Introduction
Gastric cancer is the fourth most common malignant
tumor worldwide and the second leading cause of cancer-associated
mortality (1), 70% of which occur
in developing regions, including 40% of individuals in China
(2). Despite advances in cancer
therapy, the majority of advanced malignancies remain incurable.
Radiotherapy is the main method of local control for several types
of unresectable tumor, and for controlling gastric bleeding.
Previous studies have shown that radiotherapy did not improve the
survival rate of patients with gastric cancer, whereas local
control rates were 70% (3).
Radiotherapy is considered an attractive modality for the high
incidence of locoregional failures following surgical treatment of
gastric cancer (4,5).
Tumors in humans frequently express high levels of
epidermal growth factor receptor (EGFR), which has been associated
with poor prognosis when expressed at high levels (6). In 511 cases of gastric carcinoma, the
expression of EGFR was 27.4% (7).
In several cases, including gastric cancer, the overexpression of
EGFR drives tumor cells towards uncontrolled proliferation,
allowing the cells to evade programmed death, thereby enhancing
their ability to migrate and metastasize. The activation of EGFR is
involved in the resistance of tumor cells to radiotherapy (8). In response to radiation, EGFR is
rapidly activated and induces several downstream signaling
pathways, including mitogen-activated protein kinase
(MAPK)-extracellular signal regulated kinase (ERK) and
phosphoinositide 3-kinase (PI3K)/Akt. Activation of these signaling
pathways may promote cell proliferation and apoptosis avoidance,
and the repair of radiation-induced DNA damage through homologous
and non-homologous recombination (9). Repeated exposure to radiation also
results in increased expression of EGFR (9,10).
Therefore, EGFR inhibitors are the most promising molecular
targeting agents for use in combination with radiotherapy (11–13).
Advances in the field of genetic engineering have led to the
development of various EGFR inhibitors, including monoclonal
antibodies, tyrosine kinase inhibitors (TKIs), antisense
oligonucleotides and single-domain antibody (14). In a previous study, the application
of cetuximab during primary radiotherapy in patients with head and
neck squamous cell carcinoma resulted in improved locoregional
tumor control and survival rates compared with patients who
received radiotherapy alone (11).
These pioneering findings have paved the way for the clinical use
of EGFR inhibitors in combination with radiotherapy.
In our previous study, a tumor-penetrating peptide
was constructed that was fused with an EGFR single-domain antibody
(15), termed anti-EGFR-iRGD, which
consisted of an anti-EGFR VHH, from the variable domain of the
heavy chain of the antibody, fused to iRGD. The tumor specific
binding peptide exhibited high permeability into the tumor. In
addition, the recombinant protein anti-EGFR-iRGD showed antitumor
activity in tumor cell lines, multicellular spheroids and mice
(16). Radiotherapy is widely used
in the treatment of various types of cancer. In the present study,
the effects of anti-EGFR-iRGD treatment in combination with
radiotherapy were investigated in gastric cancer with high levels
of EGFR.
Materials and methods
Cell culture, xenograft experiments
and ionizing radiation
Three human gastric adenocarcinoma cell lines
(SNU-719, BGC-823 and HGC-27) were maintained in Roswell Park
Memorial Institute (RPMI)-1640 medium (Invitrogen; Grand Island,
NY, USA) supplemented with 10% bovine calf serum (BCS; Life
Technologies/Gibco, Grand Island, NY) in 5% CO2 at 37°C.
All animal procedures were performed in compliance with the
guidelines set by the Animal Care Committee at Drum Tower Hospital
(Nanjing, China). A total of 5,000,000 BGC-823 gastric cancer cells
in 0.1 ml of PBS were subcutaneously injected in the lower right
flank of athymic nude BALB/c mice (5–6 weeks old, female, 18–22 g,
Shanghai Experimental Animal Center, Shanghai, China). BALB/c mice
were kept in climate-controlled quarters with a 12-h light and dark
cycle with food and water in cages under germ-free conditions.
Tumor volumes were calculated from two diameter measurements
according to the following formula: Tumor volume=(length ×
width2)/2. Radiotherapy was administered in vitro
using a 6 MeV X-ray linear accelerator (Elekta AB, Stockholm,
Sweden).
Cell viability assay and flow
cytometry assays
Following treatment with anti-EGFR-iRGD, cell
viability was evaluated using an MTT assay. In brief, the cells
were seeded into 96-well plates at a density of 3,000-8,000
cells/well. Subsequently, cells in the logarithmic phase were
treated with anti-EGFR-iRGD at indicated concentrations (6.3, 12.5,
25, 50, 100, 200, 400 and 800 µg/ml). Following incubation for 24 h
at 37°C, MTT reagent was added, followed by dimethyl sulfoxide
(DMSO), and the spectrophotometric absorbance was measured (490
nm). To detect apoptosis, cells in the logarithmic phase were
treated with anti-EGFR-iRGD for 24 h 37°C. The cells were
harvested, washed with PBS, and subsequently incubated in the dark
for 15 min at room temperature. Finally, the degree of apoptosis
was analyzed by FACScan laser flow cytometry (BD Aria II; BD
Biosciences, Franklin Lakes, NJ, USA) using an FITC Annexin V
Apoptosis Detection kit (Roche Applied Science, Indianapolis, IN,
USA). The number of cells analyzed for each sample was 50,000.
Clonogenic survival assay
The cells were seeded into 6-well plates at a
density of 500–8,000 cells per well. Following incubation for 24 h,
anti-EGFR-iRGD (100 µg/ml) was added into each well. The cells were
treated with anti-EGFR-iRGD for 24 h at 37°C and then exposed to
increasing doses of ionizing radiation (0, 2, 4, 6 and 8 Gy).
Following intervals of 7–10 days, cell colonies (consisting of ≥50
cells) were stained with crystal violet and counted manually using
optical microscopy.
