Introduction
Liver cancer, a fatal disease, is one of the most
common cancers and the leading cause of cancer-related mortality
worldwide (1). According to the
latest statistics, in the United States in 2017, it is estimated
that approximately 40,710 new cases of liver cancer will be
diagnosed and approximately 28,920 cases, equivalent to 71% of
these diagnosed cases, will eventually suucumb to this disease, and
the 5-year relative survival rate for liver cancer is currently
only 18% (2). Hepatocellular
carcinoma (HCC), the most common primary liver malignancy
(accounting for 70–90%), has an unsatisfactory clinical efficacy
due to the high incidence of recurrence and metastasis even after
curative resection (1,3,4). In
addition, sorafenib, a multi-kinase inhibitor, is a drug that is
available for the treatment of patients with advanced HCC, however
it only modestly improves survival, while a range of side-effects
commonly exist and most patients will eventually become
drug-resistant (5,6). Therefore, its clinical application is
restricted. The main reason for the unsatisfactory treatment for
HCC is that the underlying molecular mechanism of HCC oncogenesis
remains unclear. Hence, it is urgent to elucidate the mechanism
involved in HCC progression and to identify novel therapeutic
targets to improve the clinical outcome.
The interferon-induced transmembrane protein 3
(IFITM3, also called 1–8 U) gene (along with IFITM1 and IFITM2)
belongs to the IFITM family of genes, which are clustered on
chromosome 11 and have been identified as antiviral factors that
interfere with viral entry following endocytosis (7–9). In
recent years, an increasing number of studies have found
dysregulated expression of IFITM3 in various tumors (including
colon cancer, colorectal tumor and glioma), which is involved in
cell migration and invasion, tumorigenesis and progression
(10–12). However, the clinical significance
and underlying mechanisms of aberrant IFITM3 expression in HCC have
not been investigated and remain to be established.
MicroRNAs (miRNAs), a family of endogenous
(approximately 22 nucleotides) small noncoding RNAs, are able to
post-transcriptionally regulate gene expression by repressing
protein translation or silencing the expression of target genes by
directly binding to the 3′-untranslated region (3′-UTR) of
messenger RNAs (mRNAs) (13–15).
miRNAs play important roles in various biological and pathological
processes of HCC, including migration, invasion, carcinogenesis,
tumor growth and metastasis (16–19).
Several studies have reported aberrant expression of miRNA-29a
(miR-29a) in HCC. miR-29a is involved in multiple processes of
tumor progression, including cell proliferation, growth, migration,
and epithelial-mesenchymal transition (EMT) (20–22).
Furthermore, low expression of miR-29a is a poor prognostic marker
in HCC patients (23).
However, the expression and biological function of
IFITM3 in HCC and the association between IFITM3 and miR-29a remain
unclear. Previously, we performed a bioinformatics analysis and
found that a potential binding site for miR-29a exists in the 3′UTR
of IFITM3 mRNA. Thus, we presumed that miR-29a could affect HCC
progression by regulating IFITM3. In the present study, we aimed to
investigate the role of IFITM3 and miR-29a in HCC and to identify
whether IFITM3 is a new direct target gene of miR-29a. In the
addition, the association between IFITM3, miR-29a expression and
the clinicopathological characteristics of HCC patients was
investigated.
Materials and methods
Patients and tissue specimens
HCC and paracancerous tissue specimens (confirmed by
histological diagnosis) were obtained from 52 patients (37 males
and 15 females) who underwent hepatectomy at the Second Affiliated
Hospital of Nanchang University (Nanchang, China) from January 2012
to December 2016. Their age ranged from 31 to 74 years, and the
average age was 49 years. The sex, age, tumor size, TNM stage,
alpha-fetoprotein (AFP) levels and other clinicopathological
characteristics of patients were obtained from surgical and
pathological records. Prior to liver resection, no treatments,
including radiotherapy and chemotherapy, had been carried out in
these patients. After the tissue specimens were resected during
surgery, HCC and paracancerous tissue specimens were immediately
collected and placed in liquid nitrogen and then stored. Informed
consent was obtained from all patients for using their tissue
specimens and clinicopathological data. The study protocol was
approved by the Medical Ethics Committee of the Second Affiliated
Hospital of Nanchang University. This study was performed in
accordance with the ethical standards of the Declaration of
Helsinki.
Cell lines and cell culture
The human normal liver cell line (HL-7702) and 3 HCC
cell lines (Hep3B, HCCLM3 and Huh-7) were selected to conduct the
assays, and they were purchased from the Shanghai Institute of Cell
Biology (Shanghai, China). These cell lines were cultured in
high-glucose Dulbeccos modified Eagles medium (DMEM; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
supplemented with 10% fetal bovine serum (FBS; Biological
Industries, Kibbutz Beit-Haemek, Israel), 100 µg/ml streptomycin
and 100 U/ml penicillin at 37°C, in a 5% CO2 and 95%
humidity cell culture incubator.
Cell transfection
IFITM3 siRNA, pcDNA3.1(+)-IFITM3 plasmids and
negative control (NC) were purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). miR-29a mimics and NC miRNA were purchased
from Guangzhou RiboBio Biotechnology, Co., Ltd. (Guangzhou, China).
The HCCLM3 cell lines were eventually selected as experimental
subjects and assigned to the NC and treatment groups. Transfections
were performed using the Lipofectamine 3000 kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturers instructions. Two different IFITM3 siRNA sequences
were transfected into the cells, and the sequences were as follows:
IFITM3-s1 sense, 5′-CCAUUCUGCUCAUCGUCAUTT-3′ and antisense,
5′-AUGACGAUGAGCAGAAUGGTT-3′; IFITM3-s2 sense,
5′-GCUGAUCUUCCAGGCCUAUTT-3′ and antisense,
5′-AUAGGCCUGGAAGAUCAGCTT-3′. The sequences of pcDNA3.1(+)-IFITM3
plasmids were as follows: pcDNA3.1(+)-IFITM3 sense,
5′-AAGCTGGCTAGCGTTGCGGCCGCGCCACCATGAATCACACTGTCCAAACCTT-3′;
pcDNA3.1(+)-IFITM3 antisence,
5′-AGAGTCGGTACCGTCGGATCCCTATCCATAGGCCTGGAAGATCAG-3′.
