Introduction
Sonodynamic therapy (SDT) combines ultrasound and
sonosensitizers to treat malignancies. SDT is a type of anti-cancer
treatment with promising potential due to its excellent ability to
selectively destroy tumor cells and maximize the protection for
normal tissues, which makes the SDT method unique for treating
patients with tumors in anatomical positions that are difficult to
access surgically (1,2).
Previous studies have suggested that SDT induces
apoptosis, autophagy, and necrosis of tumor cells (3–9). Our
previous studies have demonstrated that SDT inhibits
neovascularization in tumor tissues (10), enhances the pro-inflammatory
response and reverses the passive properties of macrophages and
dendritic cells (11). However, the
effects of SDT on the immune status of the tumor microenvironment
are still unknown.
The occurrence of tumor-associated immune responses
is dependent mainly on the infiltration of reactive leukocytes in
the tumor microenvironment (12,13).
The tumor microenvironment should exhibit similar immune cell
surveillance to normal tissue. However, due to the long-term
exposure of tumor-induced immune cells to the tumor
microenvironment, they may directly or indirectly promote
malignancy. Therefore, immune cells are often ineffective in
destroying tumors because of their inability to implement their
cytotoxic effector functions, which is shown by the transformation
of M1-M2 type tumor-associated macrophages, and the restriction of
dendritic cells in the ‘immature stage’ (14).
Galon et al (15) demonstrated that the numbers of
antigen-specific immune cells within the tumor microenvironment,
such as CD8+ T cells, are highly relevant to the
clinical prognosis (15).
Morphological changes in the tumor microenvironment suggest that
SDT could mitigate the declining function of macrophages and
dendritic cells in both nude mice and BALB/c mice with healthy
immune systems (11). As SDT can
positively affect the phenotype and number of macrophages and
dendritic cells within the tumor microenvironment, it was
hypothesized that it is highly likely for SDT to beneficially
influence the numbers and phenotype of tumor-associated immune
cells, which may then improve outcomes.
In the current study, the effects of SDT on the
number of immune cell subtypes and outcomes in the BALB/c mice
xenograft model were investigated. In addition, it should be noted
that immune cell infiltration into the tumor microenvironment
occurs via the vascular system. Hence, the present study also
investigated the characteristics of blood vessels involved in the
transport of immune cells.
Materials and methods
Chemicals and reagents
5-Aminolevulinic acid (5-ALA) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rabbit
anti-intercellular adhesion molecule (ICAM)-1 (catalog no.
bs-4617R), vascular cell adhesion protein (VCAM)-1 (catalog no.
bs-0396R), E-selectin (catalog no. bs-1273R), CD68 (catalog no.
bs-20402R), CD4 (catalog no. bs-0647R), CD8 (catalog no. bs-0647R),
CTLA-4 (catalog no. bs-1179R), Foxp3 (catalog no. bs-0269R), CD80
(catalog no. bs-2211R), CD45 (catalog no. bs-0522R) antibodies were
provided by BIOSS (Beijing, China). The mouse anti-CD31 antibody
(catalog no. ab24590) was from Abcam (Cambridge, UK) and the rabbit
anti-β-actin antibody (catalog no. A1978 MSDS) was from
Sigma-Aldrich. All other chemicals and reagents were supplied by
OriGene Technologies, Inc. (Beijing, China) unless otherwise
indicated.
Cell culture
Murine melanoma B16F10 cells were purchased from the
Chinese Academy of Sciences, Shanghai Institute Cell Resource
Center, and cultured in Roswell Park Memorial Institute 1640 medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin,
and 100 g/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a 5% CO2 humidified
environment. Primary human umbilical vein endothelial cells
(HUVECs) and their appropriate medium were purchased from ScienCell
Research Laboratories (San Diego, CA, USA). Tissue culture flasks
were pre-coated with poly-L-lysine. Cells were cultured in
endothelial cell growth medium supplemented with endothelial cell
growth supplement, 5% fetal bovine serum, and
penicillin/streptomycin solution and maintained at 37°C in 5%
CO2 humidified environment. HUVECs from the 2nd to 5th
passage were used in all experiments.
Tumor xenograft mouse model
A total of forty-eight male BALB/c mice (weight,
12–14 g; age, 4 weeks) were purchased from Vital River Laboratories
(Beijing, China). All mice were bred in dedicated pathogen-free
barrier facilities in a standard animal laboratory allowing free
activity and were provided with standard food and water ad
libitum. Mice were maintained at 22–24°C in 55% humidity, on a
12-h light/12-h dark cycle. 100 µl B16F10 cell suspension
(1×106 cells/ml) was injected subcutaneously into the
flanks of the mice. The mice were ready for use when the tumors
reached 100 mm3 (~5 days after inoculation). All animal
protocols were approved by the Animal Care and Use Committee of
Harbin Medical University (Harbin, China).
Ultrasonography
The ultrasonic device was designed and manufactured
by the Harbin Institute of Technology, which contained two parts: A
RIGOL DG204A function arbitrary wave form generator and a North
Star Model SWA200ARF power amplifier. This machine was used as
previously described (7).
Treatment protocols
In the in vivo experiment, 48 mice were
divided randomly and equally into four groups: Control, 5-ALA (250
mg/kg body weight), sonication (US), and sonication plus 5-ALA (250
mg/kg) (US + ALA). The tumors were sonicated (1.0 MHz,
0.8W/cm2, 10% duty cycle) for 5 min in the dark in the
US group and US + ALA group four hours after intraperitoneal
injection of 5-ALA. This treatment was repeated twice weekly for 2
weeks (namely, four treatments in total). Half of the mice in each
group were used for histology and western blotting procedures and
the other half in each group were used for survival analysis.
