Introduction
Acute promyelocytic leukemia (APL), a distinct
subtype of acute myeloid leukemia (AML), is characterized by
reciprocal chromosomal translocation of t (15;17), which results in
the production of promyelocytic leukemia-retinoic acid receptor α
(PML-RARα) fusion protein (1). For
decades, APL has been considered as the most malignant form of AML
due to its severe bleeding tendency and high early mortality rate
(2,3). Notably, at present, APL is the most
curable form of AML and can be treated using all-trans
retinoic acid (ATRA) and arsenic trioxide (ATO), which mainly
induce cell differentiation and apoptosis (3,4).
However, APL treatment is associated with some issues such as ATRA
or ATO resistance, relapse, differentiation syndrome and adverse
effects (5–7). Therefore, it is necessary to identify
other therapeutic strategies for APL treatment.
Salinomycin (SAL), a polyether antibiotic, is widely
used as an anticoccidial drug for poultry (8). Recently, Gupta et al performed
high-throughput screening of 16,000 compounds and found that SAL
selectively killed breast cancer stem cells (CSCs) at least
100-times more effectively than conventional chemotherapeutic drug
paclitaxel (9). Further studies
have indicated that SAL exerts potential anticancer effects against
different human cancer cell types, including lung, gastric and
prostate cancer, and glioblastoma cells (10–13),
without adversely affecting healthy cells (14–16).
Accumulating evidence suggests that the presence of CSCs, which
have capability of self-renewal and tumor-initiating capacities,
are the major cause of drug resistance and relapse after therapy
(17). SAL affects the
proliferation of various CSCs including those present in breast,
gastric and ovarian cancer (9,18,19). A
previous study revealed that SAL treatment also reversed multidrug
resistance in leukemia stem cells (LSCs) such as KG-1a cells
(20). Moreover, SAL was revealed
to reverse multi-drug resistance in many cancer cell types
(21,22). Collectively, these findings revealed
that SAL is a potential anticancer drug. However, limited studies
have assessed the effect of SAL against leukemia.
SAL is also known as an inhibitor of Wnt/β-catenin
signaling (23) which plays key
roles in both normal cell development and tumorigenesis (24). Aberrant activation of Wnt/β-catenin
signaling is frequently implicated in the pathogenesis of AML.
Notably, high β-catenin expression was observed in both AML cell
lines and primary blasts (25,26).
In addition, recent studies have shown that Wnt/β-catenin signaling
was associated with leukemia cell differentiation. Attenuation of
Wnt/β-catenin signaling promoted cell differentiation (27), whereas its activation blocked
monocyte-macrophage differentiation in AML cell lines (28). Therefore, we hypothesized that SAL
induced APL cell differentiation by blocking Wnt/β-catenin
signaling.
Since limited studies have assessed the cytotoxicity
of SAL against leukemia cells and its effect on leukemia cell
differentiation, we investigated the effect of SAL in APL cell
lines NB4 and HL-60 in the present study. We found that SAL
markedly inhibited cell proliferation and induced the apoptosis and
differentiation of APL cell lines NB4 and HL-60. Our results
provide a foundation for further exploring the clinical use of
SAL.
Materials and methods
Materials
Salinomycin (HY-15597) and IWR-1 (HY-12238) were
purchased from MedChem Express (Monmouth Junction, NJ, USA). Cell
Counting Kit-8 (CCK-8) reagent was purchased from Sevenseas Futai
Biotechnology Co., Ltd. (Shanghai, China). Hoechst 33258 reagent
was purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Wright Giemsa stain solution was purchased from Beijing
Solarbio Science & Technology Co., Ltd. (Beijing, China). The
phycoerythrin (PE)-conjugated CD11b antibody (1:20; cat. no.
301306) was purchased from BioLegend, Inc., (San Diego, CA, USA).
The antibody against CD11b (1:1,000; cat. no. ab133357) was
purchased from Abcam (Cambridge, MA, USA). The antibody against
LRP6 (1:1,000; cat. no. sc-25317) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Antibodies against caspase-3
(1:1,000; cat. no. 9665),caspase-9 (1:1,000; cat. no. 9504),
cleaved caspase-9 (1:1,000; cat. no. 9509), cytochrome c
(1:1,000; cat. no. 11940), β-catenin (1:1,000; cat. no. 8480),
cyclin D1 (1:1,000; cat. no. 2922) and C-myc (1:1,000; cat. no.
5605) were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Antibodies against Bax (1:500; cat. no. wl01637) and
Bcl-2 (1:500; cat. no. wl01158), PARP (1:500; cat. no. WL01932)
were purchased from Wanleibio Co., Ltd. (Shenyang, China). Goat
anti-rabbit secondary antibody (1:4,000; cat. no. ZB-2301), goat
anti-mouse secondary antibody (1:4,000; cat. no. ZB-2305) and
anti-β-actin antibody (1:1,000; cat. no. BM0627) were purchased
from Zhongshan Golden Bridge Biotechnology; OriGene Technologies
(Beijing, China).
