Introduction
Liver cancer is a global health issue.
Hepatocellular carcinoma (HCC), a major subtype of primary liver
cancer, is a one of the most common malignant tumors and is the
third leading cause of cancer-related mortality (1,2). HCC
develops resistance to most chemotherapeutic agents (3). Significant advances have been made in
the early diagnosis and management of HCC; however, therapeutic
strategies for the treatment of HCC are still limited (4). At present, surgical techniques remain
the most common therapeutic option for the eradication of cancer
nodules. Nevertheless, most patients undergoing tumor dissection
still suffer from an unsatisfactory outcome, with respect to high
recurrence rates and distant organ invasion (5,6). Thus,
it can be seen that most HCC patients have a poor prognosis
(7). Less than 5% of patients
survive for more than 2 years (8).
Furthermore, the main anticancer drugs for HCC, such as oxaliplatin
and sorafenib, have side-effects and are associated with multidrug
resistance (9–11). As an embryonal malignancy of
hepatocellular origin, hepatoblastoma (HB) is the most common
primary liver tumor in childhood, with a poor prognosis and
aggressive behavior (12).
Therefore, it is necessary to identify a novel anticancer drug with
higher selectivity and greater efficiency against hepatic
carcinoma.
JIB-04 is a small molecular compound with two
isomers, the E- and Z-isomers (Fig. 1A
and B). It is well known that tumors have a complicated and
aberrant epigenetic landscape. Only when the cancer epigenome has a
certain degree of susceptibility can drugs be targeted for
treatment. It was reported that only the E-isomer of JIB-04 was
active in a locus de-repression (LDR) assay, which induced the
expression of a silenced transgene leading to cancer-specific cell
death. The results confirmed that JIB-04 could inhibit the growth
and metastasis of human lung cancer and prostate cancer cell lines,
and thus acted as an antitumor agent (13).
Mutations in the p53 gene have been found in most
human malignancies. In addition, mutant p53 proteins can promote
tumorigenesis (14–16). As a tumor suppressor existing in
most human tumors, p53 protein plays a crucial role in cellular
genomic stability and cellular apoptosis after exposure to various
types of stress (17,18). It has been demonstrated that p53 is
able to regulate the transcription and expression of proapoptotic
and apoptotic proteins, including Bak, Bax, caspase-3 and
caspase-9, resulting in cellular apoptosis (19,20).
Previous studies have demonstrated that cancer cells undergo
apoptotic cell death, and that the apoptosis-related caspase-3,
caspase-7 and PARP cleavage proteins are activated following
stimulation with the E-isomer of JIB-04 (13). However, it remains unknown whether
this phenomenon is via a p53 activation-dependent pathway. In
addition, the question of whether the E-isomer of JIB-04 displays
efficacy in hepatic carcinoma has not been investigated. Therefore,
the aim of the current study was to evaluate the anticancer effect
of JIB-04, and to explore the underlying mechanism of JIB-04
induced-apoptosis in MHCC97H and HepG2 cells.
Materials and methods
Medicine and reagents
JIB-04 E-isomer
(C17H13ClN4; Fig. 1) was purchased from Sigma-Aldrich
(cat. no. SML0808; Merck KGaA, Darmstadt, Germany), and then was
dissolved in dimethyl sulfoxide (DMSO) to make a stock solution at
a concentration of 50 mM. All stock solutions were stored at −20°C
in the freezer. The working solutions of JIB-04 were prepared by
further diluting the stock solutions with culture medium. The final
concentration of DMSO was below 0.1% in this study. Pifithrin-α
(PFT-α) was obtained from Sigma-Aldrich (cat. no. P4359; Merck
KGaA) and diluted to a final concentration of 30 µM. The Cell
Counting Kit-8 (CCK-8) (cat. no. CK04) and the Annexin V-FITC
apoptosis detection kit (cat. no. AD10) were obtained from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). The primary
antibodies against caspase-3 (cat. no. 9665S), caspase-9 (cat. no.
9502S), Bak (cat. no. 3814S), Bcl-2 (cat. no. 4223S), Bax (cat. no.
2772S), p53 (cat. no. 9282S), PARP (cat. no. 5542L) and GAPDH (cat.
no. 2118L) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The secondary antibody was obtained from Sino
Biological, Inc. (cat. no. SSA004; Beijing, China).