In the in vitro experiment, HUVECs were
divided into four groups: Control, ALA, US, and US + ALA. In the
ALA and US + ALA groups, the cells were incubated with 1 mM ALA in
the dark. Instead of ALA, an equivalent quantity of medium was used
for the control and US groups. After 4 h incubation, the cells in
the US and US + ALA groups were exposed to ultrasound (0.87 MHz,
0.6 W/cm2, 60% duty cycle) for 60 sec in the dark.
Evaluation of antitumor effect
Mice were examined once every 2 days after the tumor
volume reached 100 mm3. The orthogonal tumor dimensions
(A and B) were measured with Vernier calipers. The tumor volume was
calculated as follows: Volume=(π/6) × A × B2. To
minimize suffering, mice were sacrificed when the primary tumor
reached a diameter of 1.5 cm (death due to progressive tumor) or
when the mouse had lost ≥20% of its body weight and the primary
tumor was less than 1.5 cm (death due to metastatic disease). Mice
were considered cured when the tumor did not return 90 days after
corresponding treatment or their body weight remained normal.
Histology
Formalin-fixed (10%, overnight at 4°C),
paraffin-embedded tumor tissues were sectioned (2.5 µm), placed in
series on silanized glass slides, dewaxed, and rehydrated. Antigens
were retrieved following treatment in 10 mM citrate buffer (pH 6.0)
for 15 min in a pressure cooker. After rinsing with
phosphate-buffered saline, the sections were immersed in 3%
hydrogen peroxide solution for 10 min to block endogenous
peroxidases. Non-specific binding was prevented by incubation in 5%
normal goat serum for 20 min in a humidified chamber.
For immunofluorescence staining, the sections were
then incubated with antibodies for CD45, CD68, CD4, CD8 (1:100
dilution) overnight at 4°C, washed, and incubated with Alexa Fluor
488-conjugated goat anti-rabbit IgG (1:800 dilution; Abcam; catalog
no. ab150117) at 37°C for 30 min. For double staining with
antibodies for CD31 and ICAM-1, VCAM-1, or E-selectin (1:100
dilution), samples were incubated at 4°C overnight with the primary
antibodies (mouse anti-mouse CD31 and rabbit anti-mouse ICAM-1,
VCAM-1 or E-selectin, or mouse IgG1). Samples were then washed and
incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG
(1:800 dilution; Abcam; catalog no. ab150117) and Alexa Fluor
405-conjugated donkey anti-rabbit IgG (1:800 dilution; Abcam;
catalog no. ab175651) at 37°C for 30 min. Images were captured with
a fluorescence imaging microscope (Olympus BX51; Olympus
Corporation, Tokyo, Japan) equipped with Flash Point software.
For immunohistochemical staining, the sections were
incubated with antibodies for CD31 (1:100) overnight at 4°C,
washed, and incubated with secondary antibodies (immediate-use goat
anti-rabbit IgG horseradish-peroxidase polymers; OriGene
Technologies, Inc.; catalog no. PV-6001) at 37°C for 30 min.
Diaminobenzidine was used as a chromogen. Tissue sections were
counterstained with hematoxylin at room temperature for 10 sec,
dehydrated and mounted. The sections were observed under a light
microscope (Olympus BX51; Olympus Corporation).
Negative control immunostaining was carried out
using the same procedure except that the primary antibody was
replaced by nonimmunized serum.
Western blot analyses
Fresh tissue samples and cells were lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) on ice. Protein concentrations were
determined using BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of proteins (30 µg per lane) were
separated by 10% SDS-PAGE and electrophoretically transferred onto
a nitrocellulose filter membrane. After blocking in Tris-buffered
saline Tween-20 (TBST) containing 5% low-fat milk at room
temperature for 1.5 h, the membranes were incubated overnight at
4°C with primary antibodies against the target proteins CD68 (1:200
dilution; BIOSS; catalog no. bs-20402R), CD4 (1:300 dilution;
BIOSS; catalog no. bs-0647R), CD8 (1:350 dilution; BIOSS; catalog
no. bs-0647R), CTLA-4 (1:200 dilution; BIOSS; catalog no.
bs-1179R), Foxp3 (1:100 dilution; BIOSS; catalog no. bs-0269R),
CD80 (1:300 dilution; BIOSS; catalog no. bs-2211R), with β-actin
(1:5,000 dilution; Sigma-Aldrich, Merck KGaA; catalog no. A1978
MSDS) as loading control. After washing three times with TBST, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:30,000 dilution; OriGene Technologies,
Inc.; catalog no. ZB-2301) for 1 h at room temperature. After
washing twice with TBST which was then removed from the membrane,
ECL developing reagent (Beyotime Institute of Biotechnology) was
then added to the membrane. Protein levels were detected using an
ECL detection system (Tanon-5200; Tanon Technology Co., Ltd.,
Shanghai, China) (16). The
relative abundance of each band was quantified by using Quantity
One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data
were normalized to β-actin.
Transmission electron microscopy
(TEM)
For TEM study, xenografts were dissected and fixed
with 2.5% glutaraldehyde for 2 h. Then, they were postfixed in 1%
OsO4 at 4°C for 2 h, and were embedded with Epon812 (EM
Sciences, Washington, PA, USA) for 72 h at 60°C. Ultrathin sections
were cut (70 nm) and stained with 0.5% uranium acetate at room
temperature for 30 min, followed by lead citrate, and then observed
under a transmission electron microscope (JEOL 200, Hitachi, Ltd.,
Tokyo, Japan).
Reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR)
Total-RNA from the cultured cells was isolated using
TRIzol® reagent (Sigma-Aldrich; Merck KGaA) as described
in a previous work (8). To quantify
ICAM-1 mRNA, first strand cDNAs were synthesized using the
ImProm-II™ Reverse Transcription System (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. The
RT-PCR was performed using Go Taq® Colorless Master Mix
(Promega Corporation) on an Alpha Unit Block Assembly for DNA
Engine System (Bio-Rad Laboratories, Inc.). All primers were
obtained from GenScript (Nanjing, China).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
endogenous control. Primers used for mRNA detection were: Forward,
5′-CTCCTGTGACCAGCCCAAGT-3′ and reverse, 5′-GGCAGCGTAGGGTAAGGTTC-3′
for ICAM-1 and forward, 5′-AACGGATTTGGTCGTATTGG-3′ and reverse,
5′-TGGAAGATGGTGATGGGATT-3′ for GADPH. Thermocycling conditions: One
cycle at 94°C for 2 min, followed by 25 cycles at 94°C for 30 sec,
56°C for 40 sec, and 72°C for 30 sec. This was followed by a single
cycle at 72°C for 5 min to facilitate final extension. PCR products
were run on 1% agarose gels and visualized by AlphaDigiDoc digital
camera gel imaging system (ProteinSimple, San Jose, CA, USA). The
densitometry was quantified by using Quantity One software (Bio-Rad
Laboratories, Inc.).
