Introduction
Epithelial ovarian cancer (EOC) is characterized by
multifocal intraperitoneal dissemination and the accumulation of
ascitic fluid coupled with intense neovascularization (1). Similar to the majority of other solid
tumors, angiogenesis occurs in the early stages of EOC development,
and may precede neoplastic transformation (2,3). In
addition, surgical stress may enhance ovarian cancer angiogenesis
(4). Therefore, inhibition of
angiogenesis is one of the most promising approaches to EOC
treatment. Bevacizumab, a monoclonal antibody directed against
vascular endothelial growth factor (VEGF)-A, has been approved for
preliminary treatment of ovarian cancer (5). However, the clinical benefits are
limited and recurrent ovarian cancer has been reported in a
proportion of the patients (6).
Hypoxic stress generated by successful preliminary therapy may
cause upregulation of selective pro-angiogenic factors (7). Overcoming this evasive resistance
further supports the need for a novel therapeutic approach in this
field. In recent years, G-protein-coupled receptors (GPCRs) have
been implicated in the initiation and progression of a variety of
tumors (8). Thus, regulators of
GPCR signaling are likely important in the pathophysiology of
cancer (9). Regulator of G-protein
signaling-5 (RGS5) is a member of the RGS family that consists of a
diverse group of multifunctional proteins and has been reported to
be expressed in vascular, cardiac, and skeletal muscle tissues
(10). RGS5 was recently identified
as a major upregulated gene in pericytes, and is responsible for
several morphological changes in tumor vessels. Studies have found
that the levels of RGS5 can decrease following increased expression
of anti-VEGF antibody as a result of inhibition of angiogenesis. In
addition, RGS5 protein expression was increased after blocking the
VEGFR signaling pathway in mice during corneal neovascularization,
and the effects of inhibiting angiogenesis are superior to those of
blocking the VEGFR pathway (11).
Thus, targeting RGS5 may affect tumor vessels.
Endothelial cells in malignant tumors are
genetically variable and differ from endothelial cells derived from
normal vessels at the molecular as well as the functional level
(12,13). It is essential to study the precise
effect of RGS5 on the biological characteristics of ovarian
carcinoma-derived endothelial cells (ODMECs) prior to using them
experimentally and performing drug research on anti-angiogenesis in
EOC. RGS5 plays a vital role in the development of the vasculature.
RGS5 was found to be abundantly expressed in EOC compared with
normal ovaries. However, the distribution of RGS5 in EOC and its
significance require further investigation. Therefore, in the
present study, we observed the expression of RGS5 in EOC, as well
as its association with clinicopathological parameters. We also
assessed the status of RGS5 in primary endothelial cells derived
from human EOC.
The aim of the present study was to examine the RGS5
protein expression in ovarian cancer cells and microvascular
structures and determine whether the expression of RGS5 was
significantly associated with peritoneal metastasis in patients
with ovarian cancer.
Materials and methods
Patients and tissue specimens
A total of 127 human paraffin-embedded EOC tissue
samples that had been collected from consecutive patients (The
average age of the patients was approximately 50 years) undergoing
standard surgical procedures for primarily diagnosed ovarian cancer
at the Department of Obstetrics and Gynecology, Southwest Hospital
(Chongqing, China) between December 2004 and July 2009, were used
in this study. Relevant data were obtained via retrospective review
of the medical files of patients. These data included demographic
information, histopathological diagnosis, tumor grade, disease
stage, ascites status, CA125 level, chemotherapy regimen and
response to clinical treatment or chemotherapy. The tumors were
histopathologically characterized according to the WHO criteria,
tumor grade was determined based on the Gynecological Oncology
Group criteria, and disease stage was assessed according to the
International Federation of Gynecology and Obstetrics staging
system (14). None of the patients
received any preoperative anticancer treatment, including
chemotherapy, radiotherapy or biotherapy. Patients who died from
unknown causes or emergency were excluded from this study. The
Ethics Committee of the First Affiliated Hospital of the Third
Military Medical University approved the procedures and all
patients provided written informed consent.
Patients with EOC were interviewed by telephone. The
majority of the patients had been reviewed after completing
treatment every 3–6 months over a 2-year period, and annually
thereafter. Patients' follow-up information was updated until May
2016 by reviewing medical records. Progression-free survival (PFS)
and overall survival (OS) were estimated from the date of the first
cytoreduction to the date of recurrence/death or at the last
follow-up, whichever occurred first. The progression (recurrence or
metastasis) of ovarian cancer was confirmed by radiological
examination. Recurrence was indicated clinically by the appearance
of new lesions or an increase in the serum CA125 level to more than
twice the upper limit of the reference range. Patients who remained
alive without recurrence at the last follow-up were censored.
Patients in the progressive disease group were those in whom no
disease remission had been observed after treatment. Patients in
the relapsed disease group were those in whom disease remission had
been clinically documented. The extent of cytoreduction was defined
as optimal if the largest diameter of any residual lesion from
surgery was <1 cm, or as suboptimal if it was >1 cm.
Immunohistochemistry
Immunohistochemical (IHC) examination of RGS5 in the
paraffin-embedded samples was performed using a standard
streptavidin-peroxidase method as previously described (13). EOC sections (4-µm) were
deparaffinized and unmasked for 5 min. After blocking endogenous
peroxidase activity, the sections were incubated for 10 min with
20% normal rabbit serum to block non-specific binding sites. The
primary antibody used was a polyclonal antibody against human RGS5
protein (dilution 1:100; cat. no. HPA001821; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The samples were incubated overnight at
4°C in a moist chamber. Following three washes, the sections were
incubated for 30 min with goat anti-rabbit secondary IgG (dilution
1:400; cat. no. SP-9001; ZSGB-BIO, Beijing, China). The
3,5-diaminobenzidine (DAB) Detection kit (ZSGB-BIO) was used for
staining. Negative controls with phosphate-buffered saline (PBS;
0.01 mol/l, pH 7.4) replacing the primary antibody were also
included. Finally, the tissue sections were counterstained with
hematoxylin, dehydrated and mounted in resinous mountant. Digital
images were captured using a BH-2 light microscope (Olympus Corp.,
Tokyo, Japan) at an ×200 magnification.
RGS5 expression was determined semi-quantitatively
by assessing the immunostaining intensity and the percentage and
distribution of positively stained cells, as previously reported.
Briefly, the tissue sections were screened at a high power (×200)
and the mean of five visual fields was estimated. The mean
percentage of immunoreactive cells was described as follows: 0,
<5%; 1, 6–25%; 2, 26–50%; 3, 51–75% and 4, >75%. The
intensity of RGS5 immunostaining was scored as follows: 0,
negative; 1, weakly positive; 2, positive; 3, strongly positive.
For sections with heterogeneous staining, the predominant pattern
was taken into consideration for scoring. A staining index (with
values of 0–12) was obtained by multiplying the staining intensity
with the proportion of immunopositive tumor cells. For the
statistical analysis, the patients were classified into three
groups: Negative or low reactive cases (score 0–1), cases with
moderate (scores 2–5) and cases with high expression (scores 6–12).
All histological evaluations were independently performed in a
double-blinded manner by two expert pathologists (Dr Jiang Zhu and
Dr Feng Wu). Any differences in the scores were resolved by
discussion between the two pathologists.
Cell culture
Primary human ODMECs were isolated from EOC tissue
as previously described (13) and
cultured in complete EGM-2MV medium (Lonza, Basel, Switzerland).