Western blot assay
The expression levels of EGFR in gastric cancer
cells were confirmed by western blot analysis. Cell lysates were
prepared with a detergent buffer, as previously described (17). Protein concentrations were measured
with the BCA Protein Assay according to the manufacturer's manual
(Beyotime Institute of Biotechnology, Shanghai, China). The
proteins (30 µg) were separated by 10% SDS-PAGE, and transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% bovine serum albumin
(BSA; Life Technologies/Gibco) in Tris-buffered saline, containing
0.05% Tween-20 for 2 h at room temperature, and then incubated
overnight at 4°C with a 1:2,000 dilution of primary antibody
targeting EGFR (dilution 1:2,000; cat. no. 4267; Cell Signaling
Technology, Inc., Danvers, MA, USA) and β-actin (dilution 1:2,000;
cat. no. AF0003; Beyotime Institute of Biotechnology, Haimen,
China). The membranes were incubated with a 1:2,000 dilution of
horseradish peroxidase-conjugated goat anti-mouse (1:2,000; cat.
no. A0216; Beyotime Institute of Biotechnology) and goat
anti-rabbit antibodies (dilution 1:2,000; cat. no. A0208; Beyotime
Institute of Biotechnology) for 1 h at room temperature and
detected by ECL reagents (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Penetration in tumor tissue
Following radiation, the distribution of
anti-EGFR-iRGD in tumor tissues was determined by laser scanning
confocal microscopy (LSCM). Animal models were used to locate
proteins and the permeability of recombinant proteins following
radiotherapy was examined. The BALB/c mice (n=3 mice per group)
were subcutaneously injected with EGFR-overexpressing BGC-823 cells
(5,000,000 gastric cancer cells in 0.1 ml of PBS), in the right
flank (no radiation, 0 GY) and in the left flank (radiation, single
dose of 2 Gy). When the tumors reached a volume of ~150
mm3, radiotherapy was delivered to the left flank at 600
cGy/min with 6 MV X-rays. The mice received a single dose of 2 Gy.
At 24 h post-radiation treatment, rhodamine-B-labeled
anti-EGFR-iRGD was administered to the BGC-823 tumor-bearing mice
via tail vein injection. The mice were sacrificed and tumors were
harvested 1 h following the administration of anti-EGFR-iRGD. The
tumors were frozen and sections were cut. Finally, the tumor
sections (5 µm) were subjected to DAPI staining and visualized
using LSCM.
In vivo antitumor effect
The gastric cancer cells (BGC-823) were
subcutaneously injected into BALB/c mice. When the subcutaneous
tumor was ~100 mm3, the mice were randomly divided into
four groups. The day of randomization was designated as ‘Day 1’.
The mice were treated every day by intraperitoneal injection with
anti-EGFR-iRGD at 1 mg on the first day (day 1) and either 0.6 mg
during each subsequent injection (a total of five injections). At
24 h following the first injection, radiotherapy was delivered to
one field, including the tumor, with 5-mm margins, using a Clinac
2300 C/D linear accelerator. Radiation was delivered at 600 cGy/min
with 6 MV X-rays beams at doses of 10 Gy, in five fractions, with
one fraction each day. The mice were monitored daily, and tumor
volume and body weight were recorded every 3 days. The mice were
sacrificed at the end of the experiment. Following treatment,
histological observation of the heart, liver, spleen, lung and
