Introduction
Cancers are diseases involving the uncontrollable
growth of abnormal cells that overcome the usual limitations to
cell division. Cancer is now a relatively common disease. For
example, there were about 90.5 million individuals diagnosed with
cancer in 2015 (1), and more than
14.1 million new cases of cancer occur each year. Cancer is a
leading cause of death worldwide, accounting for 8.8 million deaths
in 2015; 15.7% of all deaths. The most common causes for
cancer-related death are lung, liver, colorectal, stomach and
breast cancers (2). Cancer is
becoming an enormous burden on society in high- and low-income
countries alike, costing an estimated 1.16 trillion USD worldwide
in 2010 (3).
The global incidence of cancer is increasing due to
the growth and aging of the population, as well as an increasing
prevalence of established risk factors such as hormone replacement
therapy, obesity, physical inactivity, radiation and chemical
exposure, infection, autoimmune disease, smoking and changing
reproductive patterns, as are associated with urbanization and
economic development (4). Some
hormones play a role in the development of cancer by promoting cell
proliferation. For example, a previous study indicated that there
is a positive correlation between testosterone level and prostate
cancer (5). Hormones are also
important agents in other sex-specific cancers, such as cancers of
the ovary, testis, endometrium and breast. Furthermore, sex
disparity in the incidence, aggressiveness, response to therapy and
prognosis has been observed for a variety of cancers, such as lung,
prostate, colorectal, stomach, breast and uterine cancer (6).
As cancer is widespread and lethal, it is considered
by some to be the most significant health problem faced by
humanity. The majority of cancer deaths are associated with
metastasis, but the mechanisms for metastasis and cancer spread are
poorly characterized (7). A recent
study indicated that dietary lipids may promote the metastasis of
cancer cells (8). Adipocytes and
their functionally related cells are strong candidates for the
promotion of carcinogenesis and the influencing of tumor behavior
(9). Body mass index (BMI) is used
as a general measure of mass and can serve as an unspecific means
of estimating adiposity (10).
Obesity is a growing public health issue and the second most common
preventable cause of death (11).
It is estimated that over one billion adults are overweight and 315
million are obese worldwide (12).
Economically developed countries have the highest prevalence of
obesity. In USA, around one in three (36%) adults is obese
(12). Researchers have identified
eight types of cancer linked to excess weight and obesity: Stomach,
liver, gall bladder, pancreas, ovary, thyroid cancer, meningioma
and multiple myeloma (13).
However, to the best of our knowledge, the relationship between BMI
and cancer has not been systemically investigated.
Cancer survival rates, including two- and five-year
survival, are used to estimate the prognosis for a specific cancer
(14,15). The chance of survival depends on the
type of cancer and the extent of disease at the start of treatment
(16). The early detection of
cancer is associated with a good prognosis (16,17).
Recently, with developments in the treatment of cancer, the
survival rates for patients with various types of cancer have been
improved (18–21). For example, the overall mortality
rate for cancer in the USA has dropped by at least one-fifth over
the past two decades according to new statistics from the American
Cancer Society (22). The
improvements in survival rates can also be attributed to
improvements in diagnosis and prognosis (16,23).
The importance of patient characteristics to long-term survival is
the subject of much debate (24–26).
The factors affecting cancer survival rates include sex, age,
geographical location and the pathological type of cancer (27–30).
The most significant risk factor for developing cancer is age. The
effect of aging on cancer is complicated by factors such as DNA
damage and inflammation promoting tumorigenesis, and factors such
as vascular aging and endocrine changes inhibiting it (31).
The epidemiology of cancer has changed with the
advancement of technology and social development (32). By using The Cancer Genome Atlas
database (TCGA), the present study was designed to systemically
investigate the effect of sex, BMI and age on cancer incidence and
survival rates for various cancer types. It is anticipated that
clarifying these issues will lead to a deeper understanding of
cancer, as will be necessary for the development of precision and
personalized medicine.
Materials and methods
Clinical data preparation
TCGA is a project, started in 2005, to improve the
diagnosis, treatment and prevention of cancer through a better
understanding of its genetic basis. This project is supervised by
the National Cancer Institute's Center for Cancer Genomics and the
National Human Genome Research Institute, which are funded by the
US government. Clinical data of the patients from the TCGA database
(version 2016_01_28, http://cancergenome.nih.gov/) were downloaded using
the Broad GDAC Firehose (http://gdac.broadinstitute.org/). We downloaded the
clinical data of 14,504 cancer cases, including 38 different types
of cancer. The clinical data included patient ID number, vital
status, overall survival time, age at initial pathologic diagnosis,
time to last follow-up, gender, height and weight.
Body mass index (BMI)
BMI, a measure calculated from height and weight,
was used to categorize a person as underweight, normal weight,
overweight, or obese (33). We
included only BMI measurements taken after the age of 20, assuming
that subjects had reached their adult height by this age. BMI was
computed as each patient's weight in kilograms divided by the
square of their height in meters (kg/m2). The patients
were principally divided into 3 groups on the basis of BMI:
Underweight (<18.5 kg/m2), normal (≥18.5 to <25.0
kg/m2) and high (≥25.0 kg/m2) (33). The high group included two
sub-groups: Overweight (≥25 to <30 kg/m2) and obese
(≥30 kg/m2).
Two- and five-year survival rates
The survival rate is the percentage of individuals
with a disease still alive after a given period of time after the
initial diagnosis or treatment, which can be used to estimate the
prognosis for that disease (27).
An overall survival rate for a type of cancer includes individuals
of all ages and health conditions, including those diagnosed
particularly early or late. There are various types of survival
rates, but two types of survival rates, i.e. two- and five-year
survival rates, are more commonly cited in cancer statistics
(34,35). For aggressive cancers with a shorter
life expectancy following diagnosis, two-year survival statistics
are typically used to estimate prognosis. Some cancers can recur
many years after their initial identification and treatment. If
they have not recurred by five years after the initial pathogenic
diagnosis, the chance of a later recurrence is very small (36). Therefore, the five-year survival
rate can be used to compare the effectiveness of treatment. In the
present study, two- and five-year survival rates for each of 38
types of cancers were calculated by Kaplan Meier curves and were
compared by performing the Tarone-Ware test.
Statistical analysis
Statistical analysis system (SAS) is a software
suite that can mine, alter, manage and retrieve data from a variety
of sources and perform statistical analysis (37). The SAS software suite has >200
components. The SAS/STAT component was used to perform the
statistical analysis in this present study. A t-test was used to
determine if two sets of data were significantly different from
each other, such as sex and BMI for different types of cancer
(38). The chi-squared test was
used to determine whether there was a significant difference
between the expected and observed frequencies in one or more
categories, such as incidence, two- and five-year survival rate for
different types of cancer (39).
