Introduction
Cancer cells can invade and metastasize from primary
sites through the blood and lymphatic system to other distant sites
in the body. Metastatic cells must detach from the primary site and
escape from normal defense mechanisms including cell cycle arrest
and apoptosis. Anoikis is a form of apoptosis that is triggered
when cells detach from their surrounding extracellular matrix
(ECM), resulting in the loss of cell-cell communication and growth
signals. Metastatic tumor cells; however, can survive under this
harsh condition and this adaptive process is also known as anoikis
resistance. In addition, cancer cells can grow in the absence of
anchorage to the ECM and their neighboring cells, a process termed
anchorage-independent growth. Anoikis resistance and
anchorage-independence are therefore crucial steps in a series of
changes that tumor cells undergo during malignant transformation.
Extensive studies on anoikis resistance mechanisms have been
performed in cancers to identify molecular targets for preventing
metastasis (1–3). Moreover, anchorage-independent growth
of cancer cells in vitro (colony forming capacity in soft
agar media) has been used to predict the tumor phenotype,
particularly with respect to the potential for metastasis in
primary breast and lung tumors (4).
Most cancer cells exhibit altered metabolism
characterized by increased glucose uptake. This metabolic shift can
alter glucose metabolism to produce enough energy and build the
biomolecules needed by cancer cells. This includes the hexosamine
biosynthesis pathway (HBP), a minor branch of the glycolytic
pathway. The end product of the HBP is uridine diphosphate
N-acetylglucosamine (UDP-GlcNAc), a sugar donor which is
used for classical glycosylation in the endoplasmic reticulum and
Golgi apparatus, as well as for O-GlcNAcylation in the
cytoplasm, nucleus and mitochondria. Protein O-GlcNAcylation
is a post-translational modification of serine or threonine
residues of various nuclear-cytoplasmic and mitochondrial proteins
(5). This glycosylation is not
static but dynamically regulated by two key enzymes,
O-GlcNAc transferase (OGT) (6) and O-GlcNAcase (OGA) (7), for addition and removal of
O-GlcNAc from proteins, respectively. Growing evidence
suggests that an aberrant O-GlcNAcylation level is
associated with malignancy (8–10).
Previously, we reported that the
O-GlcNAcylation level is increased in primary breast and
colorectal cancer tissues (11,12).
Although an enhanced level of this modification is observed in most
cancers, its roles in malignant transformation are still largely
unexplored. In this study, the levels of O-GlcNAcylation and
OGT were examined in six cancer cell lines including breast (MCF-7
and MDA-MB-231), colorectal (SW480 and SW620), and liver (SK-Hep1
and HepG2) and their normal cells. In addition, we investigated the
effects of reducing O-GlcNAcylation using RNA interference
against the OGT gene in these cancer cell lines under
different culture conditions including conventional monolayer,
anchorage-independent growth of cancer cells in vitro, and
anoikis resistance. Further studies were also performed to identify
the proteins affected by O-GlcNAc reduction in anoikis
resistance and the results are discussed.
Materials and methods
Cell cultures
Gibco™ Dulbecco's modified Eagle's medium (DMEM),
RPMI-1640, penicillin-streptomycin solution, and L-glutamine were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Hyclone™ fetal bovine serum (FBS) was obtained from GE Healthcare
Life Sciences (Logan, UT, USA). Human cancer cell lines including
breast (MDA-MB-231 and MCF-7), colorectal (SW480 and SW620), and
liver (HepG2 and SK-Hep-1) were purchased from the American Type
Culture Collection (ATCC; Rockville, MD, USA). MDA-MB-231, MCF-7
and HepG2 cells were cultured in DMEM supplemented with 10% FBS.
SW480, SW620 and SK-Hep-1 cells were maintained in RPMI-1640 medium
supplemented with 10% FBS. Human normal mammary epithelial cells
(HMECs) and its medium (Mammary Epithelial Cell Growth Medium,
MEGM) were purchased from Lonza (Walkersville, MD, USA) and
cultured as recommended by the manufacturer. Human normal colon
epithelial cells (CCD 841 CoN) and human normal liver epithelial
cells (THLE-3), from ATCC, were provided by Dr Jutamaad
Satayavivad, Chulabhorn Research Institute, Thailand. CCD 841 CoN
was cultured in DMEM supplemented with 10% FBS, and 1% L-glutamine
while THLE-3 was cultured in DMEM supplemented with 10% FBS and 25
mmol/l HEPES (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). All
culture media were supplemented with 100 U/ml penicillin and 100
mg/ml streptomycin. The cells were maintained in humidified
atmosphere of 95% air and 5% CO2 at 37°C.
Assessment of O-GlcNAc transferase
(OGT) and O-GlcNAcylation levels
The levels of O-GlcNAc, OGT and β-actin were
determined by immunoblotting using monoclonal mouse anti-human
O-GlcNAc antibody, RL2 (ab2739, Abcam, Cambridge, MA, UK),
monoclonal rabbit anti-human OGT antibody (O6264, Sigma-Aldrich;
Merck KGaA) and monoclonal mouse anti-human β-actin antibody
(mAb3700, Cell Signaling Technology, Beverly, MA, USA) as
previously described (13).
Briefly, cells were lysed in RIPA buffer containing 1% protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and 20 µmol/l
Thiamet-G (Sigma-Aldrich; Merck KGaA). Protein samples (20 µg) were
separated by 10% SDS-PAGE and transferred to PVDF membranes
(Millipore, Bedford, MA, USA). Duplicate gels with equal amount
protein loading were performed; one for O-GlcNAc modified
proteins and another for OGT and β-actin. The membranes were probed
with specific primary antibodies at 1:1,000. Immunoblots were
developed with WesternBright™ ECL (Advansta, Menlo Park, CA, USA).
The signals were captured and measured using an image analysis
program (ImageQuant LAS4000; GE Healthcare, Marlborough, MA, USA).
β-actin was used to compare protein loading of cell lysates.
RNA interference
siRNA oligonucleotides of O-GlcNAc
transferase (OGT) (sense, 5′-UAAUCAUUUCAAUAACUGCUUCUGC-3′ and
antisense, 5′-GCAGAAGCAGUUAUUGAAAUGAUUA-3′) and scramble negative
control medium GC duplex were designed and purchased from
Invitrogen; Thermo Fisher Scientific, Inc. For monolayer cultures,
transfection of the siRNA oligos into cancer cells was carried out
using Invitrogen™ Lipofectamine 2000™ in a forward transfection
mode as described previously (11).
For soft agar and anoikis resistance cultures, cells were
transfected using a reverse transfection mode as described
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc).
Monolayer culture and cell growth
assay
Cancer cell growth was assessed by monitoring cell
viability throughout 5 days using the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay in monolayer cultures as previously described (14). Briefly, cell suspensions were seeded
into 96-well plates, cultured for 1 day, and transfected with RNA
interference. Transfected cells were further cultured for 1–5 days.
Then, the wells were replaced and incubated with fresh culture
media containing 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) for 2 h
at 37°C. Finally, the medium was removed and replaced with DMSO
(100 µl/well). Absorbance was measured at 550 nm and subtracted
with the absorbance at 650 nm, using a microplate reader. The
number of viable cells was determined from the absorbance. Cultures
with at least three independent wells were studied. The number of
viable cells in each day was normalized by those of the Scramble
siRNA at day 1, and reported as the percent relative cell
growth.
