Introduction
Lung cancer is the most common cause of cancer death
in Japan, North America and Europe. Approximately 85% of these
cases are non-small cell lung cancer (NSCLC) (1). More than 50% of patients with NSCLC
have lymphadenopathy and/or distant metastasis and, thus, a poor
prognosis. The 5-year relative survival rate of patients with
distant metastases is less than 4% (2). The primary reason for the difficulties
associated with treating NSCLC is that patients are identified at a
very late stage and effective treatments available for advanced
NSCLC are limited. However, advances have recently been achieved in
elucidating the molecular origins of NSCLC, with the better
classification of histological subtypes and development of targeted
therapies. A molecular analysis of NSCLC has suggested many new
molecularly targeted drugs for its treatment (3,4). Thus,
suitable preclinical tumor models are required to assess whether
new anticancer drugs will be effective for NSCLC.
Preclinical tumor models are a fundamental component
for the study and design of new treatment regimens for cancer
(5). These models are either
ectopic (tumors in an abnormal site, such as lung cancer grown
subcutaneously) or orthotopic (tumors in their organ or tissues of
origin, such as lung cancer in the lung). In 1889, Paget proposed
the ‘seed and soil’ theory in which an organ-specific site provides
tumor cells with the most appropriate environment for local growth
and metastasis (6). Subcutaneously
implanted tumors in mice generally grow rapidly, which does not
reflect the slower doubling times of most human cancers; therefore,
they may be more sensitive to chemotherapy drugs than orthotopic
tumors (7). Models with
subcutaneously implanted tumors generally show a low incidence of
distant metastatic disease. The orthotopic transplantation of
tumors results in a higher incidence of distant metastases
(8), and these models show
spontaneous metastasis. Orthotopic models may provide more relevant
pharmacokinetic and pharmacodynamic information than subcutaneous
ectopic models (9). The advantage
of orthotopic models is their similar characteristics to those of
tumors in clinical settings (5).
Considerable efforts have focused on developing
clinically relevant models using orthotopic tumor implantation. We
established an orthotopically implanted lung cancer model in SCID
mice without thoracotomy (10–15).
We previously reported an animal model that consisted of the
orthotopic implantation of lung cancer cell lines and showed a high
frequency of lymphatic metastasis. This model was simple and
reproducible, enabling transplantation in many mice at once, and
metastatic patterns, exhibiting lymphatic metastases and/or distant
metastases at high frequencies, were similar to those observed
clinically in human lung cancer. However, the primary disadvantage
of orthotopic models is that changes in tumor sizes are difficult
to monitor continuously and reproducibly, and may only be assessed
at necropsy.
To conduct continuous monitoring, we employed in
vivo imaging techniques, such as computed tomography (CT) and
positron-emission tomography-computed tomography (PET/CT), for
small animals. CT and 18F-fluorodeoxyglucose
(18F-FDG) PET/CT are excellent methods for detecting
primary lesions or lymph node metastases in patients with NSCLC. CT
provides high anatomical resolution criteria for the detection of
abnormal lymph nodes, with an axial short-axis diameter of 1-cm
sensitivity (51–64%) and specificity (74–86%). PET using
18F-FDG is superior for detecting lymph node metastasis
with a sensitivity of 74% and specificity of 85% (16–18).
We previously reported the non-invasive monitoring of the
anticancer effects of cisplatin (CDDP) on lung cancer in an
orthotopic SCID mouse model using 18F-FDG PET/CT
(19). The findings obtained
supported the use of 18F-FDG-PET/CT to detect tumor
progression and the therapeutic responses of lung cancer in an
orthotopic model through non-invasive and repeated monitoring. We
consider our orthotopic models to provide suitable systems for the
preclinical evaluation of the antitumor efficacies of potential new
NSCLC therapies.
In the present study, we evaluated the usefulness of
small-animal PET/CT and CT to non-invasively and repeatedly monitor
the inhibitory effects of the conventional anticancer agents, CDDP
and erlotinib, on orthotopically implanted lung cancer in SCID
mice. The aim of the present study was to establish a standard
model to evaluate the efficacies of novel treatment regimens in
lung cancer.