Cell lines and drug treatment
The MHCC97H and HepG2 cells, obtained from the
cancer cell repository (Shanghai Cell Bank, Shanghai, China), were
cultured in DMEM (cat. no. 21885108; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (cat. no. 10099141; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a
humidified incubator with an atmosphere of 5% CO2. Both
MHCC97H and HepG2 cells were treated with various concentrations
(0, 0.25, 0.5 and 1 µM) of JIB-04 when the cell confluence reached
70–80%.
Cell inhibition and cytotoxicity
assay
CCK-8 assay: Cells were seeded at a density of
5×103 cells/well into 96-well plates. Briefly, different
concentrations of JIB-04 were used to treat MHCC97H and HepG2
cells. Following various exposure times, the supernatants were
removed. CCK-8 solution was diluted 10 times in warm assay medium.
Then, 100 µl diluent was transferred to each well. Plates were
incubated for 2 h at 37°C with 5% CO2. The absorbance
was recorded by using a plate reader (Perkin-Elmer, Inc., Waltham,
MA, USA) at a wavelength of 450 nm. The experiments were performed
independently and at least in triplicate. Inhibition rate (%) was
calculated according to the following equation: Inhibition rate (%)
= [OD450(control) -
OD450(treated)]/[OD450(control) -
OD450(blank) × 100%.
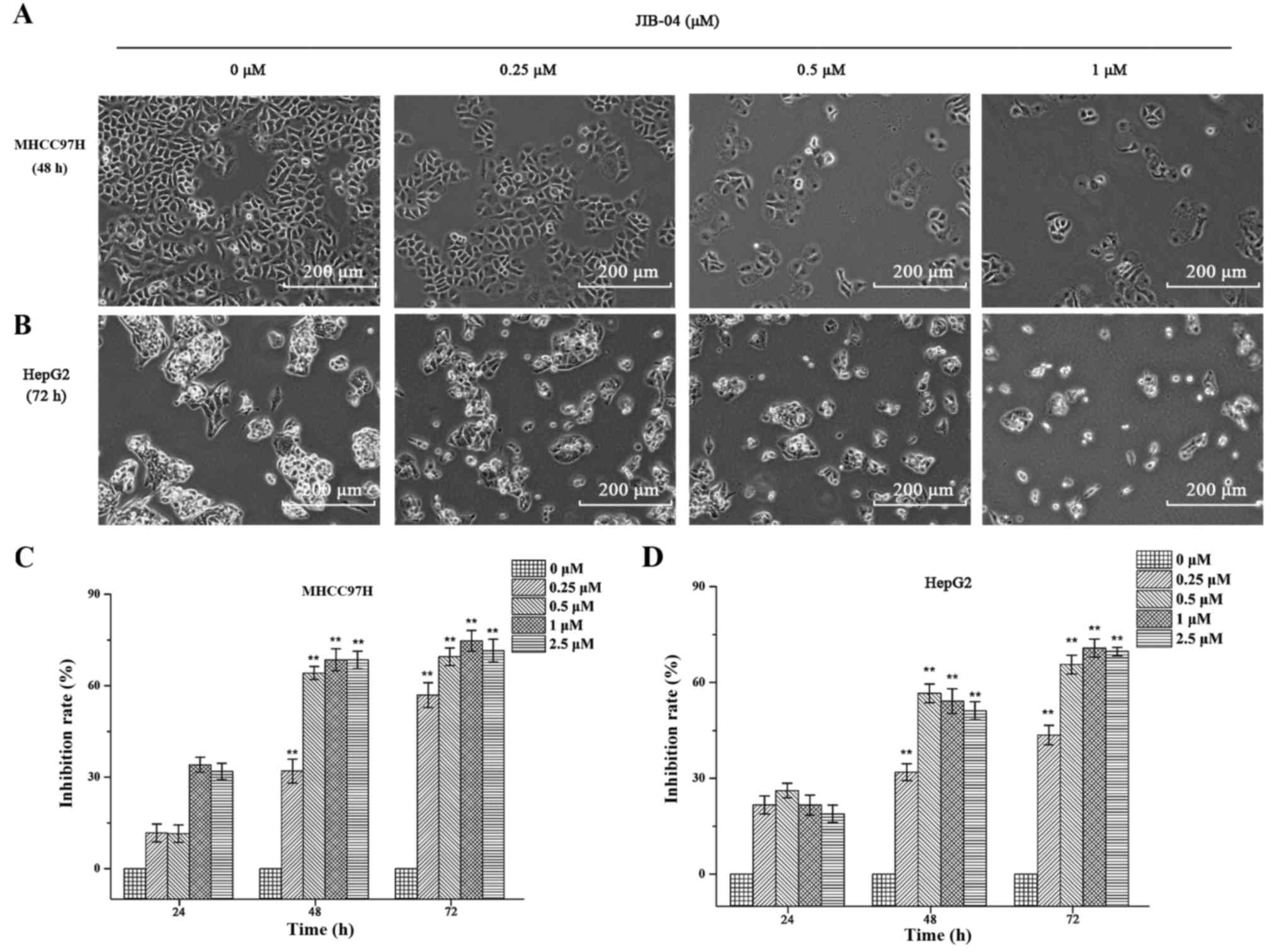
Assessment of cell morphology
Cells were seeded at a density of 1×105
cells/well into a 6-well plate. After pretreatment with different
concentrations (0, 0.25, 0.5 and 1 µM) of JIB-04 for different
exposure times (48 h for MHCC97H and 72 h for HepG2), images were
captured (scale bar, 200 µm) by an inverted microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Apoptosis assay
Apoptotic cells were detected using an Annexin
V-FITC apoptosis detection kit (Dojindo Molecular Technologies,
Inc.). Experiments were carried out using flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) and analyzed
using ModFit and CellQuest 5.1 software (BD Biosciences). According
to the manufacturer's instructions, MHCC97H and HepG2 cells were
plated at an initial concentration of 1×105 cells/well
in 6-well plates. After incubation for 12 h, the cells were treated
with different concentrations (0, 0.25, 0.5 and 1 µM) of JIB-04 for
various exposure times (48 h for MHCC97H and 72 h for HepG2), and