Anchorage-independent growth
assay
Anchorage-independent cell growth assay in
vitro was performed using soft agar cultures as described
previously (11). Briefly, 1×104
cells (OGT knockdown and scramble cells using a reverse
transfection mode) were suspended in 1 ml top agar medium (the
complete medium with 0.4% agar). The cell suspension was then
overlaid onto 1.5 ml bottom agar medium (the complete medium with
0.8% agar) in 6-well culture plates in triplicate. Fresh complete
medium was added to plates every 3 days as a feeder layer. Once
colonies were propagated (MDA-MB-231 and SW620 for 21 days, MCF-7,
SK Hep-1 and HepG2 for 18 days, and SW480 for 28 days), they were
stained with 0.005% crystal violet in 50% methanol for 1 h, and
images of the stained wells were captured. Colony number counts and
average sizes were determined by ImageJ software version 1.42I
(National Institutes of Health, Bethesda, MD, USA). All images were
saved in an 8 bit format. The measured area was selected by
elliptical selection and the threshold image was set using the
threshold tool. The mode of analyzing particles was used with
parameters of size: 1-infinity and circularity: 0.00–1.00. Cultures
with at least two independent wells were studied. The results were
reported as the average ± standard deviation of colony number and
size in OGT-knockdown cells normalized by those in the siScramble
control.
Anoikis resistance assay
Anoikis resistance of cancer cells was determined by
culturing the cells in polyHEMA-coated plates as descried
previously with some modifications (2). Briefly, 2×105 cells
(OGT-knockdown and scramble cells using a reverse transfection
mode) were cultured in polyHEMA-coated plates. The polyHEMA-coated
plates were prepared by soaking 30 mg/ml poly-HEMA (Sigma-Aldrich;
Merck KGaA) in 95% ethanol and putting this onto the plates and
drying at 37°C in an incubator, followed by extensive washing with
water and UV sterilization. After culturing for 3 days, cells were
photographed using an inverted phase-contrast microscope at ×10
magnification and harvested by centrifugation at 1,000 × g for 5
min. After harvesting, the cell pellets were resuspended in PBS
buffer, and incubated with trypsin-EDTA solution (Gibco; Thermo
Fisher Scientific, Inc.) for 10 min. The dissociated cells were
collected for measurement of OGT and O-GlcNAc levels as
described above. Aliquots of the collected cells were further
determined for viability of anoikis-resistant cells using trypan
blue dye exclusion assay. Trypsinized cells (20 µl) were mixed with
20 µl of 0.04% trypan blue. Viable and non-viable cells from four
microscopic viewing areas were counted using a hemocytometer.
Cultures with at least two independent wells were studied. The
results were reported as the average ± standard deviation of
anoikis-resistant cells (viable cells) in the OGT-knockdown cells
normalized by those in the siScramble control.
Two-dimensional gel electrophoresis
(2-DE)
2-DE was performed to measure the global protein
alteration between OGT-knockdown and scramble control groups.
Briefly, cultured cells (2×106) were lysed with 2-D
lysis buffer, 1% protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA), homogenized and harvested as described previously (11). Protein samples (100 µg) were
electro-separated by isoelectric focusing (IEF) in immobilized pH
gradient (IPG) strips, pH 3.0–10.0, followed by 10% SDS-PAGE. After
electrophoresis was performed, proteins on the gels were stained
using 0.1% Coomassie brilliant blue R-250 (CBB). Protein spots were
scanned using ImageScanner (Amersham Biosciences; GE Healthcare,
Chicago, IL, USA) and quantitatively measured by ImageMaster 2-DE
Platinum 7.0 software (GE Healthcare). Three independent
experiments were performed. The relative intensity of each protein
spot showing statistically significant difference was selected and
reported as a fold change between two groups (OGT knockdown vs.
Scramble control).
Protein identification by mass
spectrometric analysis
Protein spots with statistical difference in
expression levels were excised from the gel, destained and
enzymatically digested by trypsin (Promega, Madison, WI, USA). The
digested peptides were then identified using Nanoflow liquid
chromatography coupled with the amaZon speed ion trap mass
spectrometry (Bruker, Billerica, MA, USA) as previously described
(13). MASCOT search with NCBInr
version 20130630 sequence databases (http://www.matrixscience.com) was performed in order
to identify the protein spots. Search parameters were set as
follows: peptide mass tolerance was 1.2 Da, MS/MS ion mass
tolerance was 0.6 Da, allowance was set to 1 missed cleavage,
enzyme set as trypsin, the limit of peptide charges was 1+, 2+ and
3+. Decoy was marked. Proteins with molecular weight (MW) and pI
consistent to the spot on 2-DE gel and total ion scores >80
units (P-value <0.05) were considered positively identified.
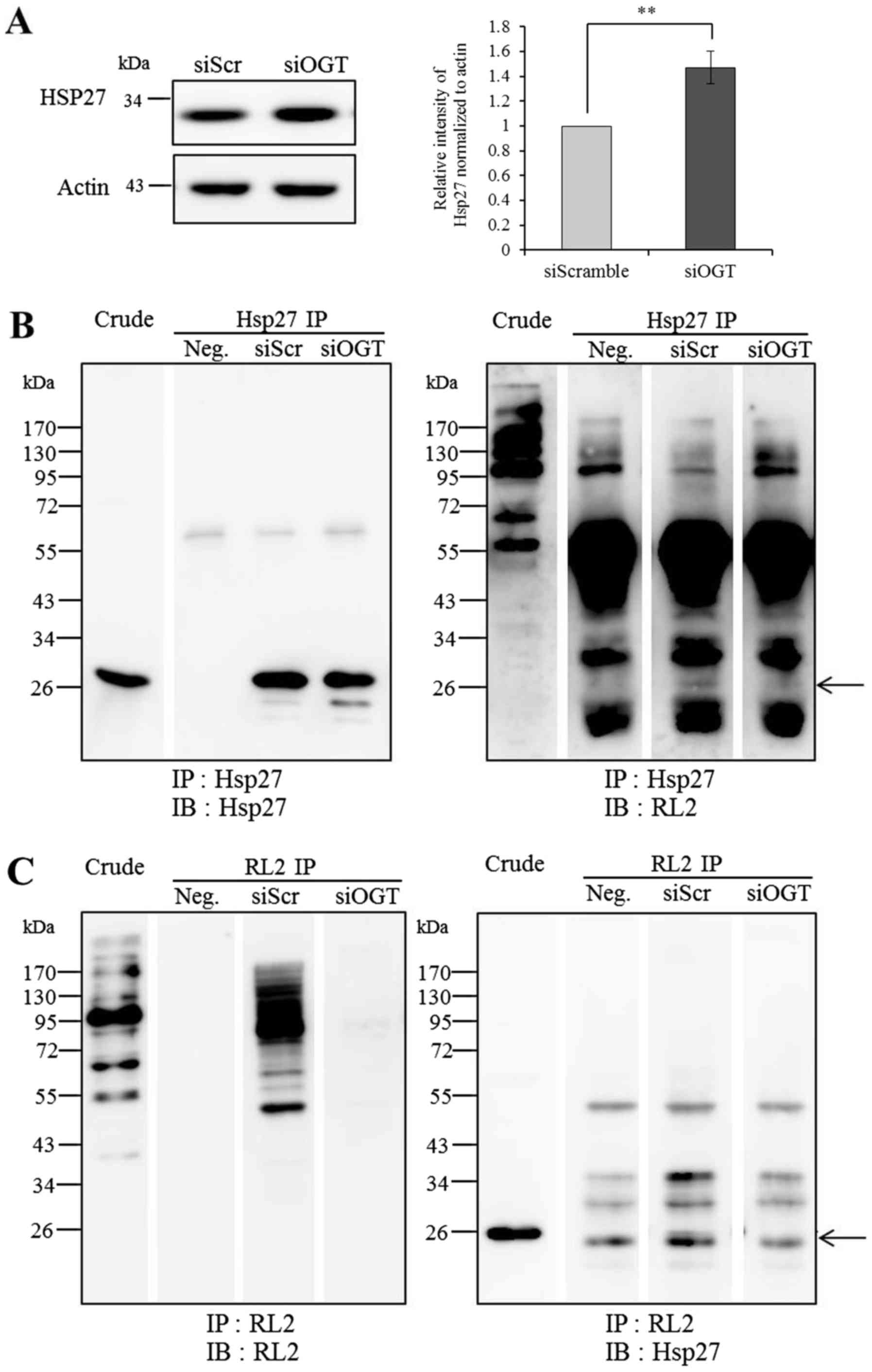
Confirmation of Hsp27 protein and
O-GlcNAc-Hsp27 levels
The expression level of Hsp27 in the siOGT and
siScramble cells was confirmed by immunoblotting (IB). Protein
samples (20 µg) were separated by 10% SDS-PAGE, transferred onto
PVDF membranes, and probed using monoclonal mouse anti-human Hsp27
antibody (1:2,000; cat. no. ab2790; Abcam, Cambridge, MA, USA).
