Introduction
Lung cancer is one of the most common malignant
tumors, with the highest rate of morbidity and mortality worldwide
(1). Non-small cell lung cancer
(NSCLC) accounts for ~80% of bronchogenic carcinomas, and ~65% of
the patients with NSCLC present locally advanced or metastatic
disease at the time of diagnosis (2,3).
Cisplatin [cis-diamminedichloroplatinum (II); DDP] is one of the
most effective anticancer drugs for the treatment of lung cancer
and other tumors. Although the combination of DDP with
third-generation anticancer drugs (gemcitabine, paclitaxel,
vinorelbine and docetaxel) has improved the efficiency and overall
survival in patients with NSCLC, the development of primary or
acquired DDP resistance often reduces the therapeutic efficacy,
even leading to treatment failure (4,5).
Therefore, it is important to explore the mechanisms underlying DDP
resistance, and to search for effective strategies that may avoid
and overcome drug-resistance in NSCLC therapy.
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling
pathway is involved in the regulation of cell survival,
proliferation, differentiation, angiogenesis and apoptosis
(6,7). Sustained activation of the
PI3K/Akt/mTOR signaling pathway promotes cancer cell proliferation,
invasion and metastasis, and inhibits cell apoptosis in different
types of cancer (8,9). In addition, excessive activation of
the PI3K/Akt/mTOR pathway confers resistance to various cancer
therapies and is often associated with a poor prognosis of various
types of cancer, including NSCLC (10–12).
Therefore, inhibition of the PI3K/Akt/mTOR pathway is one of the
more efficient strategies to potentiate the antitumor effects of
DDP-based treatment for NSCLC.
Nucleotide excision repair (NER) is a universal and
highly functionally conserved DNA repair pathway that repairs DNA
lesions that alter the helical structure of the DNA molecule and
interfere with DNA replication and transcription. Excision repair
cross complementation group 1 (ERCC1) is one of the most important
components of the NER enzyme family due to its involvement in the
excision of DNA. During the DNA repair process, ERCC1 dimerizes
with xeroderma pigmentosum complementation group F (XPF) forming an
ERCC1-XPF complex that has a structure-specific endonuclease
activity, which makes the incision 5′ to the lesion site (13). ERCC1-XPF is essential for the repair
of DDP-induced DNA adducts. When ERCCl is highly expressed, the
efficiency of DNA repair is greatly increased and cancerous cells
are able to continue to survive and grow, resulting in tumor
resistance to DDP (14,15). Several studies have revealed that
elevated expression of ERCC1 (and ERCC1-XPF complexes) is
associated with DDP resistance and may act as a prognostic marker
for poor survival in patients with various types of cancer treated
with platinum-based chemotherapy, including NSCLC, breast, gastric
and urothelial ovarian cancer (16–19).
Conversely, suppression of ERCC1 expression can enhance or restore
sensitivity to DDP in various types of cancer (20,21).
In addition, negative ERCC1 expression or low ERCC1 expression was
reported to be associated with increased survival in patients with
advanced NSCLC treated with platinum-based chemotherapy (22). Therefore, agents targeting ERCC1 may
enhance platinum activity and/or reverse resistance, although this
strategy has to be further validated.
BEZ235, a novel dual PI3K/mTOR inhibitor, can
reverse the hyperactivation of the PI3K/Akt/mTOR pathway, resulting
in antitumor activity in cancer of various origins (23–25).
Recently, studies have revealed that combined treatment with
AZD6244, Trichostatin A and BEZ235 exerted synergistic antitumor
effects on NSCLC cells (26,27).
However, few studies have extensively examined the synergistic
effect between DDP and BEZ235 in NSCLC, and the association between
the level of ERCC1 expression and the activity of the PI3K/Akt/mTOR
signaling pathway is not fully understood. Hence, in the present
study, the following issues were investigated: i) whether
combination of BEZ235 with DDP could enhance sensitivity of
A549/DDP cells to DDP; and ii) if so, whether the mechanism
underlying the synergistic effect of combination therapy was
associated with downregulation of ERCC1 expression by BEZ235 in
A549/DDP cells.
Materials and methods
Cell culture
The human lung adenocarcinoma cell line (A549) and
DDP-resistant A549 cell line (A549/DDP; final concentration of 3.33
µM DDP to maintain drug resistance) were purchased from Guangzhou
Biological Technology Co., Ltd. (Guangzhou, China) and the Central
South University Advanced Research Center (Hunan, China),
respectively. All the cell lines were cultured at 37°C and 5%
CO2 in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; ExCell, Biology, Inc., Shanghai, China) and 1%
penicillin/streptomycin (Beyotime Institute of Biotechnology,
Haimen, China). A549/DDP cells were cultured in medium without DDP
for 1 week prior to experiments. Exponentially growing cells were
used in all experiments.
Reagents
BEZ235 was purchased from Selleck Chemicals
(Houston, TX, USA) and dissolved in DMSO at 42°C to obtain a stock
concentration of 5 mM. DDP was purchased from Qilu Pharmaceutical
Co., Ltd. (Shandong, China) and dissolved in PBS to obtain a stock
concentration of 6.67 mM. BEZ235 and DDP were stored at −80°C and
−20°C, respectively, and diluted to the desired final concentration
in RPMI-1640 medium at the time of use.