were then harvested and washed twice with cold D-Hanks buffer
solution. The cells were resuspended in binding buffer
(1×106 cells/ml). Subsequently, 5 µl Annexin V-FITC and
5 µl propidium iodide (PI) were added to 100 µl cell supernatant,
and incubated in the dark for 10 min prior to analysis. The Annexin
V-positive cells were regarded as being in the early apoptosis
stage, while Annexin V and PI double-positive cells were in the
late apoptosis stage.
Western blot analysis
The MHCC97H and HepG2 cells were cultured at an
initial concentration of 1×105 cells/ml in 100 mm
culture dishes, and incubated for 12 h at 37°C for 24 h.
Subsequently, cells were pretreated with different concentrations
(0, 0.25, 0.5 and 1 µM) of JIB-04 for different exposure times (48
h for MHCC97H and 72 h for HepG2) and lysed in lysis buffer (cat.
no. P0013; Beyotime Institute of Biotechnology, Haimen, China) for
30 min on ice. The lysates were centrifuged at 10,391.81 × g at 4°C
for 10 min. Then, the supernatants were collected. The protein
expression levels of samples were measured using a BCA
concentration measurement kit (cat. no. P0010; Beyotime Institute
of Biotechnology). Lysates were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and were
then transferred to polyvinylidene difluoride membranes (PVDF; EMD
Millipore, Billerica, MA, USA). Blocking was performed with 5%
fat-free dry milk in Tris-buffered saline (cat. no. V900483;
Sigma-Aldrich, Co., Merck KGaA) containing 0.05% Tween-20 (TBST)
for 1 h. Subsequently, the membranes were incubated with the
primary antibodies, including anti-caspase-3, anti-caspase-9,
anti-Bak, anti-Bcl-2, anti-p53, anti-Bax, anti-PARP and GAPDH, at a
dilution of 1:1,000 overnight at 4°C. Following washing three times
with TBST, the membranes were incubated with HRP-conjugated goat
anti-rabbit IgG at a dilution of 1:2,000 for 4 h at 4°C. The
membranes were washed with TBST three times for 10 min each.
Detection was carried out using the enhanced chemiluminescence
method. The integrated density value of each band for each image
was calculated using ImageJ software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation (SD). Statistical analysis was performed using SPSS
software (version 17.0; IBM Corp., Armonk, NY, USA). One-way ANOVA
(Tukey's test) was used to evaluate between-group differences.