Immunoblots were developed with WesternBright ECL and the signals
were captured as described above. β-actin was used to compare
protein loading of cell lysates. The results were reported as the
average band intensity ± standard deviation of Hsp27 in the
OGT-knockdown cells normalized by those in the siScramble control.
At least three independent experiments were performed.
Hsp27 was further confirmed to be O-GlcNAc
modified using immunoprecipitation (IP). Briefly, proteins (1,000
µg) in low-salt lysis buffer were incubated with antibodies against
Hsp27 (1:250; cat. no. ab2790; Abcam) and RL2 (1:200; cat. no.
ab2739; Abcam). The suspensions were mixed gently by shaking in an
end-over-end manner at 4°C for overnight. After that, the immune
complexes were incubated with Protein A and G Sepharose (GE
Healthcare) for 2 h to perform coupling reaction. After the washing
steps, the immune complexes were eluted by adding 25 µl of 2X
sampling buffer and heating at 95–100°C for 10 min. The eluted
samples were loaded onto 12.5% SDS-PAGE and immunoblotted with RL2
and Hsp27 antibodies to determine the levels of
O-GlcNAc-Hsp27.
Double knockdown of OGT and Hsp27
siRNA-mediated gene silencing against OGT
(Invitrogen; Thermo Fisher Scientific, Inc.) and Hsp27 (sc-29350,
Santa Cruz Biotechnology, Dallas, TX, USA) was performed in MCF-7
cells. Transfections were performed in reverse transfection mode
using Lipofectamine 2000™ as described above. The effects of siRNA
of Hsp27, OGT and Hsp27/OGT on anchorage-independent cell growth
and anoikis-resistant cells were examined using soft agar cultures
and trypan blue dye exclusion assay, respectively, as described
above.
Statistical analysis
The statistical analysis was conducted using
unpaired Student's t-test to test for the difference between two
groups. One-way ANOVA was used where appropriate followed by a
Bonferroni's multiple comparison test using Prism 5.0 of GraphPad
Software Inc. (GraphPad Software, Inc., San Diego CA, USA). The
statistical significance was defined as P<0.05 and
P<0.01.
Results
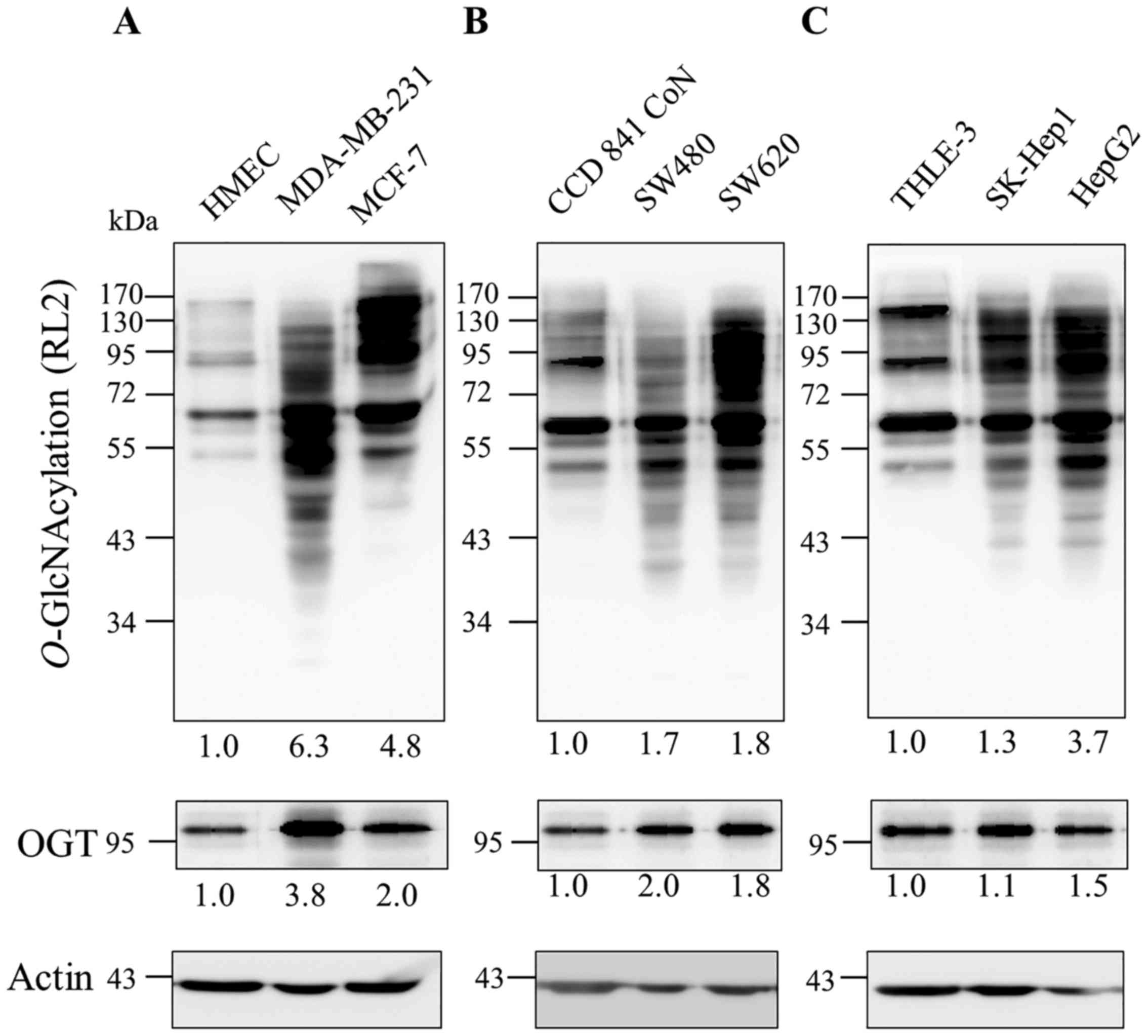
Augmentation of O-GlcNAcylation and
O-GlcNAc transferase in cancer cells
Since several reports revealed that the
O-GlcNAcylation level is increased in many types of cancer,
we examined the levels of this modification and its catalyzing
enzyme, OGT in cancer cell lines with different invasive
capability, in comparison to their normal cells including breast
(MCF-7 and MDA-MB-231 vs HMEC), colon (SW480 and SW620 vs CCD841
CoN) and liver (SK-Hep1 and HepG2 vs THLE-3).
O-GlcNAcylation and OGT levels were increased in all tested
cancer cell lines (≥1.5 fold), except SK-Hep1 in which its OGT
level was not different but many more O-GlcNAc protein bands
were obviously observed when compared to those of THLE-3 (Fig. 1). Of note, both O-GlcNAc
modification and OGT levels in breast cancer cell lines appeared to
be higher than those in colon and liver cancer cell lines.