The P-value is the probability for a given statistical model that,
when the null hypothesis is true, the statistical summary (such as
the sample mean difference between two compared groups) would be
the same as or of greater magnitude than the actual observed
results. The cut-off for statistical significance was set as
P<0.05. We performed a multiple testing correction procedure
(Bonferroni adjustment) to adjust our statistical confidence
intervals based on the number of tests performed. Origin is a
computer program for interactive scientific graphing and data
analysis. The bar charts and line charts were produced with Origin
9.0 software (https://www.originlab.com). R 3.5.0 (https://www.r-project.org/) is a software for
statistical computing and graphics (40). The pheatmap package of R (https://www.r-project.org/) was used to produce heat
maps.
Results
Sex disparity is identified in the
proportion of cancer incidence
There were 38 types of cancer and 14,504 cancer
cases in TCGA version 2016_01_28. A sex disparity in cancer
incidence, aggressiveness and prognosis has previously been
observed in a variety of cancer types. To systemically investigate
the sex disparity in cancer incidence, the number of cases for each
of the 38 types of cancers were analyzed. In this TCGA version, 2
types of cancer were specific to males, including prostate
adenocarcinoma (PRAD) and testicular germ cell tumors (TGCT) and 4
were specific to females, including cervical and endocervical
cancers (CESC), uterine corpus endometrial carcinoma (UCEC),
uterine carcinosarcoma (UCS) and ovarian serous cystadenocarcinoma
(OV).
The sex ratios (male/female) for the incidence of
all 38 cancers and the 32 non-sex-specific cancers were 1.04 and
1.2, respectively. This indicated that men are more susceptible to
cancer compared with women. However, the result was reversed for
some cancers, including breast invasive carcinoma (BRCA), thyroid
carcinoma (THCA), adrenocortical carcinoma (ACC), lung
adenocarcinoma (LUAD), pheochromocytoma and paraganglioma (PCPG)
and sarcoma (SARC) (all P-values <0.024; P<0.05). In
addition, there was no sex disparity in the proportion of
cholangiocarcinoma (CHOL), lymphoid neoplasm diffuse large B-cell
lymphoma (DLBC), kidney chromophobe (KICH), acute myeloid leukemia
(LAML), pancreatic adenocarcinoma (PAAD), rectum adenocarcinoma
(READ), thymoma (THYM) and uveal melanoma (UVM) (Fig. 1). The top 10 cancers types in males
were glioma (GBMLGG), pan-kidney cohort (KIPAN), PRAD, stomach and
esophageal carcinoma (STES), head and neck squamous cell carcinoma
(HNSC), lung squamous cell carcinoma (LUSC), glioblastoma
multiforme (GBM), kidney renal clear cell carcinoma (KIRC),
colorectal adenocarcinoma (COADREAD) and bladder urothelial
carcinoma (BLCA). Furthermore, the top 10 cancer types in females
were BRCA, OV, UCEC, GBMLGG, THCA, KIPAN, CESC, COADREAD, LUAD and
GBM (Fig. 1). This result indicates
that four types of cancers, i.e. GBMLGG, KIPAN, GBM and COADREAD,
are prevalent in both males and females.
To investigate the sex disparity in the cancer
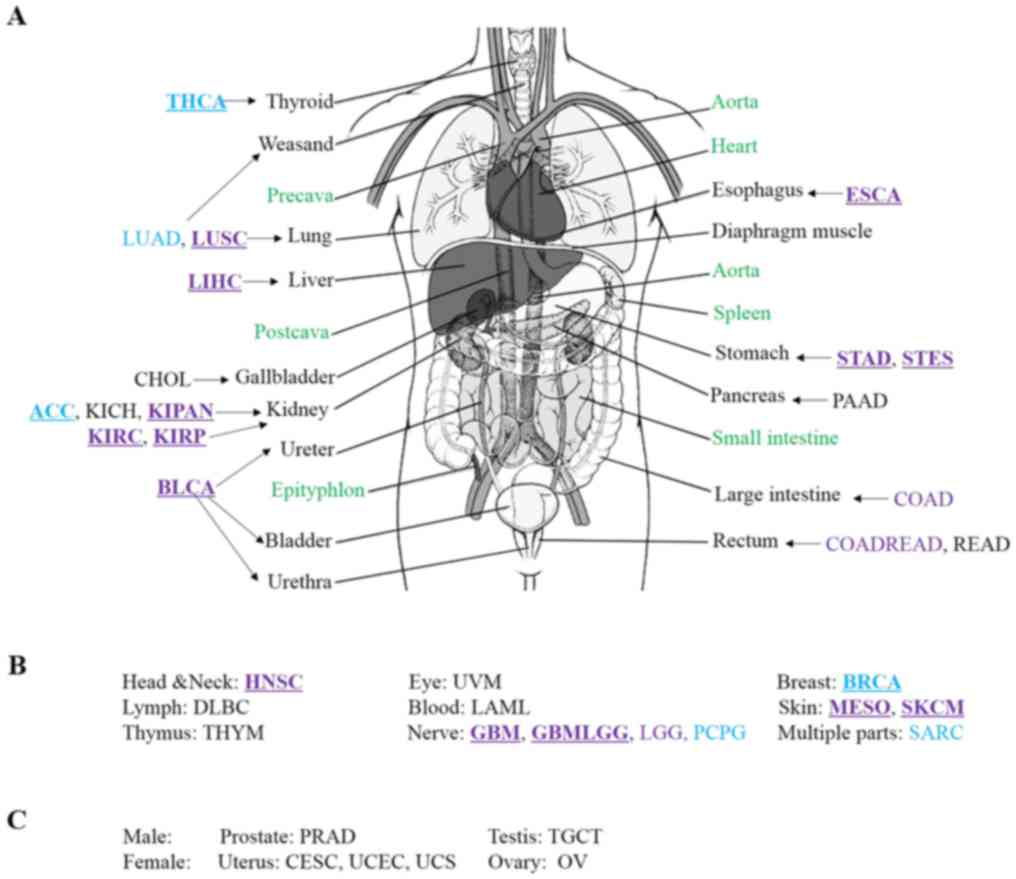
predilection site, a human body map for cancer was produced. As
shown in Fig. 2, different colors
were used to show the sex differences in the distribution of cancer
in human body. According to this data version, an interesting
phenomenon was identified, in that primary cancer did not occur in
four organs, i.e. the heart, spleen, small intestine and
epityphlon. There was also no primary cancer incidence in the
precava, postcava, aorta and diaphragm muscle (Fig. 2). These organs are not typically
associated with chronic inflammation (41). Hence, chronic inflammation may be
the most important cause of cancer, which is consistent with the
results of previous studies (41,42).
