Introduction
Colorectal cancer is one of the most common types of
tumors of the digestive tract and can seriously threaten human
health (1). Early detection,
diagnosis, and treatment may improve the long-term quality of life
of colorectal cancer patients. Considerable progress has been made
in the field of early diagnosis, however comprehensive treatment,
metastasis, and recurrence of cancer are still the most important
factors that affect prognosis (2).
Therefore, potential diagnostic markers and therapeutic targets are
still urgently required.
Lymphocyte activating gene 3 (LAG-3) is a member of
the immunoglobulin superfamily and has been shown to be a specific
marker of T helper (TH) cells (3).
As a negative costimulatory molecule, activation of LAG-3 can
negatively regulate the function of lymphocytes and inhibit the
function and life cycle of immune cells (4). Interestingly, LAG-3-positive
tumor-infiltrating lymphocytes are thought to be an independent
positive prognostic factor of non-small-cell lung cancer (5) and estrogen receptor-negative breast
cancers (6). LAG-3 selectively
increases cluster of differentiation (CD)4 on the Treg surface,
while LAG-3 antibody can reduce Treg activity in vivo.
Inhibition or knockout of LAG-3 relieves the inhibitory effect of
Treg on T cells. Programmed cell death 1 (PD-1)/ PD-ligand 1
(PD-L1) is another tumor checkpoint, and PD-1/PD-L1 activation can
promote immune suppression of the tumor microenvironment, causing
tumor cells to escape from immune surveillance and destruction
(7). Correspondingly, blocking the
PD-1/PD-L1 signaling pathway can reverse the suppression of the
tumor immune microenvironment and enhance anti-tumor activity of
the endogenous immune system (8–10).
PD-L1 binds to PD-1 on the surface of T cells to inhibit the
destructive effect of T cells on tumor cells (11). Reducing PD-L1 plays an important
role in promoting the tumor immune response and overcoming immune
escape (12). However, the effects
of PD-L1 silencing on the immune system in colorectal cancer have
not been reported.
LAG-3-positive tumor-infiltrating lymphocytes are
thought to be an independent positive prognostic factor for cancers
(5), while PD-L1 plays an important
role in promoting the tumor immune response (12). Based upon previous publications
(13), the phosphatidylinositol
3-kinase (PI3K)/RAC-α serine/threonine-protein kinase (AKT) pathway
is a cell survival pathway, supporting the development of tumors
(14,15). However, a link between PD-L1, LAG-3
and the PI3K/AKT pathway in colorectal cancer has not been
established. In the present study, the expression levels of PD-L1,
LAG-3, and the PI3K/AKT proteins of the signaling pathway were
compared between colorectal cancer and paracancerous tissues.
Thereafter, PD-L1 was silenced to investigate its effect on the
tumor growth of colorectal cancer, and to assess the mechanisms
involved. Importantly, LAG-3 activity was blocked using a specific
LAG-3 antibody to verify the checkpoint of PD-L1 in colorectal
cancer. The present study provides an experimental basis for the
use of PD-L1 inhibition in treating colorectal cancer.
Materials and methods
Clinical samples
Ethical approval for the present study was obtained
from the committee of Fujian Medical University Union Hospital
(Fuzhou, China) and informed consent was obtained from the
patients. Colorectal cancer and paracancerous tissues were
collected from ten colorectal cancer patients (male/female: 7/3)
who were diagnosed and received surgery treatment in the present
hospital from October 2016 to December 2016. These patients had not
received any chemotherapy or radiotherapy. Tissues were fixed in 4%
paraformaldehyde for 24 h at 4°C for immunofluorescence and
immunohistochemistry experiments.
Establishment of tumor models
A total of 21 female C57B/L6 (6-week-old, 20±2 g)
mice were purchased from Hunan SLAC Experimental Animal Co. Ltd.
(Shanghai, China; SCXK 2016–0002) and housed in a specific
pathogen-free environment that was automatically maintained at a
temperature of 23±2°C, a relative humidity of 45–65%, and with a
controlled 12 h light/dark cycle. The animals had free to access
food and water. All animal experiments were approved by the Ethics
Committee of Fujian Medical University Union Hospital.
The C57B/L6 mice were randomly divided into 3 groups
(n=7 in each group): control, vector control group, and PD-L1
silenced with short hairpin RNA (shPD-L1) group. CT26 cells were
purchased from the Shanghai Cell Bank of Chinese Academy of Science
(Shanghai, China) and cultured in Dulbecco's Modified Eagle's
Medium (DMEM; Thermo Fisher, Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in an environment
containing 5% CO2. Cells at 70% confluence were
transfected with empty vector (1 µg) or PD-L1 shRNA (1 µg) (both
were obtained from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 24 h after transfection, the cells
were used in the following injection. In the control group, CT26
cells in the logarithmic growth phase (1×107) were
diluted in 0.2 ml PBS and administered through subcutaneous
injection into the C57B/L6 mice. In the vector control group, CT26
cells transfected with control vector were diluted in 0.2 ml PBS
and injected into the C57B/L6 mice. In the PD-L1 silenced group,
CT26 cells transfected with viral PD-L1 shRNA were diluted in 0.2
ml PBS and injected into the C57B/L6 mice.
General conditions of the mice were monitored daily
and the tumor size was measured every 2 days. At 7 days after
injection of tumor cells, LAG-3 antibody (cat. no. ab213524; Abcam,
Cambridge, MA, USA) was injected every 3 days via intraperitoneal
injection (10 mg/kg). At the 14th day after tumor cell injection,
mice were decapitated following anesthesia (5% isoflurane), and
whole tumors were removed. Tumor specimens were fixed in 4%
paraformaldehyde (pH 7.4) at 4°C overnight and then embedded in
paraffin for tissue sectioning.
Immunohistochemistry
Tumor tissues and paracancerous tissues were fixed
in 4% paraformaldehyde for ~1 week at 4°C. Tissues were then
dehydrated, embedded, and sliced (2 µm). Thereafter, paraffin
sections were dewaxed and hydrated in 70, 75, 80, 85 and 95%
alcohol. Staining was performed using monoclonal antibodies against
PD-L1 (1:100; cat. no. ab199380; Abcam), LAG-3 (1:100; cat. no.
bs-2646R; BIOSS, Beijing, China), AKT (1:100; cat. no. bs-0115R;
BIOSS), and PI3K (1:100; cat. no. ab191606; Abcam). Endogenous
peroxidase activity was blocked with 3% (v/v)
H2O2 for 5 min at room temperature.
Subsequently, slides were incubated with primary antibodies
overnight at 4°C, followed by incubation with horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. A16104SAMPLE; Thermo Fisher Scientific, Inc.),
Alexa Fluor 593 goat anti-mouse IgG (1:100; cat. no. CW0159S; CW
Biotech, Beijing, China), or Alexa Fluor 488 goat anti-rabbit IgG
(1:100; cat. no. CW0114S; CWBIO, Beijing, China) for 30 min at room
temperature. The fluorescence intensity was analyzed by
ImageProPlus software 6.0 (National Institutes of Health, Bethesda,
MD, USA). Immunohistochemical staining was visualized with
3,3′-diaminobenzidine chromogen for 3 min at room temperature. The
nucleus was counterstained by hematoxylin at room temperature for 3
min. Both images obtained from immunohistochemistry and
immunofluorescence were obtained using a microscope (CX41; Olympus
Corporation, Tokyo, Japan). The grey density was calculated by
ImageProPlus software.
Hematoxylin and eosin (H&E)
staining
Tissues were rinsed and then dehydrated, embedded,
and sliced. Paraffin sections were dewaxed and hydrated. Sections
were stained with hematoxylin solution for 5 min and with eosin for
3 min at room temperature. Images were obtained using light
microscopy.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) assay
A TUNEL assay was carried out in the
paraffin-embedded tissues. Samples were sectioned into 2-µm slices,
dewaxed in ethanol (75, 80, 95 and 100%), and washed with PBS (3
times for 5 min each). Fresh proteinase K was prepared (2 µl
proteinase K in 98 µl PBS) and applied to cover the tissues (100
µl) at 37°C for 30 min. Finally, TdT (2 µl) and fluorescent reagent
(48 µl) were mixed and applied to stain the tissue at 37°C for 60
min (cat. no. C1088; Beyotime Institute of Biotechnology, Haimen,
China). After washing, slides were covered with anti-fading reagent
and observed at least five fields under a fluorescence
microscope.