Computer-aided morphological
analysis
Image-Pro Plus 6.0 (Media Cybernetics Inc.
Rockville, MD, USA) was used to calculate the intensity of the
detected markers. Three fields (original magnification, ×400) were
selected randomly and the optical densities of ICAM-1, VCAM-1,
E-selectin, CD68, CD4, CD8 and CD31 were calculated. Higher
integral optical density values represented higher antigen
expression.
Statistical analyses
Data are presented as the mean ± standard deviation.
Statistical differences were evaluated by unpaired Student's t-test
or one-way analysis of variance with Bonferroni post hoc test,
using SPSS software, version 20 (IBM, Armonk, NY, USA). The
Kaplan-Meier curves were analyzed with the log-rank test to assess
the significance of differences in the survival rates of the mice.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SDT inhibits growth of xenograft
tumors and improves tumor-loading mice outcomes
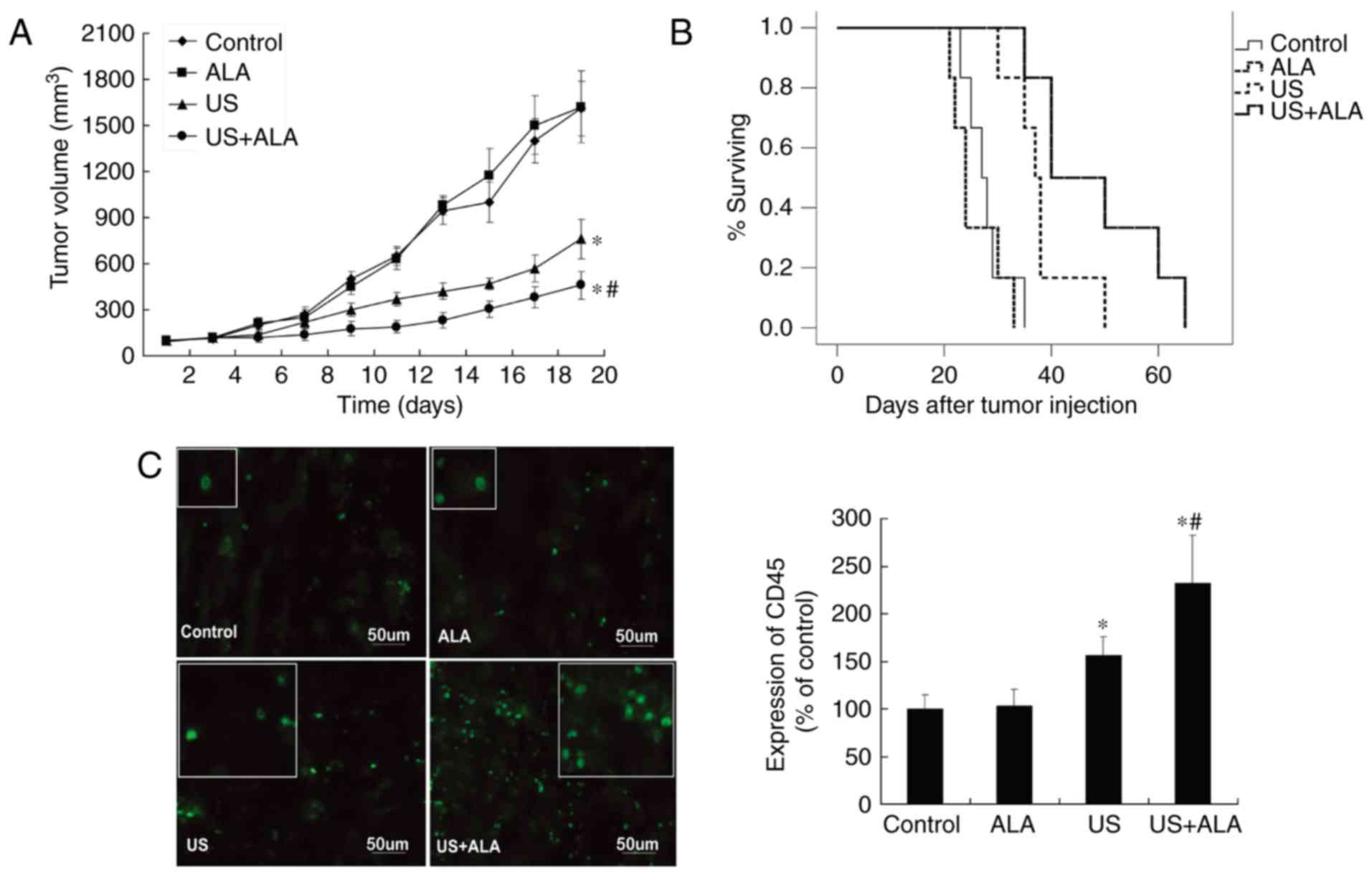
After the four treatments, tumors were observed
until the 20th day, and compared with the control group, mice
treated with ALA alone exhibited no significant tumor growth
suppression (ALA; P=0.617 vs. control). In contrast, the tumor
growth inhibition ratios with ultrasound were 22.4% (US) and 43.8%
(US + ALA), respectively (US, P=0.014; US + ALA, P<0.0001 vs.
control; Fig. 1A).
Fig. 1B shows the
survival curves of all four groups of mice. The median survival
times for the control and ALA groups were 27.5 and 24 days (ALA;
P=0.626 vs. control), respectively. The median survival times for
the US and US + ALA groups were 37.5 days (US; P=0.031 vs. control)
and 45 days (US + ALA; P=0.001 vs. control), respectively. Although
US group achieved statistical significance, SDT still had a greater
effect on the outcomes of the mice than the US treatment alone.