Males were more susceptible to cancers in the lung, liver, kidney,
ureter, bladder, urethra, esophagus, stomach, head and neck,
nervous system and skin, when compared with females. However,
females were more susceptible to cancers in the thyroid, breast and
adrenal cortex, when compared with males. These results indicated
that predilection sites of cancer were different for males and
females.
High BMI is associated with an
increased risk of cancer
In this version of TCGA, there were 3,432 patients
>20 years with recorded values for weight and height, from 18
types of cancer. According to the classification standard of the
World Health Organization (WHO), for all 18 types of cancer, 64.42%
of patients had a high BMI (≥25), including 32.34% overweight
patients (BMI ≥25 to <30) and 32.08% obese patients (BMI ≥30).
The mean percentage of patients with high BMI for each cancer type
was a minimum of 56%, except in DLBC, esophageal carcinoma (ESCA),
liver hepatocellular carcinoma (LIHC) and STES (Fig. 3). Furthermore, the mean percentage
of patients with low BMI (BMI <18.5) for all types of cancer was
2.7% and did not exceed 7% for any type of cancer (Fig. 3). These results showed that high BMI
(≥25) was likely to be a risk factor for many types of cancer
(P=9.97E-28, P<0.05), with the exception of DLBC, ESCA, LIHC and
STES and these 18 types of cancer were less likely in people with
low BMI (<18.5).
As displayed in Fig.
3, patients with high BMI accounted for a particularly high
proportion in CHOL, READ, KIPAN, kidney renal papillary cell
carcinoma (KIRP) and were most predominant in UCES (81.6%). The
result suggested that high BMI (≥25) was a high risk factor for
cancer in the gallbladder, rectum, kidney and uterus. Furthermore,
the proportion of obese patients with UCES and UCS were 59.6 and
38.5%. These results indicated that high BMI (≥30) was strongly
associated with uterine cancer and that BMI affects different types
of cancer in different ways.
The incidence of cancer demonstrates a
young age trend
The majority of entries in the TCGA database have a
record of the initial pathologic diagnosis age. The incidence of
cancer in young adults has been increasing over the last 50 years
(32). Early diagnosis is
beneficial for the treatment and prognosis of cancer. To
investigate which types of cancer are associated with younger
patients, all cases in the TCGA database were grouped by the
initial pathologic diagnosis age. They were divided into 9 groups:
Group 1 (≤10) (no >10 years old); group 2 (10–20);
group 3 (21–30); group 4 (31–40);
group 5 (41–50); group 6 (51–60);
group 7 (61–70); group 8 (71–80); and group 9 (>80 years).
Cancer occurred in every age group. According to TCGA data, 0.01%
of cancer cases occurred in patients ≤10 years old, and 4.9% of
cases occurred in patients ≤30 years old (Fig. 4). With an increase in age, the
relative distribution of cancer increased from group to group, with
a peak in group 7. After the age of 70, the proportion of the cases
of cancer decreased, likely due to the smaller proportion of the
population at this age. After the age of 80, the proportion of
cases of cancer decreased markedly, which can be attributed to the
average life expectancy (43).
 | Figure 4.The distribution of age groups for
all types of cancer. The age groups 1, 2, 3, 4, 5, 6, 7, 8 and 9
represent an initial pathologic diagnosis age of 0–10, 11–20,
21–30, 31–40, 41–50, 51–60, 61–70, 71–80 and >80,
respectively. |
There were 2, 16 and 27 types of cancers in groups
1, 2 and 3, respectively (Fig. 5),
indicating that many types of cancer occur in young people. These
cancers occurred in sites throughout the body, including the
kidney, liver, breast, stomach, uterus, gallbladder, lymph, thymus,
esophagus, nerve, head and neck, ovary, blood, skin, testis and
eye. In addition, 6 types of cancer were skewed towards occurring
in young individuals: ACC, CESC, brain lower grade glioma (LGG),
PCPG, TGCT and THCA. ACC, CESC, LGG, PCPG, TGCT and THCA were most
common group 3 (16.3%), 4 (21.82%), 3 and 4 (17.09 and 30.29%), 3
and 4 (12.85 and 18.99%), 3 and 4 (40.30 and 37.31%), and group 3
and 4 (11.53 and 23.26%), respectively. In particular, 77.61% of
TGCT patients were aged between 20 and 40. The results suggested
that TGCT predominately occurs at reproductive age, which is
potentially a significant finding for individuals of this age.