Transient knockdown of O-GlcNAc
transferase in conventional monolayer cultures
Since O-GlcNAylation and OGT levels were
upregulated in the cancer cell lines, transient knockdown of
O-GlcNAylation by RNA interference against OGT was performed
in six cancer cell lines. siOGT treatment of six cancer cell lines
showed a marked reduction of both OGT and O-GlcNAcylation
levels in comparison with the siScramble control group (Fig. 2A). Indeed, we observed that siOGT
suppressed the OGT and O-GlcNAcylation levels in our culture
system by more than 70%. However, surprisingly, OGT knockdown had
little or no effect on cell viability and growth, even though
experiments were performed up to 5 days of transfection (Fig. 2B). At some time points, fluctuation
of cell viability was observed in siOGT treatment, but this was not
consistent in a time-dependent manner.
Transient knockdown of O-GlcNAc
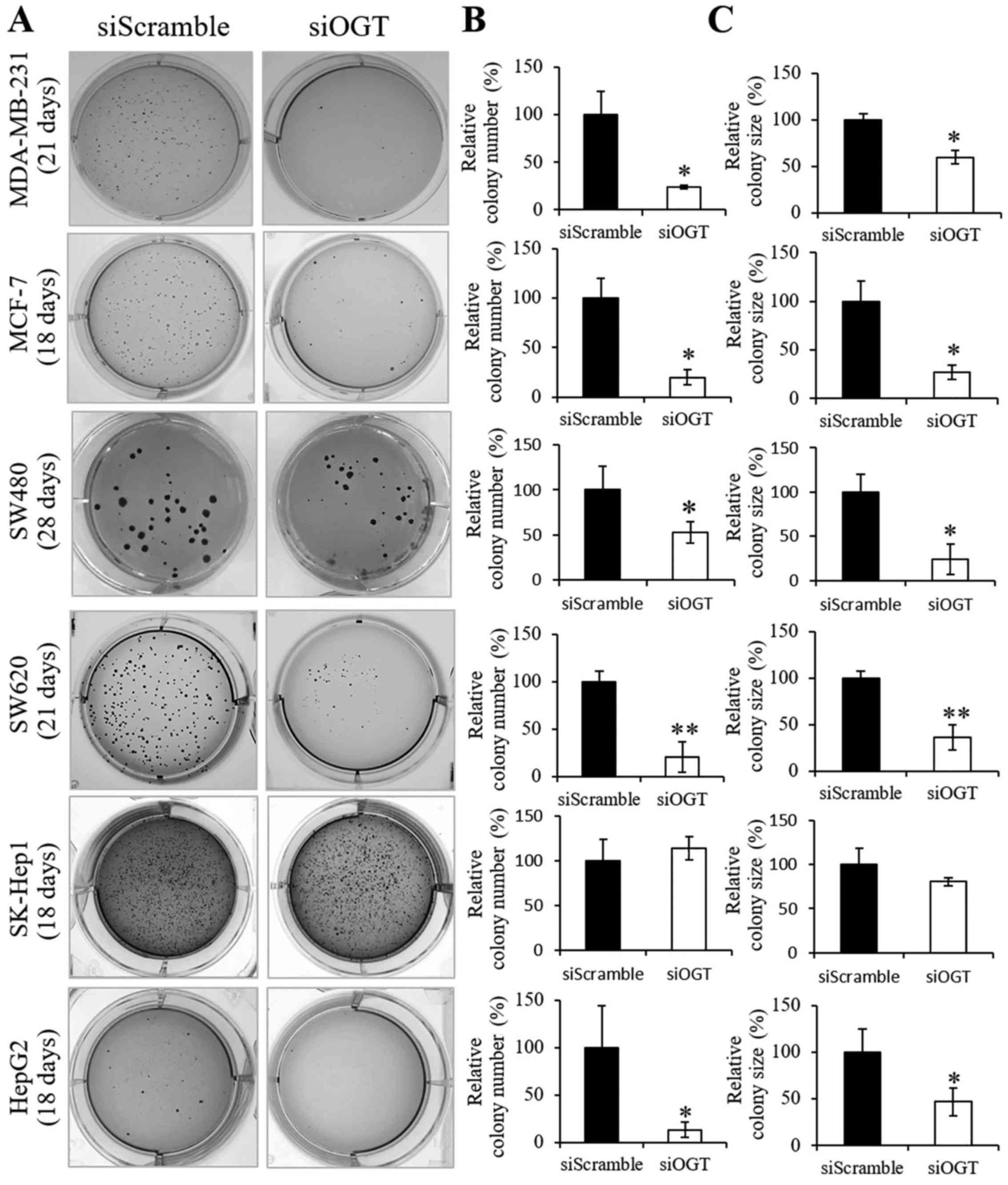
transferase in soft agar cultures
Culturing cells in soft agar is based on the colony
formation in anchorage-independent growth, which is considered the
most accurate and stringent in vitro assay for detecting
malignant transformation of cells. Since OGT knockdown had no
effect on the cell viability and growth in the monolayer culture,
we aimed to ascertain whether the reduction of this modification
may affect anchorage-independent growth in vitro.
Anchorage-independent cell growth assay of siOGT and siScramble
cells of six cancer cell lines were performed using soft agar
cultures as shown in Fig. 3A.
Notably, all cancer cell lines treated with siOGT treatment, except
SK-Hep1, displayed a significant reduction in colony number
(Fig. 3B) and colony size (Fig. 3C) when compared to those of the
siScramble cell groups, respectively. Surprisingly, OGT knockdown
was unlikely to affect colony formations in SK-Hep1 cells.