Hematoxylin and eosin (H&E) and
immunohistochemistry (IHC)
After the HCC and adjacent tissues were fixed in 10%
formalin solution, we embedded them in paraffin blocks and then cut
the tissue into paraffin sections. After deparaffnization and
hydration, some sections were stained with H&E, which was used
to detect morphological changes, and some sections were used for
immunostaining. Then, these sections were incubated with 0.3%
hydrogen peroxide for 15 min to eliminate endogenous peroxidase,
and microwave-heated at 100°C for 10 min in sodium citrate buffer
(10 mmol/l, pH 6.0) for antigen retrieval and blocked with goat
serum. Subsequently, the tissue sections were incubated with IFITM3
rabbit monoclonal antibody at a 1:200 dilution (cat. no. ab109429;
Abcam, Cambridge, MA, USA) at 4°C overnight. Subsequently, the
sections were washed with phosphate-buffered saline (PBS) 3 times
at 5-min intervals. The next step was to add the secondary antibody
biotinylated goat anti-rabbit serum IgG at a 1:2,000 dilution (cat.
no. HS101-01; TransGen Biotech, Beijing, China) to the sections and
incubate them for 30 min at 37°C. Then, the sections were stained
with diaminobenzidine (DAB) and hematoxylin dyes and were sealed
with neutral resins. Two pathologists blindly and randomly
evaluated and semi-quantitatively scored the staining intensity and
percentage of positive cells. The methods that we used to grade the
staining intensity and score the percentage of staining have
already been reported by Liu et al (24). For each immunostained section, the
overall staining index was computed by multiplying the grades and
scores to reach a value from 0 to 9, which was finally designated
as follows: 0–1, IFITM3 nonoverexpression; 2–9, IFITM3
overexpression.
Protein extraction and western blot
analysis
Western blot analysis was conducted to detect total
protein expression in tissues and treated cells after 48 h of
transfection. Total protein was extracted from tissues or cells,
which were lysed in radioimmunoprecipitation assay (RIPA) buffer
(cat. no. R0020; Solarbio Science & Technology Co., Ltd.) with
1% phenylmethanesulfonyl fluoride (PMSF), and then, the protein was
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). Protein determination was based on
bicinchoninic acid (BCA) method. A total of 12 ml protein samples
were loaded per lane. Protein samples were electrophoresed on a 15%
sodium dodecyl sulfate-polyacrylamide gel and transferred onto
polyvinylidene fluoride (PVDF) membranes. Afterwards, the membranes
were blocked with 5% Difco skim milk at room temperature for 2 h.
The membranes were then incubated with anti-IFITM3 antibody
(1:5,000 dilution) (cat. no. ab109429; Abcam) at 4°C overnight.
Tris-HCl buffer solution + Tween-20 (TBST) was used to wash the
membranes 3 times for 10 min. Subsequently, they were incubated
with horseradish peroxidase-conjugated secondary antibody at a
1:10,000 dilution (cat. no. HS101-01; TransGen Biotech) for 1 h at
room temperature. Finally, the blots were detected by enhanced
chemiluminescence (ECL) kit (cat. no. cw0049s; CWBIO, Beijing,
China), and the intensity was measured by Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reference
protein in the study is GAPDH mouse monoclonal antibody with
1:6,000 dilution (cat. no. 60004-1-Ig; Proteintech Group Inc.,
Rosemont, IL, USA).
Isolation of mRNA and quantitative
reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA of tissues and cells was isolated with an
E.Z.N.A.™ Total RNA Kit II (Omega Bio-Tek, Inc., Norcross, GA,
USA). Reverse transcription was performed with a PrimeScript RT
reagent kit with gDNA Eraser (cat. no. RR047A; Takara
Biotechnology, Co., Ltd., Dalian, China) following the protocols of
the manufacturer. qRT-PCR was performed with SYBR Premix Ex Taq™ II
(cat. no. RR820A; Takara Biotechnology, Co., Ltd.) according to the
manufacturers instructions. The levels of IFITM3 and miR-29a were
calculated with the 2−ΔΔCq method (25) and were normalized to those of
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6,
respectively. We randomly chose five non-tumor tissues of HCC
patients and selected their average ΔCq of miR-29a as an internal
control to calculate the ΔΔCq of each HCC tissue. In addition, we
selected the mean level of miR-29a relative expression as a cut-off
value according to the study of Li et al (26). The HCC patients with miR-29a
expression less than the mean value were classified as the
non-overexpression group, while the patients higher than the median
value were considered as the overexpression group. The RNA relative
expression levels of the experimental group were reported as the
fold change compared to the corresponding NC group, which was
defined as 1.0. All assays were performed in triplicate. The
specific stem-loop primer for miR-29a and U6 primer were designed
by Guangzhou RiboBio Biotechnology, Co., Ltd. (Guangzhou, China).
The primer sequences of GAPDH and IFITM3 were purchased from
GenScript Biotechnology (Nanjing, China) and were as follows: GAPDH
forward, 5′-CAGGGCTGCTTTTAACTCTGGT-3′ and reverse,
5′-GATTTTGGAGGGATCTCGCT-3′; IFITM3 forward,
5′-ACTGTCCAAACCTTCTTCTCTCC-3′ and reverse,
5′-TCGCCAACCATCTTCCTGTC-3′.
Cell wound healing assays
A wound healing assay was performed to evaluate the
cell migration capacity. Cells were seeded into 6-well plates and
incubated in DMEM with 10% FBS at 37°C. After the cells reached
90–100% confluence, artificial wounds were generated by scraping a
200 µl pipette tip across the cell surface. Subsequently, the cells
were gently washed with PBS 3 times to remove the detached cells,
and then, they were incubated in fresh complete medium for 48 h.