Materials and methods
Animals
Eighty male SCID mice (CB-17/Icr-scidJc1; CLEA
Japan, Inc., Tokyo, Japan) at 6–8 weeks of age were used in the
present study and maintained at the Laboratory for Animal
Experiments of our institution. The animals were housed in
microisolator cages on a layer of wood shavings at a temperature of
22±2°C under a fixed 12 h light/dark regime. The basic diet (MF;
Oriental Yeast Co., Ltd., Tokyo, Japan) and water were available
ad libitum. All experiments were performed in accordance
with the guidelines established by the Tokushima University
Committee on Animal Care and Use. At the end of each in vivo
experiment, mice were anesthetized with isoflurane and euthanized
humanely by dislocating the vertebrae. All experimental protocols
were reviewed and approved by the Animal Research Committee of The
University of Tokushima, Japan.
Cell lines
We used three types of NSCLC cell lines in the
present study: A549 (human adenocarcinoma lung cancer cells), FT821
(human large cell lung cancer cells) and PC-9 (human adenocarcinoma
cells). We established the FT821 cell line using a primary culture
of surgically resected tissue provided by Dr Haruhiko Fujino
(Tokushima University, Tokushima, Japan) (13). A549 (JCRB0076) cells were purchased
from the Health Science Research Resources Bank (Osaka, Japan).
PC-9 (CVCL_B260) cells were kindly provided by Professor Seiji Yano
(Division of Medical Oncology, Cancer Research Institute, Kanazawa
University, Kanazawa, Japan) (20).
PC-9 cells have an epidermal growth factor receptor (EGFR) mutation
(deletion in exon 9 of the EGFR gene, del E746_A750),
whereas the other two cell lines do not. These cell lines were
cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; BioWhittaker; Lonza, Walkersville, MD, USA) and were
maintained at 37°C in a humidified incubator equilibrated with 5%
CO2 and 95% air.
Orthotopic intrapulmonary
implantation
As described in our previous studies (10–15),
mice were fully anesthetized with 1.5% isoflurane inhalation and
placed in the right lateral decubitus position with all four limbs
restrained. A 1-cm transverse incision was made in the left lateral
skin just below the inferior border of the scapula in each mouse.
The muscles were separated from the ribs by sharp dissection and
the intercostal muscles exposed. The left lung was then visible
through the intercostal muscles. A 30-gauge needle was inserted ~5
mm into the lungs through the intercostal muscle and an inoculum of
2×106 tumor cells/ml with 400 mg/ml Matrigel
(Collaborative Biomedical Products, Bedford, MA, USA) was then
dispersed into the left lung in a final volume of 10 µl medium
(2×104 cells). The procedure required ~1 min for
completion and was easily performed. The skin incision was closed
with 3-0 silk.
18F-FDG PET/CT
measurements
On day 20 after the implantation of A549 cells, we
performed a PET/CT measurement on each mouse. Mice were then
divided into two groups: A control group [n=8, saline solution (0.7
ml), single administration intraperitoneally] and
cis-diamminedichloroplatinum (II) (CDDP) (Pfizer Japan Inc.,
Tokyo, Japan) group [n=8, 7 mg/kg CDDP (0.7 ml), single
administration intraperitoneally]. After treatments, PET/CT
measurements were evaluated every 10 days until day 50 after
implantation.
All scans were performed with a Siemens Inveon
small-animal PET scanner (Siemens Healthcare, Knoxville, TN, USA).
Mice with the orthotopic implantation of A549 cells to be monitored
by 18F-FDG PET/CT were fasted for 18–24 h, with access
to water only. Body weights were measured and mice were
anesthetized by 1.5–2.0% isoflurane inhalation and injected via a
tail-vein catheter with 10 MBq/0.1–0.2 ml 18F-FDG. The
lung field was scanned by CT [field of view (FOV): 32.0×32.0×48.1
mm3]. PET data were acquired for 20 min following a
delay of 40 min to allow for FDG uptake.