Effect of SDT on the number of
different immune cells in the tumor microenvironment
According to Galon et al, a more active
immune status implies a good prognosis (15). Therefore, the present study first
determined the effect of SDT on changes in the total number of
immune cells by using CD45 as a marker. The number of
CD45+ cells was not significantly different between the
control and ALA groups (P=0.905). However, CD45+ cell
count in both the US (P=0.039) and US + ALA (P=0.009) groups were
significantly increased compared with control group, however the US
+ ALA group demonstrated a greater increase (Fig. 1C).
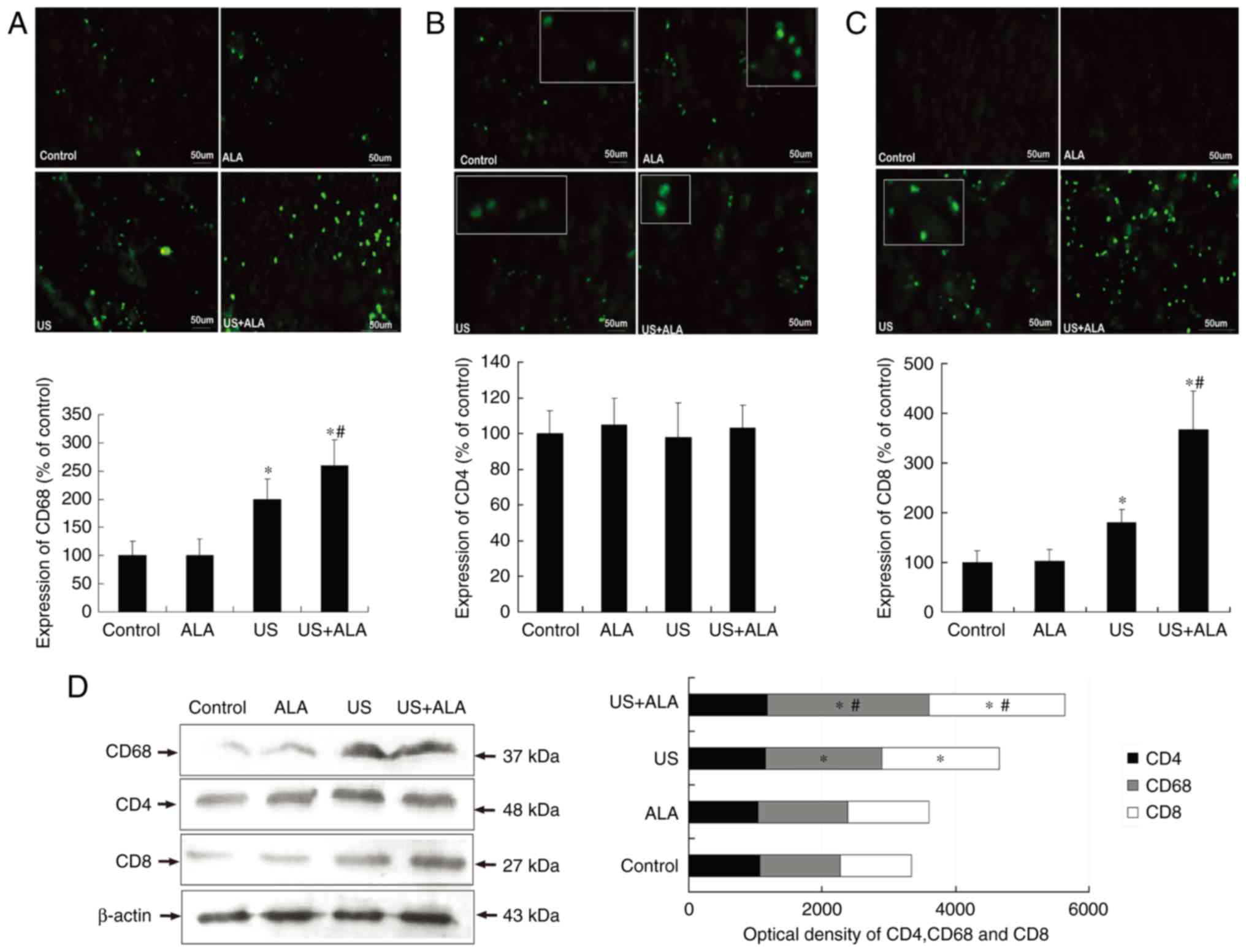
In the US + ALA group, CD68+ cells (ALA,
P=0.973; US, P=0.013; US + ALA, P<0.001 vs. control; P=0.038 for
US vs. US + ALA) and CD8+ cells (ALA, P=0.957; US,
P=0.037; US + ALA, P<0.0001 vs. control) (P=0.010 for US vs. US
+ ALA) increased significantly (Fig.
2). In addition, the extent of the increase was far greater in
the US + ALA group than in the US alone group. There were no
significant differences in the CD4+ T cells count
(Fig. 2B). In order to further
confirm these findings, western blotting was performed to measure
changes in the expression of CD4, CD8, and CD68 proteins. The
results indicated that although the expression levels of CD8 and
CD68 protein were increased in the US + ALA group, the CD4 protein
level did not change after any treatment. (Fig. 2D; ALA; P=0.698, US; P=0.876, US +
ALA; P=0.815 vs. control).
SDT reduces the immune tolerance of T
cells
The expression levels of Foxp3 and CTLA4, which are
associated with regulatory T cells and the downregulation of the
immune response, were determined, although the expression level of
the CD4 protein was similar in all four groups. Compared with the
control group, the expression levels of Foxp3 (US; P<0.0001, US
+ ALA; P<0.0001 vs. control) and CTLA-4 (US; P=0.007, US + ALA;
P=0.003 vs. control) proteins were reduced in the US and US + ALA
groups (Fig. 3). Furthermore, the
level of CD80 which is a co-stimulatory molecule related to Th1
cell activation was investigated. The results revealed that the
expression of CD80 was negligible in the control and ALA groups,
but increased significantly in US + ALA group (P<0.0001;
Fig. 3).