Cell lines and culture
The human APL cell lines NB4 and HL-60 obtained from
the Shanghai Institutes for Biological Sciences (Shanghai, China)
were then maintained in our own laboratory and cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
both from Gibco; Life Technologies, Carlsbad, CA, USA) and
penicillin (100 mg/ml) and streptomycin (100 mg/ml) in an
environment that contained 5% CO2 at 37°C.
Cell viability assay
NB4 or HL-60 cells were seeded into 96-well plates
with RPMI-1640 medium supplemented with 10% FBS. For experimental
purposes, the cells were seeded at a density of 1×104
cells/well, and then treated with different concentrations of SAL
for 24, 48 or 72 h, respectively. Then, 10 µl CCK-8 reagent was
added to each well. Following incubation for 2 h, cell viability
was assessed by detection of the absorbance at 450 nm using a
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The experiment was repeated at least three times.
Hoechst 33258 staining
Cells were treated with SAL for 48 h. Cells were
collected and washed twice using phosphate-buffered saline (PBS)
and plated onto the glass slides. After being fixed with 4%
paraformaldehyde for 20 min, the cells were permeabilized with 0.1%
Triton X-100 for 15 min. Subsequently, the cells were stained with
Hoechst 33258 reagent for 10 min at 37°C. The slides were washed
three times with PBS. Finally, the nuclear morphological changes
were observed under a fluorescence microscope (magnification,
×400).
Wright-Giemsa staining
After 72 h of treatment, cells were collected and
washed with PBS three times. Then the cells were resuspended in PBS
and fixed on slides. The morphological changes of the cells were
examined by optical microscopy (magnification, ×200 or ×1,000)
after staining with Wright-Giemsa stain solution.
NBT reduction assay
For the nitroblue tetrazolium (NBT) reduction assay,
NB4 and HL-60 cells were treated with SAL (0.6 µM) or ATRA (1 µM,
as a positive control) for 3 days. Then each cell suspension was
mixed with an equal volume of RPMI-1640 medium containing 1 mg/ml
NBT (Sigma-Aldrich, St. Louis, MO, USA) and 200 ng/ml TPA
(Sigma-Aldrich; Merck) for 30 min at 37°C. A total of 200 cells
were counted by optical microscope (magnification, ×1,000) after
staining with or without Wright-Giemsa stain solution
Western blot analysis
For protein analysis, harvested cells were washed
with ice-cold phosphate-buffered saline (PBS) three times and lysed
in RIPA solution containing protease inhibitor
phenylmethanesulfonyl fluoride (PMSF), phosphatase inhibitor NaF
and Na3VO3. Protein concentration was measured by BCA method. Equal
amounts of extracted total protein (30 or 50 µg) were separated by
10% or 12% polyacrylamide gels and then transferred to
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skim milk
for 2 h at room temperature, and then incubated with the primary
antibodies (1:1,000 or 1:500) overnight at 4°C. The membranes were
then incubated with goat anti-rabbit or goat anti-mouse secondary
antibodies (1:4,000) for 1 h at 37°C. After washing with
Tris-buffered saline containing Tween-20 (TBST), the immunoreactive
complexes were visualized using an enhanced chemiluminescence
system (GE Healthcare, Marlborough, MA, USA). β-actin was used as
the internal positive control. Each experiment was repeated at
least three times.
Flow cytometric assay
For apoptosis analysis, cells treated with different
concentrations of SAL for 48 h were harvested and washed three
times with pre-cold PBS. Cells were resuspended and stained with
Annexin V-FITC and propidium iodide (PI) (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The rate of cell apoptosis was analyzed
using a FACSorter (BD Biosciences, San Jose, CA, USA) after
incubation for 15 min at room temperature.
For the detection of cell surface differentiation
marker CD11b, cells treated with SAL or ATRA for 72 h were washed
three times with ice-cold PBS and then incubated with phycoerythrin
(PE)-conjugated CD11b antibody at 4°C for 30 min in the dark. The
cells were then washed three times with ice-cold PBS and then
analyzed using flow cytometry (BD FACSVantage; BD Biosciences) and
CellQuest Pro software version 5.1 (BD Pharmingen; BD Biosciemces,
San Diego, CA, USA).
Indirect immunofluorescence assay
The localization of β-catenin was confirmed by
indirect immunofluorescence assay. Cells were harvested,
centrifuged at 1,000 × g for 5 min at room temperature and washed
three times using PBS. Then, cells fixed with 4% paraformaldehyde
for 20 min were permeabilized with 0.1% Triton X-100 for 15 min and
then blocked with 10% goat serum for 30 min at room temperature.