Cell viability assay
The cytotoxicity of DDP and BEZ235 was determined
using MTT. Cells were seeded into 96-well plates at a density of
5×103 cells/well in 100 µl medium and cultured
overnight. Subsequently, the cells were incubated with indicated
treatments for 48 h, followed by the addition of 5 mg/ml MTT
(Biosharp, Hefei, China). Following incubation at 37°C for 4 h, the
formazan crystals in the cells were dissolved in 150 µl DMSO and
shaken for 10 min. The absorbance at 490 nm was determined using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). To
determine whether the combination of drugs was synergistic,
additive or antagonistic, the Chou-Talalay method (28) was used based on the median-effect
equation. The formulas of dose reduction index (DRI) and
combination index (CI) for two drugs were as follows:
DRI=Dx/D and
CI=(D)1/(Dx)1 +
(D)2/(Dx)2. Dx was the
dose of the drug alone that inhibited x%; D was the portion of each
drug in combination that also inhibited x%. CI<1, =1 and >1
indicated synergism, an additive effect and antagonism,
respectively; DRI>1 indicated an enhanced cytotoxicity for the
drug combination. CalcuSyn software (version 2.0; Biosoft,
Cambridge, UK) was used to realize the aforementioned process.
Wound healing assay
A549/DDP cells were seeded in 6-well plates at a
density of 5×105 cells/well in 2 ml of medium overnight.
Then the cell monolayer was scraped with a pipette tip to form a
wound and washed gently with PBS. Cells were subsequently treated
with indicated concentrations of DDP, BEZ235 or DDP combined with
BEZ235. Wounds were imaged at 0 and 24 h following the scratch
under a light microscope (magnification, ×100; Leica Microsystems
GmbH, Wetzlar, Germany).
Invasion assays
Cells in serum-free media (5×105 cells in
200 µl) were added to the upper chamber of an insert (8-µm pore
size; Costar™; Corning Inc., Corning, NY, USA) coated with Matrigel
(1:7 dilution; BD Biosciences, San Jose, CA, USA). Media (600 µl)
containing 10% FBS were added to the lower chamber. Following 24 h,
the non-invading cells were removed with a cotton wool. Cells that
invaded through the Transwell filter were fixed with methylalcohol
for 4 min, stained with 0.1% crystal violet for 7 min and imaged
using a light microscope (Olympus Corp., Tokyo, Japan) at an ×100
magnification. The number of cells in three random fields was
counted.
Hoechst 33342 staining
A549/DDP cells were cultured on glass coverslips and
treated with indicated concentrations of DDP, BEZ235 or DDP
combined with BEZ235 for 48 h. Cells were fixed with 4%
paraformaldehyde for 10 min at room temperature following
treatment, followed by staining with 1 mg/ml Hoechst 33342
(Wanleibio Co., Ltd., Shanghai, China) at room temperature for 5
min. Following washing three times with PBS, the device was mounted
with anti-fluorescence quenching agent (Beyotime Institute of
Biotechnology) and cover-slipped. The cells were then immediately
imaged using an Olympus BX43F fluorescent microscope (Olympus
Corp.) at ×400 magnification. The percentage of apoptosis rate was
determined in each culture.
Flow cytometry
The apoptotic cells were quantified using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China).
Briefly, A549/DDP cells were seeded in 6-well plates at a density
of 4×105 cells/well in 2 ml of medium overnight.
Following treatment with BEZ235 or DDP alone or in combination,
cells were detached with EDTA-free trypsin and washed twice with
cooled PBS and re-suspended in 500 µl binding buffer. The cells
were then treated with 5 µl Annexin V-FITC followed by treatment
with 5 µl PI at room temperature for 15 min in the dark.
Fluorescence was determined by flow cytometry. Flow cytometric data
were analyzed using the MACSQuantify software (version 2.4;
Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Western blot analysis
Following treatment with the selected compounds, the
cells were collected and lysed in RIPA buffer (Beyotime Institute
of Biotechnology) containing protease and phosphatase inhibitors
(Beyotime Institute of Biotechnology). Protein concentrations were
quantified using the bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology). Equal amounts of proteins (60 µg) were
separated by 8% SDS-PAGE and transferred to nitrocellulose
membranes (ExCell Biology). Following blocking with PBS containing
3% bovine serum albumin (BSA; Vicmed Biotech Co., Ltd., Xuzhou,
China) for 1 h at room temperature, the membranes were incubated
with primary antibodies, including anti-ERCC1 (1:1,000; cat. no.
4801; AbSci, Vancouver, WA, USA), anti-Akt (1:1,000; cat. no.
4685), anti-phospho (p)-Akt (Ser473; 1:1,000; cat. no. 4060),
anti-mTOR (1:1,000; cat. no. 2983), anti-p-mTOR (Ser2448; 1:1,000;
cat. no. 5536), anti-pro caspase-3 (1:1,000; cat. no. 9665) and
anti-cleaved caspase-3 (1:1,000; cat. no. 9665; Cell Signaling
Technology, Inc., Danvers, MA, USA), overnight at 4°C. Equal lane
loading was confirmed using a monoclonal antibody against β-actin
(1:1,000; cat. no. AP0060; Bioworld Technology, Inc., Minneapolis,
MN, USA). The membranes were washed three times with PBS-Tween
(PBS-T) buffer for 15 min and incubated with Near-infrared
fluorescence-conjugated secondary antibodies (1:1,000; cat. no.