kidney, and tumor tissues was performed.
Immunostaining of tumor sections and
organs
The xenografts and organs were fixed in neutral
buffered formalin, embedded in paraffin, and stained with
hematoxylin and eosin (H&E) for pathological observation. The
tissues were sectioned at a thickness of 5 µm and the sections were
evaluated using optical microscopy.
Statistical analysis
SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analysis. T-test was used to compare the means
between two groups, where their variances of both groups may be
different. One-way analysis of variance was used for multiple
comparisons. Covariance analysis was used for comparison between
four groups to remove the effects of the covariate. P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard deviation.
Results
In vitro cytotoxicity of recombinant
protein anti-EGFR-iRGD and expression of EGFR in gastric cancer
cell lines
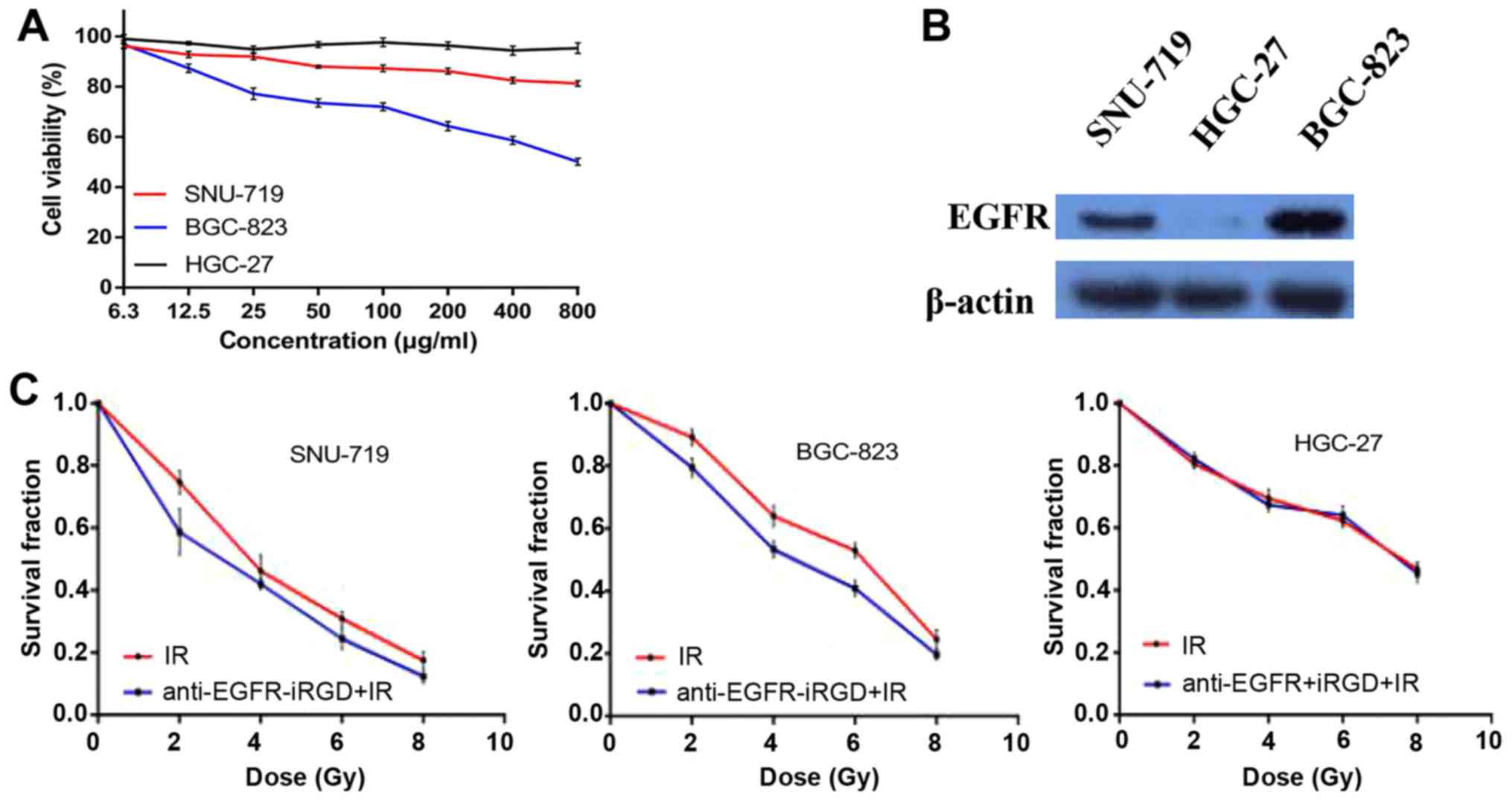
In vitro cytotoxicity was assessed using MTT
assays, which showed that, even at a low concentration,
anti-EGFR-iRGD exhibited anti-proliferative activity against the
SNU-719 cells and BGC-823 cells. Furthermore, a dose-dependent
effect of anti-EGFR-iRGD was observed in the SNU-719 cells and
BGC-823 cells. However, in the HGC-27 cells (no EGFR expression),
no anti-proliferative activity was observed, even at the highest
concentration of 800 µg/ml (Fig.
1A). To select appropriate cell lines as the study objective
and to investigate the expression levels of EGFR in different
gastric cancer cell lines, the three human gastric cancer cell
lines (SNU-719, BGC-823 and HGC-27) were evaluated by western blot
analysis. The data revealed the following descending expression
levels of EGFR: BGC-823>SNU-719>HGC-27 (Fig. 1B). These findings indicated that the
anti-proliferative activity of anti-EGFR-iRGD in human gastric
cancer cells was associated with the expression of EGFR.
Anti-EGFR-iRGD modulates
radiosensitivity
To evaluate the potential capacity of combining
anti-EGFR-iRGD with radiation in human gastric cancer cells,
experiments were performed to examine the effect of anti-EGFR-iRGD
on clonogenic survival. The clonogenic survival curves of SNU-719,
BGC-823 and HGC-27 cells are shown in Fig. 1C, in which cells were exposed to
anti-EGFR-iRGD and radiation (anti-EGFR-iRGD prior to radiation).
The data indicated that treatment with anti-EGFR-iRGD prior to
radiation induced modest but consistent radiosensitization, as
manifested by a reduction in clonogenic survival compared with
control exposure to ionizing radiation alone, in SNU-719 and
BGC-823 cells treated with 2, 4, 6 and 8 Gy.
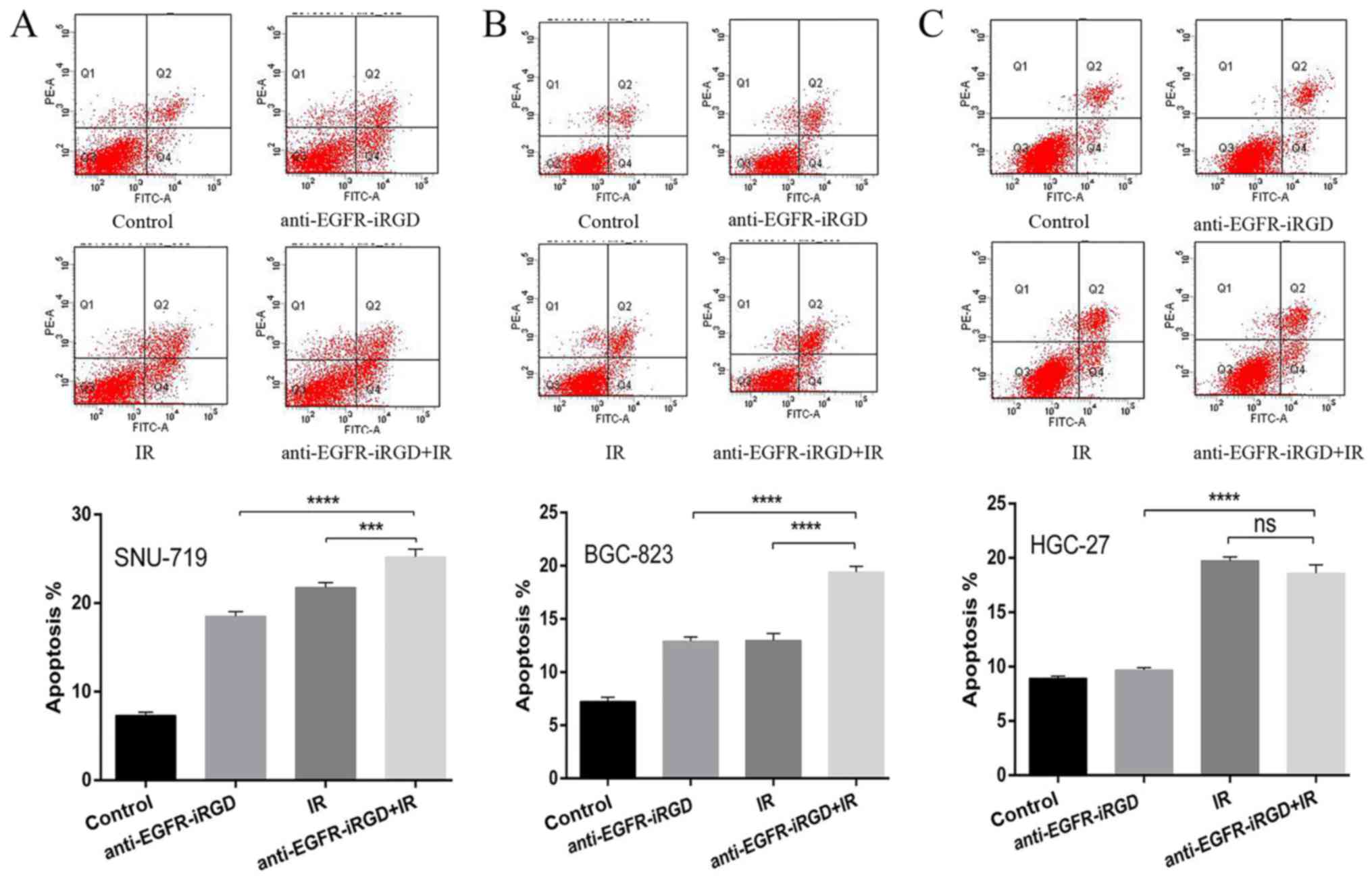
Anti-EGFR-iRGD enhances
radiation-induced apoptosis
To investigate the inhibitory effects of the
combination treatment of anti-EGFR-iRGD ± radiation on cell growth,
the apoptotic responses of recombinant proteins combined with
ionizing radiation (single dose of 6 Gy) were investigated in
SNU-719, BGC-823 and HGC-27 gastric cancer cells. Q2 represents
early apoptotic cells, Q2+Q4 indicates gastric cells that are not
viable. Compared with the cells that received ionizing radiation
alone or recombinant protein alone, the SNU-719 cells (Fig. 2A) and BGC-823 cells (Fig. 2B) pretreated with anti-EGFR-iRGD
protein showed a significant increase in apoptosis (P<0.0001).