The slides were incubated with the primary antibody β-catenin
(1:200; cat. no. 8480) at 4°C overnight. After being washed three
times with PBS, the cells were incubated with secondary antibody
goat against rabbit-IgG-FITC (1:200; cat. no. ZF0311; Zhongshan
Golden Bridge Biotechnology Co., Ltd.; OriGene Technologies) for 1
h at room temperature. Then nuclei were stained using DAPI (1:10;
Beyotime Institute of Biotechnology) for 5 min at room temperature.
Finally, the coverslips were viewed using a fluorescence microscope
(magnification, ×400) (Nikon Corp., Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Data were expressed as the
means ± standard (SD). One-way analysis of variance followed by the
Dunnett's test was performed and Student's t-test was used for
comparisons. A value of P<0.05 was considered to indicate a
statistically significant result.
Results
Salinomycin inhibits the proliferation
of APL cells
We performed CCK-8 assay to determine the viability
of NB4 and HL-60 cells which were treated with various
concentrations (0–3.2 µM) of SAL for 24, 48 and 72 h. SAL
significantly inhibited cell viability in a dose-dependent manner
after treatment for 48 and 72 h (Fig.
1). However, treatment with low concentrations (0–1.6 µM) of
SAL for 24 h did not exhibit cytotoxicity against NB4 (Fig. 1A) and HL-60 (Fig. 1B) cells. Therefore, we selected a
time-point of 48 h to investigate the effect of SAL on cell
apoptosis in subsequent experiments.
Salinomycin exerts a pro-apoptotic
effect on NB4 and HL-60 cells
To examine whether SAL-induced cell death of NB4 and
HL-60 cells was mediated by apoptosis induction, we performed
Annexin V-FITC and PI staining and flow cytometric analysis. As
shown in Fig. 2A, SAL treatment
increased the percentage of apoptotic NB4 and HL-60 cells in a
dose-dependent manner. The percentage of apoptotic NB4 cells
increased from 6.01% among control cells [treated with dimethyl
sulphoxide (DMSO)] to 32.30, 61.90 and 76.22% among cells treated
with 0.8, 1.6 and 3.2 µM SAL, respectively. The apoptotic HL-60
cells increased from 3.16% among control cells (treated with DMSO)
to 37.45, 64.46 and 85.75% among cells treated with 0.8, 1.6 and
3.2 µM SAL, respectively. Results of Hoechst 33258 staining
revealed altered morphology of NB4 and HL-60 cells treated with 1.6
µM SAL for 48 h. SAL-treated cells exhibited typical morphological
changes associated with apoptosis, such as nuclear fragmentation
and condensation (Fig. 2B). To
further explore the mechanism of apoptosis induced by SAL in NB4
and HL-60 cells we examined the expression levels of
apoptosis-associated proteins including Bcl-2, Bax, caspase-3, −8
and −9, cleaved PARP and cytochrome c. As shown in Fig. 2C, SAL increased the expression level
of Bax while it decreased the expression level of Bcl-2 and
deregulated the ratio of Bax/Bcl-2 in NB4 and HL-60 cells.
Furthermore, the increased expression levels of cleaved caspase-3,
cleaved caspase-9 (Fig. 2D) and
cleaved PARP and cytochrome c (Fig. 2E) were observed after SAL treatment.
However, cleaved caspase-8 was not detected after SAL treatment
(data not shown). Collectively, these data indicated that SAL
effectively induced the apoptosis of APL cells.
Salinomycin induces the
differentiation of APL cells
Targeting Wnt/β-catenin signaling using
6-benzylthioinosine was revealed to induce the differentiation of
leukemia cells (27). In addition,
shRNA-mediated downregulation of β-catenin promoted ATRA-induced
differentiation of HL-60 cells (29). Therefore, we determined whether SAL,
a Wnt signaling inhibitor, also induced the differentiation of
leukemia cells. For this, NB4 and HL-60 cells were incubated with
SAL (0.6 µM) or ATRA (1 µM, positive control) for 72 h, and cell
differentiation was evaluated based on morphological changes by
performing Wright-Giemsa staining. Morphological analysis revealed
that undifferentiated control (DMSO-treated) cells were
predominantly promyelocytes with round and large nuclei, whereas
cells treated with SAL or ATRA displayed morphological features of
cell differentiation, such as a smaller nucleus pattern,
cytoplasmic enlargement, lower nuclear/cytoplasmic ratio (Fig. 3A). The percentage of mature NB4
cells increased from 1.5% among control cells (treated with DMSO)
to 39.5 and 74.5% among cells treated with 0.6 µM SAL or 1 µM ATRA,
respectively. The percentage of mature HL-60 cells increased from
4.0% among control cells (treated with DMSO) to 30.5 and 63.0%
among cells treated with 0.6 µM SAL or 1 µM ATRA, respectively.
These morphological data were further confirmed by the results of
NBT testing. NBT-positive cells significantly increased after
treatment with SAL for 72 h (Fig.