V926-32211; Vicmed Biotech) for 1 h. Following washing with the
PBS-T buffer, the membranes were scanned with the Odyssey Infrared
Imaging system (LI-COR Biosciences, Lincoln, NE, USA). The
intensity of the bands was analyzed using ImageJ software (National
Institutes of Health, Bethesda, MA, USA).
Statistical analysis
All experiments were run in triplicate. All
statistical analysis was performed using SPSS 16.0 (SPSS Inc.,
Chicago, IL, USA). Data are expressed as the mean ± standard error
of the mean. The difference between the groups was analyzed using
Student's t-test when only two groups were compared or one-way
analysis of variance (ANOVA) followed by the least significant
difference/Dunett-T3 tests when more than two groups were compared.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Activation of the PI3K/Akt/mTOR
pathway and ERCC1 upregulation are involved in the chemoresistance
of A549/DDP cells
To determine the chemoresistance ability of A549/DDP
cells to DDP, an MTT assay was performed. The half-maximal
inhibitory concentration (IC50) of DDP in A549/DDP cells
was 9.21-fold higher than that of A549 cells when treated with DDP
for 48 h. Thus, A549/DDP cells demonstrated great resistance to DDP
compared with A549 cells (data not shown). To analyze whether the
PI3K/Akt/mTOR pathway and ERCC1 were involved in the
chemoresistance of A549/DDP cells, western blotting was performed.
As displayed in Fig. 1, the
activation of Akt and mTOR in A549/DDP cells was higher than that
of A549 cells (P<0.01), and the levels of ERCC1 were
significantly increased (P<0.01). The results demonstrated that
the activation of the PI3K/Akt/mTOR pathway and ERCC1 upregulation
were associated with A549/DDP cell drug resistance.
 | Figure 1.Activation of the PI3K/Akt/mTOR
signaling pathway and high expression of ERCC1 may be involved in
the drug resistance of A549/DDP cells to DDP. (A) Protein levels of
t-Akt, p-Akt, t-mTOR, p-mTOR and ERCC1 were determined by western
blotting. (B) Quantitative analysis of the protein levels of t-Akt,
p-Akt, t-mTOR, p-mTOR and ERCC1. β-actin was used as a loading
control. Data are presented as the mean ± standard error, n=3.
**P<0.01 vs. the A549-cell group. DDP, cisplatin; T, total; p,
phospho; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase
B; mTOR, mammalian target of rapamycin; ERCC1, excision repair
cross complementation group 1. |
BEZ235 inhibits A549/DDP cell
proliferation and enhances the chemotherapeutic effect of DDP
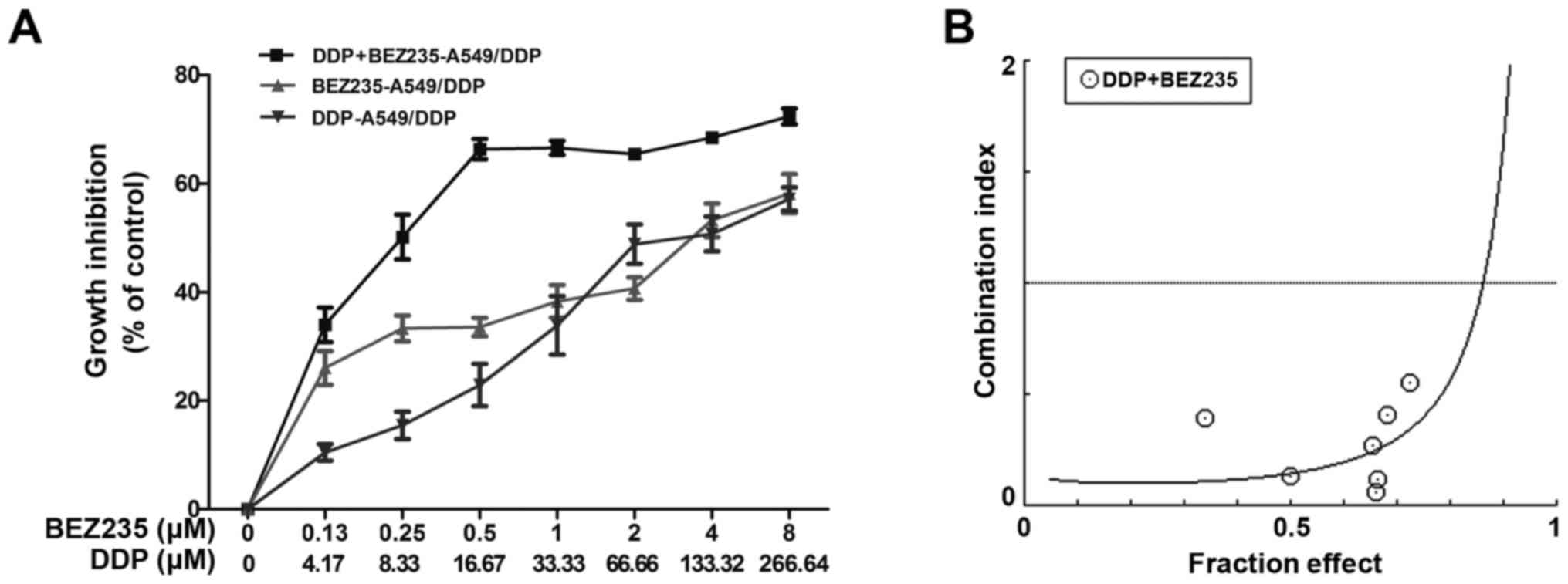
MTT assays revealed that DDP or BEZ235 treatment
significantly reduced A549/DDP cell viability. The IC50
values of DDP and BEZ235 were 118.44 and 3.62 µM, respectively. To
investigate whether BEZ235 sensitized and synergized DDP-induced
lung cancer cell death, the A549/DDP cells were treated with BEZ235
combined with DDP at a 33:1 constant ratio according to their
IC50 for 48 h. The IC50 values of BEZ235 and
DDP were 0.26 µM and 8.72 µM, respectively, and the DRI of BEZ235
and DDP were 13.82 and 13.58, respectively. (Fig. 2A). Additionally, the CI values
revealed synergistic effects between both drugs for almost all the
concentrations tested. The lowest CI values was identified at 16.67
µM DDP and 0.5 µM BEZ235 (Fig. 2B).