Statistical significance was defined as P<0.05.
Results
JIB-04 inhibits cell proliferation in
MHCC97H and HepG2 cells
In order to investigate the antitumor effects of
JIB-04, MHCC97H and HepG2 cells were treated with 0, 0.25, 0.5 and
1 µM of JIB-04 for 24, 48 and 72 h and cell viability was
determined using a CCK-8 assay. It was found that untreated cells
grew well; however, treated cells were distorted and rounded in
shape. In addition, the number of floating cells increased
significantly as the drug concentration increased. As can be seen
from Fig. 2, the results
demonstrated that JIB-04 inhibited the cell viability of MHCC97H
and HepG2 cells in a concentration-dependent manner.
JIB-04 induces apoptosis in MHCC97H
and HepG2 cells
Annexin V/PI double staining was used to detect
cellular apoptosis. Compared with the vehicle-treated control, the
Q2 and Q4 cell population in the MHCC97H and HepG2 cells,
respectively increased from 5.57 to 46.05% (48 h), and from 6.85 to
48.3% (72 h). Fig. 3 shows that
JIB-04 induced cellular apoptosis in a concentration-dependent
manner.
JIB-04 induces the expression of
p53/Bcl-2/caspase signaling pathway proteins in MHCC97H and HepG2
cells
The p53/Bcl-2/caspase signaling pathway is tightly
associated with cell apoptosis. In this study, MHCC97H and HepG2
cells were treated with 0, 0.25, 0.5 and 1 µM JIB-04 for different
exposure times and the expression levels of p53/Bcl-2/caspase
signaling pathway proteins were evaluated. Fig. 4 shows that apoptosis-related protein
expression altered following JIB-04 stimulation. After MHCC97H and
HepG2 cells were treated with different concentrations of JIB-04,
the expression levels of the apoptosis-related proteins p53,
caspase-3 and caspase-9 were significantly upregulated in a
dose-dependent manner. As an important substrate of caspase-3, PARP
was found to increase in the JIB-04-treated cells compared with the
controls. In addition, Bcl-2 expression was downregulated. Bax was
significantly upregulated in HepG2 and MHCC97H cells after
treatment with increasing concentrations of JIB-04. Therefore, the
Bcl-2/Bax protein ratio in MHCC97H and HepG2 cells was decreased in
a concentration-dependent manner. The results indicated that
intrinsic apoptosis pathways, including the
caspase-9/caspase-3-associated apoptosis pathway and the p53/Bcl-2
signaling pathway, were activated following JIB-04 treatment in
MHCC97H and HepG2 cells.
p53 plays an important role in
JIB-04-induced apoptosis in MHCC97H and HepG2 cells
In order to determine the role of p53 in
JIB-04-induced cellular apoptosis, MHCC97H and HepG2 cells were
pretreated with PFT-α (30 µM) for 6 h in the presence or absence of
JIB-04 (0.5 µM). As can be seen from Figs. 5–7,
JIB-04 triggered cellular apoptosis in MHCC97H and HepG2 cells;
however, PFT-α reversed JIB-04-induced cell growth suppression and
apoptosis, and significantly reduced the expression level of p53,
Bax, cleaved caspase-3 and −9 protein. Simultaneously, Bcl-2
expression was upregulated. The results demonstrated the key role
of p53 in JIB-04-induced cellular apoptosis in MHCC97H and HepG2
cells.
Discussion
Despite advances in therapeutic strategies for HCC,
such as transplantation, immunotherapy strategies and liver
resection, the 5-year overall survival rate remains poor (21–23).
Thus, the identification of novel anticancer drugs with higher
therapeutic efficacy against HCC is a key focus in oncology
research. JIB-04, a potential therapeutic agent against cancer, has
been confirmed to inhibit the viability of multiple cancer cell
lines (13). However, its
underlying mechanism and its efficacy in inhibiting hepatoma cell
growth in vitro is not clear. In this study, JIB-04 was
demonstrated to reduce the proliferation of MHCC97H and HepG2 cells
in a dose-dependent manner, indicating that JIB-04 may serve as a
novel candidate agent against hepatic carcinoma.
Apoptosis, a complicated physiological process,
involves complex signaling pathways, such as the activation of
cysteine proteases and p53 (24,25).