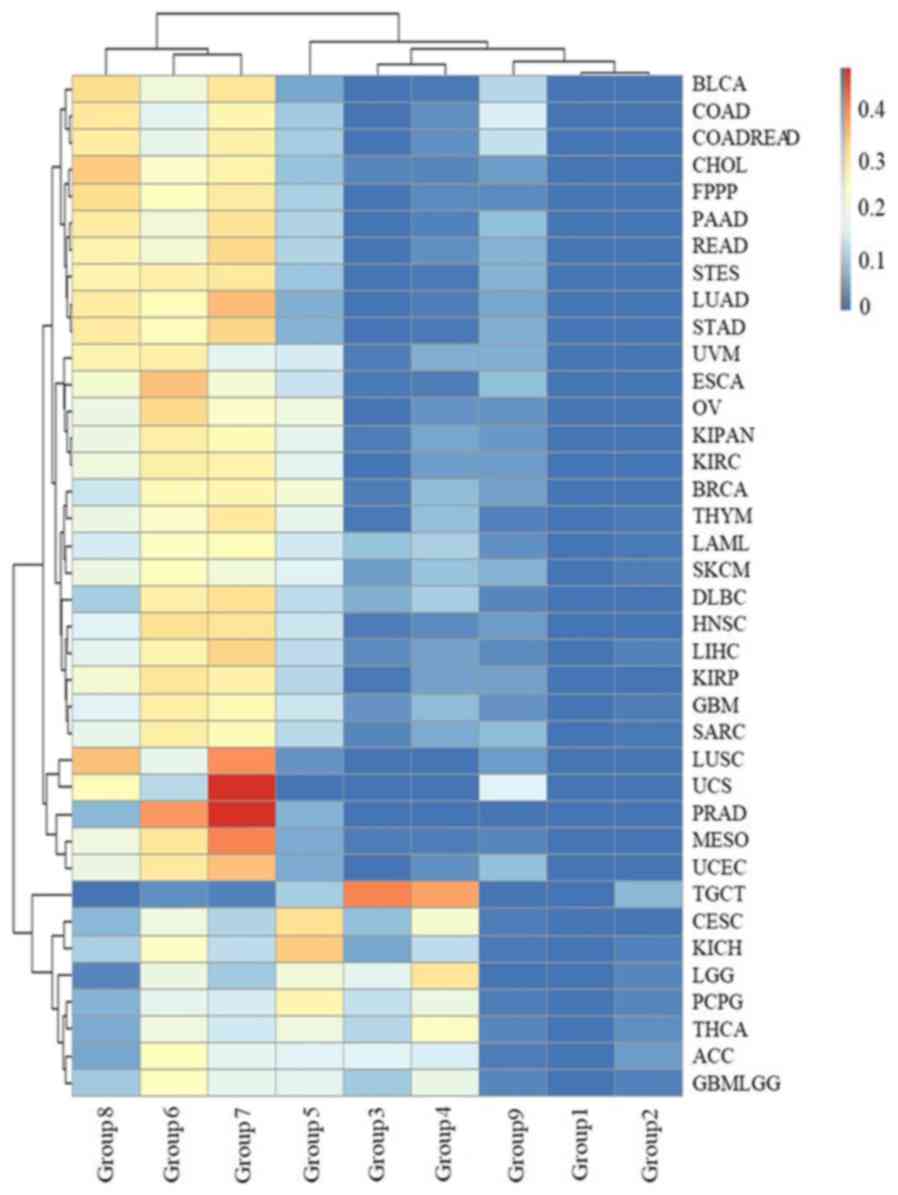 | Figure 5.The distribution of age groups for
each type of cancer. The rows and columns represent types of cancer
and age groups. The color represents the proportion of the cases
distributed in the age groups (0 to 0.4749). The age groups 1, 2,
3, 4, 5, 6, 7, 8 and 9 represent an initial pathologic diagnosis
age of 0–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80 and
>80, respectively. |
Sex, BMI and initial pathologic
diagnosis age impact the survival rate in cancer
To investigate the factors that affect the survival
rate, two- and five-year survival rates stratified by sex, BMI
ranges, initial pathologic diagnosis age groups and cancer types
were analyzed. We found that the two-year and five-year survival
rates were different for each type of cancer (Fig. 6A). GBM and PAAD had the lowest
two-year survival rates, at ≤30%. In addition, the two-year
survival rates for LAML and mesothelioma (MESO) were in the range
of 30–40%, and two-year survival rates for ESCA, STES, GBMLGG,
stomach adenocarcinoma (STAD), BLCA and UCS were in the range of
40–50% (Fig. 6A). Twenty-two types
of cancer were associated with a high (>70%) two-year survival
rate, i.e. ACC, BRCA, CESC, colon adenocarcinoma (COAD), COADREAD,
DLBC, KICH, KIPAN, KIRC, KIRP, LGG, OV, PCPG, PRAD, READ, SARC,
skin cutaneous melanoma (SKCM), TGCT, THCA, THYM, UCEC and UVM. The
10 types of cancers with the lowest two-year survival rates
occurred in the nervous system, pancreas, blood, skin, esophagus,
stomach, ureter and uterus, which develop rapidly (such as PAAD and
LAML), have previously been associated with a poor prognosis (such
as MESO), or are not easy to find in early stages (such as GBM and
GBMLGG) (16,23).
For the five-year survival rate, survival rates of
GBM were ≤5%, rates for ESCA, MESO, LAML, PAAD, STES, CHOL, and
STAD were in the 5–10% range, rates for UCS, UVM, and GBMLGG were
in the 10–20% range. However, the five-year survival rate was ≥70%
for BRCA, KICH, PCPG, PRAD, TGCT, THCA and THYM. These results
indicated there are insufficient treatment options for GBM, ESCA,
MESO, LAML, PAAD, STES, CHOL, STAD, UCS, UVM and GBMLGG. The
five-year survival rate was lower than the two-year survival rate
for all cancer types (Fig. 6B). The
differences between the two- and five-year survival rates for each
of CHOL, COAD, COADREAD, LGG, LUAD, OV, READ and UVM were ≥40%,
indicating that these types of cancer have a lack of effective
treatment available. The results suggested that the prognoses for
these eight cancers would benefit from an early diagnosis process,
as well as the development of new drugs to treat them. However, the
difference for GBM, KICH, PCPG, PRAD, TGCT and THYM was in the
5–18% range. Furthermore, there was no statistical discrepancy
between the two- and five-year survival rates for TGCT. The small
differences for these types were attributed to two separate
reasons: Firstly, the majority of cancer patients died within two
years after initial pathologic diagnosis in some types, such as
GBM. Secondly, other types of cancer in the list have an effective
treatment and prognosis, such as KICH, PCPG, PRAD, TGCT, THYM and
particularly, TGCT.
It is necessary to identify which cancers have a
large sex-specific difference, since previous studies have shown
potential sex-specific differences in the pathophysiology, clinical
presentation and treatment of cancers (44). The two- and five-year survival rates
for males and females were different for all cancer types (Fig. 6C and D). Compared with males,
females had a higher two-year survival rate for nine cancer types,
i.e. BRCA, CHOL, ESCA, GBM, GBMLGG, LUAD, LUSC, STES and UVM, but
lower two-year survival rates for three cancers, i.e. HNSC, KIRP
and SARC (Fig. 6E). For other
cancers, there was no significant difference between the two-year
survival rate for females and males (for all other cancer types,
P=0.19, P>0.05). For the five-year survival rate, the sex
distribution changed. Compared with males, females had a higher
five-year survival rate for COAD, COADREAD, ESCA, LGG, LUSC, MESO,
PCPG, READ and THCA, but a lower five-year survival rate for DLBC,
KIRP, LUAD and THYM (Fig. 6E). For
other cancer types, there was no significant difference in
five-year survival rate between females and males (for all other
cancer types, P=0.17, P>0.05).
In recent years, researchers have stressed the
importance of maintaining a healthy BMI (10,33).
Therefore, the distribution of survival rates for different BMI
ranges were analyzed for each type of cancer. We identified a
positive correlation between a high BMI and the corresponding
two/five-year survival rate in cancer samples (r=0.53, Spearman
correlation coefficient, Figs. 3
and 6A). In addition, as displayed
in Fig. 6F, there was an
interesting phenomenon in that patients with a high BMI (>25)
had a higher two/five-year survival rate compared with patients
with a normal BMI (18.5 to 25). The results may indicate that human
with high BMI, who have more energy, could better tolerate a long
process of physical deterioration (42).