Thus, the indicated DDP and BEZ235 concentrations were used in the
subsequent experiments. These results indicated that BEZ235
synergistically suppressed cell proliferation and sensitized
A549/DDP cells to DDP.
BEZ235 enhances the DDP-induced
apoptosis in A549/DDP cells
To determine whether the synergistic growth
inhibition of DDP and BEZ235 resulted from apoptosis, A549/DDP
cells were exposed to DDP and BEZ235, alone and in combination for
48 h. Evaluation of the apoptotic ratio was performed using
AnnexinV-FITC/PI staining with flow cytometry. As displayed in
Fig. 3A, compared with DDP or
BEZ235 treatment alone, DDP and BEZ235 combination treatment
induced an increase in the percentage of apoptotic cells: control,
5.68±0.59%; DDP, 15.04±1.36%; BEZ235, 10.58±0.68%; DDP and BEZ235
combination, 28.57±2.05%. To further confirm these findings, the
effect of DDP and BEZ235 on caspase activity was examined by
western blotting. The results revealed that, compared with the
control group, treatment with DDP or BEZ235 induced
cleaved-caspase-3 activation and decreased the pro-caspase-3
expression level (P<0.05 or P<0.01). Combination treatment
significantly activated cleaved-caspase-3 and decreased the levels
of pro-caspase-3 when compared with DDP or BEZ235 used alone
(P<0.01; Fig. 3B). The changes
of nuclear morphology of A549/DDP cells were also observed under a
fluorescence microscope by Hoechst 33342 DNA staining. The results
revealed that the nuclei were intact and staining was less bright
in the control group. However, A549/DDP cells treated with DDP or
BEZ235 exhibited severe chromatin condensation and apoptotic body
formation of the nuclei, and the nuclei were much brighter than
that of the control group. In addition, the combination group
exhibited significantly more apoptotic cells with condensed or
fragmented nuclei than those treated with either DDP or BEZ235
alone (Fig. 3C). These findings
indicated that co-treatment with DDP and BEZ235 synergistically
induced A549/DDP cell apoptosis.
BEZ235 restores the sensitivity of
A549/DDP cells to DDP through downregulation of the PI3K/Akt/mTOR
pathway and ERCC1 levels
To investigate the molecular mechanism underlying
the synergistic effect of DDP and BEZ235 in A549/DDP cells, western
blotting was performed to analyze the relevant targets of the
PI3K/Akt/mTOR signaling pathway and ERCC1 expression levels. Cells
were treated with DDP and BEZ235 alone or in combination for 48 h,
followed by western blot analysis. As displayed in Fig. 4A, DDP upregulated the level of
p-Akt, p-mTOR and ERCC1 (P<0.01), compared with the control
group, while BEZ235 significantly downregulated the phosphorylation
level of Akt and mTOR, and reduced the expression of ERCC1
(P<0.01). Furthermore, compared with treatment with DDP alone,
the combination of DDP and BEZ235 downregulated the level of p-Akt,
p-mTOR and ERCC1 further (P<0.01). The levels of p-Akt/p-mTOR
and ERCC1 in NSCLC were positively correlated, as determined by
Pearson's correlation analysis (Fig.
4B).
 | Figure 4.BEZ235 restores A549/DDP cell
sensitivity to DDP, and downregulates PI3K/Akt/mTOR signaling and
ERCC1 levels. A549/DDP cells were treated with the indicated
concentrations of DDP and/or BEZ235 for 48 h. (A) Western blot
analyses were performed for t-Akt, p-Akt, t-mTOR, p-mTOR and ERCC1.