However, compared wit the cells that received ionizing radiation
alone, the HGC-27 cells (Fig. 2C)
pretreated with anti-EGFR-iRGD protein did not show an increase in
apoptosis (P>0.05). These findings indicated that the
recombinant protein-enhanced apoptosis of human gastric cancer
cells was associated with the expression of EGFR.
Mechanism of recombinant protein
enhances the radiation response
Following ionizing radiation for 24 h, the
expression of EGFR in tumor tissue sections was analyzed. The
expression pattern revealed mainly membrane-bound EGFR staining,
indicating that, following ionizing radiation, EGFR was upregulated
(Fig. 3A). As shown in Fig. 3B, the increased upregulation of EGFR
was confirmed following radiation exposure (single dose of 2 Gy) in
the BGC-823 cell lines. The radiation-induced upregulation of EGFR
was inhibited by pretreatment of the tumor cells with 100 µg/ml
anti-EGFR-iRGD for 24 h. These findings showed that anti-EGFR-iRGD
treatment combined with radiotherapy effectively inhibited the
expression of EGFR in EGFR-overexpressing gastric cancer cells.
 | Figure 3.Effect of radiation on the expression
of EGFR, anti-EGFR-iRGD-induced inhibition of radiation-induced
upregulation of EGFR, and evaluation of anti-EGFR-iRGD penetration
in BGC-823 tumors. (A) Immunohistochemical staining of tumor tissue
sections from BGC-823 tumor-bearing mice following treatment with
radiation (0 or 2 Gy). Following single IR for 24 h, expression of
EGFR was upregulated. Positive (yellow) staining indicates EGFR
(magnification, ×100). (B) Effect of anti-EGFR-iRGD treatment on
expression of EGFR following radiation exposure. BGC-823 cells ± 24
h pretreatment with anti-EGFR-iRGD (100 µg/ml) were harvested 24 h
following radiation exposure (single dose of 2 Gy). Whole cell
lysates were evaluated for total levels of EGFR. (C) BALB/c mice
were subcutaneously injected with BGC-823 cells, in the right flank
(no radiation, 0 Gy) and in the left flank (radiation, 2 Gy).
Anti-EGFR-iRGD penetration was evaluated in tumors at 1 h
post-injection and following radiation of 2 Gy for 24 h. After 24 h
of radiation, penetration of anti-EGFR-iRGD into the tumor tissues
was increased. Rhodamine B-labeled proteins (red), nucleus (blue).
(magnification, ×400). EGFR, epidermal growth factor receptor. |
Evaluation of the penetration of
anti-EGFR-iRGD into tumor tissue following radiotherapy
BALB/c mice were subcutaneously injected with
BGC-823 cells, in the right flank (no radiation, 0 Gy) and in the
left flank (radiation, 2 Gy). The penetration ability of
recombinant protein anti-EGFR-iRGD following radiotherapy was then
analyzed with tumor tissue sections derived from BGC-823-bearing
mice. The penetration of anti-EGFR-iRGD was also evaluated in
BGC-823 tumors at 1 h post-injection and following radiation (2 Gy)
for 24 h. Rhodamine B-labeled proteins (red) and DAPI-labeled
nuclei (blue) were present in the images of tumor sections.
Following radiation with 2 Gy for 24 h, the penetration of
anti-EGFR-iRGD into the tumor tissues had increased (Fig. 3C).
Anti-EGFR-iRGD augments the in vivo
tumor response of gastric cancer xenografts to radiation
The in vivo activity of anti-EGFR-iRGD ±
radiation in tumor xenografts was examined. BGC-823
(5×106) cells were injected subcutaneously into the
flank of at hymic mice. The mice were treated with PBS (control) or
anti-EGFR-iRGD (1.0 mg on the first day, 0.6 mg every day from day
2–5 via intraperitoneal injection), ionizing radiation (2.0
Gy/fraction; five fractions/week; total of five fractions), or a
combination of anti-EGFR-iRGD and ionizing radiation. As shown in
Fig. 4A, treatment with radiation
alone or anti-EGFR-iRGD alone produced modest inhibition of tumor
growth in BGC-823 Xenografts. However, when combined with
radiation, anti-EGFR-iRGD enhanced the tumor growth inhibition
profile over the 24-day observation period.