After 48 h, we observed and measured the cell migration distance
from the edge of the scratch towards the center.
Cell migration and invasion
assays
To detect cell invasion and migration, 8-µm pore
size Corning Transwell chambers (Corning Inc., Corning, NY, USA)
with and without Matrigel-coating were used, respectively. At 48 h
after transfection, 4×104 cells in serum-free medium
were placed into the upper chambers for the migration assay, and
8×104 cells were seeded for invasion assays, while
medium with 10% FBS was added to the lower chamber. After 24 h of
incubation, the cells remaining in the upper surface of the
membrane were removed, and the cells that had migrated/invaded to
the lower surface of the membrane were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet. Cells were
counted in five random fields (magnification, ×200) taken by a
light microscope (Olympus Corp., Tokyo, Japan).
Cell proliferation assays
The cell proliferation ability was assessed using
the 5-ethynyl-2-deoxyuridine (EdU) proliferation assay. At 24 h
after transfection, treated cells were resuspended and seeded in
96-well plates (1×104 cells/well) and were continually
cultured for 24 h. Subsequently, after being washed 3 times with
PBS, the cells were incubated for 4 h in serum-free DMEM
supplemented with 50 µM EdU (Guangzhou RiboBio Biotechnology, Co.,
Ltd., Guangzhou, China). Then, the cells were fixed with 4%
polyformaldehyde in PBS at room temperature for 30 min. Finally,
the cells were subsequently incubated with Apollo staining solution
and Hoechst 33342 for 30 min. The percentage of EdU-positive cells
relative to the total number of cells was intended to represent the
proliferation index. A fluorescence microscope (Olympus Corp.) was
used to obtain images, and five random fields (magnification, ×200)
were selected to evaluate the proliferation rate.
Cell apoptosis assays
Cell apoptosis was assessed by fluorescence
activated cell sorting (FACS) analysis using the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit (BD Biosciences, Franklin Lakes, NJ, USA). In brief,
cells were firstly digested with trypsin and then collected and
rinsed with pre-cooled PBS at 4°C; then, they were resuspended in
100 µl of 1X binding buffer and placed into a plastic 12×75 mm test
tube, followed by the addition of 5 µl of Annexin V-FITC and 5 µl
of PI solution in the dark at room temperature. After cells were
gently vortexed and incubated for 15 min, 400 µl of 1X binding
buffer was added to the cell suspension. Finally, the cell
apoptosis rate was determined by a flow cytometry (BD
Biosciences).
Dual luciferase reporter gene
assay
The Dual-Luciferase reporter gene assay was
performed to ascertain whether IFITM3 was the direct target gene of
miR-29a. The IFITM3 3UTR dual luciferase reporter plasmids (WT and
MUT) were constructed by Guangzhou RiboBio Biotechnology, Co., Ltd.
They were co-transfected using Lipofectamine 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) with miR-29a mimics or miRNA NC
into 293T cells, respectively (Shanghai Beinuo Biotech, Co., Ltd.,
Shanghai, China). After incubation for 48 h, the luciferase
activities were detected using a Dual-Luciferase Assay System
(Promega Corp., Madison, WI, USA).
Statistical analysis
GraphPad Prism 7.0 and SPSS 22.0 software were used
for statistical analysis. Wilcoxons paired test was applied to
compare the IFITM3 expression in HCC tissues and paracancerous
tissues. The statistically significant positive correlation between
IFITM3 and miR-29a expression levels was analyzed by Spearmans
correlation analysis. Chi-square tests were used to examine the
possible associations between the expression of IFITM3 or miR-29a
and the clinicopathological characteristics of patients. The
log-rank (Mantel-Cox) test was used to assess the differences in
the overall survival rate. Comparisons between the two groups were
analyzed with t-test or Students t-test. Multiple group comparisons
were performed with the one-way analysis of variance (ANOVA), and
the post hoc test was Student-Newman-Keuls (SNK) test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
IFITM3 is upregulated and miR-29a
downregulated in HCC tissues and both are associated with HCC
progression as well as poor prognosis
To investigate the expression levels and
significance of IFITM3 in HCC tissues, a total of 52 HCC tissues
and corresponding adjacent non-tumor tissues were examined using
IHC and western blotting. The IHC results revealed that the IFITM3
protein was expressed in the cytoplasm as well as highly expressed
in 69.23% (36 of 52) of HCC tissues and only 26.92% (14 of 52) of
adjacent non-tumor tissues (Fig.
1A), which indicated that IFITM3 protein expression was
significantly upregulated in HCC tissues compared with adjacent
non-tumor tissues (P<0.01, Fig.
1B). In addition, the western blotting results revealed that
the IFITM3 protein levels were significantly elevated in 73.08% (38
of 52) of HCC tissues, whereas the IFITM3 protein levels were
elevated in only 21.15% (11 of 52) of adjacent non-tumor tissues
(Fig. 1C), which was consistent
with the IHC results, indicating that IFITM3 protein expression was
significantly upregulated in HCC tissues (P<0.01; Fig. 1D).
To explore the expression and correlation of IFITM3
and miR-29a in HCC tissues, qRT-PCR was performed for 52 HCC
tissues and adjacent non-tumor tissues. The qRT-PCR results
revealed that the average fold change of IFITM3 mRNA expression in
tumor tissues was significantly upregulated relative to that in
paired non-tumor tissues (P<0.01; Fig. 1E). However, miR-29a was
significantly decreased in tumor tissues compared with adjacent
non-tumor tissues (P<0.01; Fig.
1F). Furthermore, the expression levels of IFITM3 in HCC
tissues were negatively correlated with the miR-29a levels
(two-tailed Spearmans correlation, r=−0.502, P<0.001; Fig. 1G).
The highest and lowest relative expression of
miR-29a in 52 HCC tissues was 3.77 and 0.399, respectively. The
mean level of miR-29a relative expression (mean value=1.521) was
selected as a cut-off value. A total of 34 HCC tissues with miR-29a
expression less than the mean value were considered to have
non-overexpression of miR-29a, while the remaining 18 HCC tissues
higher than the median value were considered overexpression.