Apoptosis induction has been accepted as a mechanism of action of
anticancer drugs (26). At present,
the development of anticancer agents is mainly focused on inducing
apoptosis in tumor cells (27).
Using Annexin V/PI staining, the present study demonstrated that
JIB-04 induced MHCC97H and HepG2 cell apoptosis in a
concentration-dependent manner, indicating that apoptosis may serve
as a mechanism underlying the function of JIB-04.
In order to further explore the underlying mechanism
of JIB-04-induced apoptosis in MHCC97H and HepG2 cells, we
evaluated the common regulators of the apoptosis process. Cysteine
proteases, especially caspases, play a crucial role in regulating
cellular apoptosis (24). Owing to
their different mechanisms of action, caspases are divided into
initiator caspases, including caspase-8 and −9, and executioner
caspases, including caspase-3, −6 and −7 (28). Caspase-3 is mainly activated through
two signaling pathways: the extrinsic pathway, involving the
activation of death receptors and caspase-8; and the intrinsic
pathway, involving the mitochondria and activation of caspase-9,
which then results in cellular apoptosis (29,30).
In this study, the results demonstrated that cleaved caspase-3 and
caspase-9 were significantly upregulated after JIB-04 stimulation,
indicating that JIB-04 induced apoptosis in the MHCC97H and HepG2
cells through the mitochondrial-mediated apoptosis pathway. As a
molecular receptor of DNA damage, PARP is activated and
participates in DNA replication and transcription when DNA is
damaged (31,32). In this study, the results showed
that PARP was cleaved significantly, suggesting that JIB-04
inhibited MHCC97H and HepG2 cell growth via cleaved caspase-3 and
PARP.
Apoptosis is not only associated with the activation
of caspases, but the accumulation of apoptosis-related proteins
(Bcl-2 family) (33). To the best
of our knowledge, the apoptosis-suppressing function of Bcl-2 is
inhibited after binding with Bax protein. The apoptosis-inducing
effects are more associated with the ratio of Bcl-2/Bax than with
the quantity of Bcl-2 alone (34,35). A
previous study demonstrated that the p53/Bcl-2 pathway was closely
related to dihydromyricetin-induced HCC cell apoptosis (25). In the present study, JIB-04 induced
MHCC97H and HepG2 cell apoptosis, which was verified by the
downregulation of the ratio of Bcl-2/Bax and upregulation of p53
protein expression via the p53/Bcl-2 signaling pathway (36). Bak, a core regulator of the
intrinsic apoptosis pathway, was significantly upregulated
following JIB-04 stimulation. These results confirmed that JIB04
inhibited cell growth and induced the apoptosis of MHCC97H and
HepG2 cells.
Although many proteins are directly involved in the
regulation of p53 levels and functioning, it is generally accepted
that MDM2 is the principal negative regulator of p53 (37). Serving as an E3 ubiquitin ligase of
p53, MDM2 not only negatively regulates p53 activity through the
induction of p53 protein degradation (38), but directly inhibits p53
trans-action on chromatin (39). In
summary, MDM2 can repress p53 via both ubiquitination-dependent and
ubiquitination-independent pathways. Furthermore, MDMX can
negatively regulate the stability and activity of the p53 protein
by mediating the rapid degradation of p53 via ubiquitin-dependent
proteolysis (38,40). As a tumor-suppressor gene, p53 can
induce apoptosis by mediating several classical pathways (41). It is considered a suitable choice
for the treatment of various tumors by targeting and reactivating
p53 or enhancing its activity (42). In this study, when JIB-04 was
blocked by PFT-α, Bcl-2 suppression was reversed significantly by
decreasing p53 expression. Subsequently, the results verified that
JIB-04 induced MHCC97H and HepG2 cell apoptosis via the p53/Bcl-2
pathway.
In conclusion, the present study demonstrated that
JIB-04 effectively inhibited cell viability, and promoted MHCC97H
and HepG2 cell apoptosis via the p53/Bcl2/caspase signaling
pathway. The results provided a foundation for understanding the
anticancer effect of JIB-04 on MHCC97H and HepG2 cells, and suggest
that JIB-04 may be a promising compound for the treatment of
hepatic carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Yangfan Plan
of the Talents Recruitment Grant, Guangdong, China [Yue Ren Cai Ban
(2016) 6]; the Scientific Research Fund of Guangdong Medical
College, China (M2016031, M2016032) and Zhanjiang 2014 Annual
Financial Capital Competitive Project Science and Technology
Project (grant no. 2014A404).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design was carried out by ML and RZ.