Human aortic smooth muscle cells (HASMCs), A2780 and SKOV3 cells
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). A2780 and SKOV3 cells were cultured in
RPMI-1640 media (Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (FBS) and used as negative controls. HASMCs were
cultured in DMEM with 20% FBS. The angiogenic ability of ODMECs was
analyzed by using a two-dimensional fibrin gel assay.
Ability of ODMECs to form capillary
networks and RGS5 and endoglin (CD105) levels assay
Capillary network formation by ODMECs was analyzed
using a two-dimensional fibrin gel assay as previously described
(15). Whole-cell extracts were
prepared for the different tubular structure stages (7 and 14 days)
with mammalian protein extraction reagent (Pierce, Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Western blotting was performed
to monitor RGS5 (1:60) and endoglin (1:2,000; both from Abcam,
Shanghai, China) protein levels.
ODMEC culture under hypoxic
conditions
ODMECs confluent to ~80% in the cell culture plate
were harvested by trypsinization and EDTA. After 170 × g
centrifuging and washing, the cell re-suspension solution was
placed into the hypoxic incubator (oxygen concentration of 1%).
Then, reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting were used to detect the mRNA and
protein levels of RGS5 at 1, 3, 6, 12 and 24 h under hypoxic
conditions.
Construction and production of
lentiviral vectors
To demonstrate the specificity of siRNA against
human RGS5 (GenBank Accession no. NM_000118), the following
oligonucleotides were used: Target sequences:
5′-GAACCTTCCCTGAGCAGCT-3′, 5′-ATATTGACCACTTCACTAA-3′ and
5′-GGAAAAGGATTCTCTGCCT-3′. A scrambled siRNA was used as a negative
control. RGS5 siRNA and control siRNA bearing no sequence homology
with any known human mRNA sequences were also used. Double-stranded
DNA containing the interference sequences was synthesized and
inserted into a linearized pGenesil-GFP viral vector (Gikai Gene
Company, Shanghai, China). All the constructs were cloned and
sequenced to confirm their proper construction. Lentivirus-encoded
siRNA against RGS5 (LV-siRGS5) and control (LV-H) were produced by
co-transfecting 293T cells with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, the supernatant was
harvested and concentrated and the viral titers were measured.
Transfection of cells
ODMECs were plated into 6-well tissue culture plates
at 1×105 cells/well under hypoxic conditions (continuous
oxygen concentration of 1%). Three parallel wells were used for
each group of cells: Non-transfected cells (control group),
LV-H-transfected cells (negative control group) and
LV-R-transfected cells (knockdown group). When the cells reached
~60–70% confluence, the media was replaced with DMEM containing
lentivirus at a multiplicity of infection (MOI) of 20, and the
cells were incubated overnight (16 h). Gene transfer efficiency was
monitored by flow cytometry (FACStar plus; BD Biosciences, Franklin
Lakes, NJ, USA) or confocal microscopy (Leica TCS SP5; Leica
Microsystems, Wetzlar, Germany) at 48 h post-transfection.
RT-PCR
Total RNA was isolated at 48 h using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific). Aliquots of RNA were
reverse-transcribed to cDNA using a Superscribe First-Strand
synthesis system (Invitrogen; Thermo Fisher Scientific, Inc.). The
following PCR primers were used: RGS5: 5′-AAGATGGCTGAGAAGGCAAA-3′
and 5′-TCAGGGCATGGATTCTTTTC-3′, with a product length of 396 bp;
and GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ and
5′-GAAGATGGTGATGGGATTTC-3′, with a product length of 220 bp. The
thermal profile conditions were 30 sec at 95°C, 30 sec at 62°C and
30 sec at 72°C for 35 cycles, and a final extension at 72°C for 5
min.
Cell proliferation assays
After being transduced at an MOI of 50 under a
continuous oxygen concentration of 1%, ODMECs were grown until
confluent and plated again at an optimal density of
1×105 cells/well supplemented with EGM-2MV 20% FBS in
24-well plates. After 1, 2, 3, 4, 5 and 6 days, the cells were
stained with 20 µl MTT (5 mg/ml) (Sigma-Aldrich; Merck KGaA) at
37°C for 4 h and subsequently solubilized in 200 µl dimethyl
sulfoxide. The absorbance at 570 nm was measured using a microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Cell growth
curves were calculated as mean values of triplicates/group.
Wound healing migration assay
ODMECs were seeded in 12-well plates, infected
overnight (16 h) with either LV-siRGS5 or LV-H, and allowed to grow
until confluent under a continuous oxygen concentration of 1%. A
wound was created by using a pipette cone, and cells were allowed
to migrate in the EGM-2MV media. The wounded area was monitored 16
h after injury. Wound-induced cell migration was measured by
monitoring the distance between cells lining the wound edge and
then normalized to time 0 h.
Flow cytometric assay
ODMECs cells infected overnight (16 h) with either
LV-siRGS5 or LV-H under a continuous oxygen concentration of 1%
were harvested by trypsinization, washed in ice-cold PBS, and fixed
in 80% ice-cold ethanol in PBS. Prior to staining, the cells were
pelleted using a chilled centrifuge and re-suspended in cold PBS.
Bovine pancreatic RNase (Sigma-Aldrich; Merck KGaA) was added to a
final concentration of 2 µg/ml and the cells were incubated at 37°C
for 30 min, followed by incubation with 20 µg/ml propidium iodide
(PI; Sigma-Aldrich; Merck KGaA) for 20 min at room temperature to
analyze the cell cycle, or incubation with 61 µl FITC-Annexin V and
20 µl PI with 300 µl binding buffer for 15 min at room temperature
to analyze the apoptotic rate. The profiles of 1×104
cells were analyzed using a FACSCalibur flow cytometer (BD
Biosciences).
Western blot analysis
Whole-cell extracts were prepared with mammalian
protein extraction reagent (Pierce; Thermo Fisher Scientific,
Inc.), RIPA lysis buffer (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), and an equal amount of protein (100 µg) from each cell
line was loaded per lane and resolved by polyacrylamide gel
electrophoresis (7.5% SDS-Tris glycine). Gels were electroblotted
onto nitrocellulose membranes and blocked in 1X Tris-buffered
saline containing 0.1% Tween and 5% non-fat dry milk overnight. The
following primary antibodies were used for 1 h at room temperature:
Polyclonal mouse anti-human RGS5 antibody (dilution 1:60; cat. no.
ab196799) and rabbit anti-human CDC25A antibody (dilution 1:200;
cat. no. ab47400; both from Abcam), mouse anti-human CDK2 antibody
(dilution 1:500; cat. no. sc-6248; Santa Cruz Biotechnology, Inc.),
mouse anti-human p53 antibody (dilution 1:1,000; cat. no. AF0255)
and mouse anti-human p21 antibody (dilution 1:500; cat. no. AP021;
both from Beyotime Institute of Biotechnology, Haimen, China),
mouse anti-human p-ERK1/2 antibody (dilution 1:1,000; cat. no.
ab201015), mouse anti-human ERK1/2 antibody (dilution 1:1,000; cat.
no. ab36991), mouse anti-human p-p38 antibody (dilution 1:200; cat.
no. ab178867), mouse anti-human p38 antibody (dilution 1:200; cat.
no. ab31828; all from Abcam) and mouse anti-human GAPDH (dilution
1:10,000; cat. no. KC-5G4; KangChen Bio-Tech, Shanghai, China).