Moreover, the overall survival rate of HCC patients was both
significantly decreased in IFITM3 overexpression patients [log-rank
(Mantel-Cox) test, P=0.041; Fig.
1H] and miR-29a non-overexpression patients [log-rank
(Mantel-Cox) test, P=0.037; Fig.
1I], indicating that IFITM3 overexpression and miR-29a
non-overexpression were related to the poor prognosis of HCC
patients. In addition, the highest and lowest survival time for HCC
patients with IFITM3 overexpression or miR-29 non-overexpression
were 8 and 60 months, respectively. The median survival time for
HCC patients with IFITM3 overexpression was 37 months, and for the
miR-29 non-overexpression it was 31 months.
Finally, we analyzed the associations between IFITM3
along with miR-29a expression in HCC and clinicopathological
characteristics. The results revealed that IFITM3 overexpression
and miR-29a non-overexpression were both closely associated with
tumor size, tumor multifocal, and venous invasion (P<0.05 for
all; Table I). These data indicated
that IFITM3 overexpression and miR-29a non-overexpression were
associated with HCC aggressive behavior and metastasis.
 | Table I.Associations between miR-29a or
IFITM3 and the clinicopathological characteristics in 52 HCC
cases. |
Table I.
Associations between miR-29a or
IFITM3 and the clinicopathological characteristics in 52 HCC
cases.
|
|
| miR-29a |
| IFITM3 |
|
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathologic
characteristics | (n) | Overexpression
(n) | Nonoverexpression
(n) | P-value | Overexpression
(n) | Nonoverexpression
(n) | P-value |
|---|
| Age (years) |
|
|
| 0.686 |
|
| 0.330 |
|
≤51 | 24 | 9 | 15 |
| 15 | 9 |
|
|
>51 | 28 | 9 | 19 |
| 21 | 7 |
|
| Sex |
|
|
| 0.245 |
|
| 0.683 |
|
Male | 37 | 11 | 26 |
| 25 | 12 |
|
|
Female | 15 | 7 | 8 |
| 11 | 4 |
|
| Tumor size
(cm) |
|
|
| 0.005 |
|
| 0.005 |
| ≤5 | 21 | 12 | 9 |
| 10 | 11 |
|
|
>5 | 31 | 6 | 25 |
| 26 | 5 |
|
| TNM stage |
|
|
| 0.703 |
|
| 0.309 |
|
I–II | 27 | 10 | 17 |
| 17 | 10 |
|
|
III–IV | 25 | 8 | 17 |
| 19 | 6 |
|
| Tumor
encapsulation |
|
|
| 0.115 |
|
| 0.115 |
|
Absent | 28 | 7 | 21 |
| 22 | 6 |
|
|
Present | 24 | 11 | 13 |
| 14 | 10 |
|
| Tumor
multifocal |
|
|
| 0.001 |
|
| 0.049 |
|
Absent | 22 | 13 | 9 |
| 12 | 10 |
|
|
Present | 30 | 5 | 25 |
| 24 | 6 |
|
| Venous
invasion |
|
|
| <0.001 |
|
| <0.001 |
|
Absent | 21 | 13 | 8 |
| 9 | 12 |
|
|
Present | 31 | 5 | 26 |
| 27 | 4 |
|
| HBsAg |
|
|
|
|
|
|
|
|
Negative | 13 | 6 | 7 | 0.313 | 7 | 6 | 0.165 |
|
Positive | 39 | 12 | 27 |
| 29 | 10 |
|
| AFP (ng/ml) |
|
|
| 0.542 |
|
| 0.209 |
|
≤400 | 29 | 9 | 20 |
| 18 | 11 |
|
|
>400 | 23 | 9 | 14 |
| 18 | 5 |
|
| Cirrhosis |
|
|
| 0.278 |
|
| 0.734 |
|
Absent | 18 | 8 | 10 |
| 13 | 5 |
|
|
Present | 34 | 10 | 24 |
| 23 | 11 |
|
Knockdown of IFITM3 inhibits
migration, invasion, proliferation and promotes apoptosis of HCC
cells
To investigate the effects of IFITM3 on metastasis,
proliferation and apoptosis of HCC cells, we firstly examined the
protein expression levels of IFITM3 in normal hepatocyte cells
(HL-7702) and 3 HCC cell lines (Hep3B, HCCLM3 and Huh-7) by western
blot analysis. The results revealed that expression of the IFITM3
protein in HCC cells was higher than that in the normal hepatocyte
cell line. The HCCLM3 cells with the highest expression level of
IFITM3 were finally selected for subsequent functional assays
(P<0.01; Fig. 2A). Furthermore,
two types of IFITM3 siRNA were used to transfect the HCCLM3 cell
lines to detect the more effective sequence. At 48 h after
transfection, IFITM3 protein expression was examined using western
blotting, and the results revealed that IFITM3-s1 siRNA was more
effective than IFITM3-s2 (P<0.01 for all; Fig. 2B). Thus, IFITM3-s1 siRNA was
selected for subsequent RNA interference assays.
 | Figure 2.Knockdown of IFITM3 inhibits
invasion, migration, proliferation and promotes apoptosis of HCC
cells. (A) Western blot analysis of IFITM3 expression in human
normal hepatocyte cells (HL-7702) and 3 HCC cell lines (Hep3B,
HCCLM3 and Huh-7) which indicated that IFITM3 protein expression in
HCC cells was significantly higher than that in normal liver cells.