Administrative support was undertaken by RZ. Provision of study
materials was performed by WL and JL. All authors aided in the
performance of the experiments. Collection and assembly of data was
achieved by BL and DC. Data analysis and interpretation was carried
out by XH, YY and WL. Manuscript writing was undertaken by WL. All
authors were involved in the final approval of the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng JS, Chou CT, Liu YY, Sun WC, Shieh
P, Kuo DH, Kuo CC, Jan CR and Liang WZ: The effect of oleuropein
from olive leaf (Olea europaea) extract on Ca2+
homeostasis, cytotoxicity, cell cycle distribution and ROS
signaling in HepG2 human hepatoma cells. Food Chem Toxicol.
91:151–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng M, Gao W, Wang R, Chen W, Man YG,
Figg WD, Wang XW, Dimitrov DS and Ho M: Therapeutically targeting
glypican-3 via a conformation-specific single-domain antibody in
hepatocellular carcinoma. Proc Natl Acad Sci USA. 110:E1083–E1091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilson TR, Fridlyand J, Yan Y, Penuel E,
Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al:
Widespread potential for growth-factor-driven resistance to
anticancer kinase inhibitors. Nature. 487:505–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Q, Ma S, Liu B, Liu J, Zhu R and Li
M: Chrysin induces cell apoptosis via activation of the
p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp
Ther Med. 12:469–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Au JS and Frenette CT: Management of
hepatocellular carcinoma: Current status and future directions. Gut
Liver. 9:437–448. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Fan X, Tang M, Chen R, Wang H, Jia
R, Zhou X, Jing W, Wang H, Yang Y, et al: Osteopontin induces
autophagy to promote chemo-resistance in human hepatocellular
carcinoma cells. Cancer Lett. 383:171–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo HL, Chen J, Luo T, Wu FX, Liu JJ, Wang
HF, Chen M, Li LQ and Li H: Downregulation of macrophage-derived
T-UCR uc.306 associates with poor prognosis in hepatocellular
carcinoma. Cell Physiol Biochem. 42:1526–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruix J, Takayama T, Mazzaferro V, Chau
GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, et al: Adjuvant
sorafenib for hepatocellular carcinoma after resection or ablation
(STORM): A phase 3, randomised, double-blind, placebo-controlled
trial. Lancet Oncol. 16:1344–1354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horgan AM, Dawson LA, Swaminath A and Knox
JJ: Sorafenib and radiation therapy for the treatment of advanced
hepatocellular carcinoma. J Gastrointest Cancer. 43:344–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabernero J, Garcia-Carbonero R, Cassidy
J, Sobrero A, Van Cutsem E, Köhne CH, Tejpar S, Gladkov O,
Davidenko I, Salazar R, et al: Sorafenib in combination with
oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as
first-line treatment of metastatic colorectal cancer: The RESPECT
trial. Clin Cancer Res. 19:2541–2550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
13
|
Wang L, Chang J, Varghese D, Dellinger M,
Kumar S, Best AM, Ruiz J, Bruick R, Peña-Llopis S, Xu J, et al: A
small molecule modulates Jumonji histone demethylase activity and
selectively inhibits cancer growth. Nat Commun. 4:20352013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osborne C, Wilson P and Tripathy D:
Oncogenes and tumor suppressor genes in breast cancer: Potential
diagnostic and therapeutic applications. Oncologist. 9:361–377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freed-Pastor WA, Mizuno H, Zhao X,
Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li
W, Polotskaia A, et al: Mutant p53 disrupts mammary tissue
architecture via the mevalonate pathway. Cell. 148:244–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bailey SM, Meyne J, Chen DJ, Kurimasa A,
Li GC, Lehnert BE and Goodwin EH: DNA double-strand break repair
proteins are required to cap the ends of mammalian chromosomes.