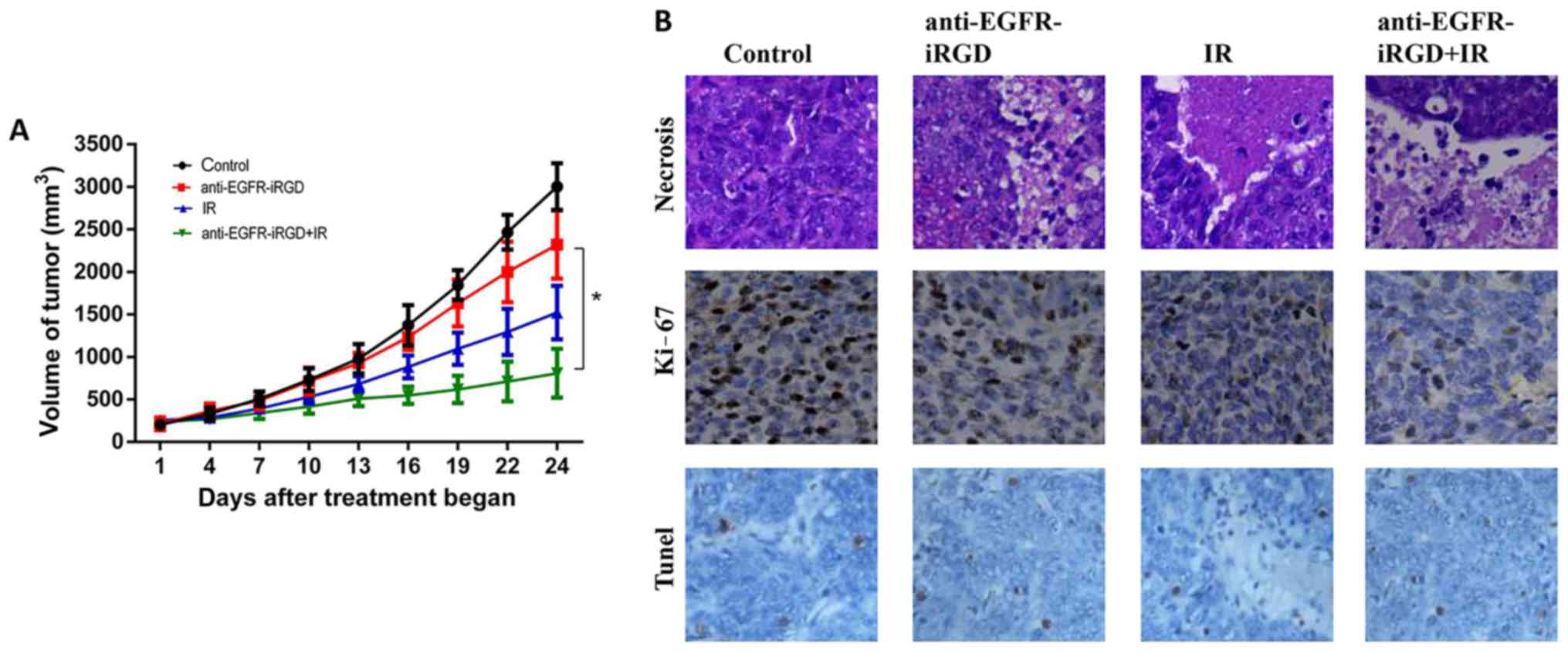 | Figure 4.Inhibitory effect of anti-EGFR-iRGD
in combination with IR on tumor growth in mice. (A) Tumor growth
curves. Mice bearing subcutaneous BGC-823 were treated with PBS,
anti-EGFR-iRGD, IR, or anti-EGFR-iRGD combined with IR. Data are
presented as the mean ± standard error of the mean (n=5). One-way
analysis of variance was used for the analysis of tumor growth
(*P<0.05). (B) Evaluation of cell necrosis, and the
antiproliferative effect of anti-EGFR-iRGD combined with radiation
in BGC-823 tumors 24 days post-treatment. Cell necrosis was
evaluated by hematoxylin and eosin staining (magnification, ×100)
of tumor sections, whereas cell proliferation was evaluated by
immunohistochemistry of Ki-67. Cell death was evaluated by
immunohistochemistry using TUNEL (magnification, ×100), there was
no statistically significant difference between three treated
groups. EGFR, epidermal growth factor receptor; IR, ionizing
radiation. |
In vivo expression of proliferating
cellular nuclear antigen, apoptosis and necrosis
BGC-823 tumor xenografts were used for evaluation of
the expression of markers of tumor proliferation (Ki-67). The
immunohistochemical staining for Ki-67 indicated that the number of
proliferating cells were the lowest in the combined treatment
group, intermediate in the groups receiving single modality
treatment with either anti-EGFR-iRGD or radiation, and highest in
the control group. Furthermore, TUNEL staining results showed that
the number of apoptotic cells in the combined treatment group was
marginally higher than that in the radiotherapy group and the
fusion protein group. However, there was no statistically
significant difference between three treated groups because the F
statistic of one-way ANOVA was 1.679 with P=0.227>0.05 (The F
statistic of the variance homogeneity test between tree groups was
0.344 with P=0.716>0.05). The pathological examination showed
tumor necrosis in all treatment groups. However, the necrotic area
of the PBS-treated control group was the smallest, whereas the
anti-EGFR-iRGD and radiation-treated groups had larger necrotic
regions. The largest necrotic regions were apparent in the combined
treatment group. These results demonstrated the capacity of
anti-EGFR-iRGD in modulating cellular proliferation and cell
necrosis (Fig. 4B).