**P<0.01 compared with the HL-7702 cells. (B) Two different
IFITM3 siRNA sequences were transiently transfected into HCCLM3
cells to knockdown expression of IFITM3. After 48 h of
transfection, western blot analysis revealed that the expression of
IFITM3 was markedly downregulated with the IFITM3-siRNA groups. (C)
Wound healing assay results revealed that wound closure was delayed
with IFITM3 knockdown in HCCLM3 cells compared with NC groups at
48-h time-points after transfection. (D) Migration and invasion
assays indicated that the migration and invasion abilities of
IFITM3-s1 transfection HCCLM3 cells were decreased compared with
the NC groups. (E) EdU proliferation assays revealed that the
proliferation abilities were significantly reduced in
IFITM3-s1-transfected HCCLM3 cells than in the NC groups. Blue,
Hoechst 33342 staining of nuclei with all cells. Red, Apollo
staining of EdU with proliferating cells. Overlay, the percentage
of proliferating cells. (F) The apoptosis rate of HCCLM3 cells
transfected with IFITM3-s1 was significantly higher than in the NC
groups. **P<0.01 compared with the NC groups. NC, negative
control; HCC, hepatocellular carcinoma; IFITM3, interferon-induced
transmembrane protein 3. |
Wound healing assays were performed to assess the
variation of the cell migration ability for IFITM3 knockdown, and
the results revealed that cell migration ability was significantly
decreased in the IFITM3-s1 group (P<0.01; Fig. 2C). In addition, the results of the
Transwell chamber assay without Matrigel for HCCLM3 cells were also
consistent with the wound healing assay results, and using a
Matrigel-coated Transwell chamber, we found that HCC cells with
IFITM3 knockdown invaded through the matrix slower than the NC
group cells (P<0.01 for all; Fig.
2D).
To explore the effects of IFITM3 knockdown on cell
viability, EdU proliferation assays were conducted. The results
revealed that growth of HCCLM3 cells was significantly decreased in
the IFITM3-s1 group (P<0.01; Fig.
2E). To confirm whether IFITM3 was involved in the apoptosis of
HCC cells, we used flow cytometry to examine the cell apoptosis
rate of the treated HCC cells. The results indicated that the
apoptosis rate of the IFITM3-s1 group was higher than that of the
NC group (P<0.01; Fig. 2F).
Upregulated IFITM3 promotes migration,
invasion and proliferation as well as inhibits apoptosis of HL-7702
cells
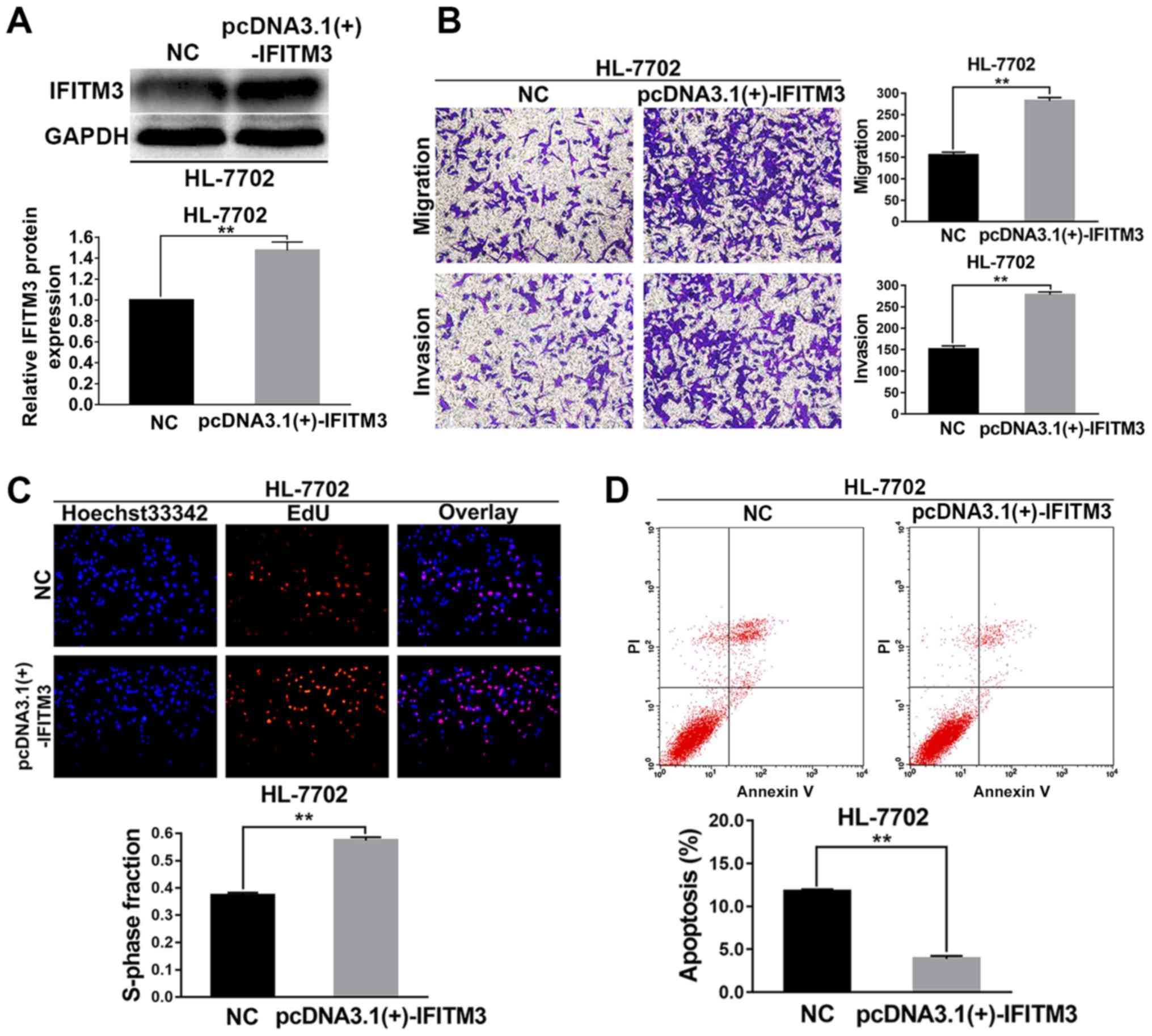
The HL-7702 cells were selected for the IFITM3
upregulated assays. pcDNA3.1(+)-IFITM3 plasmids were transiently
transfected into HL-7702 cells to enhance the expression of IFITM3.