Transient knockdown of O-GlcNAc
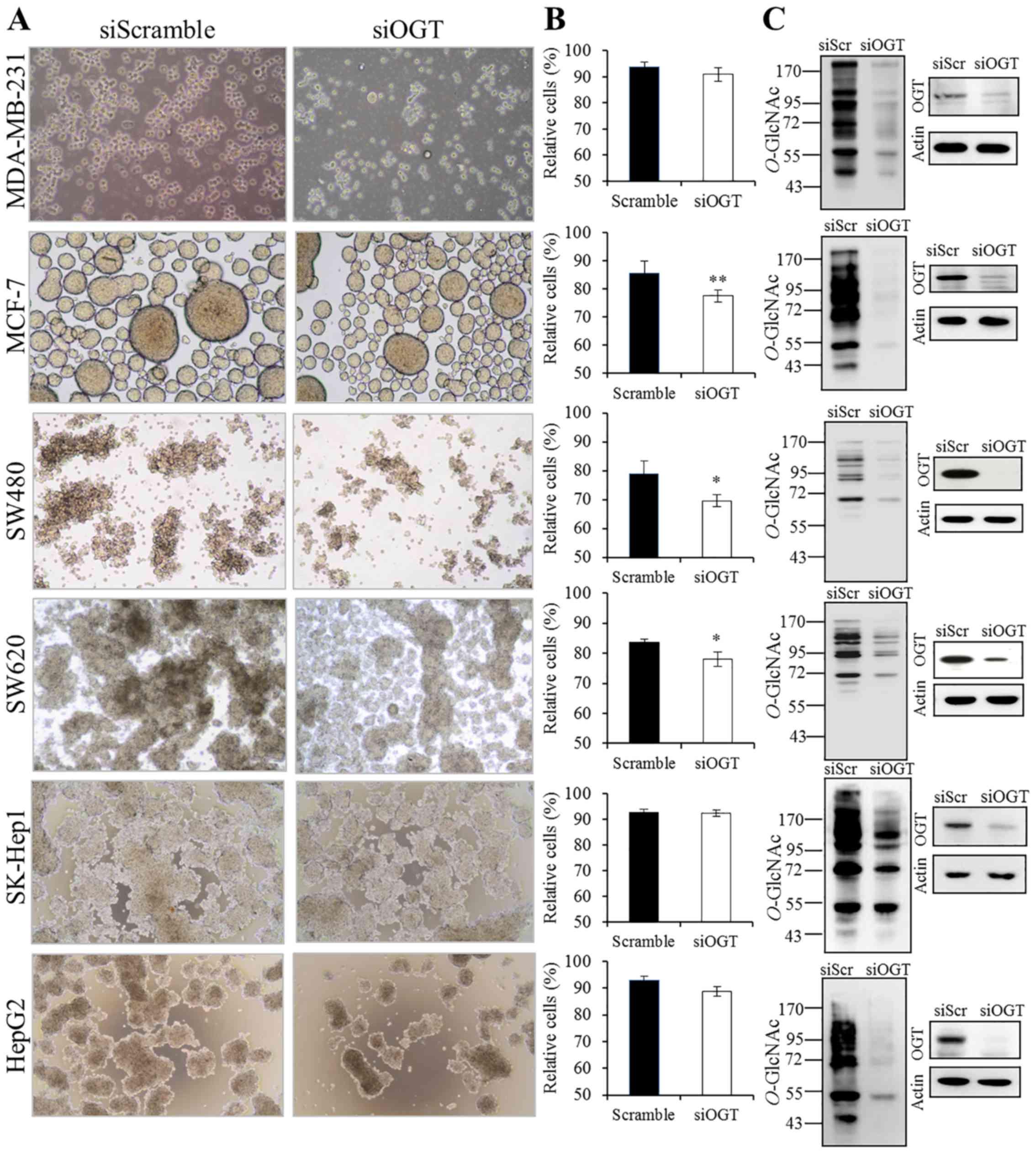
transferase in anoikis resistance cultures
Cancer cells can resist anoikis and thereby survive
after detachment from their primary site and can travel through the
circulatory systems as circulating cancer cells. In this study,
anoikis resistance was assessed in vitro by culturing cells
on polyHEMA-coated plates. As shown in Fig. 4A, all cancer cells were able to grow
as floating spheroids in the medium culture. Among the six cancer
cell lines, OGT knockdown statistically decreased viable cells only
in the MCF-7, SW480 and SW620 cell lines while it exhibited a
weaker effect on MDA-MB-231 and HepG2 cells and no effect was
observed in the SK-Hep1 cells (Fig.
4B). We also confirmed that, under the anoikis resistance
culture conditions, OGT and O-GlcNAcylation levels were
still reduced following siOGT treatment when compared to levels in
the siScramble control group (Fig.
4C).
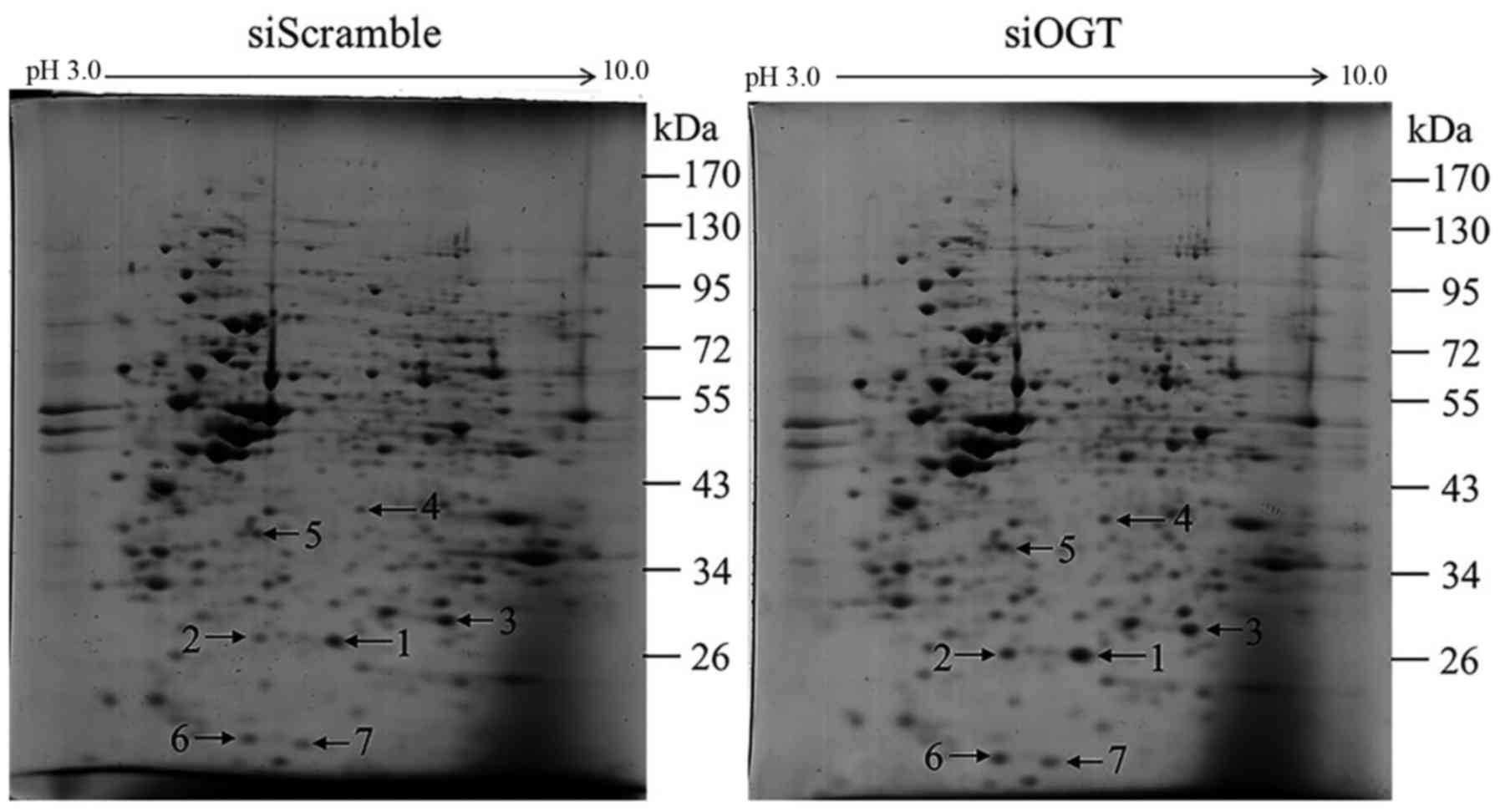
Alteration of protein expression in
anoikis-resistant cells by OGT knockdown
Since siOGT treatment caused a statistically
significant decrease in anoikis resistance of MCF-7 cells,
two-dimensional (2-DE) gel electrophoresis and mass spectrometric
analysis were used to determine which proteins would be affected
when the OGT level was reduced under anoikis-resistant conditions.
Image analysis revealed that 7 protein spots were differentially
expressed in the siOGT-transfected cells compared to those in the
siScramble control group (≥1.5 fold, P<0.05) (Fig. 5). Then, these 7 upregulated proteins
were identified by mass spectrometric analysis and the results are
shown in Table I. These identified
proteins were categorized into 3 groups based on their protein
functions. The first was heat shock protein 27 (Hsp27) (spot no. 1
and no. 2) which is involved in chaperone/stress response. The
second group was involved in energy metabolism including
triosephosphate isomerase (TPI) (spot no. 3), inorganic
pyrophosphatase (spot no. 5), PCTP-like protein (spot no. 6) and
nucleoside diphosphate kinase A (spot no. 7). The last protein was
peroxiredoxin-2 (spot no. 4) which is involved in cellular
protection/detoxification.
 | Table I.List of upregulated proteins found in
siOGT-transfected compared to siScramble-treated MCF-7 cells
culturing under aniokis resistance conditions. |
Table I.