Measurement of tumor volumes by small
animal CT
All scans were performed with a Siemens Inveon
small-animal CT scanner (Siemens Healthcare). Mice with the
orthotopic implantation of A549, FT821, or PC-9 cells were weighed
and then anesthetized by 1.5–2.0% isoflurane inhalation. CT
acquisition was performed as described in the previous section
(18F-FDG PET/CT measurements).
We orthotopically implanted mice with A549 cells. We
evaluated the success of orthotopic implantations as well as tumor
volumes in all animals by periodic CT after implantation. When
tumor volumes reached >1 mm3, mice were treated with
CDDP or erlotinib. These treatments were initiated on days 21, 50,
and 35 after implantation with A549, FT821, and PC-9 cells,
respectively. Mice implanted with A549 cells were divided into four
groups: control [n=4, saline solution (0.7 ml), single
administration intraperitoneally]; CDDP [n=4, 7 mg/kg CDDP (0.7
ml), single administration intraperitoneally]; control [n=4, 6%
sulfobutylether-β-cyclodextrin (Captisol) (ChemScene, Monmouth
Junction, NJ, USA) solution (0.2 ml)/day, oral administration 5
days/week]; and erlotinib (n=4, 25 mg/kg erlotinib dissolved in 6%
Captisol solution/day, oral administration 5 days/week) (21,22).
CDDP and erlotinib were obtained from Pfizer Japan, Inc. and
Selleck Chemicals (Houston, TX, USA), respectively. Mice implanted
with FT821 and PC-9 cells were similarly divided into four groups
(n=3/group). After treatments, mice were evaluated by CT every
third day.
Analysis of primary tumor volumes and
SUVmax
PET and CT images were analyzed using Inveon
Research Workplace software (IRW; version 3.0; Siemens Healthcare).
In all PET/CT datasets, the volume of interest (VOI) was defined
manually around the primary tumor on CT images as the tumor volume.
In fused PET images, the maximum standardized uptake value
(SUVmax) was calculated from the maximum voxel value
(Bq/ml) in the VOI.
Histological examination of lymph
nodes and lung metastasis
Among the mice examined by PET/CT and CT, all mice
in each group were used for pathological analyses. We sacrificed
mice and en bloc resected the bilateral lungs, trachea and
bronchi, heart, esophagus, and mediastinal region of the mouse.
Implanted tumors in the lungs were sectioned at a maximal cut and
the lungs were cut into 5 to 6 1- to 2-mm-thick pieces in the minor
axis direction and the mediastinum was cut in the longitudinal axis
direction. These pieces were fixed by 10% formalin and embedded in
paraffin. Paraffin sections stained with hematoxylin and eosin
(H&E) were examined by a Leica DM 2500 light microscopy (Leica
Microsystems, Wetzlar, Germany) and the sizes of tumors were
measured using the ruler of the microscope.
Statistical analysis
Tumor volumes and SUVmax numbers in the
control and CDDP groups were compared using the Mann-Whitney U
test. Relationships between tumor volumes and SUVmax
were assessed using Spearman's test (SPSS software, version 20; IBM
Inc., New York, NY, USA). A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Monitoring responses using tumor
volumes and SUVmax
Coronary CT and PET/CT images of control and CDDP
mice are presented in Fig. 1A. In
both groups, CT scans detected a lung tumor in the left lung on day
20 after implantation. The sizes of tumors in both groups increased
from day 20 to 50. In coronal PET/CT images of control and CDDP
mice, intense FDG uptake was observed in the tumors of both groups
on day 30 and the intensity of FDG uptake increased from day 30 to
50. The tumor volumes and SUVmax of the control and CDDP
groups are displayed in Fig. 1B.
Tumor volumes in both groups increased exponentially, and were
similar in the CDDP and control groups from day 20 to 50 after
implantation (Fig. 1B-a).
SUVmax increased from day 20 to 50 after implantation,
with no significant difference between the control and CDDP groups
(Fig. 1B-b). A correlation was
observed between tumor volumes and SUVmax in both groups
(control: r=0.76, P<0.01; CDDP: r=0.70, P<0.01; Fig. 1B-c).