The semi-quantitative analysis results of relative protein
expression in A549/DDP cells (right). (B) Correlation between ERCC1
and p-Akt expression, and p-mTOR expression in A549/DDP cells was
determined using Pearson's correlation analysis. Data are presented
as the mean ± standard error, n=3. *P<0.05, **P<0.01 vs. the
control group; aP<0.05 vs. BEZ235 alone;
bP<0.01 vs. DDP alone. DDP, cisplatin; T, total; p,
phospho; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase
B; mTOR, mammalian target of rapamycin; ERCC1, excision repair
cross complementation group 1. |
BEZ235 and DDP synergistically inhibit
the migration and invasion abilities of A549/DDP cells
Activation of Akt is associated with NSCLC invasion
and migration, and our study demonstrated that Akt phosphorylation
was upregulated in A549/DDP cells when treated with DDP. Several
functional studies were performed to investigate whether BEZ235
enhanced the ability of DDP, and inhibited the migration and
invasion of A549/DDP cells by downregulating Akt phosphorylation. A
wound healing assay revealed that the rate of wound closure
decreased significantly when treated with BEZ235 or DDP, and that
compared with BEZ235 or DDP treatment, the combined treatment with
BEZ235 and DDP inhibited the migration ability of A549/DDP cells
further (P<0.01; Fig. 5A).
Similarly, Transwell assays indicated that BEZ235 or DDP treatment
effectively reduced the invasion ability of A549/DDP cells, and the
combination treatment exhibited a stronger effect (P<0.01;
Fig. 5B). These results
demonstrated that BEZ235 and DDP have synergistic inhibitory
effects on NSCLC in vitro.
Discussion
DDP has been extensively used in the treatment of
various tumor types, including lung carcinoma. However, inherent
and acquired drug resistance continues to be a major clinical
problem in lung cancer management. Therefore, developing new
therapeutic strategies to overcome drug-resistance is a key
priority. In the present study, combination of BEZ235 with DDP
enhanced the antitumor effects, and increased the sensitivity of
drug-resistant lung cancer cell line A549/DDP to DDP.
BEZ235 is a synthetic low-molecular-mass
imidazoquinoline compound that potently and reversibly inhibits
PI3K and mTOR kinase activity by binding to the ATP-binding cleft
of these enzymes (29).
Accumulating preclinical studies have demonstrated that NVP-BEZ235
has beneficial pharmaceutical properties as an anticancer agent. It
was previously reported that NVP-BEZ235 exhibited promising
therapeutic activity in various types of cancer when used alone or
combined with agents other than DDP (25,30).
In the present study, BEZ235 also demonstrated strong antitumor
activity in MTT assays. The combination of the two drugs
significantly inhibited cell viability of the A549/DDP lung cancer
cell line. Consistent with its anti-proliferative effects,
co-treatment of BEZ235 with DDP was more effective in inhibiting
cell migration and invasion than DDP or BEZ235 administered alone.
In addition, the CI value was <1 for almost all the
concentrations tested. These results indicated that the PI3K/mTOR
dual inhibitor BEZ235 synergistically potentiated the antitumor
effects of DDP in A549/DDP cells.
DDP is a platinum drug. The mechanism of action of
DDP is thought to be the platinum interstrand and intrastrand
crosslinks that form DDP-DNA adducts, which interfere with DNA
replication and transcription, resulting in DNA fragmentation and
error coding, eventually triggering cell death (31). Although the mechanisms of platinum
resistance are not clearly understood, NER appears to play an
important role in mediating platinum resistance or sensitivity to
platinum chemotherapeutic agents in cancer treatment. ERCC1, an
important component required for NER, is involved in repair of the
intra and inter-strand DNA crosslink caused by platinum-based
treatment. In addition, it has been reported that ERCC1 expression
was significantly elevated by DDP treatment in several tumor types,
but data on the role of ERCC1 in NSCLC are still limited and are
contradictory (32,33). In the present study, the results
revealed that the ERCC1 expression level was significantly higher
in A549/DDP cells treated with DDP than that of the control group,
whereas BEZ235 or co-administration of BEZ235 and DDP significantly
decreased the level of ERCC1 expression compared with DDP treatment
alone, indicating that BEZ235 blocked DDP-induced ERCC1 protein
expression. Therefore, this indicated that the enhancement of DDP
sensitivity by BEZ235 was at least, partly attributed to
downregulation of ERCC1 expression in A549/DDP cells, thus leading
to the reduction in the proficiency to repair DDP-induced DNA
damage.
To explore the potential relationship between the
PI3K/Akt/mTOR pathway and ERCC1 expression, the expression of
PI3K/Akt/mTOR pathway proteins was analyzed by western blotting.
The findings revealed that the levels of p-Akt and p-mTOR in
A549/DDP cells were much higher than that of A549 cells, indicating
that the PI3K/Akt/mTOR pathway is activated in A549/DDP cell lines.