List of upregulated proteins found in
siOGT-transfected compared to siScramble-treated MCF-7 cells
culturing under aniokis resistance conditions.
| No. | Protein name | Database | Accession
number | MW/pI (kDa/pI) | Peptide
matches | Score |
Fold-changea (siOGT/siScr) | P-value |
|---|
| 1 | Heat shock protein
27 | NCBInr | gi|4504517 | 22.768/5.98 | 8 | 398 | 2.22 | <0.001 |
| 2 | Heat shock protein
27 | NCBInr | gi|4504517 | 22.768/5.98 | 5 | 207 | 2.0122 | 0.012 |
| 3 | Triosephosphate
isomerase | NCBInr | gi|136066 | 26.894/7.10 | 8 | 394 | 1.66 | 0.04 |
| 4 |
Peroxiredoxin-2 | NCBInr | gi:32189392 | 22.049/5.66 | 3 | 89 | 1.55 | 0.04 |
| 5 | Inorganic
pyrophosphatase | NCBInr | gi|11056044 | 33.095/5.54 | 2 | 92 | 1.51 | 0.04 |
| 6 | PCTP-like
protein | NCBInr | gi|116812600 | 33.427/6.67 | 3 | 103 | 1.5 | 0.05 |
| 7 | Nucleoside
diphosphate kinase A | NCBInr | gi|35068 | 17.138/5.83 | 4 | 148 | 1.5 | 0.008 |
Reduction of O-GlcNAcylation and Hsp27
expression and its O-GlcNAc modification
As determined by 2-DE and mass spectrometric
analysis, the expression level of Hsp27 was the most markedly
increased upon OGT knockdown. Therefore, Hsp27 which exerts
chaperone/stress response functions was selected for further
validation by immunoblotting. The results showed that the
expression level of Hsp27 was increased by siOGT knockdown in
comparison to this level in the siScramble control with
significantly higher relative band intensity (P<0.01) (Fig. 6A). Using Hsp27 immunoprecipitation
and immunoblot analysis of Hsp27 and O-GlcNAc (RL2)
antibodies, we found that Hsp27 was modified by O-GlcNAc
(Fig. 6B and C). The level of
O-GlcNAc-modified Hsp27 was obviously decreased following
siOGT transfection when compared to the level in the siScramble
control. The reduction in the O-GlcNAc-modified Hsp27 level
following siOGT may be the result of a global decrease in the
O-GlcNAcylation level upon OGT knockdown.
Double knockdown of OGT and Hsp27
According to previous results, it has been suggested
that the elevation of Hsp27 may be associated with the suppression
of anoikis resistance and anchorage-dependent growth of MCF-7
cells. Therefore, we determine whether the reduction in Hsp27 is
capable to regain the growth in anoikis-resistant cultures under an
OGT silencing condition. Transient knockdown of Hsp27 was performed
to diminish the Hsp27 expression level. Hsp27 immunoblotting
revealed a decreased level of Hsp27 in siHsp27 and siOGT/siHsp27
transfected cells whereas O-GlcNAc immunoblotting showed
that the O-GlcNAc level was reduced in the siOGT and
siOGT/siHsp27 cells when compared to those of siScramble controls
(Fig. 7A). Double knockdown of
siOGT/siHsp27 (siDouble) reversed the inhibitory effect in
anoikis-resistant cultures compared to that of the siOGT-transected
cells (Fig. 7B). Moreover, double
knockdown of siOGT/siHsp27 markedly restored the growth of MCF-7
cells in soft agar cultures when compared to that of the
siOGT-transected alone cells (Fig.
7C).
Discussion
Emerging evidence reveals that aberrant
glycosylation is associated with pathobiological states of various
diseases including cancer. In general, cancer cells require a high
uptake of glucose for their rapid growth. Some glucose can enter
into the hexosamine biosynthesis pathway (HBP), a minor branch of
glycosis, which is responsible for producing a sugar donor,
UDP-GlcNAc, for glycosylation reactions including
O-GlcNAcylation. Increases in the HBP flux, UDP-GlcNAc and
O-GlcNAcylation, are therefore directly related to the high
glucose uptake generally observed in malignant cells. In this
study, we examined the O-GlcNAcylation level in human cancer
cell lines originating from the breast (MDA-MB-231 and MCF-7),
colon (SW480 and SW620) and liver (SK-Hep1 and HepG2) cells. Most
cancer cell lines, except SK-Hep1, showed an increased modification
level in comparison to that of their normal cell counterparts. This
increase was also consistent with the upregulation of OGT
expression level (Fig. 1). Less
change in O-GlcNAc and OGT levels, on the other hand, were
observed in SK-Hep1 cells. This indicates that the regulation of
O-GlcNAcylation and its controlling enzymes in various
cancer cell lines may be different. However, previous research from
our laboratory and others reported that augmentation of
O-GlcNAcylation and OGT levels were associated with the
malignant phenotypes of most cancers including breast (11,15),
colon (12,16,17),
liver (18–20) and prostate (21,22).
It is noted that the regulation of O-GlcNAcylation is
dynamic and may not be only dependent on the expression levels of
its cycling enzymes (OGT and OGA) but also on its enzymatic
activities (23). Further
investigation, therefore; is needed to clarify what other factors
regulate this dynamic modification.
OGT is vital for cellular survival. Deletion of OGT
results in embryonic lethality (24). Moreover, reduction in OGT, together
with stress stimuli treatment, caused a dramatic decrease in cell
viability (25). Surprisingly,
reduction in O-GlcNAcylation through RNA interference of OGT
did not appear to alter cell growth and proliferation under
conventional monolayer cultures, but instead inhibited colony
formation of breast cancer cells under anchorage-independent growth
(15,26). From these findings, we aimed to
ascertain whether OGT may be required for anchorage-independent
growth in other cancer cell types. Interestingly, the results
showed that reducing the OGT level could not affect the growth in
monolayer cultures of the tested cancer cell lines (Fig. 2). In contrast, siOGT treatment
caused a decrease in colony formation, in terms of both numbers and
sizes, compared to those of the siScramble controls, except for
SK-Hep1 cells (Fig. 3). Consistent
with this result, other groups also reported that decreased
O-GlcNAcylation caused a reduction in colony formation, as
observed by others in lung (17),
prostate (22) and pancreatic
cancers (27). In addition, six
cancer cell lines were also cultured on polyHEMA-coated plates to
determine the anoikis resistance of the cancer cells. Under this
culture condition, reduction in the O-GlcNAcylation level
affected anoikis-resistant growth of lowly invasive adenocarcinoma
(MCF-7, SW480 and SW620), but had weaker effects on highly invasive
adenocarcinoma cells (MDA-MB-231 and SK-Hep1) and even liver cells
(HepG2) (Fig. 4). According to our
results, OGT knockdown had a high inhibitory effect on colony and
spheroid formations of MCF-7 cells whereas it had little or no
effect on SK-Hep1 cells. This indicates some correlation between
cell type and the anoikis-resistant property. Moreover, the
mechanisms underlying anchorage-independent growth and anoikis
resistance may differ, thus the role of O-GlcNAcylation may
depend on both cellular properties and cellular adaptation to tumor
microenvironmental changes.