Anticancer effects of CDDP and
erlotinib in mice with A549-, FT821-, and PC-9-implanted tumors
assessed by CT imaging
We examined the anticancer effects of CDDP in mice
with A549-, FT821-, and PC-9-implanted tumors using CT imaging. The
tumor volumes of A549 implants in the CDDP group were similar to
those in the control group, confirming the results of the previous
experiment (Fig. 2A-a). The tumor
volumes of FT821 implants in the CDDP group were significantly
smaller than those in the control group (P<0.05) (Fig. 2A-b). The tumor volumes of PC-9
implants in the CDDP group were smaller than those in the control
group, and this difference was significant on day 44 only
(P<0.05) (Fig. 2A-c).
 | Figure 2.(A) Tumor volumes and body weights in
mice implanted with: a and d, A549; b and e, FT821; or c and f,
PC-9 cells and treated, as indicated, with CDDP or vehicle
(control). (B) Tumor volumes and body weights in mice implanted
with: g and j, A549; h and k, FT821; or i and l, PC-9 cells and
treated, as indicated, with erlotinib or vehicle (control).
*P<0.05, significant difference between control and CDDP
groups. |
In the CDDP groups, the body weights of mice with
A549 and FT821 implants were significantly lower than those of the
respective control groups. In mice with PC-9 implants, this
reduction in body weight was temporary (Fig. 2A, d-f).
We examined the anticancer effects of erlotinib in
mice with A549-, FT821-, and PC-9-implanted tumors by CT imaging.
The tumor volumes of A549 implants in the erlotinib group were
similar to those in the control group (Fig. 2B-g). The tumor volumes of FT821
implants were slightly lower in the erlotinib group than in the
control group (Fig. 2B-h). The
tumor volumes of PC-9 implants were significantly lower in the
erlotinib group than in the control group (P<0.05) (Fig. 2B-i).
The body weights of mice with A549 implants were
significantly lower in the erlotinib group than in the control
group (Fig. 2B-j). The body weights
of mice with FT821 and PC-9 implants were similar with and without
the erlotinib treatment (Fig. 2B, k and
l).
Detection of lymph node metastases of