3B). Cell differentiation was further confirmed by detecting
the expression of CD11b, a surface myeloid differentiation marker,
by performing flow cytometric and western blot analyses. As shown
in Fig. 3C, SAL or ATRA treatment
significantly increased the percentage of CD11b-positive cells in a
dose-dependent manner. Results of western blot analysis also
revealed that CD11b expression increased after SAL or ATRA
treatment. Previous studies have demonstrated that CCAAT/enhancer
binding protein β (C/EBPβ) plays a crucial role in myeloid
differentiation (30). In the
present study, we found that SAL also enhanced C/EBPβ expression
(Fig. 3D). Thus, these results
revealed that SAL effectively induced leukemic-cell
differentiation.
 | Figure 3.SAL induces cell differentiation in
NB4 and HL-60 cells. (A and B) NB4 and HL-60 cells were treated
with SAL (0.6 µM) or ATRA (1 µM, as a positive control) for three
days. (A) Then, cell morphology was examined by Wright's staining
under a light microscope (magnification, ×20 and ×100). (B)
Differentiation was also assessed by NBT reduction test. A total of
200 cells were counted under a microscope to determine the
percentage of NBT-positive cells. Data is expressed as the means ±
SD of three independent experiments. (C) NB4 and HL-60 cells were
treated with 0 (DMSO), 0.2, 0.4 and 0.6 µM SAL or 1 µM ATRA for 72
h, and the percent of differentiated cells was determined by
assessing CD11b expression and analyzed by flow cytometry. (D) NB4
and HL-60 cells were treated with 0 (DMSO), 0.4 and 0.6 µM SAL or 1
µM ATRA for 72 h, and the expression of differentiation marker
CD11b and C/EBPβ were examined by western blot analysis. Each
experiment was repeated at least three times. *P<0.05,
**P<0.01, ***P<0.001 vs. the DMSO group, n=3. SAL,
salinomycin; ATRA, all-trans retinoic acid; NBT, nitroblue
tetrazolium; C/EBPβ, CCAAT/enhancer binding protein β; DMSO,
dimethyl sulphoxide. |
Salinomycin inhibits Wnt/β-catenin
signaling
Since activation of canonical Wnt signaling resulted
in low ability of cell differentiation (28), we explored whether canonical Wnt
signaling was involved in SAL-induced cell differentiation.
β-catenin is the central molecule involved in canonical Wnt
signaling, therefore we evaluated β-catenin expression in NB4 and
HL-60 cells treated with SAL (0.4 and 0.6 µM) or ATRA (1 µM) for
three days by performing western blotting. We found that the total
β-catenin level was decreased after SAL or ATRA treatment for 72 h
(Fig. 4A). It has been revealed
that after stabilization and accumulation, β-catenin translocates
into the nucleus and binds transcription factors belonging to
T-cell factor/lymphoid enhancer factor (TCF/LEF) family to
stimulate the expression of target genes such as cyclin D1 and