After 3 washes in TBS/0.1% Tween 20, the membranes were incubated
for 1 h at room temperature with horseradish peroxidase-conjugated
secondary antibody (dilution 1:10,000; cat. no. KC-MM-035; KangChen
Bio-Tech). Protein was detected using chemiluminescence (Santa Cruz
Biotechnology, Inc.) and autoradiography (Kodak, Rochester, NY,
USA).
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software package (version 13.0; SPSS, IBM., Chicago,
IL, USA). Associations of RGS5 expression with the clinical
parameters of the patients were assessed by the Chi-squared test.
Fisher's exact test was also used when necessary. The Kaplan-Meier
method was used to estimate the probability of overall and
disease-free survival, and the log-rank test was used to compare
different survival curves. Multivariate survival analysis was
performed on all factors using the Cox regression model. Receiver
operating characteristics curve analysis was used to compare the
clinicopathological characteristics for estimation of the survival
prediction. All P-values were the results of two-sided tests, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of RGS5 is associated with
cancer metastasis in ovarian carcinoma
RGS5 was mainly expressed in the cytoplasm of
ovarian carcinoma cells, and the highest levels were in the regions
infiltrated by the tumor cells (Fig.
1A). In this study, according to the staining index described
above, the protein expression with a scoring index of ≥4 (median
score of RGS5 protein expression in the primary ovarian lesions)
was defined as positive expression. A total of 42 cases were
excluded due to significant discrepancies in the staining. Negative
or low reactivity was observed in 55 cases (scores 0–1 and 2–5,
respectively), and high expression in 30 cases (scores 6–12). RGS5
expression was not associated with younger age at diagnosis (<50
years; P=0.168), histological type (serous vs. not serous;
P=0.325), early-stage disease (I and II vs. III and IV; P=0.466),
low ascites incidence (P=0.198), and low-grade tumors (1 and 2 vs.
3; P=0.820). We examined the peritoneal metastasis of ovarian
carcinoma with different expression of RGS5: A strong association
was identified between the expression of RGS5 and intraperitoneal
metastasis in ovarian carcinoma (P=0.004). Tumors highly expressing
RGS5 were generally associated with a low incidence of
intraperitoneal metastasis (Table
I).
 | Table I.Association of RGS5 protein
expression with clinicopathological parameters. |
Table I.
Association of RGS5 protein
expression with clinicopathological parameters.
| Variables | All cases | Low expression | High
expression | P-value |
|---|
| Age at surgery
(years) |
|
|
| 0.168 |
|
<50 | 34 | 23 | 11 |
|
|
≥50 | 51 | 32 | 19 |
|
| Histological
type |
|
|
| 0.325 |
|
Serous | 59 | 34 | 24 |
|
|
Other | 25 | 18 | 7 |
|
| Histological grade
(Silveberg) |
|
|
| 0.820 |
| G1 | 8 | 4 | 4 |
|
| G2 | 32 | 20 | 12 |
|
| G3 | 42 | 27 | 15 |
|
| FIGO stage |
|
|
| 0.466 |
|
I–II | 27 | 15 | 12 |
|
|
III–IV | 52 | 34 | 18 |
|
| Ascites |
|
|
| 0.198 |
| No | 22 | 11 | 11 |
|
|
Yes | 63 | 43 | 20 |
|
| CA125 (U/ml) |
|
|
| 0.625 |
|
<500 | 42 | 25 | 18 |
|
|
≥500 | 29 | 19 | 10 |
|
| Lymph node
metastasis |
|
|
| 0.467 |
|
Absent | 40 | 29 | 15 |
|
|
Present | 30 | 16 | 12 |
|
| Intraperitoneal
metastasis |
|
|
| 0.004 |
|
Absent | 27 | 11 | 15 |
|
|
Present | 45 | 36 | 10 |
|
Association between RGS5 expression
and the 5-year survival rate
The 5-year survival rate of patients with ovarian
cancer has important clinical implications and was estimated using
Kaplan-Meier survival curves. The survival time was 72.34±8.41
months for patients with RGS5-positive tumors and 43.56±5.41 months
for those with RGS5-negative tumors. Then, the survival rates were
compared using the log-rank method in univariate survival analysis.
The results of our survival analysis indicated that patients with
RGS5-high tumors had higher survival rates (P<0.001) compared
with patients with RGS5-low tumors (Fig. 1B).
Expression of RGS5 protein in ovarian
cancer cells and its association with microvascular density (MVD)
marker CD34
MVD is a measure of the degree of tumor
angiogenesis, reflected by the expression of CD34 antibody in the
vascular endothelial cell membrane as brown-yellow particles. We
evaluated the activity of angiogenesis in ovarian cancer by
continuous counting of the microvessels in 5 high-power microscopic
fields (×200). In order to study whether there is a statistical
association between the expression of MVD and RGS5, we classified
RGS5 expression in ovarian cancer cells according to the level and
compared it with the difference in MVD within the respective
tumors. The results demonstrated that the tumors expressing high
levels of RGS5 also exhibited a significantly lower MVD, which
co-localized with CD34 expression. The opposite was observed in
tissues with low expression of RGS5, which indicated that the
expression of RGS5 in ovarian cancer cells was negatively
associated with MVD (P<0.05; Table
II).
 | Table II.The relationship between RGS5 protein
and MVD. |
Table II.
The relationship between RGS5 protein
and MVD.
| Variable | All cases | MVD (mean ±
SD) |
P-valuea |
|---|
| Expression of
RGS5 |
|
| 0.037 |
|
High | 18 | 17.64±11.29 |
|
|
Low | 33 | 28.82±17.30 |
|
Expression of RGS5 protein in ovarian
cancer microvasculature is associated with blood vessels
co-localized with endoglin, but not with blood vessels co-localized
with CD34
RGS5 was expressed in the ovarian cancer
microvasculature, whereas weaker expression, by comparison of
serial sections, was observed for CD34. RGS5- and CD34-positive
blood vessels rarely overlap (Fig.
1C). However, they are visible in some microvessels where
endoglin expression is observed, but the expression of the two
genes is weaker (Fig. 1D and E).
Even in the tube-like structures, expression of CD34 or endoglin is
not usually detected together with expression of RGS5 (Fig. 1C).
ODMECs do not stably express RGS5 in a
conventional in vitro culture
There is normally increased expression of VEGF,
transforming growth factor-β, platelet-derived growth factor
(PDGF)-BB and other growth factors in tumor cells and their
microenvironment. However, it is highly likely that the
microenvironment of ODMECs cultured in vitro lacks these
factors. Previous studies reported that the expression of endoglin
was significantly higher in ODMECs (15), but in the present study we found
that in vitro ODMECs did not significantly express RGS5,
which is a crucial factor for their biological function with
respect to PDGF-BB (16) (Fig. 2A). Some scholars believe that the
high expression of RGS5 in the tumor microenvironment in
vivo is a result of the presence of high levels of angiogenic
factors. Hence, in the cell culture system, when
angiogenesis-related factors, such as VEGF and PDGF-BB, are added
at a concentration of 10 ng/ml, it leads to stimulation of RGS5
expression, which can be detected in ODMECs and human aortic smooth
muscle cells (HASMCs) after 24 h. However, the results of the
present study revealed no promoting effect of VEGF or PDGF-BB on
the expression of RGS5 (Fig. 2A).
This indicated that the high expression of RGS5 in tumor tissue may
not depend on regulation via the VEGFR and PDGF-BB signaling
pathways in this model system.