Flow cytometry
Tumor tissues were ground and passed through a
200-mesh. Following centrifugation (4°C, 400 × g, 3 min), cells
were collected. Antibodies against CD4-fluorescein isothiocyanate
(1:400; cat. no. 561833; BD Biosciences, Franklin Lakes, NJ, USA)
and CD8-phycoerythrin (1:400; cat. no. 561949; BD Biosciences) were
added to each tube. After incubation at room temperature for 10 min
in the dark, cells were detected by flow cytometer (NovoCyte 2060R;
ACEA Biosciences, Inc., Hangzhou, China) and data were analyzed by
FlowJo 10 (FlowJo, LLC., Portland, OR, USA).
Cell Counting Kit (CCK)-8 assay
The CD4+ and CD8+ T cells in
the tumor tissues were sorted using a flow sorting apparatus, and
then placed on a 96-well plate and cultured for 24 h. 10 µl CCK-8
(Gibco; Thermo Fisher Scientific, Inc.) was added to each well.
After an additional incubation for 4 h in a CO2
incubator at 37°C, the absorbance was detected by a microplate
reader (Thermo Fisher Scientific, Inc.) at a wavelength of 490 nm.
Cell viability was defined by optical density (OD) values.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Following the various treatments, total RNA was
extracted using TRIzol® (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. mRNA purity was confirmed
by OD280/OD260 using a spectrophotometer and amplified by a
one-step RT-PCR kit (cat. no. DRR046A; Takara Bio, Inc., Otsu,
Japan). The primers were added into a 25-µl PCR reaction system
following a protocol of 94°C denaturation 45 sec, 56°C annealing 45
sec, and 72°C extension 60 sec for 40 cycles. Primers are listed in
Table I.
 | Table I.Primer sequences for the target
genes. |
Table I.
Primer sequences for the target
genes.
| Genes | Primer sequences
(5′-3′) | Primer length
(bp) | Product length
(bp) | Annealing
temperature (°C) |
|---|
| PD-L1 |
|
| 111 | 57 |
| F |
CCATCTTATTATGCCTTGGTGTAG | 24 |
|
|
| R |
TTTGCTTCTTTGAGTTTGTATCTTG | 25 |
|
|
| PI3K |
|
| 140 | 54 |
| F |
CCAACACCTTCATCATCC | 18 |
|
|
| R |
CTCCTCCTCCTGCTTCTT | 18 |
|
|
| AKT |
|
| 231 | 58.7 |
| F |
TGTGATCTTAATGTGCCCGTC | 21 |
|
|
| R |
TACTCTTTCCAGACCCACGAC | 21 |
|
|
| GAPDH |
|
| 100 | 58.6 |
| F |
GAAGGTCGGAGTCAACGGAT | 20 |
|
|
| R |
CCTGGAAGATGGTGATGGG | 19 |
|
|
Western blotting
Following treatments, protein was extracted using a
protein isolation kit (cat. no. 28-9425-44, ReadyPrep; GE
Healthcare Life Sciences) (containing phenylmethane sulfonyl
fluoride), as previously described (16). Protein samples were heated at 100°C
for 10 min, and the protein concentration was quantified using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Proteins (25 µg/well) were separated by SDS-PAGE (12% gel), and
protein was then transferred onto a nitrocellulose membrane.
Membrane was blocked with 5% nonfat milk in phosphate-buffered
saline with Tween-20 at room temperature for 2 h. Primary
antibodies against, PD-L1 (1:1,000; cat. no. ab199380; Abcam), AKT
(1:1,000; cat. no. bs-0115R; BIOSS), PI3K (1:1,000; cat. no.
ab191606; Abcam) and GAPDH (1:1,000; cat. no. ab24071; Abcam) were
incubated with membranes overnight at 4°C. After washing (3 times
for 10 min each), membranes were incubated with the goat
Anti-Rabbit IgG H&L (HRP) (1:10,000; cat. no. ab131368; Abcam)
for 2 h at room temperature. Chemiluminescent substrate detection
reagent (cat. no. RPN2133; GE Healthcare Life Sciences) was applied
to reveal the signals. Target bands were analyzed by ImageJ
software for grayscale analysis.
Statistical analysis
Data were expressed as mean and standard deviation
(n=7 in each group) and analyzed using SPSS software, version 19.0
(IBM SPSS, Armonk, NY, USA). Statistical significance was assessed
using a paired Student's t-test (parametric) or one-way analysis of
variance with Newman-Keuls as the post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
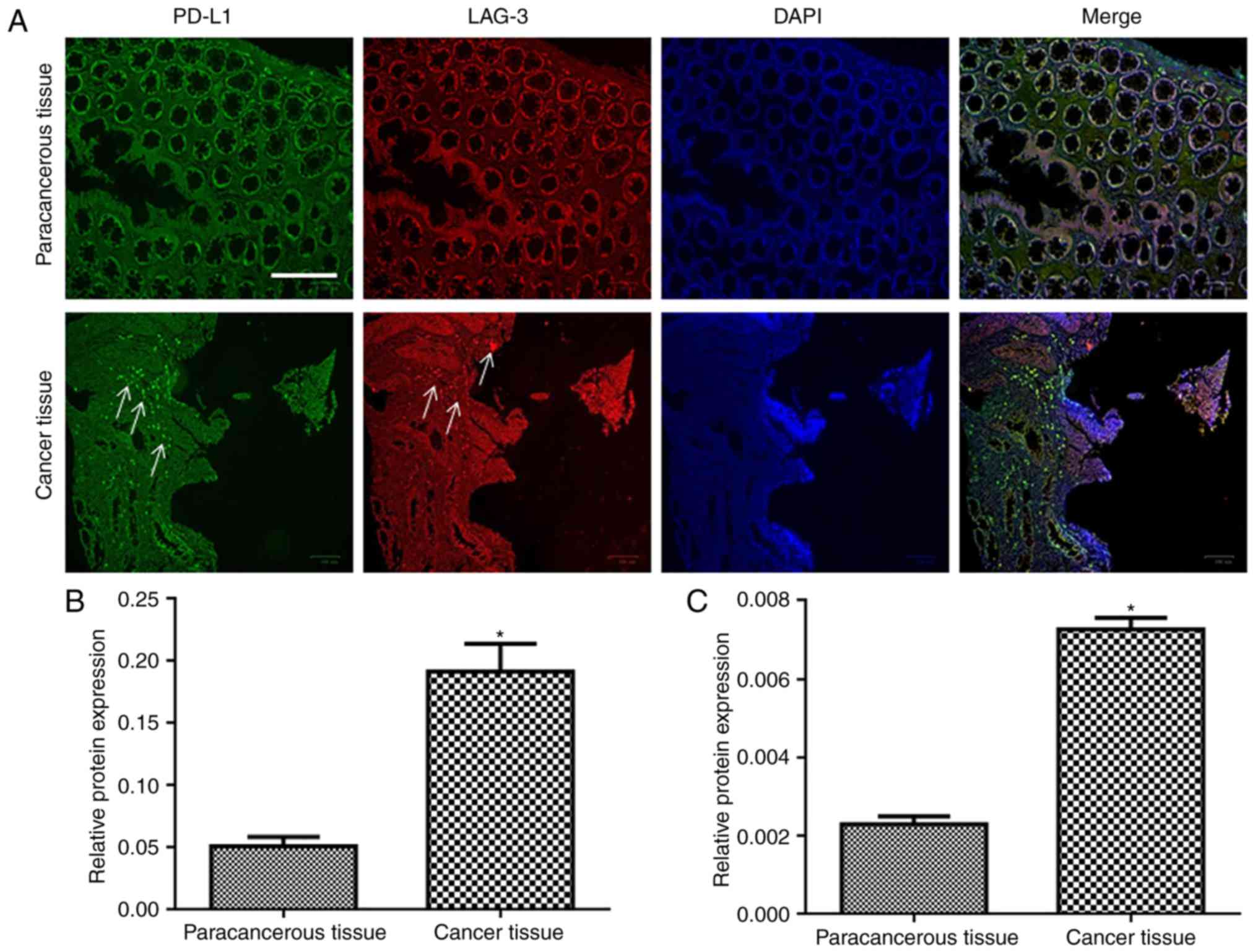
Expression of PD-L1 and LAG-3 is
increased in cancerous tissue compared with paracancerous
tissue
PD-L1 expression was extensively observed in cancer
tissues (Fig. 1A). LAG-3 expression
was observed at the edge of tumors. Conversely, very little PD-L1
expression was observed in paracancerous tissues. LAG-3 expression
was not observed in paracancerous tissues. Quantitative results
demonstrated that the expression of PD-L1 and LAG-3 in the
carcinoma tissue was significantly increased compared with in the
paracancerous tissue (P<0.05; Fig.