The cancer survival rate was found to be related to
the initial pathologic diagnosis age, i.e. the younger the
pathologic diagnosis age, the better prognosis and the smaller
mortality for all types of cancer with the exception of TGCT
(Fig. 7). The age of patient death
for TGCT was ≤40 years of age in all cases. Furthermore, >40% of
deceased patients with LGG, CESC, PCPG and ACC were in age groups
1–5, and 30% deceased patients with DLBC, SKCM and GBMLGG were
<50 years of age. These results indicated that more attention
should be paid to the biochemical indicators of nerve, kidney,
uterus, lymph and skin tumors in younger individuals. However, the
initial pathologic diagnosis ages for deceased patients with most
types of cancer were distributed in age groups 6, 7 and 8 (Fig. 7), including >80% for LUSC, PRAD,
MESO, THYM, STAD, LUAD, READ, STES, BLCA, CHOL, KIRP, KIRC, KIPAN
and ESCA.
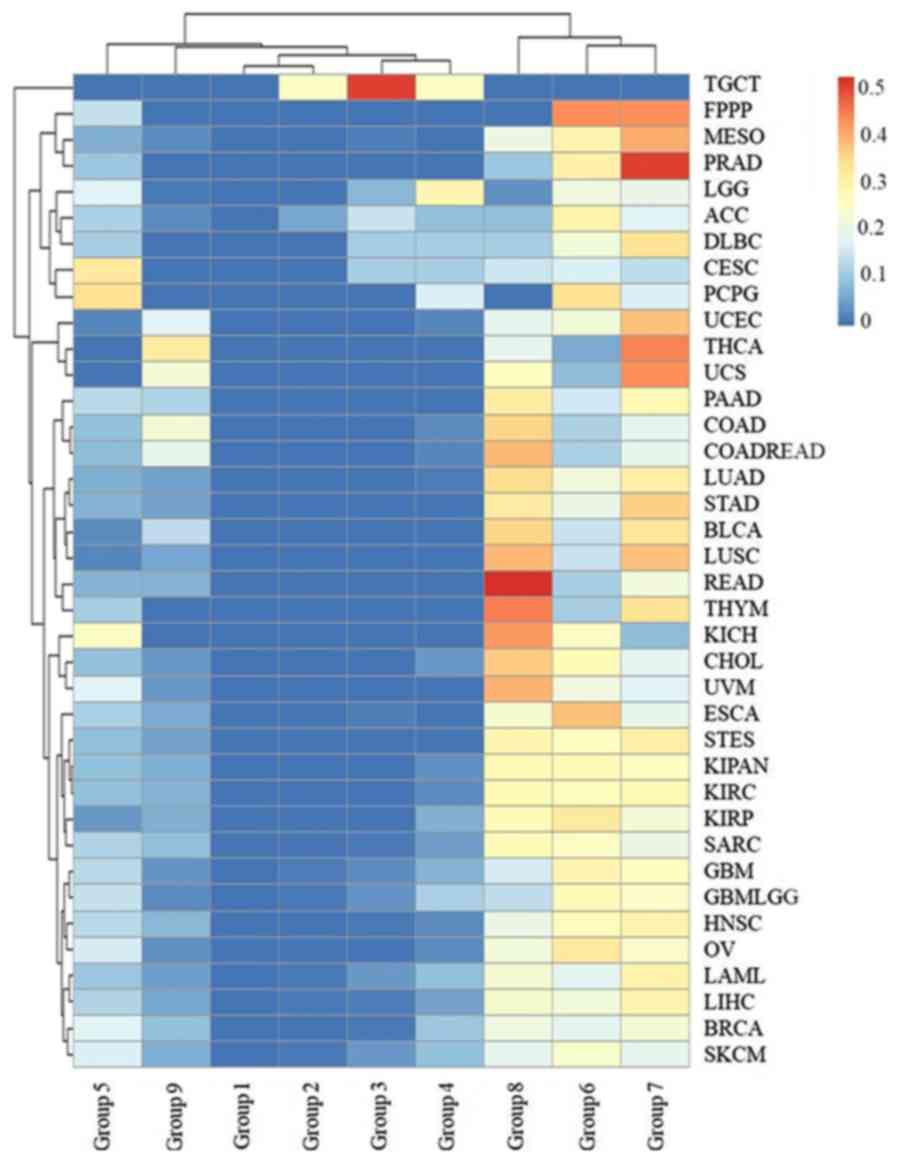 | Figure 7.The distribution of the initial
pathologic diagnosis age groups for the deceased patients with each
type of cancer. The rows and columns represent the types of cancer
and age groups. The color represents the proportion of the cases
distributed in the age group (0–0.5185). The age groups 1, 2, 3, 4,
5, 6, 7, 8 and 9 represent an initial pathologic diagnosis age of
0–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80 and >80,
respectively. |
Discussion
Previous studies have indicated a sex disparity in
cancer incidence, aggressiveness, response to therapy and prognosis
in a variety of cancers, including breast, endometrial, prostate,
ovarian and testicular cancer (45,46).
In the present study, we systemically observed the cancer gender
disparity for 38 types of cancer. Cancer sex disparity was not only
identified for sex-related cancers, including cancers of the
prostate, testis, uterus, ovary and breast, but also cancers of
other organs, including the lung, liver, kidney, digestive tract,
urethra, nerves, skin and head. The causes for the disparity may be
the distinctive sex hormone expression between males and females,
which plays an important role in cancer cell proliferation,
apoptosis and differentiation, indicating a likely involvement in
carcinogenesis. For example, high levels of testosterone, and
estrogen and progesterone are risk factors for prostate cancer and
breast cancer, respectively (47,48).
The reasons of the disparity for other cancer types are not fully
understood, and are likely due to lifestyle, psychological distress
(anxiety, depression), social distress (family problems,
job-related problems) and spiritual distress (spiritual pain,
spiritual alienation) (46). For
example, compared with females, males are more susceptible to
cancers in the lung and liver, likely due to their increased
smoking and alcohol consumption rates (49). These results indicated that it is
necessary to differentiate cancer screening practices and
treatments between females and males. During regular physical
examinations, we should pay more attention to the KIPAN, PRAD,
STES, HNSC, LUSC, KIRC and BLCA for men, and the BRCA, OV, UCEC,
THCA, KIPAN, CESC and LUAD for women.
The adult BMI of many countries is trending higher.