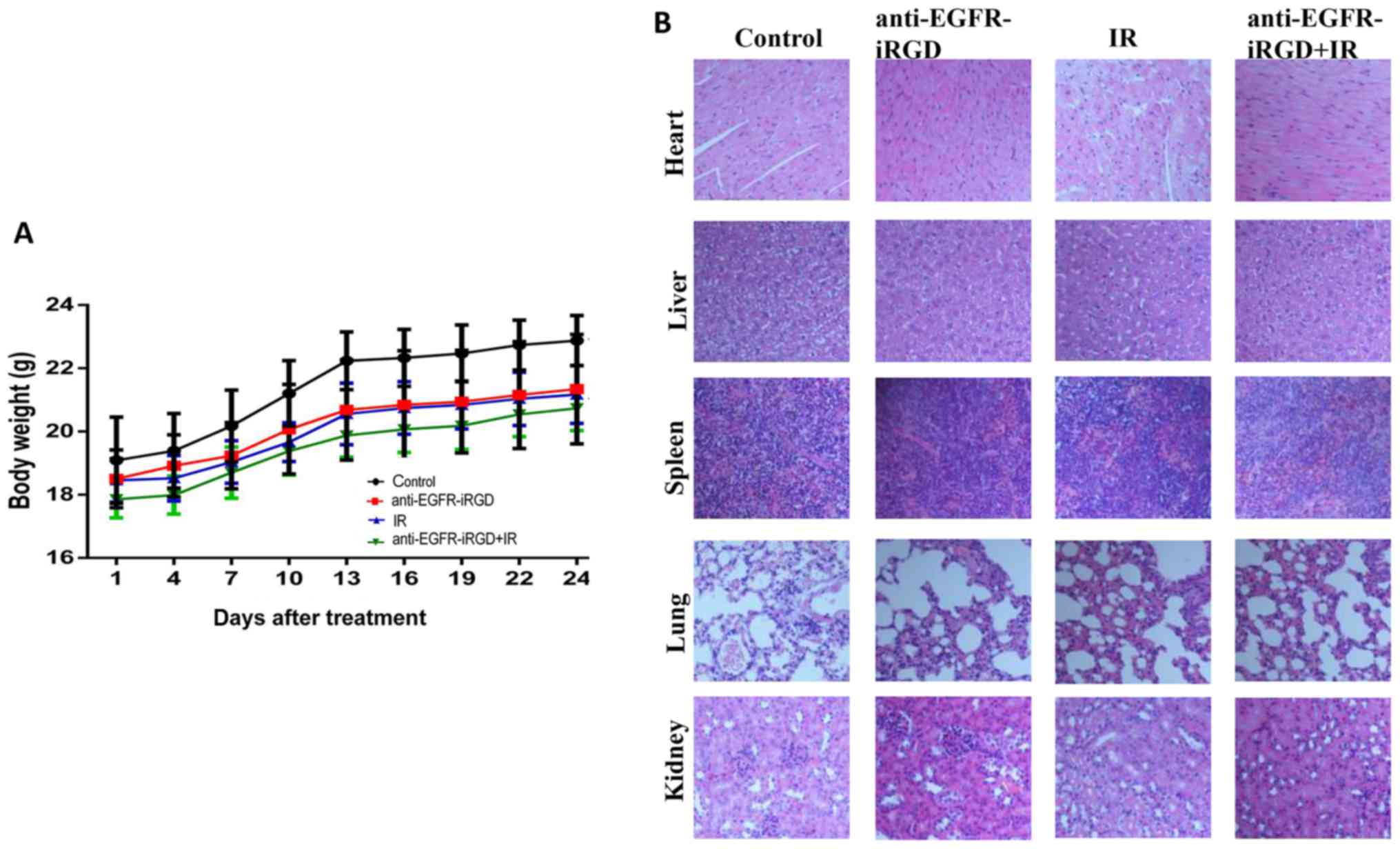
Side effects of anti-EGFR-iRGD with
ionizing radiation
As shown in Fig. 5A,
none of the mice treated with anti-EGFR-iRGD, ionizing radiation or
combination treatment showed any body weight loss. The mean body
weights of mice in the three treatment groups were marginally lower
than that of mice in the control group, however, no significant
differences were observed in body weight between the four groups by
covariance analysis (P=0.174>0.05). The H&E staining of the
organs (Fig. 5B) showed that tissue
changes comprised only the presence of inflammatory cells that
infiltrated the spleen. No significant damage was observed in the
heart, liver, lung or kidney.
Discussion
In our previous study, it was demonstrated that the
recombinant protein anti-EGFR-iRGD exhibited antitumor activity in
gastric cancer cell lines, multicellular spheroids, and mice
(16). In the present study, the
capacity of anti-EGFR-iRGD to modulate the radiation response of
human gastric cancer cell lines and xenografts was investigated.
Previous studies have indicated a favorable antitumor interaction
between radiation and EGFR inhibitors (18–20).
It was suggested that this enhanced effect may explain the levels
of EGFR activation during cell cycle kinetics and radiation, which
may contribute to the inhibition of accelerated cellular
repopulation.
EGFR is a receptor tyrosine kinase that belongs to
the ErbB family. The overexpression or upregulation of EGFR is
generally associated with an adverse outcome (21–23).
EGFR can also be activated by radiotherapy (24,25).
Mechanistically, high levels of EGFR are reported to enable tumor
cells to be more radioresistant for the activation of downstream
signals (26). The EGFR downstream
signal transduction pathways, through the PI3K/AKT or Ras/Raf/MAPK
pathways, have proven to be efficient regulators of cancer gene
expression, cell cycle progression, cell proliferation,
angiogenesis, invasion and metastasis (27). Therefore, EGFR has been considered
as a key target in anticancer treatments, particularly in
combination with radiotherapy. Two classes of pharmacological EGFR
inhibitors have been used clinically: TKIs and monoclonal
antibodies (28). In several
studies, it was reported that the overexpression of EGFR was
correlated with lower tumor control rates following radiation
(29,30), however, conflicting results have
also been reported (31–33).
The interaction between radiation and levels of EGFR
was first described >20 years ago. Early studies showed that
prolonged exposure to EGF increased the cytotoxic effects of
radiation (34,35). Translational studies in patients
have shown that the overexpression of EGFR was correlated with
radioresistance (36). The
mechanism of EGFR inhibitors combined with radiosensitization is
complex. In response to radiation, three distinct phases in the
effect of EGFR have been elucidated. These phases include
activation of pro-survival pathways, enhanced cell proliferation,
and the role of EGFRs in DNA repair (37,38).
An explanation for radiosensitization may be that the tumor
repopulation is limited by the cytostatic effect of EGFR inhibition
during fractionation radiotherapy. Other studies have suggested
that radiosensitivity may be more complex than the induction of
cell cycle arrest alone. In previous studies, it was shown that
cetuximab promoted radiation-induced apoptosis and impaired
sublethal damage to DNA repair, thereby affecting the nuclear
translocation of DNA-PK (39). The
effect of radiation on the activation of EGFR is most pronounced in
serum-starved or confluent cells (24). Studies have shown that the
radiosensitivity of quiescent and proliferating cells is different
from that of the inhibition of EGFR. Specifically, in quiescent
cells radiation induces the transient activation of EGFR, resulting
in S phase progression, impaired DNA repair and enhanced cell death
(40). The inhibition of EGFR may
protect cells in the first few hours following radiation, whereas
the combined effects of G1 arrest and DNA repair inhibition may
result in increased sensitization 24 h following inhibition.