1B and C).
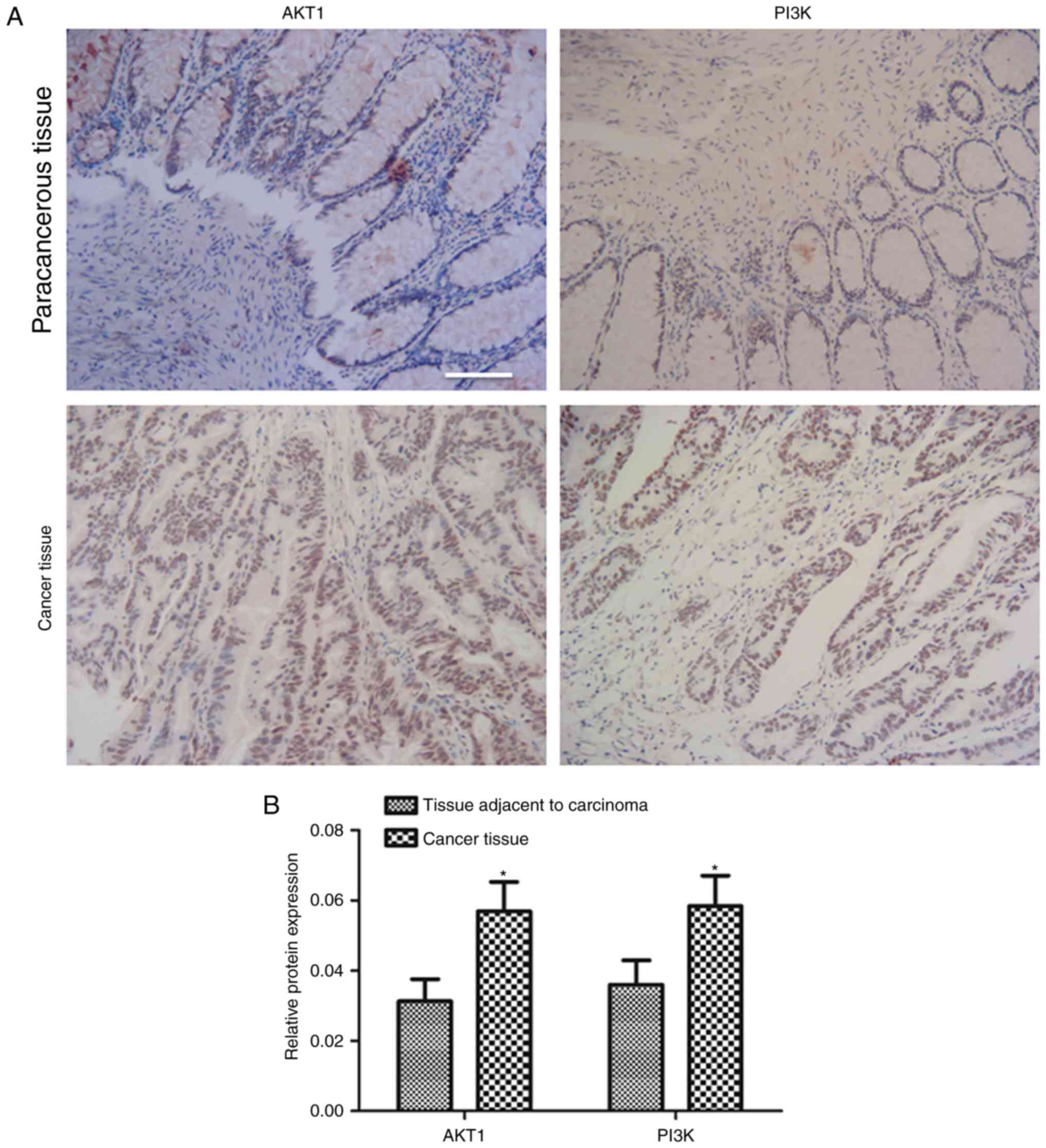
Expression of PI3K and AKT is
increased in cancer tissue compared with paracancerous tissue
The present study detected the expression levels of
AKT and PI3K in carcinoma tissue. Expression of AKT and PI3K was
extensively observed in cancer tissues, but rarely observed in
paracancerous tissue (Fig. 2A).
Quantitative results showed that the expression of AKT and PI3K in
carcinoma tissues was significantly increased compared with
paracancerous tissue (P<0.05; Fig.
2B).
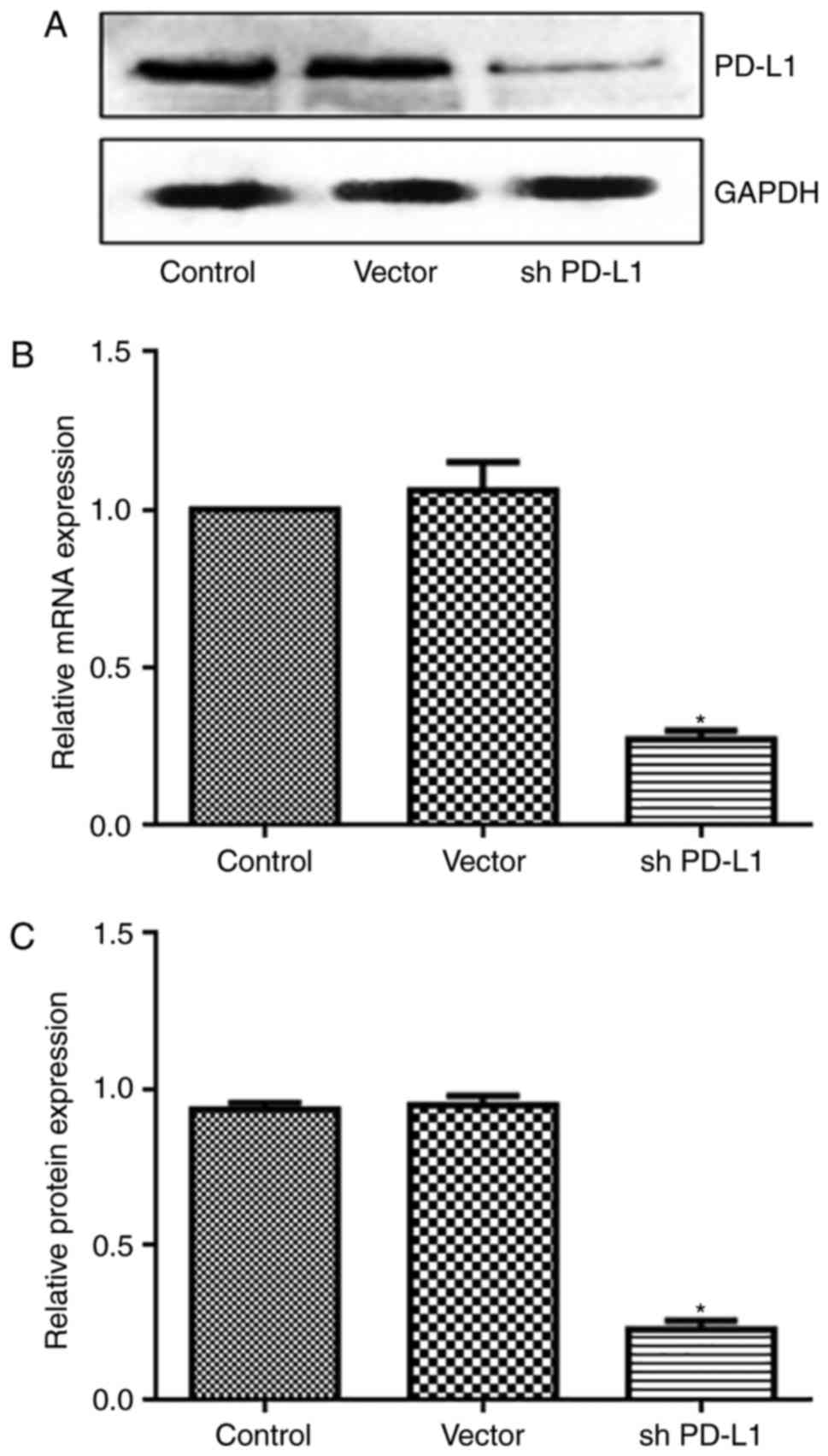
PD-L1 silencing reduces tumor
growth
Based upon the aforementioned results, the present
study designed experiments to inhibit PD-L1 by shRNA. In addition,
to avoid the effect of LAG-3, its activity was blocked by using an