A previous study indicated that the global obesity prevalence will
reach 18% for men and 21% for women. Severe obesity will surpass 6%
for men and 9% for women by 2025 (50). Being underweight, and severe and
morbid obesity, are associated with elevated risks of adverse
health outcomes (51). In the
present study, we found that high BMI (≤25) is potentially a risk
factor for many types of cancer. However, there were four types of
cancer, i.e., DLBC, ESCA, LIHC and STES, which were associated with
a low average BMI, which may be because the initial clinical
symptoms for these cancers included weight loss, resulting in a low
weight at initial pathologic diagnosis. Patients with a low BMI
(<18.5) had a reduced incidence for all 38 types of cancer.
These results indicated that adipocytes and their functionally
related cells likely play important roles in the promotion of
carcinogenesis. For example, adipocytes are thought to interact
with cancer cells in the breast to promote the malignant growth of
breast tumors (52). Furthermore,
more and more research has confirmed a relationship between lipid
metabolism and the proliferation of cancers (53). However, the mechanism of adipocytes
and their functionally related cells in the promotion of
carcinogenesis is not fully established and will be the core of our
future work.
There is an increasing trend in young people in many
types of cancer. For example, there was a large increase in breast
cancer among young women in Brazil between 1988 and 2003 (54). The reason for the increase is
uncertain. In the present study, we systemically investigated the
incidence in young people in 38 types of cancer. A percentage of
4.9% of all cancer cases occurred in patients ≤30 years old. ACC,
CESC, LGG, PCPG, TGCT and THCA were particularly associated with
young patients. A total of 77.61% of the cases of TGCT occurred in
patients that were 20–40 years old. ACC has a bimodal age
distribution by age which is clustered in children under 5 years
old and adults aged 30–40 years. CESC may be identified earlier due
to the universal application of cervical cytology screening
(55). LGG is the most common
childhood brain tumor (56). PCPG
and TCGT mostly occur in young or middle-age adults and TGCT most
commonly occurs in between the ages of 35 and 65.
Survival for all types of cancer has markedly
improved since the last century (57). A number of improvements in treatment
during this period have undoubtedly contributed to the improved
survival rate. Previous studies have shown sex, BMI and initial
pathologic diagnosis age differences in the pathophysiology,
clinical presentation and treatment response of cancers, such as
head and neck cancer, endometrial cancer and breast cancer
(10,44,58).
In the present study, we systemically investigated the effect of
sex, BMI and initial pathologic diagnosis age on two- and five-year
survival rates for each of the 38 different types of cancer in
TCGA. We first identified that two- and five-year survival rates
were different for different types of cancer. Nine types of cancer,
i.e., GBM, PAAD, LAML, MESO, ESCA, STES, GBMLGG, STAD and UCS, were
associated with particularly low two- and five-year survival rates,
indicating that the treatment of these types of cancer should be
improved. In addition, females generally had a higher survival rate
than males, with the exception of two-year survival rates for HNSC,
KIRP and SARC, and five-year survival rates for DLBC, KIRP, LUAD
and THYM. Survival rates for these specific cancers vary widely
between men and women, potentially due to biologically and
socioculturally determined factors. The role of sex and gender in
cancer etiology, prevention and treatment is determined by complex,
interacting variables that differ by cancer site. A comprehensive
understanding requires the consideration of physiology, anatomy,
hormones, behavior, lifestyle, environment and access to medical
care.
Furthermore, there was a positive relationship
between high BMI and survival rate. High BMI association with
cancer is conflicting, as high BMI is a risk factor for many types
of cancer. However, this result is consistent with the previous
hypothesis that adipocytes not only play a critical role in the
promotion of carcinogenesis, but that leukemia cell proliferation
is inhibited when adipocytes in the bone marrow increase (59). This conflict may arise from
adipocytes providing sufficient energy for normal and abnormal
metabolic activity during cancer.
Regarding the initial pathologic diagnosis age, it
is known that an earlier pathologic diagnosis of a cancer is
associated with a better prognosis (60). The initial pathologic diagnosis age
of the deceased cancer patients in the TCGA dataset was
predominately distributed between the ages of 60 and 80. These
results suggest that there is an increased chance of survival for
most types of cancer when they are identified earlier. Cancers are
associated with relatively low mortality when the initial
pathologic diagnosis age is below 40, with the exception of TGCT.
Furthermore, the initial pathologic diagnosis age for >30% of
deceased patients in seven cancers, i.e., LGG, CESC, PCPG, ACC,
DLBC, SKCM and GBMLGG, was below 50. These types of cancer were
consistent with the types of cancers that were associated with a
young age of diagnosis. For these types of cancers, we should
strengthen early detection and rapid response systems to improve
their prognosis.
The results of this study indicated that many
factors impact the prognosis of cancers, such as sex, BMI and
initial pathologic diagnosis age. Different types of cancer, sex
and age groups may require different treatments. These results
provide new insights, which may inform the development of precision
and personalized medicine.
Acknowledgements
The authors thank the National Cancer Institute and
the National Human Genome Research Institute for sharing the
clinical dataset of cancers in the TCGA database.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81701440), the
Natural Science Foundation of Jiangsu Province (grant no.
BK20170620), the China Postdoctoral Science Foundation (grant no.