Although EGFR has often been described as a cell
surface receptor, it is closely associated with several nuclear
processes. In addition, resistance to radiation has been associated
with nuclear levels of EGFR (38).
Nuclear EGFR signaling is important in gene regulation, but also
affects DNA repair. Nuclear EGFR is involved in resistance to
EGFR-targeted therapies. In addition to the classic mechanism of
DNA damage, high dose per fraction radiation (>8 Gy) may
generate stromal effects that are not accounted for in traditional
radiobiological modeling (41).
Anti-EGFR-iRGD, which specifically targets EGFR, spreads
extensively throughout the tumor mass. Furthermore, following
radiation for 24 h, anti-EGFR-iRGD appear to permeate even further
into the tumor tissue. The combination of an increased degree
and/or different modes of DNA damage and injury to the tumor
microenvironment arising from the use of hypofractionation may work
synergistically to cause irreparable and lethal injuries to
irradiated tumor cells (41,42).
For EGFR-targeted therapies to be successful,
appropriate patient selection is required to optimize efficacy. The
RTOG 0617 study showed the importance of patient selection when
EGFR-targeted therapy with radiotherapy was used (43). Significant progress has been made in
the development of novel radiation approaches. However, the
integration of targeted therapy and radiotherapy has raised several
unresolved questions, including the identification of patients,
optimal dose and time of radiation, treatment sequence, and side
effects of treatment. Therefore, further investigations are
required to better analyze targeted therapies and, in particular,
the combination of antibodies and radiotherapy.
In conclusion, given the importance of EGFR in
several types of cancer and the well-defined role of EGFR in the
response to radiotherapy, this receptor is an important target when
treatment is combined with radiotherapy. The present study
demonstrated that anti-EGFR-iRGD was an effective radiosensitizer
in EGFR-overexpressing gastric cancer cells and xenografts. The
radioenhancement in gastric cancer cells and xenografts was
associated with inhibited radiation-induced upregulation of EGFR,
inhibited cell proliferation and promotion of cell apoptosis. In
conclusion, anti-EGFR-iRGD was a selective and effective
radiosensitizer in gastric cancer, which makes it a potential
superior EGFR-targeted therapy for further preclinical and clinical
use.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2017YFC1308900), the National Natural Science Foundation of China
(grant nos. 81502037, 81572601, 81672367 and 81220108023) and the
Natural Science Foundation of Jiangsu Province (grant no.
BK20151095).
Availability of data and materials
The datasets analyzed in the present study are
available from the corresponding author on reasonable request.
Authors' contributions
BL, QL, HS and FJ conceived and designed the study.
FM, AZ, ND, HZ, HQ and LY were involved in the experiments and
drafted the manuscript. HX performed the statistical analysis. BL,
QL, HS and FJ wrote and revised the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Ethics Committee of Nanjing Drum Tower Hospital, The Affiliated
Hospital of Nanjing University Medical School.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
TKI
|
tyrosine kinase inhibitor
|
|
LSCM
|
laser scanning confocal microscopy
|
References
|
1
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henning GT, Schild SE, Stafford SL,
Donohue JH, Burch PA, Haddock MG and Gunderson LL: Results of
irradiation or chemoirradiation for primary unresectable, locally
recurrent, or grossly incomplete resection of gastric
adenocarcinoma. Int J Radiat Oncol Biol Phys. 46:109–118. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansen EP, Boot H, Verheij M and van de
Velde CJ: Optimal locoregional treatment in gastric cancer. J Clin
Oncol. 23:4509–4517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smalley SR, Gunderson L, Tepper J,
Martenson JA Jr, Minsky B, Willett C and Rich T: Gastric surgical
adjuvant radiotherapy consensus report: Rationale and treatment
implementation. Int J Radiat Oncol Biol Phys. 52:283–293. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: Prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS: Review of epidermal growth
factor receptor biology. Int J Radiat Oncol Biol Phys. 59 Suppl
2:S21–S26. 2004. View Article : Google Scholar
|
|
9
|
Baumann M and Krause M: Targeting the
epidermal growth factor receptor in radiotherapy: Radiobiological
mechanisms, preclinical and clinical results. Radiother Oncol.
72:257–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harari P and Huang S: Radiation combined
with EGFR signal inhibitors: Head and Neck cancer focus. Semin
Radiat Oncol. 16:38–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lammering G: Molecular predictor and
promising target: Will EGFR now become a star in radiotherapy?
