic NF-κB signaling and restrains stemness features in liver
cancer. J Hepatol. 63:661–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang Y, Zheng T, Song R, Wang J, Yin D,
Wang L, Liu H, Tian L, Fang X, Meng X, et al: Hypoxia-mediated
sorafenib resistance can be overcome by EF24 through Von
Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in
hepatocellular carcinoma. Hepatology. 57:1847–1857. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu
Y, Chang SS, Lin WC, Hsu JM, Hsu YH, et al: Deubiquitination and
Stabilization of PD-L1 by CSN5. Cancer Cell. 30:925–939. 2016.
View Article : Google Scholar : PubMed/NCBI
|