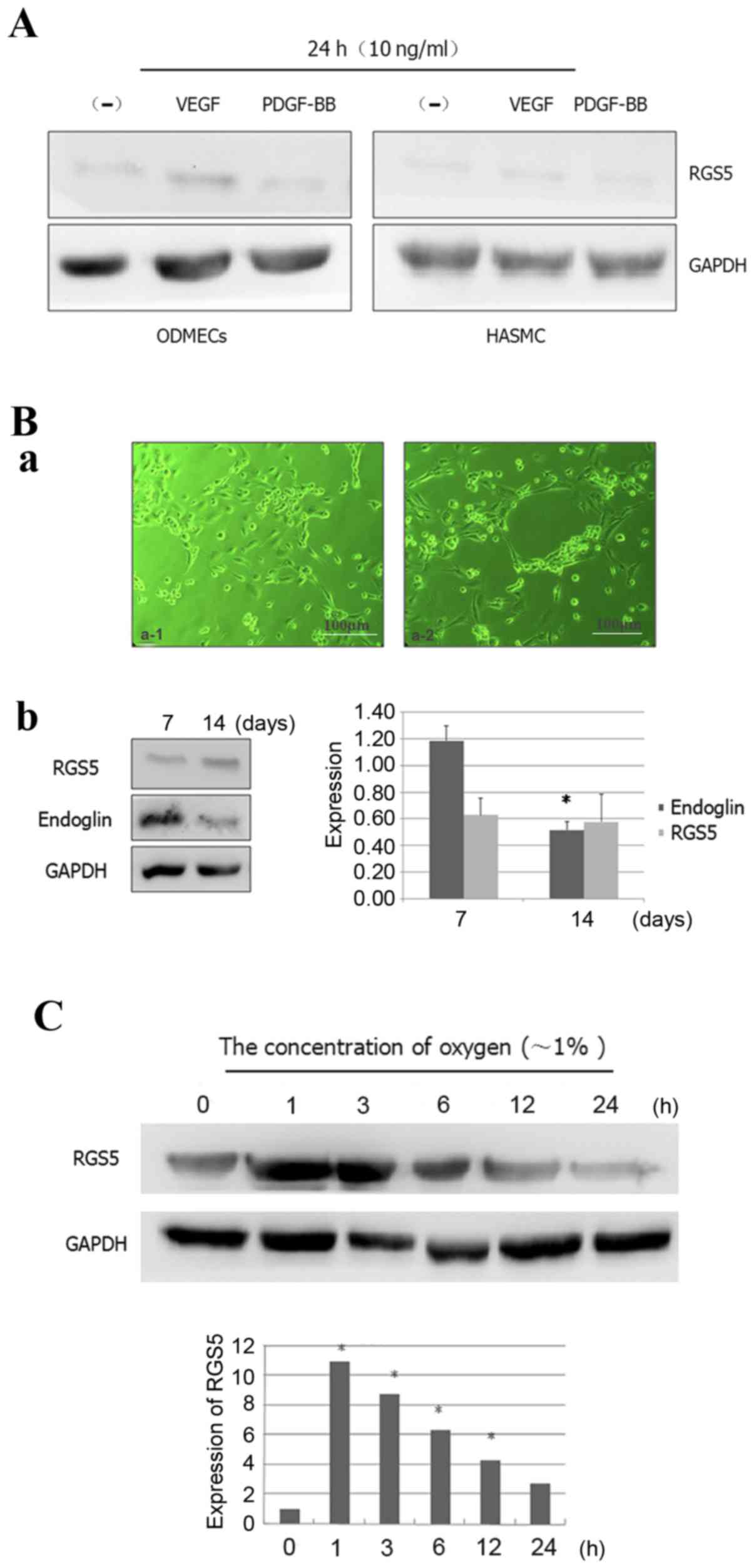 | Figure 2.Expression patterns of RGS5 in
ODMECs. (A) RGS5 was not obviously expressed in ODMECs and HASMCs
in a conventional in vitro culture, since VEGF and PDGF-BB
cannot effectively stimulate the expression of RGS5. (B) RGS5 did
not play a decisive role in the formation of the lumen-like
structures by ODMECs. (a) ODMECs form lumen-like structures on the
FN-gel (phase contrast microscope, ×100). (b) The expression of
endoglin was different at different stages of lumen-like structure
formation by ODMECs (n=3, P<0.05), but the expression of RGS5
exhibited no statistically significant difference (n=3, P>0.05).
(C) The expression of RGS5 was detected in ODMECs at different
time-points (1, 3, 6, 12 and 24 h) under hypoxic conditions (~1%
oxygen). *P<0.05. RGS5, regulator of G-protein signaling 5;
ODMECs, ovarian carcinoma-derived endothelial cells; HASMCs, human
aortic smooth muscle cells; FN, fibronectin; VEGF, vascular
endothelial growth factor; PDGF, platelet-derived growth
factor. |
RGS5 does not play a decisive role in
the formation of the lumen-like structures by ODMECs
In ODMECs, high expression of the
angiogenesis-related proteins VEGFR-2 and endoglin was observed,
regardless of the enhancement of the in vitro angiogenesis
ability or lack thereof. In the preliminary study, we found that
ODMECs spontaneously formed lumen-like structures on a single cell
layer in a culture plate with no extracellular matrix, such as
gelatin or fibronectin (FN), when subjected to long-term culture of
5–6 weeks. Therefore, this part of the experiment aimed to further
investigate the tube-forming ability of ODMECs in vitro by
the FN-collagen system (fibrin gel-based) (17). In the FN-gel package, serum-free
EGM-2MV culture medium was used for 3–4 days, by which time ODMECs
exhibited budding growth of adjacent formations (Fig. 2B-a). Then, the cells appeared to
join and form tubular structures after ~1 week, and connected to
form a network which was observed for ~10–12 days, after which time
they began to break into the lumen (Fig. 2B-a). Under the same conditions,
human umbilical vein endothelial cells failed to form any complete
lumen-like structures. The size of the tube-like formations of
ODMECs was not uniform, with large-diameter lumina or tufted cell
masses both visible.
In order to determine the role of RGS5 in ODMECs in
in vitro culture, we detected the expression of RGS5 and
endoglin at different stages of lumen-like structure formation by
ODMECs. FN-gel cells were seeded on the seventh day, when adjacent
cells exhibited budding growth and connected into a tubular
structure. The results revealed that the expression of endoglin was
found to be significantly higher compared with that of cells seeded
on FN-gels for ~14 days, when the formation of lumen-like
structures was stable and had started to disintegrate (n=3,
P<0.05; Fig. 2B). In addition,
no significant change in the expression of RGS5 was observed with
prolonged incubation time. This indicated that endoglin was
involved in tube formation by ODMECs in vitro, while RGS5
did not play a decisive role in the formation of the lumen-like
structures by ODMECs.
Expression of RGS5 increases in ODMECs
during hypoxia
The expression of RGS5 in vitro exhibited
little change with time prolongation. This is presumably due to the
tumor vascular endothelial cells being extracted from the tumor
microenvironment, and the high expression of the pro-angiogenic
factors VEGF and PDGF-BB in the tumor microenvironment, which in
turn decreased the expression of RGS5 (Fig. 2). This indicated that the high
expression of RGS5 in tumor tissue may not rely on regulation via
the VEGFR and PDGF-BB signaling pathways.