Radiother Oncol. 74:89–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yewale C, Baradia D, Vhora I, Patil S and
Misra A: Epidermal growth factor receptor targeting in cancer: A
review of trends and strategies. Biomaterials. 34:8690–8707. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bell A, Wang ZJ, Arbabi-Ghahroudi M, Chang
TA, Durocher Y, Trojahn U, Baardsnes J, Jaramillo ML, Li S, Baral
TN, et al: Differential tumor-targeting abilities of three
single-domain antibody formats. Cancer Lett. 289:81–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sha H, Zou Z, Xin K, Bian X, Cai X, Lu W,
Chen J, Chen G, Huang L, Blair AM, et al: Tumor-penetrating peptide
fused EGFR single-domain antibody enhances cancer drug penetration
into 3D multicellular spheroids and facilitates effective gastric
cancer therapy. J Control Release. 200:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: Common themes and therapeutic
opportunities. Semin Cancer Biol. 22:1–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SM, Li J, Armstrong EA and Harari
PM: Modulation of radiation response and tumor-induced angiogenesis
after epidermal growth factor receptor inhibition by ZD1839
(Iressa). Cancer Res. 62:4300–4306. 2002.PubMed/NCBI
|
|
19
|
Milas L, Mason K, Hunter N, Petersen S,
Yamakawa M, Ang K, Mendelsohn J and Fan Z: In vivo enhancement of
tumor radioresponse by C225 antiepidermal growth factor receptor
antibody. Clin Cancer Res. 6:701–708. 2000.PubMed/NCBI
|
|
20
|
Bianco C, Tortora G, Bianco R, Caputo R,
Veneziani BM, Caputo R, Damiano V, Troiani T, Fontanini G, Raben D,
et al: Enhancement of antitumor activity of ionizing radiation by
combined treatment with the selective epidermal growth factor
receptor-tyrosine kinase inhibitor ZD1839 (Iressa). Clin Cancer
Res. 8:3250–3258. 2002.PubMed/NCBI
|
|
21
|
Eriksen JG, Steiniche T and Overgaard J;
Danish Head and Neck Cancer study group (DAHANCA), : The influence
of epidermal growth factor receptor and tumor differentiation on
the response to accelerated radiotherapy of squamous cell
carcinomas of the head and neck in the randomized DAHANCA 6 and 7
study. Radiother Oncol. 74:93–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bentzen SM, Atasoy BM, Daley FM, Dische S,
Richman PI, Saunders MI, Trott KR and Wilson GD: Epidermal growth
factor receptor expression in pretreatment biopsies from head and
neck squamous cell carcinoma as a predictive factor for a benefit
from accelerated radiation therapy in a randomized controlled
trial. J Clin Oncol. 23:5560–5567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt-Ullrich RK, Mikkelsen RB, Dent P,
Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK and Chen
PB: Radiation-induced proliferation of the human A431 squamous
carcinoma cells is dependent on EGFR tyrosine phosphorylation.
Oncogene. 15:1191–1197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sturla LM, Amorino G, Alexander MS,
Mikkelsen RB, Valerie K and Schmidt-Ullrichr RK: Requirement of
Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth
factor receptor by ionizing radiation and modulation by SHP2. J
Biol Chem. 280:14597–14604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang SM, Bock JM and Harari PM: Epidermal
growth factor receptor blockade with C225 modulates proliferation,
apoptosis, and radiosensitivity in squamous cell carcinomas of the
head and neck. Cancer Res. 59:1935–1940. 1999.PubMed/NCBI
|
|
27
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciardiello F and Tortora G: A novel
approach in the treatment of cancer: Targeting the epidermal growth
factor receptor. Clin Cancer Res. 7:2958–2970. 2001.PubMed/NCBI
|
|
29
|
Gibson MK, Abraham SC, Wu TT, Burtness B,
Heitmiller RF, Heath E and Forastiere A: Epidermal growth factor
receptor, p53 mutation, and pathological response predict survival
in patients with locally advanced esophageal cancer treated with
preoperative chemoradiotherapy. Clin Cancer Res. 9:6461–6468.
2003.PubMed/NCBI
|
|
30
|
Giralt J, de las Heras M, Cerezo L, Eraso
A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majó J, et
al: The expression of epidermal growth factor receptor results in a
worse prognosis for patients with rectal cancer treated with
preoperative radiotherapy: A multicenter, retrospective analysis.
Radiother Oncol. 74:101–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chakravarti A, Seiferheld W, Tu X, Wang H,
Zhang HZ, Ang KK, Hammond E, Curran W Jr and Mehta M:
Immunohistochemically determined total epidermal growth factor
receptor levels not of prognostic value in newly diagnosed
glioblastoma multiforme: Report from the radiation therapy oncology
group. Int J Radiat Oncol Biol Phys. 62:318–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chakravarti A, Winter K, Wu CL, Kaufman D,
Hammond E, Parliament M, Tester W, Hagan M, Grignon D, Heney N, et
al: Expression of the epidermal growth factor receptor and Her-2
are predictors of favorable outcome and reduced complete response
rates, respectively, in patients with muscle-invading bladder
cancers treated by concurrent radiation and cisplatin-based
chemotherapy: A report from the Radiation Therapy Oncology Group.
Int J Radiat Oncol Biol Phys. 62:309–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CM, Shrieve DC, Zempolich KA, Lee RJ,
Hammond E, Handrahan DL and Gaffney DK: Correlation between human
epidermal growth factor receptor family (EGFR, HER2, HER3, HER4),
phosphorylated Akt (P-Akt), and clinical outcomes after radiation
therapy in carcinoma of the cervix. Gynecol Oncol. 99:415–421.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bonner JA, Maihle NJ, Folven BR,
Christianson TJ and Spain K: The interaction of epidermal growth
factor and radiation in human head and neck squamous cell carcinoma
cell lines with vastly different radiosensitivities. Int J Radiat
Oncol Biol Phys. 29:243–247. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwok TT and Sutherland RM: Enhancement of
sensitivity of human squamous carcinoma cells to radiation by
epidermal growth factor. J Natl Cancer Inst. 81:1020–1024. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu A, Shaeffer J, Leslie S, Kolm P and
El-Mahdi AM: Epidermal growth factor receptor: An independent
predictor of survival in astrocytic tumors given definitive
irradiation. Int J Radiat Oncol Biol Phys. 34:809–815. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nyati MK, Morgan MA, Feng FY and Lawrence
TS: Integration of EGFR inhibitors with radiochemotherapy. Nat Rev
Cancer. 6:876–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen DJ and Nirodi CS: The epidermal
growth factor receptor: A role in repair of radiation-induced DNA
damage. Clin Cancer Res. 13:6555–6560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harari PM and Huang SM: Epidermal growth
factor receptor modulation of radiation response: Preclinical and
clinical development. Semin Radiat Oncol. 12 Suppl 2:S21–S26. 2002.
View Article : Google Scholar
|
|
40
|
Ahsan A, Hiniker SM, Davis MA, Lawrence TS
and Nyati MK: Role of cell cycle in epidermal growth factor
receptor Inhibitor-mediated radiosensitization. Cancer Res.
69:5108–5114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brown JM and Koong AC: High-dose
single-fraction radiotherapy: Exploiting a new biology? Int J
Radiat Oncol Biol Phys. 71:324–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gerszten PC, Mendel E and Yamada Y:
Radiotherapy and radiosurgery for metastatic spine disease: What
are the options, indications, and outcomes? Spine (Phila Pa 1976).
34 Suppl 22:S78–S92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with or
without cetuximab for patients with stage IIIA or IIIB
non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|