LAG-3 antibody in all the groups. PD-L1 expression was
significantly reduced following transfection with shRNA PD-L1
(P<0.05; Fig. 3). The
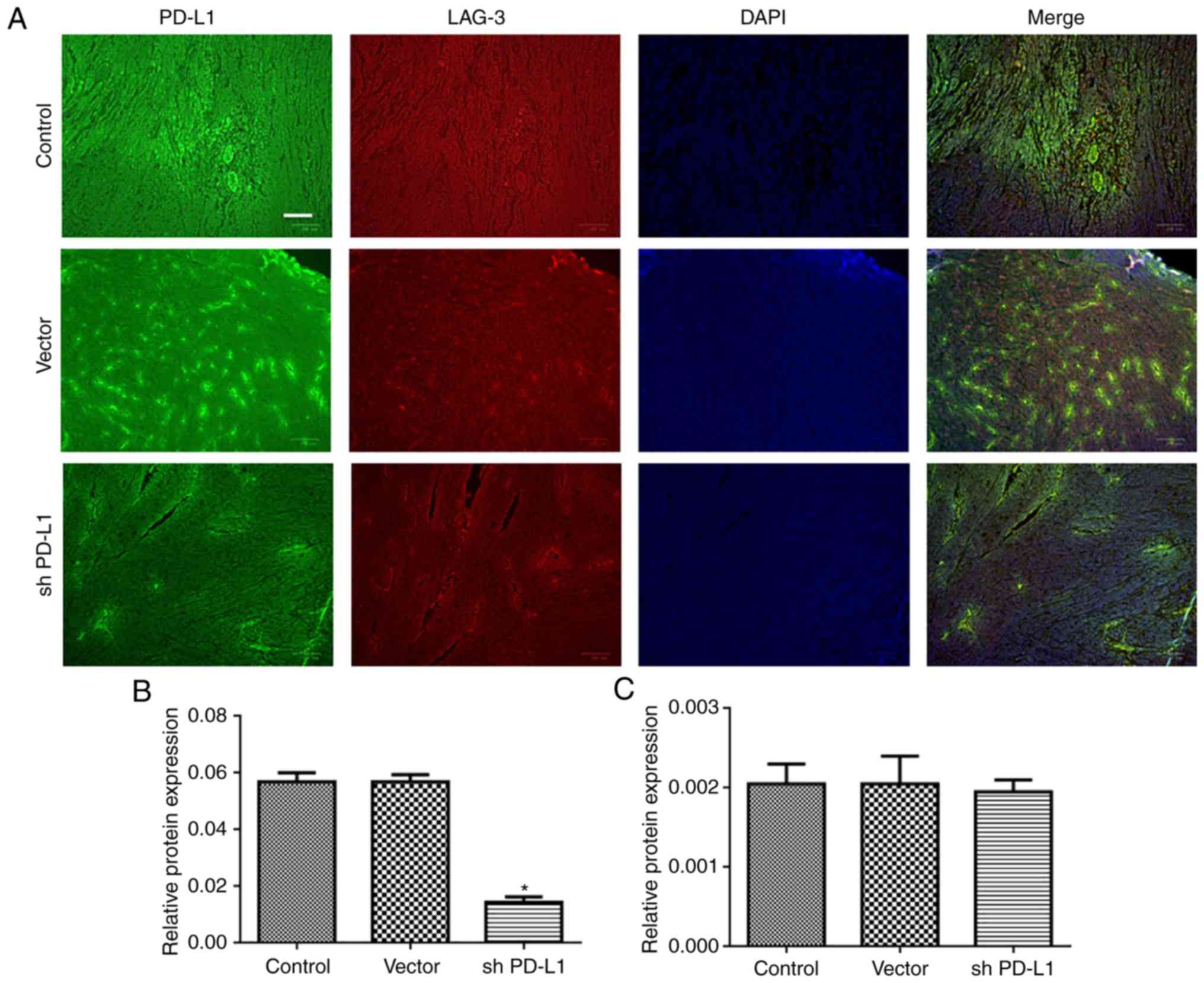
established cell lines were injected into C57B/L6 mice, and tumor
growth was evaluated. PD-L1 and LAG-3 expression was also detected
in tumor tissues (Fig. 4A). PD-L1
expression was significantly reduced in the PD-L1 silenced group,
compared with the control group (P<0.05; Fig. 4B), whereas LAG-3 expression in the 3
groups was comparable (Fig.
4C).
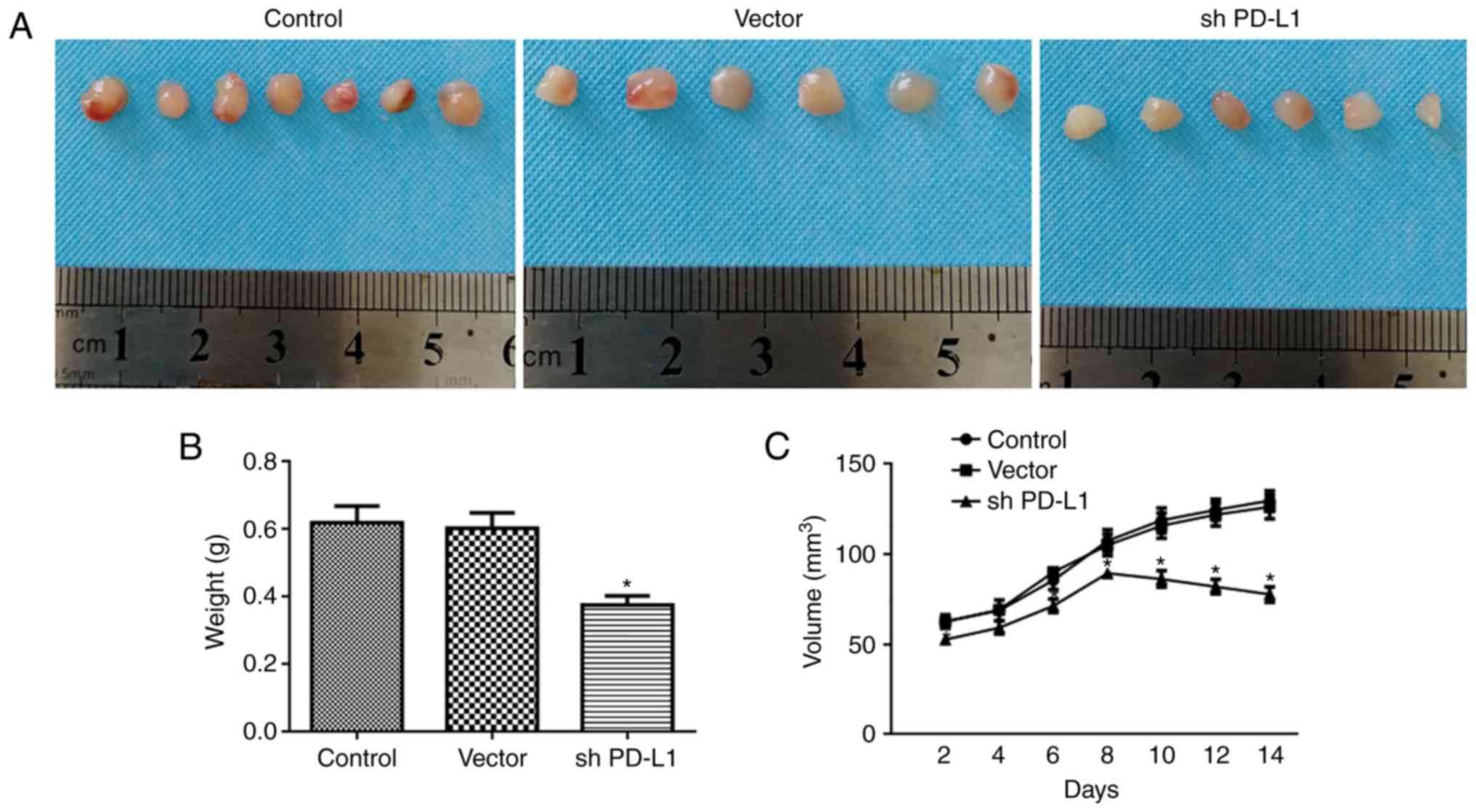
Tumors weighed less in the PD-L1 silenced group,
compared with the control group (P<0.05; Fig. 5A and B). Tumor growth was
significantly inhibited in the PD-L1 silenced group, compared with
the control group (P<0.05; Fig.