Firstly, western blot analysis revealed that the IFITM3 protein was
increased after transfection with the pcDNA3.1(+)-IFITM3 plasmid,
which indicated that the plasmid was effective (P<0.01; Fig. 3A). After IFITM3 was upregulated with
pcDNA3.1(+)-IFITM3 plasmids, the results of the Transwell chamber
assay revealed that the migration and invasion abilities of the
HL-7702 cells were significantly increased (P<0.01; Fig. 3B). The results of the EdU
proliferation assays revealed that growth ability of the HL-7702
cells was significantly increased after upregulation of IFITM3
(P<0.01; Fig. 3C). The results
of flow cytometry indicated that the apoptosis rate of HL-7702
cells in the pcDNA3.1(+)-IFITM3 group was lower than the NC group
(P<0.01; Fig. 3D).
Upregulated miR-29a suppresses
migration, invasion and proliferation as well as promotes apoptosis
of HCC cells
As shown in Fig. 4,
the effects of upregulated miR-29a in HCC cells on the biological
behaviors of those cells were consistent with the effects of IFITM3
knockdown. To investigate the function and mechanism of miR-29a in
HCC, the expression level of miR-29a was first examined in 3 HCC
cell lines (Hep3B, HCCLM3 and Huh-7) and normal hepatocyte cells
(HL-7702) using qRT-PCR. As shown in Fig. 4A, the level of miR-29a in these HCC
cell lines was decreased compared with the HL-7702 cells; and the
HCCLM3 cell lines had the lowest expression levels of miR-29a
(P<0.001; Fig. 4A). Hence,
HCCLM3 cells were selected for subsequent assays and were
transfected with miR-29a mimics. At 36 h post-transfection, a
satisfactory transfection efficiency was observed by qRT-PCR, and
using western blot analysis, we found that the IFITM3 protein
expression was reduced after 48 h of transfection (P<0.01;
Fig. 4B).
Wound healing assays were performed to detect the
variation of migration capacity of HCCLM3 cells after they were
transfected with miR-29a mimics. The results revealed that the
migration capacity of HCCLM3 cell lines was decreased after miR-29a
was upregulated (P<0.05; Fig.
4C). Transwell chamber migration and invasion assays
demonstrated that the invasion and metastatic abilities of HCCLM3
cells were reduced after miR-29a was upregulated (P<0.01 for
all; Fig. 4D).
To investigate the effects of miR-29a upregulation
on the cell proliferation capacity, EdU proliferation assays were
carried out. The results indicated that the growth of HCCLM3 cells
was significantly decreased in the miR-29a mimics group (P<0.01;
Fig. 4E). Flow cytometry was
performed to examine the cell apoptosis rate change of the miR-29a
mimic-treated HCCLM3 cells. The results revealed that the apoptosis
rate of the miR-29a mimic group was higher than that of the NC
groups (P<0.01; Fig. 4F).
IFITM3 is the target gene of
miR-29a
We focused on the post-transcriptional regulation of
IFITM3 and investigated the effects of miRNAs on IFITM3. Next, we
employed bioinformatics analysis to predict potential miRNAs that
targeted the 3′UTR of IFITM3 mRNA by using 2 websites, including
TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do). Then, a
total of 14 candidate miRNAs (miR-15a, miR-15b, miR-16, miR-29a,
miR-125a, miR-195, miR-221, miR-424, miR-486, miR-497, miR-552,
miR-1185, miR-1207 and miR-1294) with good scores were selected for
further screening and their corresponding mimics were respectively
transfected into HCCLM3 cells. Finally, western blot analysis
revealed that miR-29a could markedly suppress the protein
expression of IFITM3 (P<0.01; Fig.
5A and B), which indicated that miR-29a was involved in the
regulation of IFITM3. As shown in Fig.
5C, the 3′-UTR of IFITM3 contained a binding site for miR-29a.
Subsequently, we performed a dual luciferase reporter gene assay to
confirm the regulation relationship between miR-29a and IFITM3. The
results revealed that the miR-29a mimics could reduce the
luciferase activities of the wild-type IFITM3 reporter vector
(IFITM3-WT) rather than the mutant-type IFITM3 reporter vector
(IFITM3-MUT) (P<0.01; Fig. 5D),
indicating that IFITM3 was the target gene of miR-29a.
Discussion
HCC is one of the most common and aggressive
cancers. Although surgical resection, chemotherapy and radiation
have been used to treat patients with HCC, the strong invasive and
metastatic properties of HCC result in a high incidence of
recurrence, metastasis and poor survival for patients (1–4,27).
Thus, there is a pressing need to reveal the molecular mechanisms
of HCC to develop novel therapeutic strategies for treating this
disease.
Initiation and progression of HCC have been
demonstrated to involve the dysregulation of various genes and
miRNAs (28,29). The human IFITM protein family,
including IFITM1 (also named 9–27), IFITM2 (also named 1–8D), and
IFITM3 (also named 1-8U), initially received attention because
human IFITM3 was found to limit influenza A virus (IAV) infection
in cultured cells. Then, they were identified as antiviral factors
and possess antiviral activities that limit the infection of
cultured cells by many viruses (7,9,30).
However, recently, accumulating evidence has shown that aberrant
expression of IFITM3 is associated with the progression of various
cancers. For example, Li et al (10) revealed that IFITM3 was expressed at
higher levels in colon cancer and played a critical role in its
progression and metastasis. The study of Andreu et al
(11) confirmed that IFITM3 gene
expression was significantly upregulated in colorectal tumors and
could be rapidly induced after activation of the β-catenin
signaling pathway. Bing et al (12) also demonstrated that IFITM3 played
an important role in glioma cell growth and migration and was
associated with tumorigenesis. In addition, overexpression of
IFITM3 in gastric cancer has been observed and related to tumor
metastasis (31). For patients with
esophageal squamous cell carcinoma, overexpression of IFITM3
indicated a high risk of lymphatic metastatic recurrence after
Ivor-Lewis esophagectomy (32).