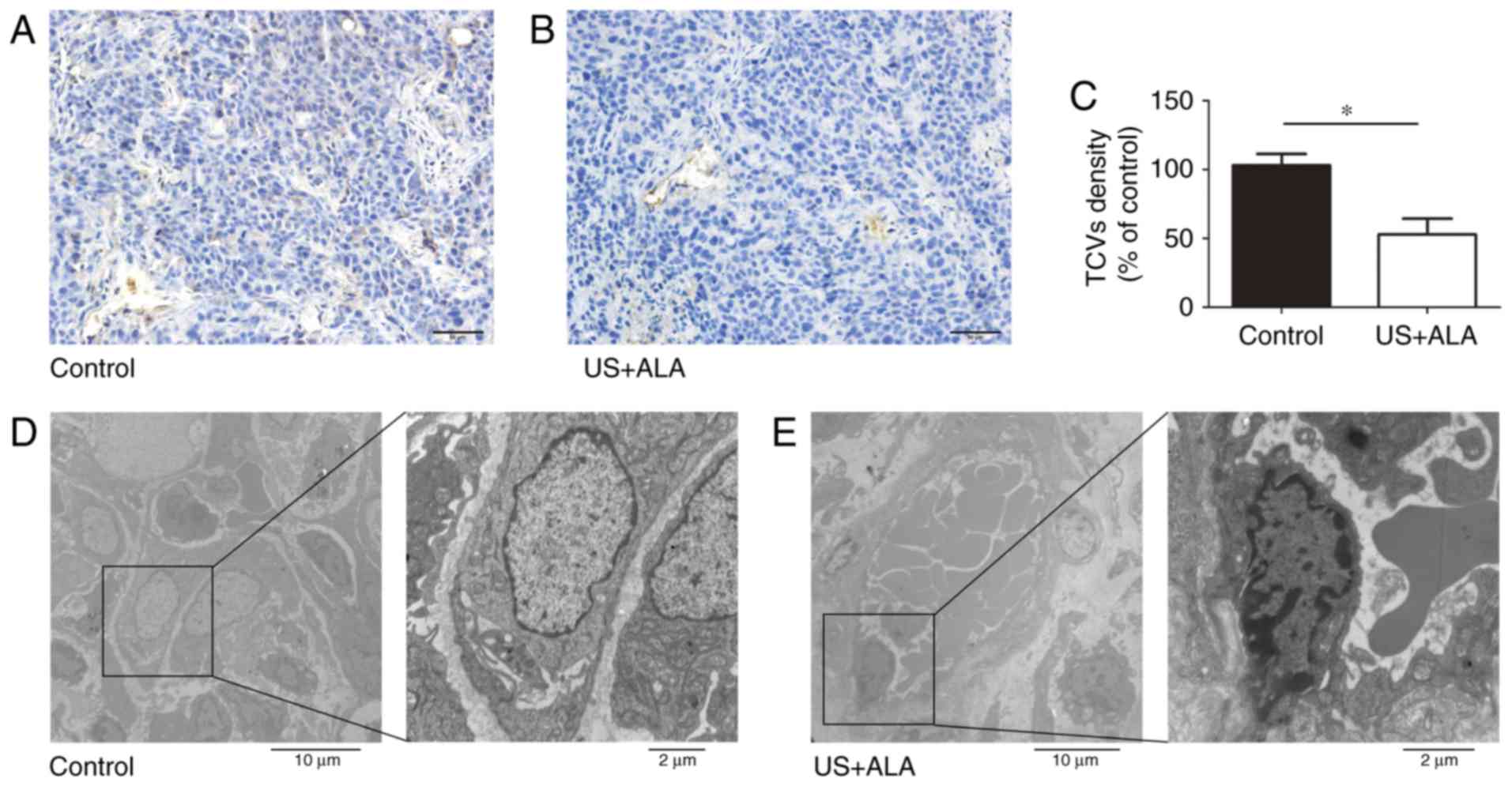
Effect of SDT on blood vessels in the
central and peripheral area of tumor
CD31 was used as a marker of blood vessels. Compared
with the control group, the number of tumor central vessels (TCVs)
was significantly reduced in US + ALA group (Fig. 4A-C). TEM revealed that the there was
no damage of TCVs in control group, whereas endothelial cells of
TCVs in US + ALA group were damaged with mitochondrial swelling,
chromatin aggregation, and the appearance of platelets and red
blood cells in proximity to the vascular endothelium (Fig. 4D and E).
Compared with the control group (1619±153.1
µm2), the mean lumen cross-sectional area of tumor
peripheral vessels (TPVs) was increased in the US + ALA group (4138
± 379.3 µm2; Fig. 5A and
B) and this difference was statistically significant (P=0.003;
Fig. 5C). TEM revealed that many
lymphocytes were present in the lumen of TPVs (Fig. 5D-F). Immunofluorescence double
staining results showed that ICAM-1, VCAM-1 and E-selectin, which
were associated with lymphocyte adhesion, were co-expressed with
vascular endothelial marker CD31 respectively in the TPVs of the US
+ ALA group (Fig. 5G).