2017M613434) and the Foundation for Key Medical Talents in Jiangsu
Province (grant no. ZDRCA2016096).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XH and CS designed and carried out the study and
drafted the manuscript. LC designed the study and helped write the
manuscript. BY and LC conceived the study and were the lead writers
of the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adrenocortical carcinoma
|
|
BLCA
|
bladder urothelial carcinoma
|
|
BRCA
|
breast invasive carcinoma
|
|
CESC
|
cervical and endocervical cancers
|
|
CHOL
|
cholangiocarcinoma
|
|
COAD
|
colon adenocarcinoma
|
|
COADREAD
|
colorectal adenocarcinoma
|
|
DLBC
|
lymphoid neoplasm diffuse large B-cell
lymphoma
|
|
ESCA
|
esophageal carcinoma
|
|
FPPP
|
FFPE Pilot Phase II
|
|
GBM
|
glioblastoma multiforme
|
|
GBMLGG
|
glioma
|
|
HNSC
|
head and neck squamous cell
carcinoma
|
|
KICH
|
kidney chromophobe
|
|
KIPAN
|
pan-kidney cohort (KICH+KIRC+KIRP)
|
|
KIRC
|
kidney renal clear cell carcinoma
|
|
KIRP
|
kidney renal papillary cell
carcinoma
|
|
LAML
|
acute myeloid leukemia
|
|
LGG
|
brain lower grade glioma
|
|
LIHC
|
liver hepatocellular carcinoma
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
MESO
|
mesothelioma
|
|
OV
|
ovarian serous cystadenocarcinoma
|
|
PAAD
|
pancreatic adenocarcinoma
|
|
PCPG
|
pheochromocytoma and paraganglioma
|
|
PRAD
|
prostate adenocarcinoma
|
|
READ
|
rectum adenocarcinoma
|
|
SARC
|
sarcoma
|
|
SKCM
|
skin cutaneous melanoma
|
|
STAD
|
stomach adenocarcinoma
|
|
STES
|
stomach and esophageal carcinoma
|
|
TGCT
|
testicular germ cell tumors
|
|
THCA
|
thyroid carcinoma
|
|
THYM
|
thymoma
|
|
UCEC
|
uterine corpus endometrial
carcinoma
|
|
UCS
|
uterine carcinosarcoma
|
|
UVM
|
uveal melanoma
|
References
|
1
|
GBD 2015 Disease and Injury Incidence and
Prevalence Collaborators, : Global, regional, and national
incidence, prevalence, and years lived with disability for 310
diseases and injuries, 1990–2015: A systematic analysis for the
Global Burden of Disease Study 2015. Lancet. 388:1545–1602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2015 Mortality and Causes of Death
Collaborators, : Global, regional, and national life expectancy,
all-cause mortality, and cause-specific mortality for 249 causes of
death, 1980–2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enkhtaivan G, Kim DH and Pandurangan M:
Cytotoxic effect of TDZ on human cervical cancer cells. J Photochem
Photobiol B. 173:493–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sud A, Kinnersley B and Houlston RS:
Genome-wide association studies of cancer: Current insights and
future perspectives. Nat Rev Cancer. 17:692–704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herati AS, Kohn TP, Butler PR and
Lipshultz LI: Effects of testosterone on benign and malignant
conditions of the prostate. Curr Sex Health Rep. 9:65–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edgren G, Liang L, Adami HO and Chang ET:
Enigmatic sex disparities in cancer incidence. Eur J Epidemiol.
27:187–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Micalizzi DS, Maheswaran S and Haber DA: A
conduit to metastasis: Circulating tumor cell biology. Genes Dev.
31:1827–1840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pascual G, Avgustinova A, Mejetta S,
Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blee AM and Huang H: Fat lure: Adipocytes
attract cancer cells out of the prostate. Translational Cancer Res.
5 Suppl 1:S123–S125. 2016. View Article : Google Scholar
|
|
10
|
Rojas KE, Matthews N, Raker C, Clark MA,
Onstad M, Stuckey A and Gass J: Body mass index (BMI),
postoperative appearance satisfaction, and sexual function in
breast cancer survivorship. J Cancer Surviv. 12:127–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amadou A, Ferrari P, Muwonge R, Moskal A,
Biessy C, Romieu I and Hainaut P: Overweight, obesity and risk of
premenopausal breast cancer according to ethnicity: A systematic
review and dose-response meta-analysis. Obes Rev. 14:665–678. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Finucane MM, Stevens GA, Cowan MJ, Danaei
G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN,
et al: National, regional, and global trends in body-mass index
since 1980: Systematic analysis of health examination surveys and
epidemiological studies with 960 country-years and 9.1 million
participants. Lancet. 377:557–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rafee S, Flavin A and O'Reilly S: Body
fatness and cancer. N Engl J Med. 375:20082016.PubMed/NCBI
|
|
14
|
Montazeri A: Quality of life data as
prognostic indicators of survival in cancer patients: An overview
of the literature from 1982 to 2008. Health Qual Life Outcomes.
7:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moghimi-Dehkordi B and Safaee A: An
overview of colorectal cancer survival rates and prognosis in Asia.
World J Gastrointest Oncol. 4:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Y, Qiao G, Xu E, Xuan Y, Liao M and
Yin G: Biomarkers for early diagnosis, prognosis, prediction, and
recurrence monitoring of non-small cell lung cancer. Onco Targets
Ther. 10:4527–4534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gesthalter YB, Vick J, Steiling K and
Spira A: Translating the transcriptome into tools for the early
detection and prevention of lung cancer. Thorax. 70:476–481. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Wang MM, Guo XW, Wu CY, Li DR, Zhang
X and Zhang PT: Different survival benefits of Chinese medicine for
pancreatic cancer: How to choose? Chin J Integr Med. 24:178–184.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wills B, Cardona AF, Rojas L, Ruiz-Patino
A, Arrieta O, Reguart N, Carranza H, Vargas C, Otero J, Corrales L,
et al: Survival outcomes according to TIMP1 and EGFR expression in
heavily treated patients with advanced non-small cell lung cancer
who received biweekly irinotecan plus bevacizumab. Anticancer Res.
37:6429–6436. 2017.PubMed/NCBI
|
|
20
|
Mercier J and Voutsadakis IA: A Systematic
review and Meta-analysis of retrospective series of regorafenib for
treatment of metastatic colorectal cancer. Anticancer Res.
37:5925–5934. 2017.PubMed/NCBI
|
|
21
|
Sineshaw HM, Jemal A, Thomas CJ and Mitin
T: Changes in treatment patterns for patients with locally advanced
rectal cancer in the United States over the past decade: An
analysis from the National Cancer Data Base. Cancer. 122:1996–2003.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiani A, Narayanan S, Pena L and Friedman
M: The role of diagnosis and treatment of underlying liver disease
for the prognosis of primary liver cancer. Cancer Control. 24:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chennubhotla C, Clarke LP, Fedorov A,
Foran D, Harris G, Helton E, Nordstrom R, Prior F, Rubin D, Saltz
JH, et al: An assessment of imaging informatics for precision
medicine in cancer. Yearb Med Inform. 26:110–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brunner M, Olschewski M, Geibel A, Bode C
and Zehender M: Long-term survival after pacemaker implantation.
Prognostic importance of gender and baseline patient
characteristics. Eur Heart J. 25:88–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burney IA and Lakhtakia R: Precision
medicine: Where have we reached and where are we headed? Sultan
Qaboos Univ Med J. 17:e255–e258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yavas O, Hayran M and Ozisik Y: Factors
affecting survival in breast cancer patients following bone
metastasis. Tumori. 93:580–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chok KS and Law WL: Prognostic factors
affecting survival and recurrence of patients with pT1 and pT2
colorectal cancer. World J Surg. 31:1485–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Labastida R, Dexeus S, Fábregas R,
Tresserra F and Fernández A: Endometrial cancer: Factors affecting
survival. Eur J Gynaecol Oncol. 24:381–383. 2003.PubMed/NCBI
|
|
30
|
Kojima F, Yamamoto K, Matsuoka K, Ueda M,
Hamada H, Imanishi N and Miyamoto Y: Factors affecting survival
after lobectomy with pulmonary artery resection for primary lung
cancer. Eur J Cardiothorac Surg. 40:e13–e20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Magalhães JP: How ageing processes
influence cancer. Nat Rev Cancer. 13:357–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
May M: Statistics: Attacking an epidemic.