C-myc (31). Therefore, we
investigated the subcellular localization of β-catenin using
immunofluorescence assay. As shown in Fig. 4B, β-catenin preferentially
accumulated in the nucleus of the control (DMSO-treated) cells. SAL
or ATRA treatment decreased β-catenin levels in both the nucleus
and cytoplasm. Furthermore, the expression of LRP6, C-myc and
cyclin D1 also decreased after treatment with SAL or ATRA for 72 h
(Fig. 4C). These data indicated
that Wnt/β-catenin signaling was involved in SAL or ATRA
induced-cell differentiation.
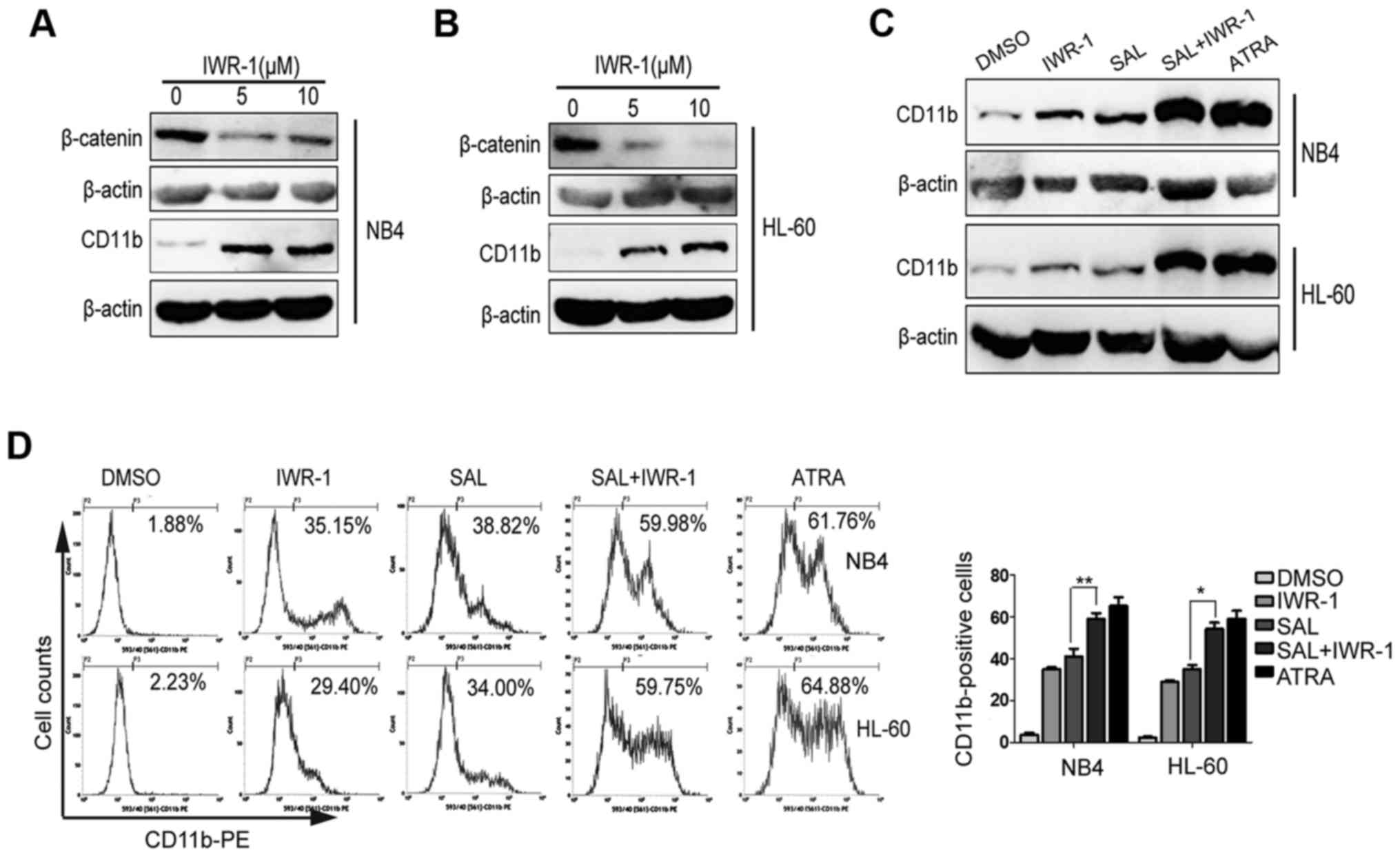
IWR-1, a Wnt inhibitor, promotes
salinomycin-induced cell differentiation
NB4 and HL-60 were treated with Wnt inhibitor IWR-1
(5 or 10 µM) for 72 h. We observed that IWR-1 treatment decreased
β-catenin expression in NB4 (Fig.
5A) and HL-60 (Fig. 5B) cells.
Next, we evaluated whether IWR-1 also induced cell differentiation.
As shown in Fig. 5A and B, IWR-1
treatment promoted cell differentiation, as indicated by increased
CD11b expression. To further determine whether SAL-induced
differentiation of leukemic-cells were involved the canonical Wnt
signaling, we investigated the effect of combined treatment with
SAL and IWR-1 on NB4 and HL-60 cells. For this, NB4 and HL-60 cells
were treated with IWR-1 (10 µM), SAL (0.4 µM) or both or with ATRA
(1 µM) for three days. We observed that compared with SAL treatment
alone, the combination treatment with IWR-1 and SAL enhanced CD11b
expression as determined by performing flow cytometric and western
blot analyses (Fig. 5C and D).
These results indicated that combined treatment with SAL and IWR-1
increased cell differentiation and that SAL induced cell
differentiation by suppressing Wnt signaling.
Discussion
Although prognosis of patients with APL has
significantly improved since the introduction of ATRA and ATO, the
current treatment of APL is associated with some issues such as
drug toxicity, resistance and relapse. Therefore, it is necessary
to determine novel alternative therapeutic strategies to overcome
these issues and to improve the outcome of patients with APL. It
was determined that SAL is a potential agent for the elimination of
LSCs (20). In addition, it was
revealed that SAL exerts non-toxic effects on normal peripheral
blood cells (14,16). Therefore, SAL may be a potential
drug for the treatment of leukemia and we thus further investigated
its effect on apoptosis and differentiation in APL cells in this
study.
We firstly evaluated the effect of SAL on cell
viability and found that SAL significantly inhibited the growth of
NB4 and HL-60 cells (Fig. 1), which
was consistent with the result of previous studies assessing the
effect of SAL on leukemia cell proliferation (14,16).