Breast cancer cell growth and colony formation could be suppressed
through the downregulation of IFITM3 expression (33).
To date, however, the expression and potential
biological function of IFITM3 in HCC have not been elucidated. The
present study revealed that IFITM3 expression was significantly
increased in human HCC tissues and HCC cells. In addition,
knockdown of IFITM3 inhibited migration, invasion and proliferation
as well as promoted apoptosis of HCC cells. Furthermore, after
upregulation of IFITM3, the invasion, migration and proliferation
abilities of HL-7702 cells were increased, but the apoptosis rate
was decreased. These results indicated that IFITM3 plays important
roles in the tumorigenesis and development of HCC. However, the
upstream molecular mechanism remains unclear.
miRNAs are a class of endogenous ~22 nucleotide RNAs
that have central roles in gene regulatory networks. Generally,
miRNAs control gene expression by regulating the mRNA or
translation of target genes (34).
miRNAs always play important roles in various biological and
pathological processes of HCC. Thus, we focused on the
post-transcriptional regulation of IFITM3 and investigated the
effects of miRNAs on IFITM3. Therefore, we employed two
bioinformatics analysis websites to predict the potential miRNAs
targeting the 3′UTR of IFITM3 mRNA. Initially, 14 candidate miRNAs
(miR-15a, miR-15b, miR-16, miR-29a, miR-125a, miR-195, miR-221,
miR-424, miR-486, miR-497, miR-552, miR-1185, miR-1207 and
miR-1294) with good scores were selected for additional screening.
Eventually, only miR-29a was identified as a functional target gene
of IFITM3 by using dual luciferase reporter gene assay and western
blot analysis. Previously, some studies have already reported
aberrant expression of miR-29a in HCC that was associated with
multiple processes involved in tumor progression. For example, the
study of Zhu et al (20)
noted that miR-29a was downregulated in HCC and could suppress cell
proliferation by targeting SPARC. Mahati et al (21) also reported that miR-29a was
downregulated in HCC and that it could suppress growth and
migration of HCC by regulating CLDN1. Additionally, miR-29a could
also regulate TGF-β-induced EMT in HCC (22). However, the effect of miR-29a on
IFITM3 remains to be established, and the biological significance
of miR-29a in HCC has not been fully elucidated.
In the present study, we investigated the effects of
upregulated miR-29a in HCC cells on the IFITM3 expression levels
and on the biological behaviors of HCC cells. We found that IFITM3
protein expression was reduced after cells were transfected with
miR-29a mimics, indicating that miR-29a could downregulate the
expression of IFITM3. The results also indicated that the invasion,
migration and proliferation capabilities of HCC cells were
suppressed and the apoptosis rate was increased after miR-29a
upregulation, which were consistent with the effects of IFITM3
knockdown. The results provided further evidence that IFITM3 is the
target of miR-29a.
Our results also revealed that the level of miR-29a
was significantly upregulated in HCC tissues and was negatively
correlated with IFITM3 expression. Furthermore, the overall
survival rate of HCC patients was significantly decreased in
patients with IFITM3 overexpression and miR-29a non-overexpression,
indicating that they were related to poor prognosis. Moreover,
IFITM3 overexpression and miR-29a non-overexpression were both
closely correlated with tumor size, tumor multifocal and venous
invasion, which are always associated with tumor growth and
metastasis. In general, multifocal HCC can be caused by two
distinct biological processes. One is intrahepatic metastasis (IM)
which represents that a primary lesion occurs firstly and then
spreads to additional locations in the liver. The other one is
multicentric carcinogenesis (MC) which represents multiple lesions
that occur independently due to underlying pathogenesis (35). The results indicated that miR-29a
and IFITM3 may be involved in the progression of HCC
carcinogenesis. Therefore, HCC with miR-29a non-overexpression and
IFITM3 overexpression may be more invasive with higher malignancy,
and patients may be more inclined to tumor multifocal and
intrahepatic recurrence.
In summary, the results of the present study
collectively indicated that miR-29a suppressed migration, invasion,
and proliferation as well as promoted apoptosis of HCC via IFITM3.
Thus, IFITM3 was identified as a novel target of miR-29a. These
data laid the foundation for using miR-29a and IFITM3 regulatory
pathways for the diagnosis and treatment of HCC patients.
Acknowledgements
The authors are especially grateful to ‘Jiangxi
Provincial Key Laboratory of Molecular Medicine in the Second
Affiliated Hospital of Nanchang University’ for providing
the experimental facilities.
Funding
The present study was supported by grants from the
Natural Science Foundation of Jiangxi Province, China (no.
20171BAB205063), the Youth Science Foundation of Jiangxi Province,
China (no. 20171BAB215037) and the Graduate Innovation Special
Foundation of Nanchang University (no. cx2016397).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YL, EL, WL, LW conceived and designed the study. YL,
EL, CG, JG and JA contributed to the acquisition of the data. YL,
EL, JM, WL and LW analyzed and interpreted the data. YL, EL, JM,
CG, JG, JA, WL and LW wrote, reviewed, and/or revised the
manuscript. WL and LW supervised the study. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Ethics Committee of the Second Affiliated Hospital of Nanchang
University. This study was performed in accordance with the ethical
standards of the Declaration of Helsinki. Informed consent was
obtained from all patients for using their tissue specimens and
clinicopathological data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ochiai T, Ikoma H, Okamoto K, Kokuba Y,
Sonoyama T and Otsuji E: Clinicopathologic features and risk
factors for extrahepatic recurrences of hepatocellular carcinoma
after curative resection. World J Surg. 36:136–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Nagano H, Ota H, Morimoto O,
Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A,
et al: Patterns and clinicopathologic features of extrahepatic
recurrence of hepatocellular carcinoma after curative resection.
Surgery. 141:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bailey CC, Zhong G, Huang IC and Farzan M:
IFITM-family proteins: The cells first line of antiviral defense.