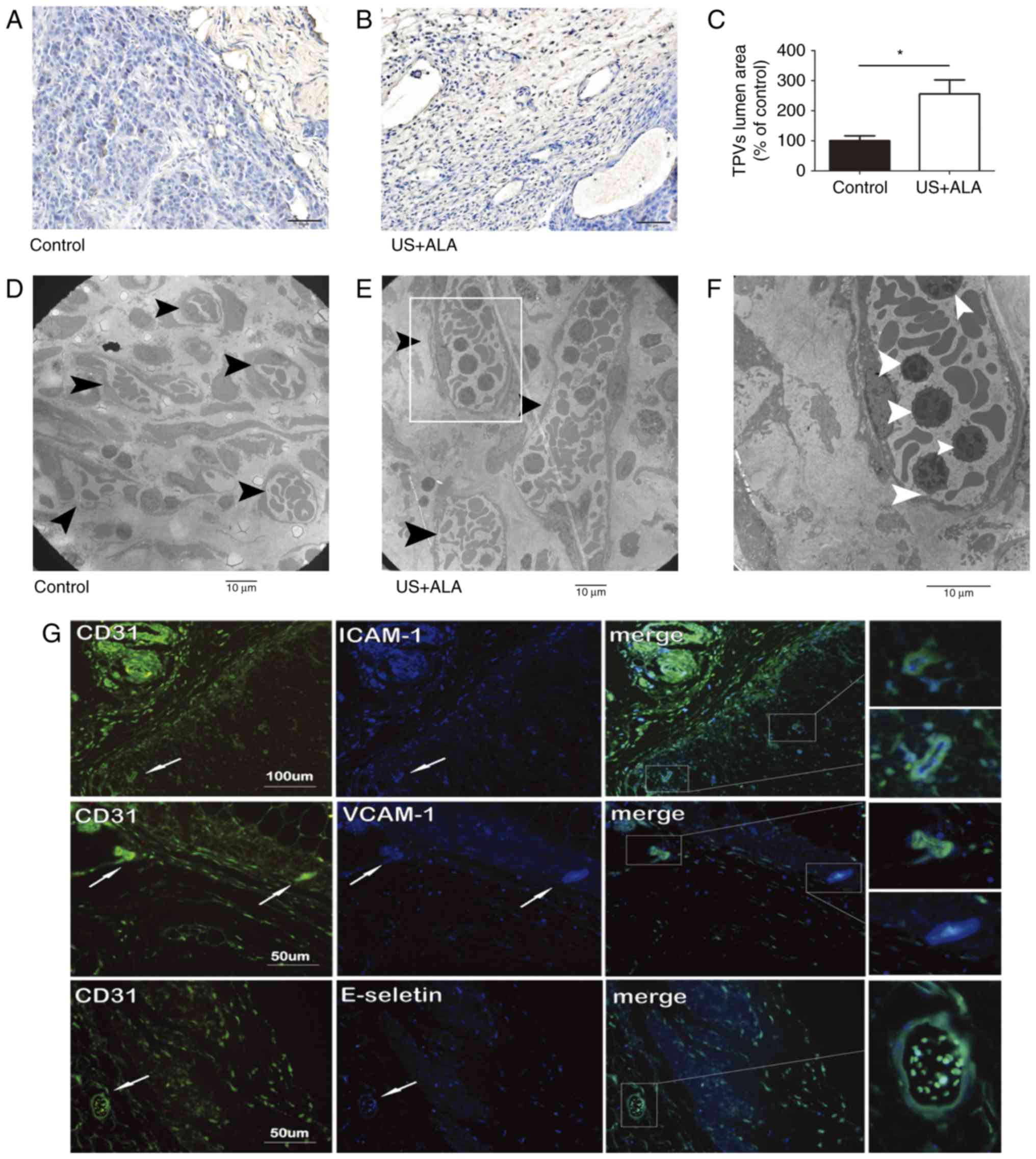 | Figure 5.Effect of sonodynamic therapy on TPVs.
Immunohistochemical staining of TPVs in (A) control and (B) US +
ALA groups, using mouse anti-CD31. (C) Statistical significance was
determined by an unpaired t-test. Data are presented as the mean ±
standard deviation. *P<0.05 vs. control. (D-F) TEM images of the
TPVs. Compared with the control group, the lumen diameter of TPVs
(black arrowheads) in US + ALA group was increased, and many
lymphocytes (white arrowheads) were shown to be present in the
lumen of these vessels. (F) Magnified areas of images presented in
(E). Magnification, (D and E) ×2000, (F) ×4000. Scale bars, 10 µm.
(G) Immunofluorescence double staining of the tumor vasculatures in
the US + ALA group. ICAM-1-CD31, VCAM-1-CD31, and E-selectin-CD31
were located mainly in vascular endothelial cells distributed
around the tumor periphery. TPVs, tumor peripheral blood vessels;
US, sonication; ALA, 5-aminolevulinic acid; ICAM-1, intercellular
adhesion molecule-1; VCAM-1; vascular cell adhesion protein-1. |
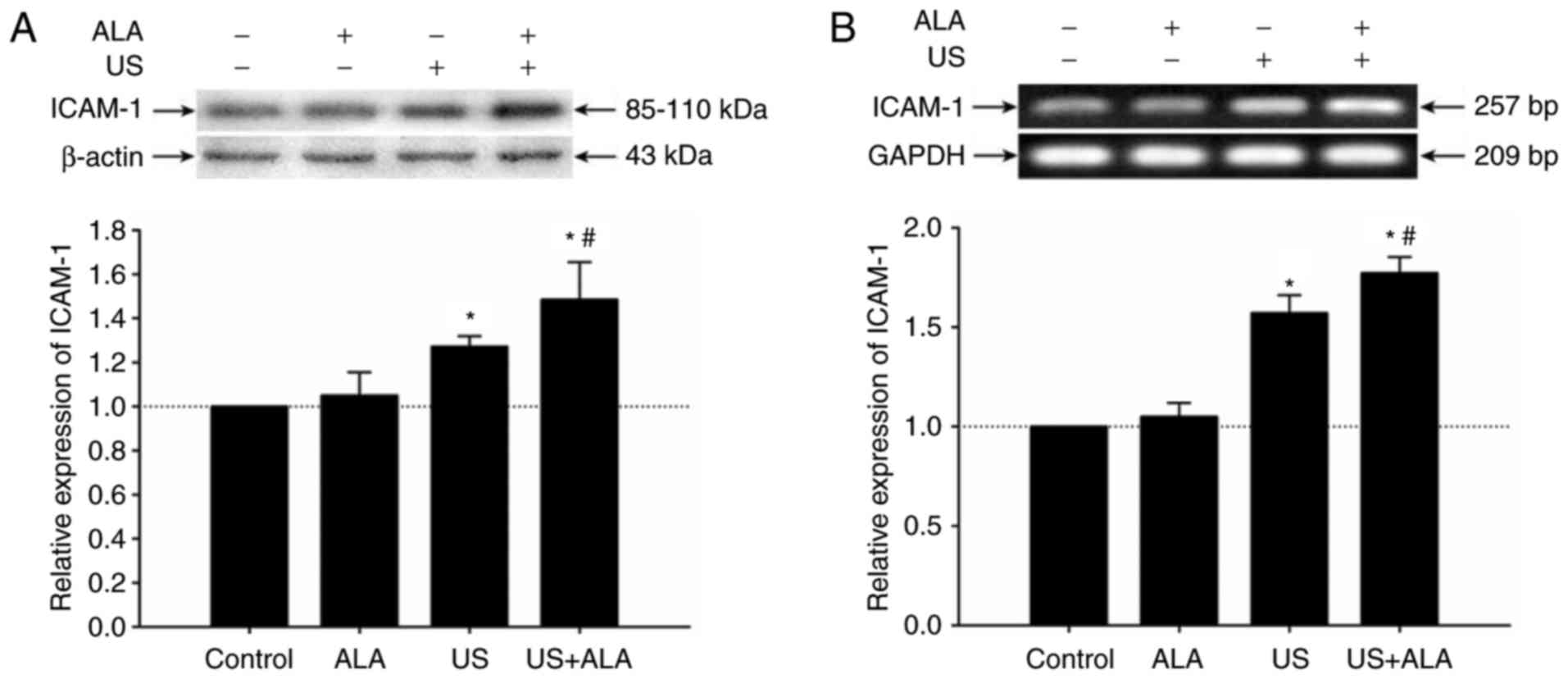
Effect of SDT on ICAM-1 expression on
HUVECs
To investigate whether SDT affects ICAM-1 expression
on endothelial cells, the present study detected the expression
level of ICAM-1 protein on HUVECs by western blotting. The results
indicated that compared with the US group, the expression level of
ICAM-1 protein was significantly increased in the US + ALA group
(P=0.038; Fig. 6A). There was no
significant difference between the ALA and control groups (P=0.984;
Fig. 6A). In order to further
confirm the results of western blotting, the mRNA level of ICAM-1
was detected by RT-sqPCR. Results demonstrated that the mRNA level
of ICAM-1 was increased in US + ALA group (P<0.0001, Fig. 6B).
Discussion
SDT significantly inhibited the tumor growth and
increased the survival rate in the murine xenograft model. It was
demonstrated that the number of CD45+, CD68+
and CD8+ T cells were noticeably higher in the SDT group
compared with other groups. In contrast, the number of
CD4+ T cells did not demonstrate a significant
alteration. Regulatory T cells, which are a subtype of
CD4+ cells, can inhibit the destruction of tumor cells
by the immune system, and thus reduce immune responses. Then, the
expression levels of Foxp3 and CTLA-4 proteins, which are
associated with T regulatory cells were investigated.
T regulatory cell-associated Foxp3 functions as a
master regulator (transcription factor) in the development and
function of regulatory T cells (17,18)
and is able to inhibit the activating effect of T cell receptors
(19). CTLA-4 is expressed on the
surface of T cells and regulates the cellular immune process. The
activation of T cells to attack foreign cells occurs via the
activation of CD28 receptors, and this activity is terminated by
CTLA-4 (20). The results revealed
that expression levels of CTLA-4 and Foxp3 decreased significantly
following SDT.
During the immune process, co-stimulating molecules
such as CD80 and CD86 are important adhesion molecules, expressed
on antigen-presenting cells and bind with CD28 expressed on T cells
(21). It is reported that the
expression level of CD80, but not CD86, plays a key role in the