Tumor vasculature is usually heterogeneous, with
luminal enlargement and distortion, while tumor neovascularization
is prominent, but is often in a state of hypoxia. Hence, we
detected the expression of RGS5 at different time-points (1, 3, 6,
12 and 24 h) under hypoxic conditions (1% oxygen). The results
demonstrated that hypoxia can enhance the expression of RGS5 in
ODMECs. The expression of RGS5 was significantly higher in hypoxia
at 1 h, but it decreased as hypoxia was prolonged (Fig. 2C), which indicated that the
expression of RGS5 was significantly induced in the early stages of
hypoxia.
LV-siRGS5 effectively inhibits the
expression of RGS5 at the mRNA and protein level in primary
ODMECs
After 48 h, the total RNA and protein were extracted
from the blank control group as well as the transfected LV-siRGS5
and negative control LV-H ODMECs after hypoxia for 6 h. Using qPCR
and western blotting, the expression level of RGS5 mRNA and
protein, respectively, was measured. The results demonstrated that
LV-siRGS5 inhibited the expression of RGS5 mRNA and protein in
ODMECs. At the mRNA level, the blank group and negative control
group LV-H-siRNA and LV-siRGS5 three groups of quantitative
analysis were 1.07±0.05, 1.32±0.09 and 0.52±0.06, 0.60±0.04 and
0.54±0.02, respectively, with the expression of negative control
LV-H not shown to affect the RGS5 mRNA (n=3, P<0.05; Fig. 3A). At the protein level, the blank
group and negative control group LV-H-siRNA and LV-siRGS5
quantitative analysis were 0.92±0.08, 0.95±0.21 and 0.17±0.04,
0.22±0.08 and 0.19±0.12, respectively, with the negative control of
LV-H-siRNA not affecting the expression of RGS5 (n=3, P<0.05;
Fig. 3B). As the RGS5 protein
belongs to the B/R4 subfamily, and the family includes RGS1, RGS2,
RGS3, RGS4, RGS5, RGS8, RGS13, RGS16, RGS 18 and RGS21, the
expression of members of the same subfamily of RGS proteins is
basically identical; therefore, to elucidate the effect of
LV-siRGS5 on the expression of RGS4, RGS2 and RGS16 in endothelial
cells, we detected the expression of RGS5, RGS2, RGS4 and RGS16 in
ODMECs following transfection with LV-siRGS5 and negative control
LV-H. The results demonstrated that LV-siRGS5 did not interfere
with the expression of RGS2, RGS4 or RGS16 (Fig. 3C).
 | Figure 3.LV-siRGS5 effectively inhibits the
expression of RGS5 at the mRNA and protein level in ODMECs. (A) At
the mRNA level, the blank group, the negative control group
LV-H-siRNA and the LV-siRGS5 three groups of quantitative analysis
were 1.07±0.05, 1.32±0.09 and 0.52±0.06, 0.60±0.04, 0.54±0.02,
respectively; the expression of negative control LV-H did not
affect the RGS5 mRNA (n=3, P<0.05). (B) At the protein level,
the blank group, the negative control group LV-H-siRNA and the
LV-siRGS5 three groups of quantitative analysis was 0.92±0.08,
0.95±0.21 and 0.17±0.04, 0.22±0.08, 0.19±0.12, respectively; the
negative control LV-H-siRNA did not affect the expression of RGS5
(n=3, P<0.05). (C) LV-siRGS5 did not interfere with the
expression of RGS2, RGS4 and RGS16 in the B/R4 subfamily. RGS5,
regulator of G-protein signaling 5; ODMECs, ovarian
carcinoma-derived endothelial cells; LV, lentiviral vector. |
RGS5 inhibits the proliferation
ability of ODMECs
A hypoxic environment can induce changes of protein
expression in vascular endothelial cells, in order to modify the
corresponding biological characteristics. The high expression of
RGS5 in tumor angiogenesis overlaps with the vascular endoglin
marker. The vascular endothelial cells express highly the ‘active’
state of the vascular pro-growth factor receptor. Although it was
previously found that RGS5 does not play a decisive role in the
formation of the lumen-like structures observed when ODMECs are
grown in vitro, endoglin is known to participate in
angiogenesis, and is associated with cell proliferation. Therefore,
this experiment determined the effect of RGS5 on the proliferation
ability of ODMECs by MTT method under hypoxic conditions. The
results demonstrated that the proliferation ability of the
LV-siRGS5 ODMEC group increased by 61.36% (n=3, P<0.05; Fig. 4A) compared with the negative control
group (Fig. 4A), indicating that
the RGS5 protein was able to inhibit the proliferative ability of
ODMECs.
RGS5 does not affect the migration of
ODMECs under hypoxic conditions
The ultrastructure of ODMECs was observed under an
electron microscope, and the cytoplasm was found to contain
abundant microtubules and microfilaments. This finding indicated
that ODMECs may possess a strong ability for movement. In order to
determine whether the overexpression of RGS5 induced by hypoxia
would affect the migration ability of ODMECs, we detected the rate
of cell migration using the scratch test. At 16 h after scratching,
the healing rate in the LV-H-ODMEC and LV-siRGS5-ODMEC groups were
52.62±5.63 and 55.83±6.89%, respectively, with no statistically
significant difference (n=3, P>0.05; Fig. 4B), indicating that RGS5 did not
participate in ODMEC migration under hypoxic conditions.
RGS5 can induce apoptosis of ODMECs
under hypoxic conditions
Tumor hypoxia often promotes angiogenesis and is
conducive to tumor development and metastasis; on the other hand,
it also induces cell apoptosis, thereby inhibiting tumor growth.
The balance of these two effects is important in tumor development
and outcome. Hypoxia can induce the expression of RGS5 and it is
crucial to understand whether RGS5 participates in the apoptosis of
ODMECs. This may be achieved by interfering with the expression of
RGS5 and detection of the ODMEC apoptosis rate in the LV-H and
LV-siRGS5 groups under normoxic and hypoxic conditions at 12 h. The
present study revealed that hypoxia can induce apoptosis of ODMECs
(0.020 vs. 3.09%), and the apoptosis rate of the LV-H and LV-siRGS5
groups at 12 h after hypoxia was 3.09 and 0.606%, respectively
(Fig. 4C), indicating that RGS5
promoted the apoptosis of ODMECs under hypoxic conditions.
RGS5 enables ODMECs to remain in the
G1 phase of the cell cycle under anoxic conditions
By interfering with the expression of RGS5 under
hypoxia, observations of the ODMEC cell cycle in the LV-H and
LV-siRGS5 groups revealed that, under hypoxic conditions, RGS5 can
arrest ODMECs in the G1 phase of the cell cycle (43.02 vs. 77.75%),
The number of G1 cells in the LV-H and LV-siRGS5 groups was
74.24±8.33 and 46.33±4.53%, respectively (n=3, P<0.05; Fig. 4D), indicating that RGS5 can arrest
the cell cycle at the transition of the G1 to the S phase by
reducing the proliferation rate of ODMECs under anaerobic
conditions.
RGS5 inhibits the generation of CDC25A
mediated by the MAPK/ERK signaling pathway, which causes cell cycle
arrest at the G1 phase
Under hypoxic conditions, RGS5 arrested ODMECs at
the G1 phase, and the rate of cell proliferation was decreased. The
regulation of the cell cycle is strict and orderly, and the cell
division cycle protein, CDC25A (ras-GRF1), plays a key role in the
regulation of the cell cycle and the response to DNA damage.