5C).
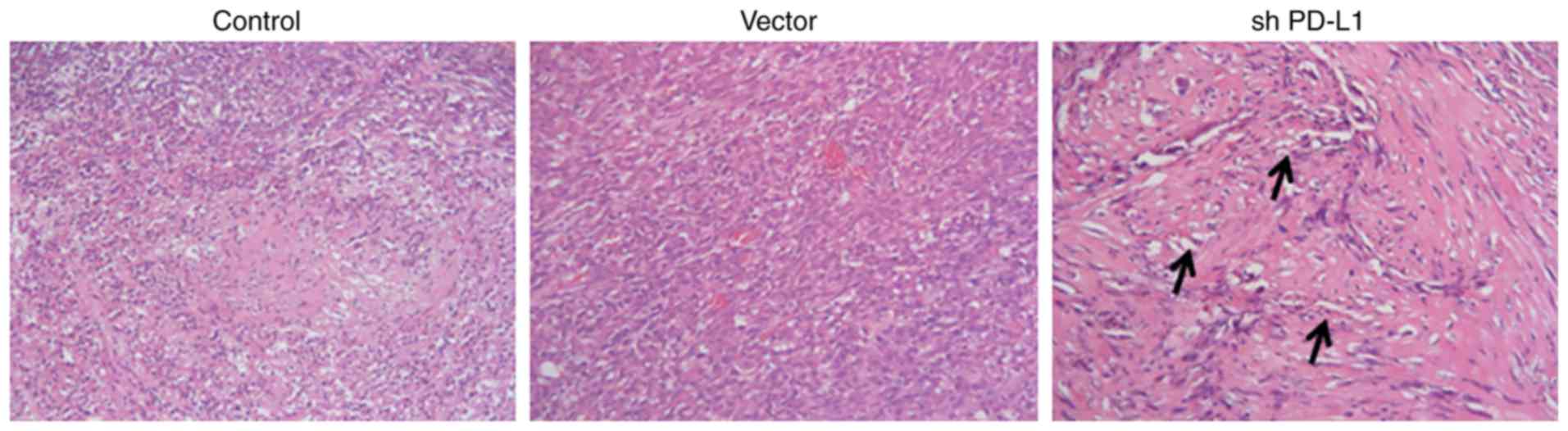
Tumor cells in the PD-L1 silenced group had a loose
arrangement and were inactive in cell growth (Fig. 6). In control groups, tumor cells
were tightly arranged, and the size was relatively uniform.
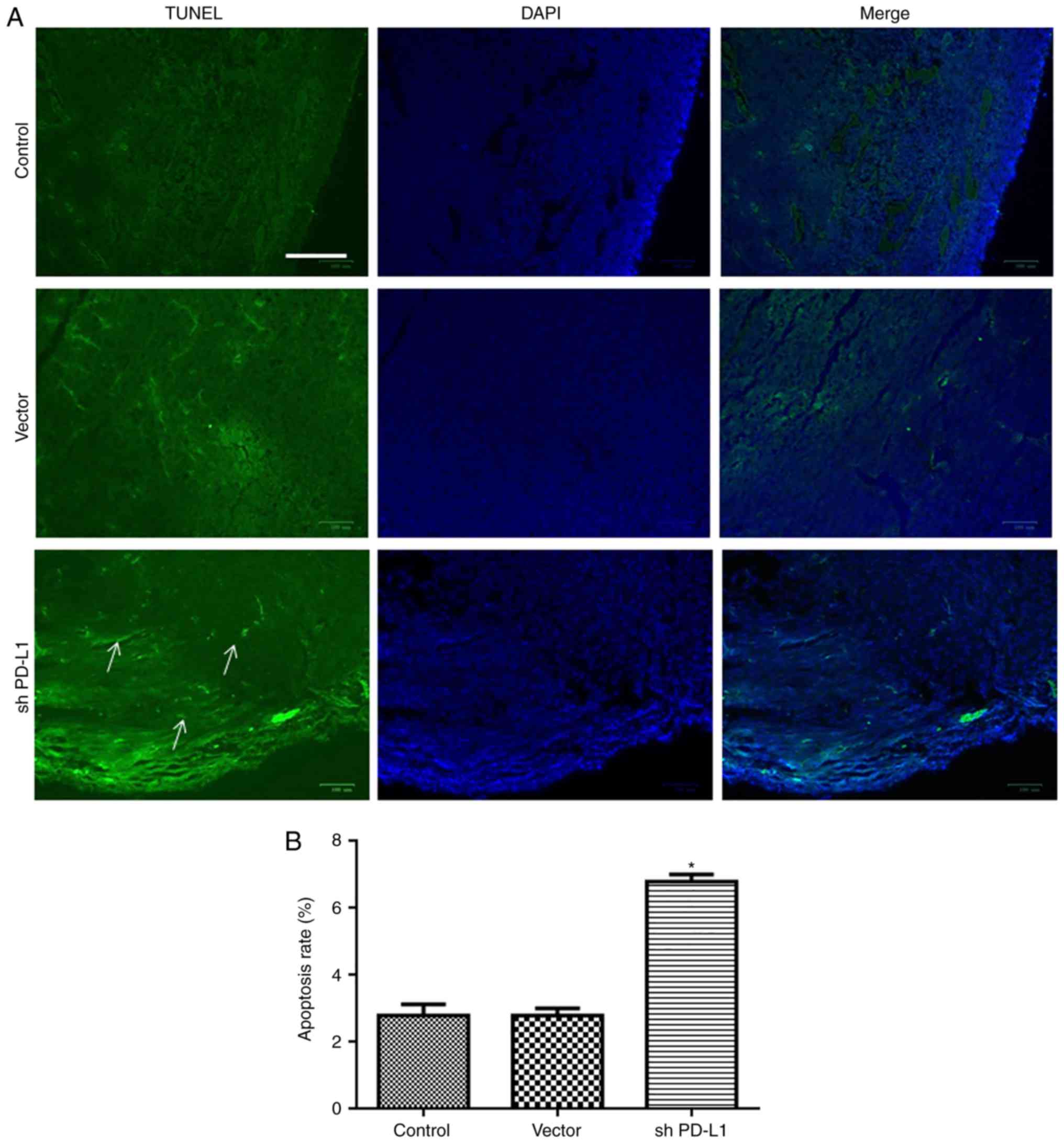
The present study also detected the apoptosis of
tumor cells. Apoptosis was increased in the shPD-L1 group compared
with control groups (P<0.05; Fig.
7).