induction of CD4+ cytotoxic T cells (CTLs) (22). The results revealed that the
expression levels of CD80 increased significantly following SDT.
The aforementioned data demonstrated that SDT may strengthen
inflammatory responses in the tumor microenvironment by limiting T
regulatory cell activity, increasing the proportion of activated
CD4+ T cells and enhancing antigen presentation.
The enhancement and maintenance of anti-tumor
immunity occurs via recognition of the tumor antigens and killing
of the tumor cells, by specific CTL cells. T cells are activated in
the tumor draining lymph node by corresponding tumor antigens, then
are drawn to the tumor site, and adhere to the vascular endothelial
cells, and migrate into the tumor microenvironment to exert
antitumor immunity (23). It is
well known that tumor vascular endothelial cells exhibit anergy in
the tumor microenvironment, which reduces the adhesion of T cells
to vascular endothelial cells, and consequently decreases the
accumulation of specific T cells in the tumor microenvironment
(24). The results demonstrated
that compared with the control group, the number of TCVs in the SDT
group decreased, and notably the lumen of TPVs was dilated with a
large number of lymphocytes present. Furthermore, CD31 colocalized
with ICAM-1, VCAM-1 or E-selectin respectively in the TPVs and the
expression level of mRNA and protein of ICAM-1 in SDT group
increased, which showed that SDT may not only activate T cells but
also enhance the adhesion ability of vascular endothelial cells and
thus accelerate the migration of T cells into the tumor
microenvironment. The aforementioned data indicated that SDT
improved the antitumor immune response in the murine B16F10
melanoma xenograft model. Current research regarding the impact of
SDT on the immune response, particularly the adaptive immune
response, is still at the initial stage (11,25,26).
These results need to be verified through different tumor models in
the future studies.
The present study demonstrated that SDT inhibited
tumor growth, effectively activated CD8+ T cells and
inhibited the activity of T regulatory cells, which consequently
resulted in a beneficial outcome. Furthermore, TPVs were dilated in
murine xenograft and the expression of ICAM-1 was upregulated on
HUVECs following SDT, which may facilitate the recruitment of
immune cells. These results demonstrated that the future prospects
of research on anti-tumor immunity derived from SDT are
promising.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272503).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YP and LJ performed the xperiments, analyzed the
data, wrote and edited the manuscript. SW participated in the
design of methodology and completed certain experiments and data
analysis. JZ and WC designed the research goals and aims, provided
thoughtful discussion and edited the manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the Animal
Care and Use Committee of Harbin Medical University (Harbin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy - a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.PubMed/NCBI
|
|
2
|
Shibaguchi H, Tsuru H and Kuroki M and
Kuroki M: Sonodynamic cancer therapy: A non-invasive and repeatable
approach using low-intensity ultrasound with a sonosensitizer.