Next, we investigated whether SAL-induced cell death was
accompanied by induction of cell apoptosis. Both flow cytometric
analysis and morphological changes revealed that SAL effectively
induced APL cells apoptosis (Fig. 2A
and B). Apoptosis is regulated by two central apoptotic
pathways: the extrinsic pathway (death receptor-mediated pathway)
and the intrinsic pathway (mitochondrial-mediated pathway). The
extrinsic pathway is activated via ligation of death receptors on
the cell surface membrane leading to activation of caspase-8,
followed by caspase-3. The intrinsic pathway is mediated by
different apoptotic stimuli. Most intrinsic signals induce
depolarization of the mitochondrial membrane and the release of
cytochrome c into the cytoplasm. The release of cytochrome
c activates caspase-9. This results in activation of
caspase-3, and commitment to cell death. This pathway is regulated
by the B-cell lymphoma 2 family of proteins comprised of 25 pro-
and anti-apoptotic members such as Bcl-2 and Bax (32). To determine the apoptotic pathway
induced by SAL in NB4 and HL-60 cells, we further evaluated Bcl-2,
Bax, cytochrome c, caspase-3, −8 and −9 and PARP expression.
We found that the expression of Bax/Bcl-2, cytochrome c,
cleaved caspase-9, cleaved caspase-3 and cleaved PARP increased
following SAL treatment (Fig. 2).
However, cleaved caspase-8 was not observed in our study. These
results revealed that SAL induced APL cell apoptosis through the
intrinsic pathway. Studies have revealed that inhibition of
Wnt/β-catenin signaling induces apoptosis of leukemic cells
(14,33). To determine whether β-catenin
signaling is involved in SAL-induced apoptosis, we detected the
levels of some Wnt-related proteins. We found that the expression
of LRP6, β-catenin and C-myc were also reduced after treatment with
0.8 and 1.6 µM SAL (inducing apoptosis; data not shown). Thus,
Wnt/β-catenin signaling was also involved in SAL-induced
apoptosis.
Since APL is characterized by the accumulation of
cells blocked in the promyelocytic stage, targeting cell
differentiation is an effective therapy for APL. However, little
information is available on role of SAL in modulating leukemia cell
differentiation. Therefore, we investigated the potential of SAL to
induce the differentiation of APL cell lines. We found that cells
treated with SAL exhibited typical morphological changes associated
with differentiation. Moreover, SAL treatment markedly increased
the percentage of NBT-positive and CD11b-positive cells and protein
levels of CD11b and C/EBPβ (Fig.
3). These results indicated that SAL effectively induced
leukemia cell differentiation.
Deregulation of Wnt signaling plays a critical role
in the pathogenesis of various types of cancers including AML
(34). Moreover, recent studies
have revealed that Wnt/β-catenin signaling is associated with
leukemia cell differentiation (27–29).
Therefore, we hypothesized that cell differentiation induced by SAL
involves the inhibition of Wnt/β-catenin signaling. β-catenin is at
the core of Wnt/β-catenin signaling. In the absence of Wnts,
cytoplasmic β-catenin is targeted for ubiquitination and
proteasomal degradation and is maintained at a low level. However,
the presence of Wnts which bind to Frizzled (Fzd) receptors and
lipoprotein receptor-related protein 5/6 (LRP5/6) leads to the
formation of the Wnt/Fzd/LRP5/6 complex on the cell surface. This
leads to stabilization of cytosolic β-catenin, which then
translocates into the nucleus to bind to transcription factors of
the TCF/LEF family and stimulates the expression of target genes
such as cyclin D1 and C-myc (31,35,36).
Results of western blot analysis performed in the present study
revealed that SAL blocked β-catenin, C-myc and cyclin D1 expression
(Fig. 4). Immunofluorescence
analysis revealed that the β-catenin level was decreased in both
the nucleus and cytoplasm of SAL- or ATRA-treated NB4 and HL-60
cells. These results indicated that SAL blocked Wnt/β-catenin
signaling in NB4 and HL-60 cells. This was consistent with a
previous study which revealed that SAL inhibited LRP6, a
co-receptor for Wnt ligands and activated Wnt/β-catenin signaling,
thus inhibiting Wnt/β-catenin signaling in breast and prostate
cancer cells (37). The present
study revealed that the LRP6 level was also reduced in SAL-treated
NB4 and HL-60 cells (Fig. 4C). To
further confirm whether cell differentiation induced by SAL was
associated with blocking Wnt/β-catenin signaling, we further
determined the effect of IWR-1, another Wnt inhibitor (38), on NB4 and HL-60 cells. We found that
IWR-1 also enhanced CD11b expression (Fig. 5B). Moreover, compared with SAL
treatment alone, the combination treatment with SAL and IWR-1
synergistically triggered the differentiation of NB4 and HL-60
cells (Fig. 5C and D).
Collectively, these results indicated that SAL induced leukemia
cell differentiation by inhibiting Wnt/β-catenin signaling.
Autophagy is a well-known cellular process that
plays an important role in the regulation of leukemia cell
differentiation. It was previously reported that a high β-catenin
level inhibited autophagy, thus decreasing the differentiation of
AML cells (39), and autophagy was
upregulated during ATRA-mediated APL cell differentiation (40). Recent studies have revealed that
autophagy plays a vital role in regulating PML-RARα degradation by
p62/SQSTM1 and APL cell differentiation (41). Notably, a recent study revealed that
SAL upregulated p62/SQSTM1 expression and activated an autophagic
response in AML cell lines (16).