As A549/DDP cells were treated with BEZ235 and DDP alone or in
combination for 48 h, the phosphorylation levels of Akt and mTOR
were upregulated when treated with DDP alone. However, treatment
with BEZ235 alone or in combination with DDP significantly
decreased the phosphorylation levels of Akt and mTOR in A549/DDP
cells compared with DDP treatment alone. Notably, the combination
of DDP and BEZ235 did not further reduce the phosphorylation levels
of Akt and mTOR in A549/DDP cells. In addition, the phosphorylation
levels of Akt and mTOR and ERCC1 in A549/DDP cells were positively
correlated as determined by Pearson's correlation coefficient
analysis. The current findings have not determined the mechanism
regarding the effects of BEZ235, however there are two
possibilities: i) downregulation of ERCC1 protein expression may be
an important event subsequent to the inhibition of the Akt/mTOR
signaling pathway by BEZ235 in A549/DDP cells; and ii) BEZ235 may
decrease the ERCC1 protein expression by other or unknown
mechanisms. Based on these observations, inhibition of the
PI3K/Akt/mTOR signaling pathway by BEZ235 may result in a
downregulation of ERCC1 expression that enhances the
chemosensitivity of A549/DDP cells, and overcome resistance to DDP,
but the detailed relationships involved warrant further
elucidation.
Apoptosis is an ordered and orchestrated cellular
process that occurs in physiological and pathological conditions.
Numerous studies have illustrated that disordered apoptosis has
been associated with the development of many types of solid cancer,
including lung, breast, prostate, bladder and ovarian cancer
(34–36). Besides enhancement of the repair of
DDP-caused DNA damage by ERCC1, impaired DDP-induced apoptosis has
also been implicated in the development of the DDP-resistance in
the treatment of solid malignancies (37,38).
Therefore, apoptosis induction has become a popular target of many
treatment strategies in a wide variety of tumor cells (39,40).
To determine whether the synergistic growth inhibition of BEZ235
and DDP resulted from apoptosis, A549/DDP cells were exposed to
BEZ235 and DDP, alone and in combination for 48 h, and apoptosis
was evaluated by flow cytometric analysis and Hoechst 33342 DNA
staining. Consistent with its anti-proliferative effects,
co-administration of BEZ235 with DDP significantly increased the
apoptosis of A549/DDP cells compared with cells treated with either
BEZ235 or DDP alone, indicating that combination of BEZ235 and DDP
was more effective in inducing apoptosis of A549/DDP cells. It is
generally accepted that synergy is achieved through a cooperation
of two agents functioning via distinct mechanisms. The activation
of the PI3K/Akt/mTOR pathway in A549/DDP cells promoted cell
growth, survival and proliferation, and inhibited cell apoptosis.
BEZ235 treatment induced A549/DDP cell apoptosis via inhibition of
the PI3K/Akt/mTOR pathway. Notably, in the present study DDP
treatment activated the Akt/mTOR signaling pathway as reflected by
the increased levels of Akt and mTOR phosphorylation in A549/DDP
cells, and the combination of DDP and BEZ235 did not further reduce
the phosphorylation levels of Akt and mTOR when compared with
BEZ235 treatment alone, indicating that inhibition of the Akt/mTOR
signaling pathway by BEZ235 is not the only mechanism involved in
promoting cell apoptosis. DDP is known to induce apoptosis
following DNA damage by propagation of DNA damage recognition
signals to downstream pathways, including nuclear factor-κB, p53,
p73, MAPK and mitochondria-related apoptosis signaling pathways
(38,41–42).
Therefore, treatment with BEZ235 and DDP may synergistically induce
apoptosis via the activation of complex signaling cascades in
A549/DDP cells, and the exact mechanism of this synergy requires
further investigation.
Caspases are central to the mechanism of apoptosis
as they are both initiators and executioners. Caspase-3 is involved
in both the intrinsic and extrinsic apoptotic pathways. It cleaves
the inhibitor of the caspase-activated deoxyribonuclease, which is
responsible for nuclear apoptosis (43). To further investigate the mechanism
of co-treatment in the promotion of apoptosis of A549/DDP cells,
the activity of caspase-3 was examined by western blotting. The
results of the present study revealed that the combination of
BEZ235 and DDP significantly enhanced cleaved-caspase-3 activation
and decreased pro-caspase-3 expression level when compared with DDP
or BEZ235 used alone. Consistent with this observation, Yu et
al (44) reported that BEZ235
can increase the expression of caspase-3 in human glioma stem
cells. Bhende et al (45)
reported that BEZ235-treated follicular lymphoma cells had a
1.6–2-fold increase in caspase-3 activation following 24 h of
incubation with the drug, compared with cells treated with the
vehicle alone. The results of the present study supported this
notion that BEZ235 synergistically induced apoptosis via a
caspase-3-dependent pathway in A549/DDP cells. In addition, the
results in the present study revealed that A549/DDP cells, which
have a high level of ERCC1 expression, exhibited decreased
apoptosis, and that A549/DDP cells co-treated with BEZ235 and DDP,
in which the level of ERCC1 expression was reduced, exhibited an
increased susceptibility to apoptosis. This observation indicated
that the level of ERCC1 expression may be associated with the
antitumor effects of DDP chemotherapy.
In summary, the findings of the present study
demonstrated that BEZ235 increased the chemosensitivity to DDP in
DDP-resistant lung cancer cell line A549/DDP, and BEZ235 combined
with DDP may be a promising NSCLC therapy. The results may help in
overcoming resistance to DDP and developing novel therapeutic
strategies for patients with advanced NSCLC.
Acknowledgements
Not applicable.