Proc Natl Acad Sci USA. 96:14899–14904. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Degenhardt K, Chen G, Lindsten T and White
E: BAX and BAK mediate p53-independent suppression of
tumorigenesis. Cancer Cell. 2:193–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henry H, Thomas A, Shen Y and White E:
Regulation of the mitochondrial checkpoint in p53-mediated
apoptosis confers resistance to cell death. Oncogene. 21:748–760.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mauer K, O'Kelley R, Poddar N, Flanagan S
and Gadani S: Erratum to: New treatment modalities for
hepatocellular cancer. Curr Gastroenterol Rep. 17:492015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Deng T, Zeng L and Chen W:
Efficacy and safety of radiofrequency ablation and transcatheter
arterial chemoembolization for treatment of hepatocellular
carcinoma: A meta-analysis. Hepatol Res. 46:58–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding G, Peng Z, Shang J, Kang Y, Ning H
and Mao C: LincRNA-p21 inhibits invasion and metastasis of
hepatocellular carcinoma through miR-9/E-cadherin cascade signaling
pathway molecular mechanism. Onco Targets Ther. 10:3241–3247. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alenzi FQ, Alenazi BQ, Al-Anazy FH,
Mubaraki AM, Salem ML, Al-Jabri AA, Lotfy M, Bamaga MS, Alrabia MW
and Wyse RK: The role of caspase activation and mitochondrial
depolarisation in cultured human apoptotic eosinophils. Saudi J
Biol Sci. 17:29–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powell CB, Fung P, Jackson J, Dal l'Era J,
Lewkowicz D, Cohen I and Smith-McCune K: Aqueous extract of herba
Scutellaria barbatae, a chinese herb used for ovarian cancer,
induces apoptosis of ovarian cancer cell lines. Gynecol Oncol.
91:332–340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan CH, Filippova M and Duerksen-Hughes
P: Modulation of apoptotic pathways by human papillomaviruses
(HPV): Mechanisms and implications for therapy. Viruses.
4:3831–3850. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poku RA, Salako OO, Amissah F, Nkembo AT,
Ntantie E and Lamango NS: Polyisoprenylated cysteinyl amide
inhibitors induce caspase 3/7- and 8-mediated apoptosis and inhibit
migration and invasion of metastatic prostate cancer cells. Am J
Cancer Res. 7:1515–1527. 2017.PubMed/NCBI
|
|
30
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Booth L, Cruickshanks N, Ridder T, Dai Y,
Grant S and Dent P: PARP and CHK inhibitors interact to cause DNA
damage and cell death in mammary carcinoma cells. Cancer Biol Ther.
14:458–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ciccarone F, Klinger FG, Catizone A,
Calabrese R, Zampieri M, Bacalini MG, De Felici M and Caiafa P:
Poly(ADP-ribosyl)ation acts in the DNA demethylation of mouse
primordial germ cells also with DNA damage-independent roles. PLoS
One. 7:e469272012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen
X, Cao Y, Zhang C, Lu C, Li M, et al: Dihydromyricetin promotes
hepatocellular carcinoma regression via a p53 activation-dependent
mechanism. Sci Rep. 4:46282014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reed JC: Bcl-2 family proteins: Regulators
of apoptosis and chemoresistance in hematologic malignancies. Semin
Hematol. 34 4 Suppl 5:S9–S19. 1997.
|
|
35
|
Pettersson F, Dalgleish AG, Bissonnette RP
and Colston KW: Retinoids cause apoptosis in pancreatic cancer
cells via activation of RAR-gamma and altered expression of
Bcl-2/Bax. Br J Cancer. 87:555–561. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carr MI and Jones SN: Regulation of the
Mdm2-p53 signaling axis in the DNA damage response and
tumorigenesis. Transl Cancer Res. 5:707–724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minsky N and Oren M: The RING domain of
Mdm2 mediates histone ubiquitylation and transcriptional
repression. Mol Cell. 16:631–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mu R, Lu N, Wang J, Yin Y, Ding Y, Zhang
X, Gui H, Sun Q, Duan H, Zhang L, et al: An oxidative analogue of
gambogic acid-induced apoptosis of human hepatocellular carcinoma
cell line HepG2 is involved in its anticancer activity in vitro.
Eur J Cancer Prev. 19:61–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Papazoglu C and Mills AA: p53: At the
crossroad between cancer and ageing. J Pathol. 211:124–133. 2007.
View Article : Google Scholar : PubMed/NCBI
|