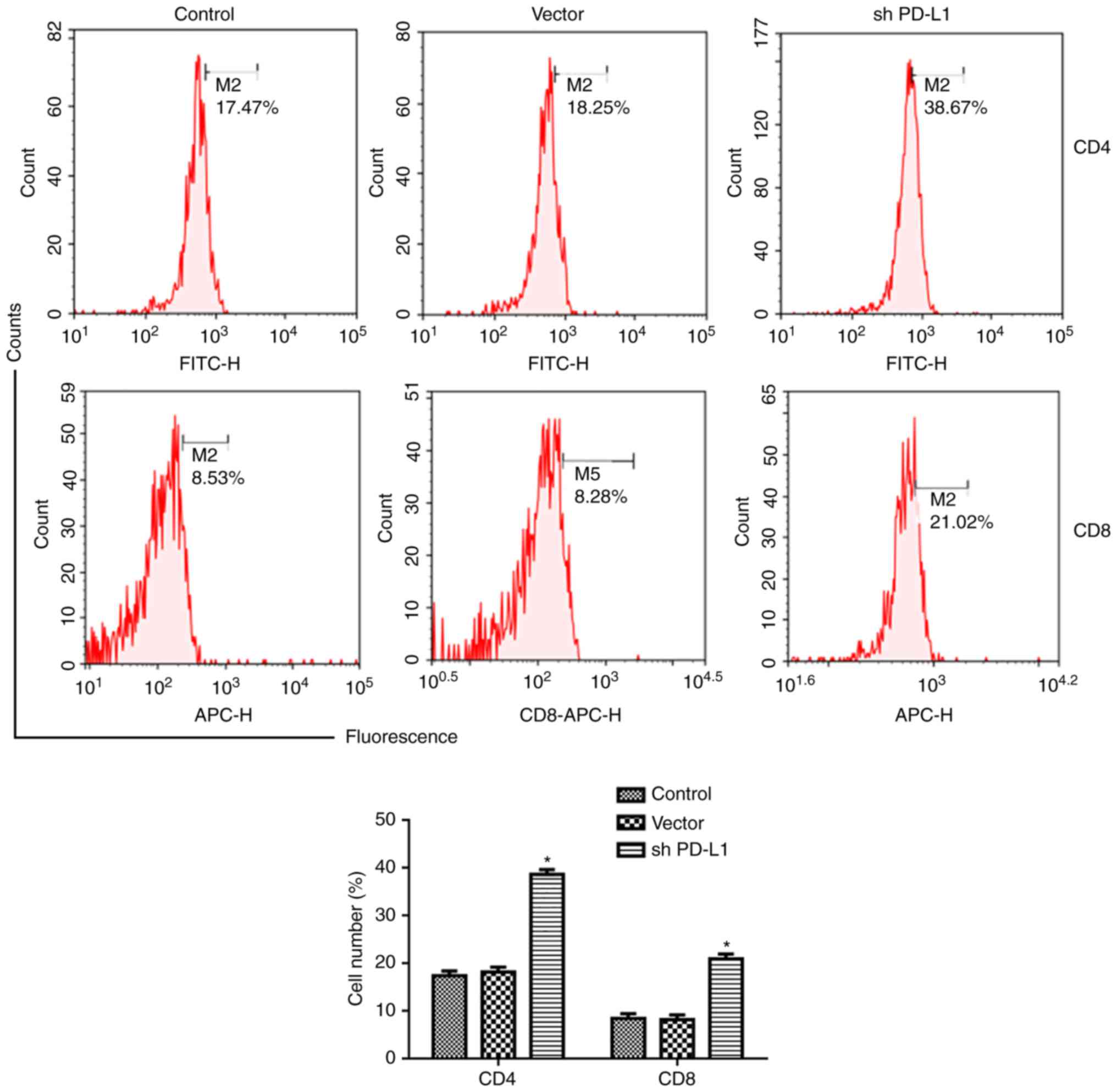
PD-L1 silencing promotes
CD8+ and CD4+ T cells in tumor tissue
Flow cytometry was used to detect the expression of
CD8+ and CD4+ T cells in tumor tissue.
Results showed that PD-L1 silencing increased the number of
CD4+ T cells and CD8+ T cells in the tumor,
compared with controls (P<0.05; Fig.
8).
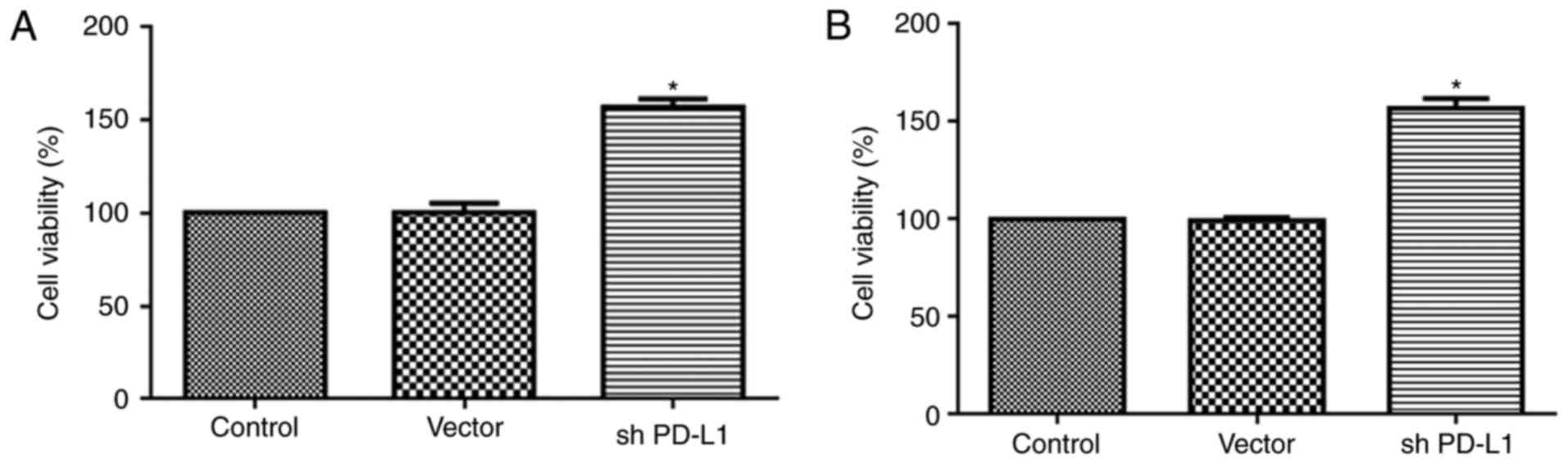
The CCK-8 assay also showed that cell viability of
CD8+ and CD4+ T cells was significantly
increased in the PD-L1 silenced group compared with controls
(P<0.05; Fig. 9).
PD-L1 silencing reduced PI3K and AKT
expression in tumor tissue
Expression of PD-L1, PI3K, and AKT in tumor tissues
of each group is shown in Fig. 10.
Compared with the control group, the expression of PD-L1, PI3K, and
AKT in the PD-L1 silenced group was significantly decreased
(P<0.05).