Funding
XW received research grants from the Pharmaceutical
research project Aosai Kang Hospital Grant, Jiangsu Province
Pharmaceutical Association (grant no. 201516) and from the Project
of Science and Technology Development fund of Nanjing Medical
University (grant no. 2016NJMU139).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AX, XW and HL conceived and designed the study. HL
performed the experiments. AX and HL wrote the manuscript. RL and
LL analyzed the data of experiments. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A National Cancer Database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma D, Wang J, Hao X, Wang Y, Hu X, Xing P
and Li J: Gemcitabine combined with cisplatin as adjuvant
chemotherapy for non-small cell lung cancer: A retrospective
analysis. Thorac Cancer. 8:482–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia Y, Zhou D, Jia Q, Ying Y and Chen S:
Synergistic and attenuated effect of HSS in combination treatment
with docetaxel plus cisplatin in human non-small-cell lung SPC-A-1
tumor xenograft. Biomed Pharmacother. 79:27–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Müller A, Zang C, Chumduri C, Dörken B,
Daniel PT and Scholz CW: Concurrent inhibition of PI3K and
mTORC1/mTORC2 overcomes resistance to rapamycin induced apoptosis
by down-regulation of Mcl-1 in mantle cell lymphoma. Int J Cancer.
133:1813–1824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasore T, Reynolds AL and Kennedy BN:
Targeting the PI3K/Akt/mTOR pathway in ocular neovascularization.
Adv Exp Med Biol. 801:805–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Zhang B, Liu Y and Yin C: EBP50
inhibits the migration and invasion of human breast cancer cells
via LIMK/cofilin and the PI3K/Akt/mTOR/MMP signaling pathway. Med
Oncol. 31:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A,
Takahashi H, Wakasugi T, Funahashi H, Sato M and Takeyama H: IGF-1
mediates PTEN suppression and enhances cell invasion and
proliferation via activation of the IGF-1/PI3K/Akt signaling
pathway in pancreatic cancer cells. J Surg Res. 160:90–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu JL, Wang ZW, Hu LM, Yin ZQ, Huang MD,
Hu ZB, Shen HB and Shu YQ: Genetic variants in the
PI3K/PTEN/AKT/mTOR pathway predict platinum-based chemotherapy
response of advanced non-small cell lung cancers in a Chinese
population. Asian Pac J Cancer Prev. 13:2157–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsodikov OV, Enzlin JH, Schärer OD and
Ellenberger T: Crystal structure and DNA binding functions of
ERCC1, a subunit of the DNA structure-specific endonuclease
XPF-ERCC1. Proc Natl Acad Sci USA. 102:11236–11241. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Y, Yan X, Zhang G, Zhao W and Jiao S:
The predictive value of ERCC1 and p53 for the effect of
panobinostat and cisplatin combination treatment in NSCLC.
Oncotarget. 6:18997–19005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HW, Choi YW, Han JH, Kim JH, Jung JH,
Jeong SH, Kang SY, Choi JH, Oh YT, Park KJ, et al: Expression of
excision repair cross-complementation group 1 protein predicts poor
outcome in advanced non-small cell lung cancer patients treated
with platinum-based doublet chemotherapy. Lung Cancer. 65:377–382.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palomba G, Atzori F, Budroni M, Ombra M,
Cossu A, Sini M, Pusceddu V, Massidda B, Frau B, Notari F, et al:
ERCC1 polymorphisms as prognostic markers in T4 breast cancer
patients treated with platinum-based chemotherapy. J Transl Med.
12:2722014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yun J, Kim KM, Kim ST, Kim JH, Kim JA,
Kong JH, Lee SH, Won YW, Sun JM, Lee J, et al: Predictive value of
the ERCC1 expression for treatment response and survival in
advanced gastric cancer patients receiving cisplatin-based
first-line chemotherapy. Cancer Res Treat. 42:101–106. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Pan H, Liu D, Mao N, Zuo C, Li L,
Xie T, Huang D, Huang Y, Pan Q, et al: Excision repair cross
complementation group 1 is a chemotherapy-tolerating gene in
cisplatin-based treatment for non-small cell lung cancer. Int J
Oncol. 46:809–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song W and Ma H: The expression of ERCC1
and BRCA1 predicts prognosis of platinum-based chemotherapy in
urothelial cancer. Onco Targets Ther. 9:3465–3471. 2016.PubMed/NCBI
|
|
20
|
Usanova S, Piée-Staffa A, Sied U, Thomale
J, Schneider A, Kaina B and Köberle B: Cisplatin sensitivity of
testis tumour cells is due to deficiency in interstrand-crosslink
repair and low ERCC1-XPF expression. Mol Cancer. 9:2482010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang IY, Kim MH, Kim HB, Lee DY, Kim SH,
Kim HY and You HJ: Small interfering RNA-induced suppression of
ERCC1 enhances sensitivity of human cancer cells to cisplatin.