We downregulated the expression of the RGS5 protein
with RNAi under hypoxic conditions and demonstrated that the RGS5
inhibited the expression of CDC25A (Fig. 5A). The expression of CDC25A was
higher under normoxic conditions compared with that under hypoxia
(Fig. 5A) and it was positively
associated with cell proliferation. Furthermore, upon assessment of
the CDK2 and cyclin E proteins, which are closely linked to the
cell cycle, it was found that both proteins increased when the RGS5
protein was decreased (Fig. 5B),
indicating that the high expression of RGS5 was associated with
cell cycle arrest.
 | Figure 5.RGS5 inhibits the expression of the
CDC25A protein induced by the MAPK/ERK signaling pathway. (A) The
expression of the CDK2, CDC25A and cyclin E proteins was higher
compared with the LV-H negative control group under hypoxic
conditions, and the expression of the p53 and p21 proteins
exhibited no change; the expression of phosphorylated ERK protein
in the LV-siRGS5 group was markedly decreased and phosphorylation
of p38 was not affected. (B) When the activity of ERK1/2 was
inhibited with PD98059, the expression of the CDC25A, CDK2 and
cyclin E proteins was decreased compared with the control group,
and the effect of PD98059 on downregulating the expression of these
proteins was in coordination with RGS5. (C) Treating ODMECs with
PD98059 and LV-siRGS5 concurrently significantly reduced the cell
proliferation rate (n=3; *P<0.05). RGS5, regulator of G-protein
signaling 5; ODMECs, ovarian carcinoma-derived endothelial cells;
MAPK, mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; LV, lentiviral vector. |
The MAPK signaling pathway generally participates in
cell proliferation and differentiation. In the present study, we
found that the expression of the phosphorylated ERK protein
significantly decreased when the RGS5 protein was decreased with
RNAi, but p38 phosphorylation exhibited no obvious changes
(Fig. 5A). To determine whether
RGS5 participates in cell proliferation and cell cycle regulation
of ODMECs by ERK kinase, we inhibited the ERK1/2 phosphorylation
protein using the ERK1/2 inhibitor, PD98059. When the activity of
ERK1/2 was inhibited, the expression of the CDC25A, CDK2 and cyclin
E proteins were decreased compared with the control group, and the
effect of PD98059 on downregulating the expression of these
proteins was associated with the expression levels of RGS5
(Fig. 5B). In addition, treatment
of ODMECs with PD98059 and LV-siRGS5 concurrently significantly
reduced the cell proliferation rate (Fig. 5C), indicating that RGS5
downregulated the expression of the downstream proteins CDC25A,
CDK2 and cyclin E, and that this effect was mediated by the
MAPK/ERK pathway. In this manner, the ODMEC cell cycle was arrested
at the G1 phase, which decreased the cell proliferation ability. In
addition, careful surveillance of the DNA integrity during the G1
phase also revealed that the cell DNA repair apparatus and the
apoptosis-inducing p53 protein were not affected by the change in
the expression of RGS5 (Fig. 5A).
The expression of cyclin-dependent kinase inhibitor, p21, which is
located downstream of the p53 gene, and that can coordinate the
association between the cell cycle, DNA replication and repair, did
not significantly differ between the LV-H and LV-siRGS5 groups
(Fig. 5A).
Discussion
RGS5 was recently revealed to be involved in tumor
angiogenesis and metastasis (18,24).
Thus, targeting RGS5 may affect both tumor cells and tumor vessels.
In this study, using IHC, we found that RGS5 was weakly expressed
in EOC microvessels expressing endoglin, with no expression in
CD34-labeled blood vessels. Similarly, weak expression of both CD34
and endoglin was also found in lumen-like structures. Some scholars
have reported that the expression of RGS5 is consistent with CD31
expression in the vasculature, and also that the expression of RGS5
and CD31 in blood vessels overlap (19,25).
However, we used CD34 as a marker for the blood vessels in EOC, and
there was no overlap between the RGS5 and CD34 markers. Although
CD31 and CD34 are markers of macrovascular and microvascular
endothelial cells, the two were revealed to be mainly expressed in
mature endothelial cells, indicating that RGS5 is only expressed
during early angiogenesis.
Studies have confirmed that the expression of RGS5
in mature or large blood vessels was significantly reduced, but was
still higher compared with that in normal vascular endothelial
cells, suggesting that RGS5 may be used as a potential
anti-angiogenic target. Endoglin is highly expressed in
tumor-derived vascular endothelial cells, and is of great value as
a marker of tumor angiogenesis (20,26).
Using IHC, we found that endoglin was mainly expressed in new
microvascular endothelial cells in the peripheral regions of EOC
compared with CD34-labeled blood vessels, whereas
endoglin-expressing blood vessels in the central part of the tumor
were significantly fewer, or even absent. In addition, RGS5 and
endoglin staining of EOC microvessels overlapped, indicating that
RGS5 may be involved in the regulation of early tumor angiogenesis.
Although studies have revealed that RGS5 is highly expressed in
early angiogenesis, participates in pericyte accumulation and
differentiation through the PDGF-BB/PDGFR pathway and plays an
important role in vascular remodeling, the mechanisms by which
tumor vascular endothelial cells are regulated remain unclear.
MVD is a measure of the degree of tumor angiogenesis
(21). Therefore, we found that MVD
(reflected by CD34 expression) in tissues with high expression of
RGS5 was significantly lower compared with that in tissues with low
expression of RGS5. The two proteins were inversely correlated,
indicating that the RGS5 protein may be involved in the regulation
of ovarian cancer angiogenesis, thus affecting the MVD in ovarian
cancer. This result is consistent with the expression of RGS5 and
MVD in gastric carcinoma. The increase of the mean MVD was revealed
to be associated with tumor metastasis. Although the expression of
RGS5 was not found to be associated with ovarian cancer lymph node
metastasis, it was associated with peritoneal metastasis,
indicating that RGS5 plays an important role in the invasion and
metastasis of ovarian cancer to the peritoneum.
Further analysis of the association between the
expression of the RGS5 protein and clinicopathological
characteristics revealed that the expression of the RGS5 protein in
ovarian cancer cells exhibited no significant correlation with
patient age, level of serum tumor markers such as CA125, the
presence of ascitic fluid, tumor size, tumor differentiation,
histological type or clinical stage of ovarian carcinoma. Ovarian
cancer patients were followed up for 5–10 years, and using a
univariate survival analysis we demonstrated that the expression of
the RGS5 protein was associated with prognosis. The postoperative
5-year survival rate in patients with high expression of the RGS5
protein was higher compared with that in patients with low RGS5
expression. The log-rank test demonstrated that there were
significant differences between the two groups of patients, with
those exhibiting high expression of RGS5 having a better prognosis.
The prognosis of ovarian cancer patients was associated with tumor
histological type, pathological grade, clinical stage, patient age,
as well as several other factors. Previous studies also found that
intratumoral MVD was a prognostic risk factor for ovarian cancer
(21,22,27,28),
although MVD was an independent factor in the prognosis of patients
with endometrial carcinoma (23),
cervical cancer (24,29), and kidney and breast cancer
(25,30). This indicated that the regulation of
MVD is of major research value in ovarian cancer.