Nature. 509 Suppl:S50–S51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eveno C, Parc Y, Laurent A, Ducreux M and
Pocard M: Body-mass index, cancer, and implications for screening.
Lancet Oncol. 16:e102–e103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Warren JL, Harlan LC, Trimble EL, Stevens
J, Grimes M and Cronin KA: Trends in the receipt of guideline care
and survival for women with ovarian cancer: A population-based
study. Gynecol Oncol. 145:486–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jensen JS, Jensen DH, Grønhøj C, Karnov
KKS, Nørregaard C, Agander TK, Specht L and von Buchwald C:
Incidence and survival of oropharyngeal cancer in Denmark: A
nation-wide, population-based study from 1980 to 2014. Acta Oncol.
57:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Welch HG, Schwartz LM and Woloshin S: Are
increasing 5-year survival rates evidence of success against
cancer? JAMA. 283:2975–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hallahan C: Data Analysis using SAS.
Sociological Methods Res. 23:373–391. 1995. View Article : Google Scholar
|
|
38
|
Xu M, Fralick D, Zheng JZ, Wang B, Tu XM
and Feng C: The differences and similarities between Two-sample
T-Test and Paired T-Test. Shanghai Arch Psychiatry. 29:184–188.
2017.PubMed/NCBI
|
|
39
|
Kim HY: Statistical notes for clinical
researchers: Chi-squared test and Fisher's exact test. Restor Dent
Endod. 42:152–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cuff J and Higgins JP: Statistical
analysis of surgical pathology data using the R program. Adv Anat
Pathol. 19:131–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schwartz L, Supuran CT and Alfarouk KO:
The Warburg effect and the hallmarks of cancer. Anticancer Agents
Med Chem. 17:164–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hofseth LJ and Wargovich MJ: Inflammation,
cancer, and targets of ginseng. J Nutr. 137 Suppl 1:183S–185S.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao B, Bray F, Beltran-Sanchez H, Ginsburg
O, Soneji S and Soerjomataram I: Benchmarking life expectancy and
cancer mortality: Global comparison with cardiovascular disease
1981–2010. BMJ. 357:j27652017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
May M, Bastian PJ, Brookman-May S,
Fritsche HM, Tilki D, Otto W, Bolenz C, Gilfrich C, Trojan L,
Herrmann E, et al: Gender-specific differences in cancer-specific
survival after radical cystectomy for patients with urothelial
carcinoma of the urinary bladder in pathologic tumor stage T4a.
Urol Oncol. 31:1141–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Corbett JB and Mori M: Gender-specific
cancers, Gender-specific reporters? Twenty-Four years of network TV
coverage. Sci Communication. 20:395–408. 1999. View Article : Google Scholar
|
|
46
|
Koyama A, Matsuoka H, Ohtake Y, Makimura
C, Sakai K, Sakamoto R and Murata M: Gender differences in
cancer-related distress in Japan: A retrospective observation
study. Biopsychosoc Med. 10:102016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Key T, Appleby P, Barnes I and Reeves G;
Endogenous Hormones and Breast Cancer Collaborative Group, :
Endogenous sex hormones and breast cancer in postmenopausal women:
Reanalysis of nine prospective studies. J Natl Cancer Inst.
94:606–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hall SA, Araujo AB, Kupelian V, Maserejian
NN and Travison TG: Testosterone and breast cancer. J Sex Med.
7:1035–1037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
NCD Risk Factor Collaboration (NCD-RisC),
: Trends in adult body-mass index in 200 countries from 1975 to
2014: A pooled analysis of 1698 population-based measurement
studies with 19.2 million participants. Lancet. 387:1377–1396.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bosy-Westphal A, Reinecke U, Schlörke T,
Illner K, Kutzner D, Heller M and Müller MJ: Effect of organ and
tissue masses on resting energy expenditure in underweight, normal
weight and obese adults. Int J Obes Relat Metab Disord. 28:72–79.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dirat B, Bochet L, Dabek M, Daviaud D,
Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S,
et al: Cancer-associated adipocytes exhibit an activated phenotype
and contribute to breast cancer invasion. Cancer Res. 71:2455–2465.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Omabe M, Ezeani M and Omabe KN: Lipid
metabolism and cancer progression: The missing target in metastatic
cancer treatment. J Appl Biomed. 13:47–59. 2015. View Article : Google Scholar
|
|
54
|
Freitas R Jr, Freitas NM, Curado MP,
Martins E, Silva CM, Rahal RM and Queiroz GS: Incidence trend for
breast cancer among young women in Goiania, Brazil. Sao Paulo Med
J. 128:81–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wiener HG, Klinkhamer P, Schenck U, Arbyn
M, Bulten J, Bergeron C and Herbert A: European guidelines for
quality assurance in cervical cancer screening: Recommendations for
cytology laboratories. Cytopathology. 18:67–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arnautovic A, Billups C, Broniscer A,
Gajjar A, Boop F and Qaddoumi I: Delayed diagnosis of childhood
low-grade glioma: Causes, consequences, and potential solutions.
Child Nerv Syst. 31:1067–1077. 2015. View Article : Google Scholar
|
|
57
|
Clegg LX, Li FP, Hankey BF, Chu K and
Edwards BK: Cancer survival among US whites and minorities: A SEER
(Surveillance, Epidemiology, and End Results) Program
population-based study. Arch Intern Med. 162:1985–1993. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weinstock C, Bigenwald R, Hochman T, Sun
P, Narod SA and Warner E: Outcomes of surveillance for
contralateral breast cancer in patients less than age 60 at the
time of initial diagnosis. Curr Oncol. 19:e160–e164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Boyd AL, Reid JC, Salci KR, Aslostovar L,
Benoit YD, Shapovalova Z, Nakanishi M, Porras DP, Almakadi M,
Campbell CJV, et al: Acute myeloid leukaemia disrupts endogenous
myelo-erythropoiesis by compromising the adipocyte bone marrow
niche. Nat Cell Biol. 19:1336–1347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Casartelli G, Dorcaratto A, Ravetti JL,
Sola S, Vitali A, Merlo DF and Frosina G: Survival of high grade
glioma patients depends on their age at diagnosis. Cancer Biol
Ther. 8:1719–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|