Anticancer Res. 31:2425–2429. 2011.PubMed/NCBI
|
|
3
|
Li Y, Wang P, Zhao P, Zhu S, Wang X and
Liu Q: Apoptosis induced by sonodynamic treatment by protoporphyrin
IX on MDA-MB-231 cells. Ultrasonics. 52:490–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Leung AW, Jiang Y, Yu H, Li X and
Xu C: Hypocrellin B-mediated sonodynamic action induces apoptosis
of hepatocellular carcinoma cells. Ultrasonics. 52:543–546. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Liu Q, Wang Z, Wang P, Zhao P,
Zhao X, Yang L and Li Y: Role of autophagy in sonodynamic
therapy-induced cytotoxicity in S180 cells. Ultrasound Med Biol.
36:1933–1946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su X, Wang P, Yang S, Zhang K, Liu Q and
Wang X: Sonodynamic therapy induces the interplay between apoptosis
and autophagy in K562 cells through ROS. Int J Biochem Cell Biol.
60:82–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lv Y, Zheng J, Zhou Q, Jia L, Wang C, Liu
N, Zhao H, Ji H, Li B and Cao W: Antiproliferative and
Apoptosis-inducing effect of exo-Protoporphyrin IX based
sonodynamic therapy on human oral squamous cell carcinoma. Sci Rep.
7:409672017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Z, Fan H, Lv G, Zhou Q, Yang B, Zheng J
and Cao W: 5-Aminolevulinic acid-mediated sonodynamic therapy
induces anti-tumor effects in malignant melanoma via
p53-miR-34a-Sirt1 axis. J Dermatol Sci. 79:155–162. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Zhou Q, Hu Z, Yang B, Li Q, Wang J,
Zheng J and Cao W: 5-Aminolevulinic acid-based sonodynamic therapy
induces the apoptosis of osteosarcoma in mice. PLoS One.
10:e01320742015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Z, Zheng J, Yang B, Wang Z, Fan H, Lv
Y, Li H, Jia L and Cao W: Sonodynamic therapy inhibits angiogenesis
and tumor growth in a xenograft mouse model. Cancer Lett.
335:93–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Hu Z, Wang X, Gu C, Gao Z, Cao W
and Zheng J: 5-Aminolevulinic acid-mediated sonodynamic therapy
reverses macrophage and dendritic cell passivity in murine melanoma
xenografts. Ultrasound Med Biol. 40:2125–2133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffith KD, Read EJ, Carrasquillo JA,
Carter CS, Yang JC, Fisher B, Aebersold P, Packard BS, Yu MY and
Rosenberg SA: In vivo distribution of adoptively transferred
indium-111-labeled tumor infiltrating lymphocytes and peripheral
blood lymphocytes in patients with metastatic melanoma. J Natl
Cancer Inst. 81:1709–1717. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galon J, Mlecnik B, Bindea G, Angell HK,
Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et
al: Towards the introduction of the ‘Immunoscore’ in the
classification of malignant tumours. J Pathol. 232:199–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv Y, Fang M, Zheng J, Yang B, Li H,
Xiuzigao Z, Song W, Chen Y and Cao W: Low-intensity ultrasound
combined with 5-aminolevulinic acid administration in the treatment
of human tongue squamous carcinoma. Cell Physiol Biochem.
30:321–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanchot C, Terme M, Pere H, Tran T,
Benhamouda N, Strioga M, Banissi C, Galluzzi L, Kroemer G and
Tartour E: Tumor-infiltrating regulatory T cells: Phenotype, role,
mechanism of expansion in situ and clinical significance. Cancer
Microenviron. 6:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L and Zhao Y: The regulation of
Foxp3 expression in regulatory CD4(+)CD25(+)T cells: Multiple
pathways on the road. J Cell Physiol. 211:590–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marson A, Kretschmer K, Frampton GM,
Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von
Boehmer H and Young RA: Foxp3 occupancy and regulation of key
target genes during T-cell stimulation. Nature. 445:931–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Son CH, Bae JH, Shin DY, Lee HR, Choi YJ,
Jo WS, Jung Ho M, Kang CD, Yang K and Park YS: CTLA-4 blockade
enhances antitumor immunity of intratumoral injection of immature
dendritic cells into irradiated tumor in a mouse colon cancer
model. J Immunother. 37:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharpe AH: Mechanisms of costimulation.
Immunol Rev. 229:5–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mauri D and Pichler WJ: Involvement of
CD80 in the generation of CD4+ cytotoxic T cells.
Immunol Res. 15:126–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carriere V, Colisson R, Jiguet-Jiglaire C,
Bellard E, Bouche G, Al Saati T, Amalric F, Girard JP and M'Rini C:
Cancer cells regulate lymphocyte recruitment and
leukocyte-endothelium interactions in the tumor-draining lymph
node. Cancer Res. 65:11639–11648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Griffioen AW, Damen CA, Martinotti S,
Blijham GH and Groenewegen G: Endothelial intercellular adhesion
molecule-1 expression is suppressed in human malignancies: The role
of angiogenic factors. Cancer Res. 56:1111–1117. 1996.PubMed/NCBI
|
|
25
|
Bandyopadhyay S, Quinn TJ, Scandiuzzi L,
Basu I, Partanen A, Tomé WA, Macian F and Guha C: Low-intensity
focused ultrasound induces reversal of tumor-induced T cell
tolerance and prevents immune escape. J Immunol. 196:1964–1976.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu HL, Hsieh HY, Lu LA, Kang CW, Wu MF
and Lin CY: Low-pressure pulsed focused ultrasound with
microbubbles promotes an anticancer immunological response. J
Transl Med. 10:2212012. View Article : Google Scholar : PubMed/NCBI
|