OGT may regulate O-GlcNAcylation and protein
levels of target proteins. In this study, since siOGT treatment
strongly affected both anchorage-independent growth and anoikis
resistance of MCF-7 cells, we examined total protein expression
levels in anoikis-resistant cells to determine those affected by
OGT reduction. Results from 2-DE images and mass spectrometric
analysis demonstrated that at least 7 proteins were upregulated in
the MCF-7 cells with decreased OGT (Fig. 5 and Table I). These upregulated proteins are
involved in many cellular processes including chaperone/stress
response (Hsp27), cellular metabolism (PCTP-like protein, inorganic
pyrophosphatase, triosephosphate isomerase (TPI), and nucleoside
diphosphate kinase A), and protection/detoxification
(peroxiredoxin-2). Previously, we demonstrated that
O-GlcNAcylation and OGT levels were increased in primary
breast malignant tumors and the O-GlcNAc modification levels
of 29 proteins including TPI were altered in breast cancer tissues
when compared to those of adjacent tissues (11). In parallel, Chaiyawat et al
reported that, in colon cancer cell lines, pyruvate kinase M2
(PKM2) was O-GlcNAcylated and its protein level was
increased while its O-GlcNAc level was decreased upon OGT
knockdown (13). In the present
study, the TPI level was upregulated upon OGT knockdown. In
addition to TPI, we found that the Hsp27 level was increased but
its O-GlcNAc level was decreased in siOGT cells when
compared to levels in the siScramble control cells. From these
changes observed in PKM2, TPI and Hsp27, it is possible that OGT
knockdown not only reduced the O-GlcNAc modification of
target proteins but also affected the level of target proteins by
controlling at transcription/translation levels. Further
investigation is needed to clarify how O-GlcNAcylation
regulates protein expression levels.
Hsp27 is a chaperone of the small heat shock protein
group. We found that the Hsp27 protein level was most highly
increased upon OGT knockdown under anoikis resistance conditions
(Figs. 5 and 6). Hsp27 was reported to be implicated in
various cellular processes or even under pathologic disease
conditions including cancer (28).
Its expression level affects cell proliferation, migration and
invasion in many types of cancer i.e., liver, prostate and breast
cancer (29–31). Generally, Hsp27 is involved in the
stress response mechanism which restores protein homeostasis
(adaptive mechanism) in cancer cells. It is therefore possible that
a decreased global O-GlcNAcylation level (by siOGT
knockdown) may induce harsh conditions resulting in an accumulation
of misfolded proteins and/or other stress-responsive factors, as
shown by the upregulation of Hsp27 in this study. In contrast, a
number of reports suggest that Hsp27 can serve as a tumor
suppressor which acts against cancer progression and metastasis.
For instance, overexpression of Hsp27 is sufficient to inhibit
pulmonary fibrosis and lung tumorigenesis by diminishing
endothelial-to-mesenchymal transition (EndMT) (32). Additionally, increased expression of
Hsp27 could efficiently suppress lung metastasis of colorectal
cancer in vivo (33). Hsp27
expression in salivary gland tumor tissues has been reported to be
higher in benign tumors than in malignant tumors (34). As demonstrated in both soft agar and
spheroid conditions (Fig. 7),
double knockdown of Hsp27 and OGT led to a decrease in the Hsp27
level, resulting in the reversal of this inhibitory effect of MCF-7
growth. An increased Hsp27 level upon OGT knockdown, therefore, was
likely to be causal for inhibiting cancer cell formation. However,
how increased Hsp27 is able to suppress the malignancy of MCF-7
cells is an unsolved mystery worthy of further investigation.
The gene/protein regulation of Hsp27 is complicated.
Hsp27 expression level is regulated by specificity protein 1 (Sp1)
(35), which is an ubiquitous
transcription factor that can activate or repress transcription of
many genes in response to physiological and pathological stimuli.
Sp1 is reported to be O-GlcNAc-modified and overexpression
of OGT is shown to inhibit Sp1 transcriptional activity (36). Therefore, reduction of
O-GlcNAcylation by OGT knockdown may increase Sp1 activity
and Hsp27 expression levels. Guo et al reported that Hsp27
is modified by O-GlcNAc (37). They suggested that increased
O-GlcNAc modification of Hsp27 enhanced the translocation of
Hsp27 from the cytoplasm into the nucleus and this may be a novel
regulatory state of Hsp27 functions. In our study, we found that
Hsp27 was O-GlcNAc modified (Fig. 6). Although the overall level of
Hsp27 was increased, the O-GlcNAc-Hsp27 level was decreased
upon OGT knockdown in anoikis resistance conditions (Fig. 6). Further studies, therefore; are
needed to clarify whether OGT knockdown causes less Hsp27 entry
into the nucleus.
In summary, an increase in the
O-GlcNAcylation level modulated by high glucose uptake and
overexpression of OGT may be an important malignant phenotype
observed in most cancers. Many researchers are trying to block or
reduce the action of OGT using inhibitors and/or knockdown of the
OGT gene so as to inhibit cancer progression and
development. However, the precise roles of O-GlcNAcylation
in cancer development and progression are still elusive. Anoikis
resistance and anchorage-independent growth are vital steps for
metastatic tumors. Our data suggest that, in MCF-7 cells,
O-GlcNAcylation is required for both processes, and blocking
this glycosylation by OGT knockdown affected these processes, at
least in part, via the regulation of Hsp27 expression and its
O-GlcNAc modification. This information may further
elucidate the potential mechanism of O-GlcNAc modification
associated with cancer progression, especially in breast cancer.
Nevertheless, further studies are required to determine this
precise mechanism.
Acknowledgements
The authors would like to thank Dr Jutamaad
Satayavivad, Chulabhorn Research Institute, Thailand, for culturing
of human normal colon epithelial cells (CCD 841 CoN) and human
normal liver epithelial cells (THLE-3).
Funding
The present study was supported by the Thailand
Research Fund (grant no. TRG5580006), the Chulabhorn Research
Institute and the Chulabhorn Graduate Institute, Thailand.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
PN and VC conceived and designed the study. PN and
PC performed the experiments in cell cultures, gel proteomics and
immunoblotting. DC performed the LC-MS/MS. KL and CS interpreted
the results and reviewed the manuscript. JS reviewed and edited the
manuscript and was involved in the conception of the study. PN and
VC wrote and drafted the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howard EW, Leung SC, Yuen HF, Chua CW, Lee
DT, Chan KW, Wang X and Wong YC: Decreased adhesiveness, resistance
to anoikis and suppression of GRP94 are integral to the survival of
circulating tumor cells in prostate cancer. Clin Exp Metastasis.
25:497–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khongmanee A, Lirdprapamongkol K, Tit-oon
P, Chokchaichamnankit D, Svasti J and Srisomsap C: Proteomic
analysis reveals important role of 14-3-3σ in anoikis resistance of
cholangiocarcinoma cells. Proteomics. 13:3157–3166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park
SY, Shin I, Han W and Noh DY: The alkaloid Berberine inhibits the
growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell
lines by inducing cell cycle arrest. Phytomedicine. 17:436–440.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori S, Chang JT, Andrechek ER, Matsumura
N, Baba T, Yao G, Kim JW, Gatza M, Murphy S and Nevins JR:
Anchorage-independent cell growth signature identifies tumors with
metastatic potential. Oncogene. 28:2796–2805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kreppel LK, Blomberg MA and Hart GW:
Dynamic glycosylation of nuclear and cytosolic proteins. Cloning
and characterization of a unique O-GlcNAc transferase with
multiple tetratricopeptide repeats. J Biol Chem. 272:9308–9315.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Y, Wells L, Comer FI, Parker GJ and
Hart GW: Dynamic O-glycosylation of nuclear and cytosolic proteins:
Cloning and characterization of a neutral, cytosolic
beta-N-acetylglucosaminidase from human brain. J Biol Chem.