In order to determine whether RGS5 is associated
with the tube-forming ability of ODMECs in vitro, the
expression of RGS5 was detected at different stages of lumen-like
structure formation. However, although the expression of RGS5 was
high in tumor vessels, it was low in ODMECs in vitro,
suggesting that it may be associated with the high expression of
angiogenic factors in the tumor microenvironment. This revealed
that RGS5 does not play a key regulatory role in the formation of
lumen-like structures by ODMECs in vitro, but does not
exclude the possibility of its involvement in the regulation of
angiogenesis in other ways. A previous study reported that the
expression of RGS5 in tumors highly expressing VEGF was higher
compared with that in tumors with low VEGF expression in mice. In a
mouse corneal neovascularization model, blocking the VEGF-mediated
signaling pathway before the upregulation of the RGS5 gene
effectively reduced neovascularization, whereas blocking the
signaling pathway after upregulation of the RGS5 gene did not have
the same effect, indicating that there is a connection between RGS5
and VEGF (11). In addition, the
expression profiles of RGS5 and the PDGF-BB receptor are consistent
in solid tumors (30). However,
adding the angiogenesis-related factors, VEGF and PDGF-BB, at
concentrations of 10 ng/ml for 24 h to the culture system
stimulated ODMECs. The results indicated that VEGF and PDGF-BB
cannot significantly promote the expression of RGS5 in ODMECs and
HASMCs, suggesting that the high expression of RGS5 in tumor tissue
does not depend entirely on the regulation of the VEGFR and PDGF-BB
signaling pathways.
Animal experiments have confirmed that RGS5 gene
expression is significantly increased in the brain cortex and
hippocampus of rats under chronic hypoxic conditions. The response
to hypoxia when the hypoxia inducible factor-1 (HIF-1) gene was
knocked down in mice in vivo was to decrease the expression
of RGS5. This revealed that the expression of RGS5 was regulated by
hypoxia in tumor tissues and may be regulated by the HIF-1α
signaling pathway (31,35). Hence, through regulation of the
balance between angiogenic and anti-angiogenic factors, RGS5 may
affect the progression of tumor vascularization under anoxic
conditions.
In the present study, we downregulated the
expression of the RGS5 protein in ODMECs under hypoxic conditions
with a specific RGS5-siRNA lentiviral vector in order to explore
the mechanism underlying its role in the angiogenesis of ovarian
cancer. We found that ODMECs contained abundant microtubules and
microfilaments in the cytoplasm when examined under an electron
microscope, indicating strong motility. Subsequently, by using the
scratch test we investigated whether the high expression of RGS5
induced by hypoxia was involved in cell migration. The healing rate
at 16 h after scratching in the LV-H-ODMEC and LV-siRGS5-ODMEC
groups did not differ significantly, indicating that the high
expression of RGS5 does not affect the migration ability of ODMECs
under hypoxic conditions. The tumor microenvironment contains
numerous factors, and the function of proteins in tumors is often
affected by the tumor microenvironment. We found that RGS5 does
play a decisive role in the formation of lumen-like structures by
ODMECs; therefore, it may affect ODMEC angiogenesis through other
mechanisms, such as regulation of cell proliferation (32,36).
Upon investigating the effect of RGS5 on ODMECs by MTT method under
anoxic conditions, it was found that the RGS5 protein inhibited the
proliferation of ODMECs. Upon further investigation, it was
revealed that RGS5 also promoted cell apoptosis under hypoxic
conditions, arresting ODMECs at the G1 phase of the cell cycle,
thus inhibiting progression into the S phase.
The regulation of the cell cycle is strict and
orderly (33,37), and the cell division cycle protein,
CDC25A (ras-GRF1), plays a key role in the regulation of the cell
cycle and the response to DNA damage (34,38).
The CDC25 gene is highly conserved and has three subtypes CDC25A,
CDC25B and CDC25C, among which CDC25A is considered to be involved
in the G1/S and G2/M checkpoints, whereas CDC25B and CDC25C are
mainly involved in the regulation of the G2/M checkpoint (35,39).
We determined that RGS5 inhibited the expression of CDC25A through
the downregulation of RGS5 protein expression with an RGS5-specific
siRNA slow virus vector. The expression of CDC25A was higher under
normoxic conditions compared with hypoxia, and it was positively
associated with cell proliferation. CDK2 and cyclin E are closely
linked to the cell cycle, and we found that the expression of the
RGS5 protein was inversely associated with both proteins,
indicating that a high expression of RGS5 was associated with cell
cycle arrest. The MAPK signaling pathway, including the ERK, p38
and JNK pathways, participates in cell proliferation and
differentiation (36). The JNK
pathway is mainly involved in cell inflammatory reactions, whereas
ERK-2 and p38 act through the receptors of a variety of growth
factors, mainly mediating the signal of peptide growth factors.
Their phosphorylation can promote cell proliferation,
differentiation and migration (37,40).
To determine whether RGS5 participated in the regulation of ODMEC
proliferation and the cell cycle by ERK kinase, we used the ERK1/2
inhibitor, PD98059, to inhibit the phosphorylation of ERK1/2. The
results demonstrated that the expression of the CDC25A, CDK2 and
cyclin E proteins was decreased compared with that in the control
group, and the effect of PD98059 on the downregulation of these
proteins was coordinated with RGS5. Furthermore, treating ODMECs
with PD98059 and LV-siRGS5 simultaneously can significantly reduce
the cell proliferation rate, indicating that RGS5 can downregulate
the expression of the CDC25A, CDK2 and cyclin E downstream
proteins, which is mediated by the MAPK/ERK pathway, causing ODMEC
cycle arrest at the G1 phase, thereby decreasing cell proliferation
ability.
Acknowledgements
The authors are grateful to Mrs. YuDi Li and Mrs.
ChunDong Lu for the collection of epithelial ovarian carcinoma
samples, and to Professor XueFeng Jiang and Dr Qiao Wu for
technical assistance. We would like to thank Mr. Jiang Zhu and Mr.
Feng Wu for their help in evaluating the immunohistochemistry
results. The authors would like to thank Dr Dev Sooranna, Imperial
College London, for helping to edit the manuscript.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81101994 and
81072125).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW, PYa and ZL conceived and designed the study. DW
and YX performed the experiments. LF and PYi collected the patient
samples and the information. SSS and FW contributed to the
statistical analysis. PYi wrote the manuscript and YX and PYa
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of the Third Military Medical University approved the
procedures and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ODMECs
|
ovarian carcinoma-derived endothelial
cells
|
|
EOC
|
epithelial ovarian cancer
|
|
RGS5
|
regulator of G-protein signaling 5
|
|
MVD
|
microvascular density
|
|
RNAi
|
RNA interference
|
|
CDK2
|
cyclin-dependent protein kinase 2
|
|
MAPK/ERK
|
mitogen-activated protein
kinase/extracellular signal-regulated kinase
|
|
GPCRs
|
G-protein-coupled receptors
|
|
HASMCs
|
human aortic smooth muscle cells
|
References
|
1
|
Chen Q, Su Y, He X, Zhao W, Wu C, Zhang W,
Si X, Dong B, Zhao L, Gao Y, et al: Plasma long non-coding RNA
MALAT1 is associated with distant metastasis in patients with
epithelial ovarian cancer. Oncol Lett. 12:1361–1366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landen CN Jr, Birrer MJ and Sood AK: Early
events in the pathogenesis of epithelial ovarian cancer. J Clin
Oncol. 26:995–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang HS, Feng YJ and Yao LQ: Angiogenesis,
vasculogenesis, and vasculogenic mimicry in ovarian cancer. Int J
Gynecol Cancer. 19:605–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JW, Shahzad MM, Lin YG, Armaiz-Pena G,
Mangala LS, Han HD, Kim HS, Nam EJ, Jennings NB, Halder J, et al:
Surgical stress promotes tumor growth in ovarian carcinoma. Clin
Cancer Res. 15:2695–2702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kroep JR and Nortier JW: The role of
bevacizumab in advanced epithelial ovarian cancer. Curr Pharm Des.