Annu Rev Virol. 1:261–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bailey CC, Kondur HR, Huang IC and Farzan
M: Interferon-induced transmembrane protein 3 is a type II
transmembrane protein. J Biol Chem. 288:32184–32193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feeley EM, Sims JS, John SP, Chin CR,
Pertel T, Chen LM, Gaiha GD, Ryan BJ, Donis RO, Elledge SJ and
Brass AL: IFITM3 inhibits influenza a virus infection by preventing
cytosolic entry. PLoS Pathog. 7:e10023372011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Peng Z, Tang H, Wei P, Kong X, Yan
D, Huang F, Li Q, Le X, Li Q and Xie K: KLF4-mediated negative
regulation of IFITM3 expression plays a critical role in colon
cancer pathogenesis. Clin Cancer Res. 17:3558–3568. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andreu P, Colnot S, Godard C, Laurent-Puig
P, Lamarque D, Kahn A, Perret C and Romagnolo B: Identification of
the IFITM family as a new molecular marker in human colorectal
tumors. Cancer Res. 66:1949–1955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bing Z, Wang H, Gang Z and Ping L: The
role of IFITM3 in the growth and migration of human glioma cells.
BMC Neurol. 13:2102013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Place RF, Li LC, Pookot D, Noonan EJ and
Dahiya R: MicroRNA-373 induces expression of genes with
complementary promoter sequences. Proc Natl Acad Sci USA.
105:1608–1613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kota J, Chivukula RR, ODonnell KA, Wentzel
EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M,
Clark KR, et al: Therapeutic microRNA delivery suppresses
tumorigenesis in a murine liver cancer model. Cell. 137:1005–1017.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y and
Zheng X: miR-34a inhibits migration and invasion by down-regulation
of c-Met expression in human hepatocellular carcinoma cells. Cancer
Lett. 275:44–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong CC, Wong CM, Tung EK, Au SL, Lee JM,
Poon RT, Man K and Ng IO: The microRNA miR-139 suppresses
metastasis and progression of hepatocellular carcinoma by
down-regulating Rho-kinase 2. Gastroenterology. 140:322–331. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL,
Ye QH, Qin LX and Wu XZ: microRNA-29a suppresses cell proliferation
by targeting SPARC in hepatocellular carcinoma. Int J Mol Med.
30:1321–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahati S, Xiao L, Yang Y, Mao R and Bao Y:
miR-29a suppresses growth and migration of hepatocellular carcinoma
by regulating CLDN1. Biochem Biophys Res Commun. 486:732–737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kogure T, Kondo Y, Kakazu E, Ninomiya M,
Kimura O and Shimosegawa T: Involvement of miRNA-29a in epigenetic
regulation of transforming growth factor-beta-induced
epithelial-mesenchymal transition in hepatocellular carcinoma.
Hepatol Res. 44:907–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho HJ, Kim SS, Nam JS, Kim JK, Lee JH,
Kim B, Wang HJ, Kim BW, Lee JD, Kang DY, et al: Low levels of
circulating microRNA-26a/29a as poor prognostic markers in patients
with hepatocellular carcinoma who underwent curative treatment.
Clin Res Hepatol Gastroenterol. 41:181–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Liang B, Liu H, Huang Y, Yin X,
Zhou F, Yu X, Feng Q, Li E, Zou Z and Wu L: Overexpression of
nonSMC condensin I complex subunit G serves as a promising
prognostic marker and therapeutic target for hepatocellular
carcinoma. Int J Mol Med. 40:731–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li C, Miao R, Liu S, Wan Y, Zhang S, Deng
Y, Bi J, Qu K, Zhang J and Liu C: Down-regulation of miR-146b-5p by
long noncoding RNA MALAT1 in hepatocellular carcinoma promotes
cancer growth and metastasis. Oncotarget. 8:28683–28695.
2017.PubMed/NCBI
|
|
27
|
Yuan R, Wang K, Hu J, Yan C, Li M, Yu X,
Liu X, Lei J, Guo W, Wu L, et al: Ubiquitin-like protein FAT10
promotes the invasion and metastasis of hepatocellular carcinoma by
modifying beta-catenin degradation. Cancer Res. 74:5287–5300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fabbri M, Calore F, Paone A, Galli R and
Calin GA: Epigenetic regulation of miRNAs in cancer. Adv Exp Med
Biol. 754:137–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu G, Chen X, Chen S, Ye W, Hou K and
Liang M: miR-19a, miR-122 and miR-223 are differentially regulated
by hepatitis B virus X protein and involve in cell proliferation in
hepatoma cells. J Transl Med. 14:1222016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lewin AR, Reid LE, McMahon M, Stark GR and
Kerr IM: Molecular analysis of a human interferon-inducible gene
family. Eur J Biochem. 199:417–423. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J, Wang S, Zhao Y, Guo Q, Zhang D, Chen
J, Li J, Fei Q and Sun Y: Mechanism and biological significance of
the overexpression of IFITM3 in gastric cancer. Oncol Rep.
32:2648–2656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia Y, Zhang M, Jiang W, Zhang Z, Huang S
and Wang Z: Overexpression of IFITM3 predicts the high risk of
lymphatic metastatic recurrence in pN0 esophageal squamous cell
carcinoma after Ivor-Lewis esophagectomy. PeerJ. 3:e13552015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Gao H, Chen P, Jia J and Wu S:
Knockdown of interferon-induced transmembrane protein 3 expression
suppresses breast cancer cell growth and colony formation and
affects the cell cycle. Oncol Rep. 30:171–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meister G: miRNAs get an early start on
translational silencing. Cell. 131:25–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chianchiano P, Pezhouh MK, Kim A, Luchini
C, Cameron A, Weiss MJ, He J, Voltaggio L, Oshima K, Anders RA and
Wood LD: Distinction of intrahepatic metastasis from multicentric
carcinogenesis in multifocal hepatocellular carcinoma using
molecular alterations. Hum Pathol. 72:127–134. 2018. View Article : Google Scholar : PubMed/NCBI
|