276:9838–9845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Z and Vosseller K: O-GlcNAc in
cancer biology. Amino Acids. 45:719–733. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fardini Y, Dehennaut V, Lefebvre T and
Issad T: O-GlcNAcylation: A New Cancer Hallmark? Front
Endocrinol (Lausanne). 4:992013.PubMed/NCBI
|
|
10
|
Chaiyawat P, Netsirisawan P, Svasti J and
Champattanachai V: Aberrant O-GlcNAcylated proteins: New
perspectives in breast and colorectal cancer. Front Endocrinol
(Lausanne). 5:1932014.PubMed/NCBI
|
|
11
|
Champattanachai V, Netsirisawan P,
Chaiyawat P, Phueaouan T, Charoenwattanasatien R,
Chokchaichamnankit D, Punyarit P, Srisomsap C and Svasti J:
Proteomic analysis and abrogated expression of
O-GlcNAcylated proteins associated with primary breast
cancer. Proteomics. 13:2088–2099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phueaouan T, Chaiyawat P, Netsirisawan P,
Chokchaichamnankit D, Punyarit P, Srisomsap C, Svasti J and
Champattanachai V: Aberrant O-GlcNAc-modified proteins
expressed in primary colorectal cancer. Oncol Rep. 30:2929–2936.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaiyawat P, Chokchaichamnankit D,
Lirdprapamongkol K, Srisomsap C, Svasti J and Champattanachai V:
Alteration of O-GlcNAcylation affects serine phosphorylation
and regulates gene expression and activity of pyruvate kinase M2 in
colorectal cancer cells. Oncol Rep. 34:1933–1942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiablaem K, Lirdprapamongkol K,
Keeratichamroen S, Surarit R and Svasti J: Curcumin suppresses
vasculogenic mimicry capacity of hepatocellular carcinoma cells
through STAT3 and PI3K/AKT inhibition. Anticancer Res.
34:1857–1864. 2014.PubMed/NCBI
|
|
15
|
Caldwell SA, Jackson SR, Shahriari KS,
Lynch TP, Sethi G, Walker S, Vosseller K and Reginato MJ: Nutrient
sensor O-GlcNAc transferase regulates breast cancer
tumorigenesis through targeting of the oncogenic transcription
factor FoxM1. Oncogene. 29:2831–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steenackers A, Olivier-Van Stichelen S,
Baldini SF, Dehennaut V, Toillon RA, Le Bourhis X, El
Yazidi-Belkoura I and Lefebvre T: Silencing the nucleocytoplasmic
O-GlcNAc transferase reduces proliferation, adhesion, and
migration of cancer and fetal human colon cell lines. Front
Endocrinol (Lausanne). 7:462016.PubMed/NCBI
|
|
17
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of
lung and colon cancer malignancy. Biochim Biophys Acta.
1812:514–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu
X, Zhu G, Zhao Y, Chen Y, Yu Y, et al: The essential role of YAP
O-GlcNAcylation in high-glucose-stimulated liver
tumorigenesis. Nat Commun. 8:152802017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Q, Tao T, Liu F, Ni R, Lu C and Shen
A: Hyper-O-GlcNAcylation of YB-1 affects Ser102
phosphorylation and promotes cell proliferation in hepatocellular
carcinoma. Exp Cell Res. 349:230–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu G, Tao T, Zhang D, Liu X, Qiu H, Han
L, Xu Z, Xiao Y, Cheng C and Shen A: O-GlcNAcylation of
histone deacetylases 1 in hepatocellular carcinoma promotes cancer
progression. Glycobiology. 26:820–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Itkonen HM, Gorad SS, Duveau DY, Martin
SE, Barkovskaya A, Bathen TF, Moestue SA and Mills IG: Inhibition
of O-GlcNAc transferase activity reprograms prostate cancer
cell metabolism. Oncotarget. 7:12464–12476. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu Y, Gao J, Han C, Zhang X, Liu H, Ma L,
Sun X and Yu W: O-GlcNAcylation is increased in prostate
cancer tissues and enhances malignancy of prostate cancer cells.
Mol Med Rep. 10:897–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X and Qian K: Protein
O-GlcNAcylation: Emerging mechanisms and functions. Nat Rev
Mol Cell Biol. 18:452–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Donnell N, Zachara NE, Hart GW and Marth
JD: Ogt-dependent X-chromosome-linked protein glycosylation is a
requisite modification in somatic cell function and embryo
viability. Mol Cell Biol. 24:1680–1690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zachara NE, O'Donnell N, Cheung WD, Mercer
JJ, Marth JD and Hart GW: Dynamic O-GlcNAc modification of
nucleocytoplasmic proteins in response to stress. A survival
response of mammalian cells. J Biol Chem. 279:30133–30142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Z, Vocadlo DJ and Vosseller K:
Hyper-O-GlcNAcylation is anti-apoptotic and maintains
constitutive NF-κB activity in pancreatic cancer cells. J Biol
Chem. 288:15121–15130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arrigo AP and Gibert B: HspB1, HspB5 and
HspB4 in human cancers: Potent oncogenic role of some of their
client proteins. Cancers (Basel). 6:333–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hung CS, Huang CY, Lee CH, Chen WY, Huang
MT, Wei PL and Chang YJ: IGFBP2 plays an important role in heat
shock protein 27-mediated cancer progression and metastasis.
Oncotarget. 8:54978–54992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cordonnier T, Bishop JL, Shiota M, Nip KM,
Thaper D, Vahid S, Heroux D, Gleave M and Zoubeidi A: Hsp27
regulates EGF/β-catenin mediated epithelial to mesenchymal
transition in prostate cancer. Int J Cancer. 136:E496–E507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gibert B, Eckel B, Gonin V, Goldschneider
D, Fombonne J, Deux B, Mehlen P, Arrigo AP, Clézardin P and
Diaz-Latoud C: Targeting heat shock protein 27 (HspB1) interferes
with bone metastasis and tumour formation in vivo. Br J Cancer.
107:63–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi SH, Nam JK, Kim BY, Jang J, Jin YB,
Lee HJ, Park S, Ji YH, Cho J and Lee YJ: HSPB1 inhibits the
endothelial-to-mesenchymal transition to suppress pulmonary
fibrosis and lung tumorigenesis. Cancer Res. 76:1019–1030. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YJ, Lee HJ, Choi SH, Jin YB, An HJ,
Kang JH, Yoon SS and Lee YS: Soluble HSPB1 regulates VEGF-mediated
angiogenesis through their direct interaction. Angiogenesis.
15:229–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang G, Gu X, Chen L, Wang Y and Cao B; E
Q, : Comparison of the expression of 5 heat shock proteins in
benign and malignant salivary gland tumor tissues. Oncol Lett.
5:1363–1369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mo XM, Li L, Zhu P, Dai YJ, Zhao TT, Liao
LY, Chen GG and Liu ZM: Up-regulation of Hsp27 by ERα/Sp1
facilitates proliferation and confers resistance to apoptosis in
human papillary thyroid cancer cells. Mol Cell Endocrinol.
431:71–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Su K, Roos MD, Chang Q, Paterson
AJ and Kudlow JE: O-linkage of N-acetylglucosamine to Sp1
activation domain inhibits its transcriptional capability. Proc
Natl Acad Sci USA. 98:6611–6616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo K, Gan L, Zhang S, Cui FJ, Cun W, Li
Y, Kang NX, Gao MD and Liu KY: Translocation of HSP27 into liver
cancer cell nucleus may be associated with phosphorylation and
O-GlcNAc glycosylation. Oncol Rep. 28:494–500. 2012.
View Article : Google Scholar : PubMed/NCBI
|