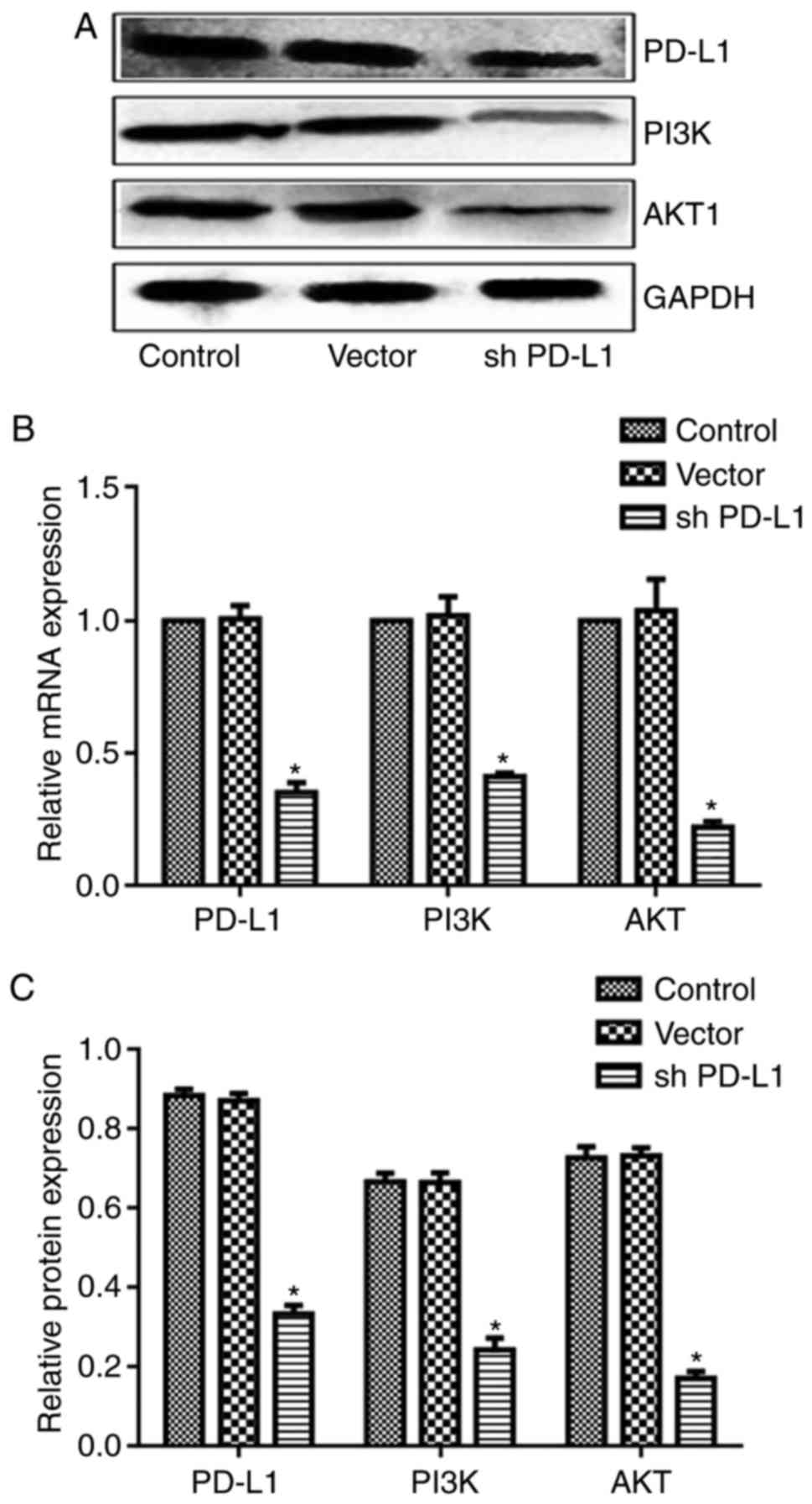 | Figure 10.PD-L1 silencing reduces PI3K and AKT
expression in tumor cells. (A) Representative blots of PD-L1, PI3K,
and AKT. (B) mRNA expression of PD-L1, PI3K, and AKT. (C) Protein
level of PD-L1, PI3K, and AKT showing that expression of PD-L1,
PI3K, and AKT in the PD-L1 silenced group decreased. *P<0.05 vs.
control. (One-way analysis of variance with Newman-Keuls). Sh,
short hairpin; PD-L1, programmed cell death ligand 1; AKT1, RAC-α
serine/threonine-protein kinase 1; PI3K, phosphatidylinositol
3-kinase. |
Discussion
Colorectal cancer seriously threatens human health,
although great progress has been made in its treatment. Metastasis
and recurrence of colorectal cancer are still the primary causes of
poor prognosis. As reported, colorectal cancer is an evolutionary
process involving multiple genes and multi-stages, particularly
oncogene activation and tumor suppressor gene inactivation
(17). PD-L1 is highly expressed in
many malignant tumors (18,19). Studies have shown that PD-L1 may be
regulated by oncogenes and various signaling pathways (20–23).
High expression of PD-L1 can reduce the immune effect of T cells in
the local microenvironment of the tumor, thus facilitating tumor
escape and promoting tumor growth (24,25).
LAG-3 is a super immunoglobulin IgG family member (26), which has a similar structure to CD4,
and can combine with major histocompatibility complex (MHC) class
II molecules. In the present study, it was observed that PD-L1 was
highly expressed in colorectal cancer tissue, while LAG-3 was
mainly expressed at the edge of tumor tissues. These results
suggested that LAG-3 is expressed on tumor-infiltrating lymph node
cells but not in colorectal cancer cells. Therefore, the
immunoregulation of PD-L1 in colorectal cancer was investigated by
blocking LAG-3 activity.
The PI3K/AKT signaling pathway is one of the most
important cell survival pathways, and is closely associated with
various malignant biological activities (27). Activation of PI3K can regulate the
growth of tumor cells and prevent apoptosis (28) by activating a variety of downstream
signals, including AKT, also known as PKB (protein kinase B), a
serine/threonine kinase (13). AKT
plays a key role in protein synthesis, cell metabolism, cell growth
and proliferation, and angiogenesis (29,30).
It has previously been demonstrated that PI3K/AKT signaling pathway
can affect cell apoptosis through a variety of mechanisms,
including promoting P53 nucleus translocation (28,31).
In addition, PI3K/AKT can also modulate B cell lymphoma-2
associated agonist of cell death (Bad) activity, cause
phosphorylation of caspase-9, block P53, and release apoptosis
factors into the mitochondria (32). These mechanisms work together to
block the process of programmed cell death, thus protecting the
cells from apoptosis, promoting cell survival, and leading to the
proliferation of tumor cells (33).
The present study demonstrated that expression of PI3K and AKT in
cancer tissues was significantly increased compared with
paracancerous tissues, indicating that PI3K and AKT were activated
in colorectal cancer tissues, and the expression levels of PI3K and
AKT could reflect the growth and proliferation of tumor cells.
In the present study, a lentivirus encoding PD-L1
shRNA was used to transfect the CT26 cell line. PD-L1 expression
significantly decreased following transfection in CT26 cells, which
indicated the efficacy of the silencing, ensuring their suitable
use in subsequent experiments. LAG-3 is expressed in a variety of
immune types, and is mainly expressed on activated T cells and
natural killer cells. LAG-3 has also been observed in activated B
cells, CD4+ and CD8+ T cells activated by
tumor tissue (34). LAG-3 plays an
important immunomodulatory role in the occurrence and development
of tumors, indicating that the growth of tumor cells is associated
with an abnormal immune state (34). In the present study, the LAG-3
antibody was used to block the effect of LAG-3 on tumor growth.
Thereafter, the effects of PD-L1 were compared in different groups.
It was revealed that the weight of tumors was significantly
decreased after blocking PD-L1, and tumor volume also decreased
significantly; furthermore, the apoptosis of tumor cells increased,
and the number and activity of CD4+ and CD8+
cells were also promoted after PD-L1 silencing. This may indicate
that inhibition of PD-L1 affects the immune system and produces a
large number of immune cells that destroy the tumor cells.
In order to demonstrate that the PI3K/AKT signaling
pathway plays a regulatory role in tumor apoptosis, animal
experiments were used to detect the effects of blocking PD-L1 on
the PI3K/AKT signaling pathway. It was demonstrated that PD-L1
silencing inhibited PI3K and AKT expression in tumor cells. These
results indicated that blocking PD-L1 enhanced the activity of T
cells that destroy tumor cells, in a PI3K/AKT signaling-mediated
manner, thereby inducing the apoptosis of tumor cells.
Nevertheless, the potential oncogenes and tumor suppressor genes,
such as P53, cyclin D1, and Bad should be investigated to confirm
these effects. In addition, apoptosis-associated gene expression
involved in both mitochondria-dependent and -independent signaling
pathways still requires clarification.
In conclusion, blocking PD-L1 can inhibit tumor
growth by activating CD4+ and CD8+ T cells,
involved in the immune response. These data reveal critical
immunomodulatory anticancer mechanisms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YC, YH, XL and GW did the experiments and analyzed
the data. YC and PC designed the study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Fujian Medical University Union Hospital and
written informed consent was obtained from all patients.