Biochem Biophys Res Commun. 327:225–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin X, Yao W, Li W, Feng X, Huo X, Yang S,
Zhao H and Gu X: ERCC1 and BRCA1 mRNA expressions are associated
with clinical outcome of non-small cell lung cancer treated with
platinum-based chemotherapy. Tumour Biol. 35:4697–4704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera VA, Zeindl-Eberhart E, Jung A,
Huber RM and Bergner A: The dual PI3K/mTOR inhibitor BEZ235 is
effective in lung cancer cell lines. Anticancer Res. 31:849–854.
2011.PubMed/NCBI
|
|
24
|
Chen L, Jin T, Zhu K, Piao Y, Quan T, Quan
C and Lin Z: PI3K/mTOR dual inhibitor BEZ235 and histone
deacetylase inhibitor Trichostatin A synergistically exert
anti-tumor activity in breast cancer. Oncotarget. 8:11937–11949.
2017.PubMed/NCBI
|
|
25
|
Xie G, Wang Z, Chen Y, Zhang S, Feng L,
Meng F and Yu Z: Dual blocking of PI3K and mTOR signaling by
NVP-BEZ235 inhibits proliferation in cervical carcinoma cells and
enhances therapeutic response. Cancer Lett. 388:12–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu Y, Wu X, Yin Y, Yang Y, Ma D and Li H:
Antitumor activity of selective MEK1/2 inhibitor AZD6244 in
combination with PI3K/mTOR inhibitor BEZ235 in gefitinib-resistant
NSCLC xenograft models. J Exp Clin Cancer Res. 33:522014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piao J, Chen L, Quan T, Li L, Quan C, Piao
Y, Jin T and Lin Z: Superior efficacy of co-treatment with the dual
PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor
Trichostatin A against NSCLC. Oncotarget. 7:60169–60180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maira SM, Stauffer F, Brueggen J, Furet P,
Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker
K, et al: Identification and characterization of NVP-BEZ235, a new
orally available dual phosphatidylinositol 3-kinase/mammalian
target of rapamycin inhibitor with potent in vivo antitumor
activity. Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torki S, Soltani A, Shirzad H, Esmaeil N
and Ghatrehsamani M: Synergistic antitumor effect of NVP-BEZ235 and
CAPE on MDA-MB-231 breast cancer cells. Biomed Pharmacother.
92:39–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eastman A: The formation, isolation and
characterization of DNA adducts produced by anticancer platinum
complexes. Pharmacol Ther. 34:155–166. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torii Y, Kato R, Minami Y, Hasegawa K,
Fujii T and Udagawa Y: ERCC1 expression and chemosensitivity in
uterine cervical adenocarcinoma cells. Anticancer Res. 34:107–115.
2014.PubMed/NCBI
|
|
33
|
Li Q, Tsang B, Bostick-Bruton F and Reed
E: Modulation of excision repair cross complementation group 1
(ERCC-1) mRNA expression by pharmacological agents in human ovarian
carcinoma cells. Biochem Pharmacol. 57:347–353. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fong MY and Kakar SS: The role of cancer
stem cells and the side population in epithelial ovarian cancer.
Histol Histopathol. 25:113–120. 2010.PubMed/NCBI
|
|
35
|
Yang H and Dou QP: Targeting apoptosis
pathway with natural terpenoids: Implications for treatment of
breast and prostate cancer. Curr Drug Targets. 11:733–744. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Souza PM and Lindsay MA: Apoptosis as a
therapeutic target for the treatment of lung disease. Curr Opin
Pharmacol. 5:232–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QQ, Lee RX, Liang H, Zhong Y and Reed
E: Enhancement of cisplatin-induced apoptosis by β-elemene in
resistant human ovarian cancer cells. Med Oncol. 30:4242013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun A, Chia JS, Chiang CP, Hsuen SP, Du
JL, Wu CW and Wang WB: The chinese herbal medicine Tien-Hsien
liquid inhibits cell growth and induces apoptosis in a wide variety
of human cancer cells. J Altern Complement Med. 11:245–256. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neuzil J, Tomasetti M, Mellick AS, Alleva
R, Salvatore BA, Birringer M and Fariss MW: Vitamin E analogues: A
new class of inducers of apoptosis with selective anti-cancer
effects. Curr Cancer Drug Targets. 4:355–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Z, Schumaker LM, Egorin MJ, Zuhowski
EG, Guo Z and Cullen KJ: Cisplatin preferentially binds
mitochondrial DNA and voltage-dependent anion channel protein in
the mitochondrial membrane of head and neck squamous cell
carcinoma: Possible role in apoptosis. Clin Cancer Res.
12:5817–5825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan M, Ni J, Song D, Ding M and Huang J:
Activation of unfolded protein response protects osteosarcoma cells
from cisplatin-induced apoptosis through NF-κB pathway. Int J Clin
Exp Pathol. 8:10204–10215. 2015.PubMed/NCBI
|
|
43
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu Y, Yu X, Ma J, Tong Y and Yao J:
Effects of NVP-BEZ235 on the proliferation, migration, apoptosis
and autophagy in HT-29 human colorectal adenocarcinoma cells. Int J
Oncol. 49:285–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhende PM, Park SI, Lim MS, Dittmer DP and
Damania B: The dual PI3K/mTOR inhibitor, NVP-BEZ235, is efficacious
against follicular lymphoma. Leukemia. 24:1781–1784. 2010.
View Article : Google Scholar : PubMed/NCBI
|