18:3775–3783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato S and Itamochi H: Bevacizumab and
ovarian cancer. Curr Opin Obstet Gynecol. 24:8–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tejpar S, Prenen H and Mazzone M:
Overcoming resistance to antiangiogenic therapies. Oncologist.
17:1039–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurst JH and Hooks SB: Regulator of
G-protein signaling (RGS) proteins in cancer biology. Biochem
Pharmacol. 78:1289–1297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lappano R and Maggiolini M: G
protein-coupled receptors: Novel targets for drug discovery in
cancer. Nat Rev Drug Discov. 10:47–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perschbacher KJ, Deng G, Fisher RA,
Gibson-Corley KN, Santillan MK and Grobe JL: Regulators of
G-protein signaling in cardiovascular function during pregnancy.
Physiol Genomics. 50:590–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitchell TS, Bradley J, Robinson GS, Shima
DT and Ng YS: RGS5 expression is a quantitative measure of pericyte
coverage of blood vessels. Angiogenesis. 11:141–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aird WC: Endothelial cell heterogeneity.
Cold Spring Harb Perspect Med. 2:a0064292012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hida K, Hida Y, Amin DN, Flint AF,
Panigrahy D, Morton CC and Klagsbrun M: Tumor-associated
endothelial cells with cytogenetic abnormalities. Cancer Res.
64:8249–8255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Comprehensive Cancer Network
(NCCN): NCNN clinical practice guidelines in Oncology. Ovarian
cancer including fallopian tube cancer and primary peritoneal
cancer (V2.2018). https://www.nccn.org/patientsMarch 9–2018
|
|
15
|
Xu Y, Wang D, Zhao LM, Zhao XL, Shen JJ,
Xie Y, Cao LL, Chen ZB, Luo YM, Bao BH and Liang ZQ: Endoglin is
necessary for angiogenesis in human ovarian carcinoma-derived
primary endothelial cells. Cancer Biol Ther. 14:937–948. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho H, Kozasa T, Bondjers C, Betsholtz C
and Kehrl JH: Pericyte-specific expression of Rgs5: Implications
for PDGF and EDG receptor signaling during vascular maturation.
FASEB J. 17:440–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosh K, Thodeti CK, Dudley AC, Mammoto A,
Klagsbrun M and Ingber DE: Tumor-derived endothelial cells exhibit
aberrant Rho-mediated mechanosensing and abnormal angiogenesis in
vitro. Proc Natl Acad Sci USA. 105:11305–11310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Z, Zuo Y, Wang J, Yu Z, Peng F, Chen Y,
Dong Y, Hu X, Zhou Q, Ma H, et al: Overexpression of the regulator
of G-protein signaling 5 reduces the survival rate and enhances the
radiation response of human lung cancer cells. Oncol Rep.
33:2899–2907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng WL, Wang PX, Wang T, Zhang Y, Du C,
Li H and Ji Y: Regulator of G-protein signalling 5 protects against
atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol.
172:5676–5689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan L, Yang H, Xu C, Chen S, Meng Z, Li K
and Chen H: ZNF750 inhibited the malignant progression of oral
squamous cell carcinoma by regulating tumor vascular
microenvironment. Biomed Pharmacother. 105:566–572. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mills GB and Moolenaar WH: The emerging
role of lysophosphatidic acid in cancer. Nat Rev Cancer. 3:582–591.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abramow-Newerly M, Roy AA, Nunn C and
Chidiac P: RGS proteins have a signalling complex: Interactions
between RGS proteins and GPCRs, effectors, and auxiliary proteins.
Cell Signal. 18:579–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tesmer JJ, Berman DM, Gilman AG and Sprang
SR: Structure of RGS4 bound to AlF4-activated G(i alpha1):
Stabilization of the transition state for GTP hydrolysis. Cell.
89:251–261. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang JH, Huang WS, Hu CR, Guan XX, Zhou HB
and Chen LB: Relationship between RGS5 expression and
differentiation and angiogenesis of gastric carcinoma. World J
Gastroenterol. 16:5642–5646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silini A, Ghilardi C, Figini S, Sangalli
F, Fruscio R, Dahse R, Pedley RB, Giavazzi R and Bani M: Regulator
of G-protein signaling 5 (RGS5) protein: A novel marker of cancer
vasculature elicited and sustained by the tumor's proangiogenic
microenvironment. Cell Mol Life Sci. 69:1167–1178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nassiri F, Cusimano MD, Scheithauer BW,
Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K and Lloyd RV:
Endoglin (CD105): A review of its role in angiogenesis and tumor
diagnosis, progression and therapy. Anticancer Res. 31:2283–2290.
2011.PubMed/NCBI
|
|
27
|
Taskiran C, Erdem O, Onan A, Arisoy O,
Acar A, Vural C, Erdem M, Ataoglu O and Guner H: The prognostic
value of endoglin (CD105) expression in ovarian carcinoma. Int J
Gynecol Cancer. 16:1789–1793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raspollini MR, Amunni G, Villanucci A,
Baroni G, Boddi V, Rossi Degl'innocenti D and Taddei GL:
Microvessel density in ovarian carcinoma: Computer image analysis
in patients with shorter and longer survival. Int J Gynecol Cancer.
15:844–849. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cantu De León D, Lopez-Graniel C, Frias
Mendivil M, Chanona Vilchis G, Gomez C and De La Garza Salazar J:
Significance of microvascular density (MVD) in cervical cancer
recurrence. Int J Gynecol Cancer. 13:856–862. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: A systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang G, Song H, Wang R, Han X and Chen L:
The relationship between RGS5 expression and cancer differentiation
and metastasis in non-small cell lung cancer. J Surg Oncol.
105:420–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ladds G, Goddard A, Hill C, Thornton S and
Davey J: Differential effects of RGS proteins on G alpha(q) and G
alpha(11) activity. Cell Signal. 19:103–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurrey NK, K A and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Y, An X, Ye Z, Cully B, Wu J and Li J:
RG S5, a hypoxia-inducible apoptotic stimulator in endothelial
cells. J Biol Chem. 284:23436–23443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cimpean AM, Saptefrati L, Ceausu R and
Raica M: Characterization of endoglin and Ki-67 expression in
endothelial cells from benign and malignant lesions of the uterine
cervix. Pathol Int. 59:695–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Qiu H, Song Y, Liu Y, Wang H, Lu M,
Deng M, Gu Y, Yin J, Luo K, et al: Cip2a promotes cell cycle
progression in triple-negative breast cancer cells by regulating
the expression and nuclear export of p27Kip1. Oncogene.
36:1952–1964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao S, Wang Y, Guo T, Yu W, Li J, Tang Z,
Yu Z, Zhao L, Zhang Y, Wang Z, et al: YBX1 regulates tumor growth
via CDC25a pathway in human lung adenocarcinoma. Oncotarget.
7:82139–82157. 2016.PubMed/NCBI
|
|
39
|
Nilsson I and Hoffmann I: Cell cycle
regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res.
4:107–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Anger T, El-Chafchak J, Habib A, Stumpf C,
Weyand M, Daniel WG, Hombach V, Hoeher M and Garlichs CD: Statins
stimulate RGS-regulated ERK 1/2 activation in human calcified and
stenotic aortic valves. Exp Mol Pathol. 85:101–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|



