Patient consent for publication
Written informed consent was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao Y, Wang J, Zhou Y, Sheng S, Qian SY
and Huo X: Evaluation of serum CEA, CA19-9, CA72-4, CA125 and
ferritin as diagnostic markers and factors of clinical parameters
for colorectal cancer. Sci Rep. 8:27322018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang M, Miao F, Huang R, Liu W, Zhao Y,
Jiao T, Lu Y, Wu F, Wang X, Wang H, et al: RHBDD1 promotes
colorectal cancer metastasis through the Wnt signaling pathway and
its downstream target ZEB1. J Exp Clin Cancer Res. 37:222018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mao X, Ou MT, Karuppagounder SS, Kam TI,
Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al:
Pathological α-synuclein transmission initiated by binding
lymphocyte-activation gene 3. Science. 353:aah33742016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen N, Liu Y, Guo Y, Chen Y, Liu X and
Liu M: Lymphocyte activation gene 3 negatively regulates the
function of intrahepatic hepatitis C virus-specific CD8+
T cells. J Gastroenterol Hepatol. 30:1788–1795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hald SM, Rakaee M, Martinez I, Richardsen
E, Al-Saad S, Paulsen EE, Blix ES, Kilvaer T, Andersen S, Busund
LT, et al: LAG-3 in non-small-cell lung cancer: Expression in
primary tumors and metastatic lymph nodes is associated with
improved survival. Clin Lung Cancer. 19:249–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burugu S, Gao D, Leung S, Chia SK and
Nielsen TO: LAG-3+ tumor infiltrating lymphocytes in breast cancer:
Clinical correlates and association with PD-1/PD-L1+ tumors. Ann
Oncol. 28:2977–2984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Liechty B, Patel S, Weber JS,
Hollmann TJ, Snuderl M and Karajannis MA: Programmed death ligand 1
expression and tumor infiltrating lymphocytes in neurofibromatosis
type 1 and 2 associated tumors. J Neurooncol. 138:183–190. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carbognin L, Pilotto S, Milella M, Vaccaro
V, Brunelli M, Caliò A, Cuppone F, Sperduti I, Giannarelli D,
Chilosi M, et al: Differential activity of nivolumab, pembrolizumab
and MPDL3280A according to the tumor expression of programmed
death-ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma,
lung and genitourinary cancers. PLoS One. 10:e01301422015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armand P, Shipp MA, Ribrag V, Michot JM,
Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose
S, et al: Programmed death-1 blockade with pembrolizumab in
patients with classical hodgkin lymphoma after brentuximab vedotin
failure. J Clin Oncol. 34:3733–3739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert C, Ribas A, Wolchok JD, Hodi FS,
Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et
al: Anti-programmed-death-receptor-1 treatment with pembrolizumab
in ipilimumab-refractory advanced melanoma: A randomised
dose-comparison cohort of a phase 1 trial. Lancet. 384:1109–1117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lambert E, Fuselier E, Ramont L, Brassart
B, Dukic S, Oudart JB, Dupont-Deshorgue A, Sellier C, Machado C,
Dauchez M, et al: Conformation-dependent binding of a Tetrastatin
peptide to αvβ3 integrin decreases melanoma
progression through FAK/PI3K/Akt pathway inhibition. Sci
Rep. 8:98372018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian J and Yuan L: Sirtuin 6 inhibits
colon cancer progression by modulating PTEN/AKT signaling. Biomed
Pharmacother. 106:109–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Chen H, Wu S, Cheng Y, Li Q, Wang J
and Zhu G: MPP+ inhibits mGluR1/5-mediated long-term
depression in mouse hippocampus by calpain activation. Eur J
Pharmacol. 795:22–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Very N, Lefebvre T and El Yazidi-Belkoura
I: Drug resistance related to aberrant glycosylation in colorectal
cancer. Oncotarget. 9:1380–1402. 2017.PubMed/NCBI
|
|
18
|
Santini FC and Hellmann MD: PD-1/PD-L1
axis in lung cancer. Cancer J. 24:15–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mathew M, Enzler T, Shu CA and Rizvi NA:
Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther.
186:130–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Chen S, Yuan W, Wang H, Chen K and
Li D and Li D: PD-1/PD-L1 interaction up-regulates MDR1/P-gp
expression in breast cancer cells via PI3K/AKT and MAPK/ERK
pathways. Oncotarget. 8:99901–99912. 2017.PubMed/NCBI
|
|
21
|
Almozyan S, Colak D, Mansour F, Alaiya A,
Al-Harazi O, Qattan A, Al-Mohanna F, Al-Alwan M and Ghebeh H: PD-L1
promotes OCT4 and Nanog expression in breast cancer stem cells by
sustaining PI3K/AKT pathway activation. Int J Cancer.
141:1402–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopez-Rivera E, Jayaraman P, Parikh F,
Davies MA, Ekmekcioglu S, Izadmehr S, Milton DR, Chipuk JE, Grimm
EA, Estrada Y, et al: Inducible nitric oxide synthase drives mTOR
pathway activation and proliferation of human melanoma by
reversible nitrosylation of TSC2. Cancer Res. 74:1067–1078. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Zeng Y, Qu Q, Zhu J, Liu Z, Ning
W, Zeng H, Zhang N, Du W, Chen C, et al: PD-L1 induced by IFN-γ
from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT
signaling pathways promoted progression of lung cancer. Int J Clin
Oncol. 22:1026–1033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blank C, Brown I, Peterson AC, Spiotto M,
Iwai Y, Honjo T and Gajewski TF: PD-L1/B7H-1 inhibits the effector
phase of tumor rejection by T cell receptor (TCR) transgenic
CD8+ T cells. Cancer Res. 64:1140–1145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Francisco LM, Salinas VH, Brown KE,
Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates
the development, maintenance, and function of induced regulatory T
cells. J Exp Med. 206:3015–3029. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen LT and Ohashi PS: Clinical blockade
of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol.
15:45–56. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu G, Wang X, Wu S and Li Q: Involvement
of activation of PI3K/Akt pathway in the protective effects of
puerarin against MPP+-induced human neuroblastoma
SH-SY5Y cell death. Neurochem Int. 60:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maugeri G, D'Amico AG, Rasà DM, Saccone S,
Federico C, Magro G, Cavallaro S and D'Agata V: Caffeine inhibits
angiogenesis in human glioblastoma cells via HIFs modulation.
Anticancer Agents Med Chem. Feb 9–2018.(Epub ahead of print). doi:
10.2174/1871520618666180209151750. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Yang S and Zhu G: Postnatal calpain
inhibition elicits cerebellar cell death and motor dysfunction.
Oncotarget. 8:87997–88007. 2017.PubMed/NCBI
|
|
31
|
Suh DS, Park SE, Jin H, Lee K and Bae J:
LRIG2 is a growth suppressor of Hec-1A and Ishikawa endometrial
adenocarcinoma cells by regulating PI3K/AKT- and EGFR-mediated
apoptosis and cell-cycle. Oncogenesis. 7:32018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Xiong YQ, Xu J, Wang JP, Meng ZL
and Hong YQ: Juglanin inhibits lung cancer by regulation of
apoptosis, ROS and autophagy induction. Oncotarget. 8:93878–93898.
2017.PubMed/NCBI
|
|
33
|
Okamura T, Fujio K, Shibuya M, Sumitomo S,
Shoda H, Sakaguchi S and Yamamoto K:
CD4+CD25−LAG3+ regulatory T cells
controlled by the transcription factor Egr-2. Proc Natl Acad Sci
USA. 106:13974–13979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okamura T, Sumitomo S, Morita K, Iwasaki
Y, Inoue M, Nakachi S, Komai T, Shoda H, Miyazaki J, Fujio K and
Yamamoto K: TGF-β3-expressing
CD4+CD25−LAG3+ regulatory T cells
control humoral immune responses. Nat Commun. 6:63292015.
View Article : Google Scholar : PubMed/NCBI
|