Thus, these findings indicated that autophagy may be involved in
SAL-induced cell differentiation. However, additional studies are
needed to investigate the effect of autophagy on SAL-mediated cell
differentiation. In addition, a previous study demonstrated that
SAL activated the Toll-like receptor pathway in AML cells (16). Activation of Toll-like receptor
pathways has been revealed to promote differentiation and growth
inhibition in AML cells (42).
Therefore, cell differentiation induced by SAL may be related to
Toll-like receptor pathways.
In summary, we found that SAL effectively inhibited
the proliferation and induced the apoptosis of NB4 and HL-60 cells.
To the best of our knowledge, this is the first study to reveal
that SAL induced the differentiation of APL cells, possibly by
blocking Wnt/β-catenin signaling. Our results provide a foundation
to broaden the clinical application of SAL which may be a promising
agent for treatment of APL or other AML types. Further studies are
warranted to investigate the combination of ATRA and SAL on APL
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation Project of CQ CSTC (grant no. 2011BA5037).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and BZL conceived and designed the study. YZ, LL,
SFY, MC, LWL, ZLS, CLX, LGG and TX performed the experiments. YZ
wrote the paper. YZ, LZ and BZL reviewed and edited the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lafage-Pochitaloff M, Alcalay M, Brunel V,
Longo L, Sainty D, Simonetti J, Birg F and Pelicci PG: Acute
promyelocytic leukemia cases with nonreciprocal PML/RARa or
RARa/PML fusion genes. Blood. 85:1169–1174. 1995.PubMed/NCBI
|
|
2
|
Rodeghiero F and Castaman G: The
pathophysiology and treatment of hemorrhagic syndrome of acute
promyelocytic leukemia. Leukemia. 8 (Suppl 2):S20–S26.
1994.PubMed/NCBI
|
|
3
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang ZY: Mechanism of action of all-trans
retinoic acid and arsenic trioxide in the treatment of acute
promyelocytic leukemia. Gan To Kagaku Ryoho. 29 (Suppl 1):214–218.
2002.PubMed/NCBI
|
|
5
|
Tallman MS: Treatment of relapsed or
refractory acute promyelocytic leukemia. Best Pract Res Clin
Haematol. 20:57–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomita A, Kiyoi H and Naoe T: Mechanisms
of action and resistance to all-trans retinoic acid (ATRA) and
arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int J
Hematol. 97:717–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanz MA and Montesinos P: How we prevent
and treat differentiation syndrome in patients with acute
promyelocytic leukemia. Blood. 123:2777–2782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Butaye P, Devriese LA and Haesebrouck F:
Antimicrobial growth promoters used in animal feed: Effects of less
well known antibiotics on gram-positive bacteria. Clin Microbiol
Rev. 16:175–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Z, Sperl B, Ullrich A and Knyazev P:
Metformin and salinomycin as the best combination for the
eradication of NSCLC monolayer cells and their alveospheres (cancer
stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status.
Oncotarget. 5:12877–12890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li T, Liu X, Shen Q, Yang W, Huo Z, Liu Q,
Jiao H and Chen J: Salinomycin exerts anti-angiogenic and
anti-tumorigenic activities by inhibiting vascular endothelial
growth factor receptor 2-mediated angiogenesis. Oncotarget.
7:26580–26592. 2016.PubMed/NCBI
|
|
12
|
Mirkheshti N, Park S, Jiang S, Cropper J,
Werner SL, Song CS and Chatterjee B: Dual targeting of androgen
receptor and mTORC1 by salinomycin in prostate cancer. Oncotarget.
7:62240–62254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xipell E, Gonzalez-Huarriz M, Martinez de
Irujo JJ, García-Garzón A, Lang FF, Jiang H, Fueyo J, Gomez-Manzano
C and Alonso MM: Salinomycin induced ROS results in abortive
autophagy and leads to regulated necrosis in glioblastoma.
Oncotarget. 7:30626–30641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niwa AM, D Epiro GF, Marques LA, Semprebon
SC, Sartori D, Ribeiro LR and Mantovani MS: Salinomycin efficiency
assessment in non-tumor (HB4a) and tumor (MCF-7) human breast
cells. Naunyn Schmiedebergs Arch Pharmacol. 389:557–571. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roulston GD, Burt CL, Kettyle LM, Matchett
KB, Keenan HL, Mulgrew NM, Ramsey JM, Dougan C, McKiernan J,
Grishagin IV, et al: Low-dose salinomycin induces anti-leukemic
responses in AML and MLL. Oncotarget. 7:73448–73461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HG, Shin SJ and Chung HW: Salinomycin
reduces stemness and induces apoptosis on human ovarian cancer stem
cell. J Gynecol Oncol. 28:e142017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou ZZ, Nie PP, Li YW, Hou BX, Rui-Li, Shi
XP, Ma ZK, Han BW and Luo XY: Synergistic induction of apoptosis by
salinomycin and gefitinib through lysosomal and mitochondrial
dependent pathway overcomes gefitinib resistance in colorectal
cancer. Oncotarget. 8:22414–22432. 2017.PubMed/NCBI
|
|
22
|
Hermawan A, Wagner E and Roidl A:
Consecutive salinomycin treatment reduces doxorubicin resistance of
breast tumor cells by diminishing drug efflux pump expression and
activity. Oncol Rep. 35:1732–1740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li R, Dong T, Hu C, Lu J, Dai J and Liu P:
Salinomycin repressed the epithelial-mesenchymal transition of
epithelial ovarian cancer cells via downregulating Wnt/β-catenin
pathway. Onco Targets Ther. 10:1317–1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simon M, Grandage VL, Linch DC and Khwaja
A: Constitutive activation of the Wnt/beta-catenin signalling
pathway in acute myeloid leukaemia. Oncogene. 24:2410–2420. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luis TC, Ichii M, Brugman MH, Kincade P
and Staal FJ: Wnt signaling strength regulates normal hematopoiesis
and its deregulation is involved in leukemia development. Leukemia.
26:414–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zang S, Liu N, Wang H, Wald DN, Shao N,
Zhang J, Ma D, Ji C and Tse W: Wnt signaling is involved in
6-benzylthioinosine-induced AML cell differentiation. BMC Cancer.
14:8862014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheng Y, Ju W, Huang Y, Li J, Ozer H, Qiao
X and Qian Z: Activation of wnt/β-catenin signaling blocks
monocyte-macrophage differentiation through antagonizing
PU.1-targeted gene transcription. Leukemia. 30:2106–2109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gandillet A, Park S, Lassailly F,
Griessinger E, Vargaftig J, Filby A, Lister TA and Bonnet D:
Heterogeneous sensitivity of human acute myeloid leukemia to
β-catenin down-modulation. Leukemia. 25:770–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SC, Kim OH, Lee SK and Kim SJ: IWR-1
inhibits epithelial-mesenchymal transition of colorectal cancer
cells through suppressing Wnt/β-catenin signaling as well as
survivin expression. Oncotarget. 6:27146–27159. 2015.PubMed/NCBI
|
|
31
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F,
Maestro R, et al: Multifaceted roles of GSK-3 and Wnt/β-catenin in
hematopoiesis and leukemogenesis: Opportunities for therapeutic
intervention. Leukemia. 28:15–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu G and Shi Y: Apoptosis signaling
pathways and lymphocyte homeostasis. Cell Res. 17:759–771. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Liu ZH, Xia J, Li XP, Li KQ, Xiong
W, Li J and Chen DL: 20(S)-ginsenoside Rh2 inhibits the
proliferation and induces the apoptosis of KG-1a cells through the
Wnt/β-catenin signaling pathway. Oncol Rep. 36:137–146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Staal FJ, Famili F, Garcia Perez L and
Pike-Overzet K: Aberrant Wnt Signaling in Leukemia. Cancers
(Basel). 8:82016. View Article : Google Scholar :
|
|
35
|
King TD, Suto MJ and Li Y: The
Wnt/β-catenin signaling pathway: A potential therapeutic target in
the treatment of triple negative breast cancer. J Cell Biochem.
113:13–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmadzadeh A, Norozi F, Shahrabi S,
Shahjahani M and Saki N: Wnt/β-catenin signaling in bone marrow
niche. Cell Tissue Res. 363:321–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu W and Li Y: Salinomycin suppresses LRP6
expression and inhibits both Wnt/β-catenin and mTORC1 signaling in
breast and prostate cancer cells. J Cell Biochem. 115:1799–1807.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kühn K, Cott C, Bohler S, Aigal S, Zheng
S, Villringer S, Imberty A, Claudinon J and Römer W: The interplay
of autophagy and β-Catenin signaling regulates differentiation in
acute myeloid leukemia. Cell Death Discov. 1:150312015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Orfali N, O'Donovan TR, Nyhan MJ,
Britschgi A, Tschan MP, Cahill MR, Mongan NP, Gudas LJ and McKenna
SL: Induction of autophagy is a key component of all-trans-retinoic
acid-induced differentiation in leukemia cells and a potential
target for pharmacologic modulation. Exp Hematol. 43:781–793. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Cao L, Kang R, Yang M, Liu L, Zhao
Y, Yu Y, Xie M, Yin X, Livesey KM, et al: Autophagy regulates
myeloid cell differentiation by p62/SQSTM1-mediated degradation of
PML-RARα oncoprotein. Autophagy. 7:401–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ignatz-Hoover JJ, Wang H, Moreton SA,
Chakrabarti A, Agarwal MK, Sun K, Gupta K and Wald DN: The role of
TLR8 signaling in acute myeloid leukemia differentiation. Leukemia.
29:918–926. 2015. View Article : Google Scholar : PubMed/NCBI
|