the mediastinum and lung metastases using CT imaging and
histopathology
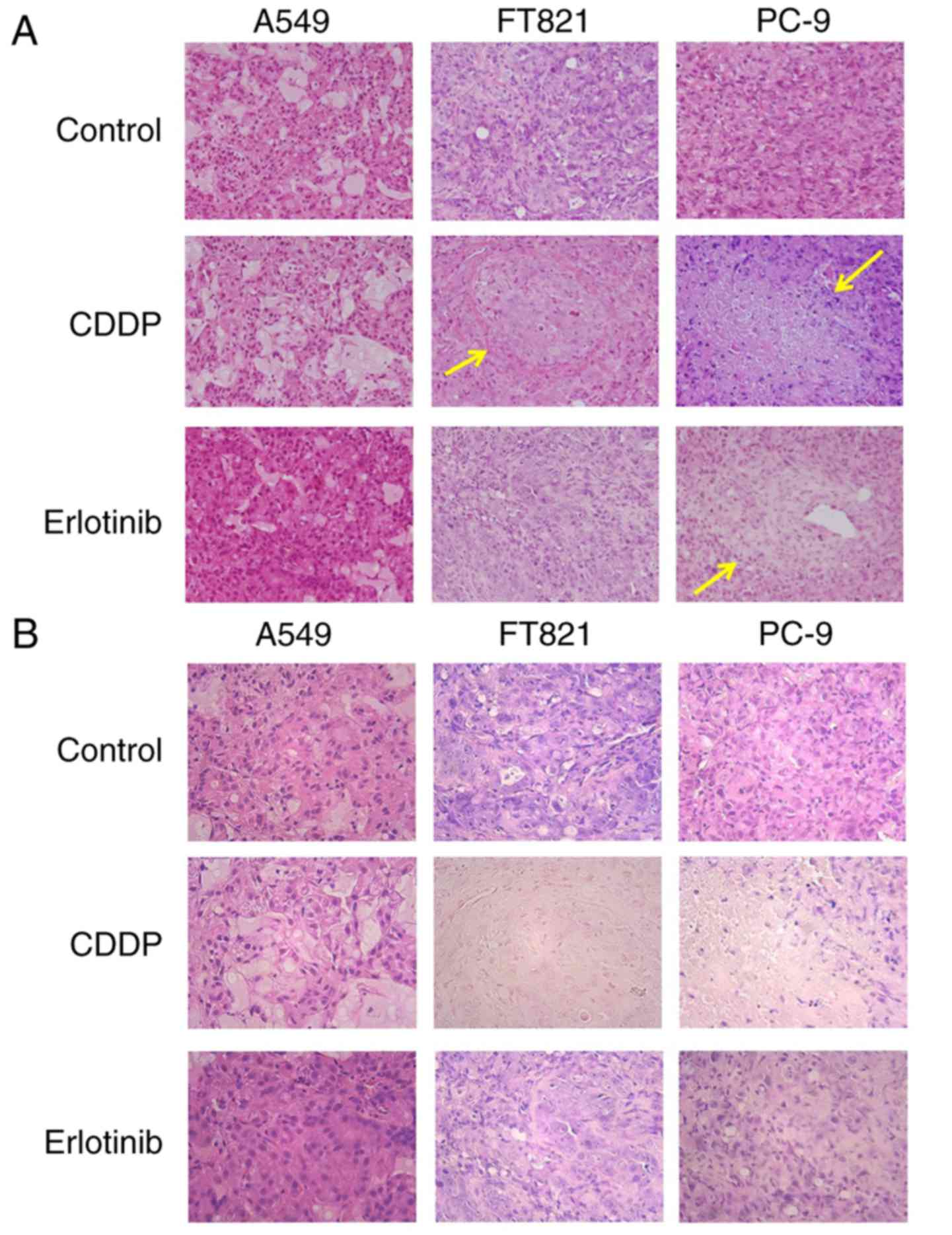
Primary tumors were histopathologically confirmed in
the lungs of all mice (Fig. 3). In
the control groups, no necrotic lesions were observed in the
tumors. In the CDDP groups, there were some necrotic lesions in
mice with PC-9 and FT821 tumors, but not in those with A549 tumors.
There were some necrotic lesions in erlotinib-treated mice with
PC-9, but not A549 or FT821 tumors.
Some mice exhibited the accumulation of FDG in the
mediastinum in the control and CDDP groups. Representative sagittal
and axial PET/CT images of the mediastinum on day 50 after
implantation are revealed in Fig. 4A
and B, with high FDG uptake in the mediastinum (white arrows).
Histopathology revealed lymph node metastases of the mediastinum
microscopically (Fig. 4E and F). In
CT images, lung metastases were detectable, whereas
18F-FDG uptake was not (Fig.
4D).
Metastases of the lung and mediastinal lymph nodes,
as detected by CT and histopathology, in mice treated with or
without CDDP and with erlotinib are presented in Table I. CT imaging detected lung
metastases in A549-, but not FT821- or PC-9-implanted mice. CT
imaging did not detect lymph node metastasis in any of the cell
lines.
 | Table I.Metastases of the lungs and the
mediastinal lymph nodes detected by CT and pathological analysis in
implanted mice with or without CDDP or erlotinib treatment. |
Table I.
Metastases of the lungs and the
mediastinal lymph nodes detected by CT and pathological analysis in
implanted mice with or without CDDP or erlotinib treatment.
|
|
| Treated with
CDDP | Treated with
erlotinib |
|---|
|
|
|
|
|
|---|
|
|
| CT imaging | Pathology | CT imaging | Pathology |
|---|
|
|
|
|
|
|
|
|---|
|
|
| Control | CDDP | Control | CDDP | Control | Erlotinib | Control | Erlotinib |
|---|
| A549 (n=4) | Lung metastases | 2/4 (50%) | 4/4 (100%) | 3/4 (75%) | 4/4 (100%) | 2/4 (50%) | 3/4 (75%) | 2/4 (50%) | 2/4 (50%) |
|
|
| 1.03±0.44 | 1.03±0.42 | 0.78±0.41 | 0.87±0.65 | 0.80±0.53 | 0.64±0.21 | 0.44±0.15 | 0.51±0.15 |
|
| Mediastinal lymph
nodes | None detected | 3/4 (75%) | 4/4 (100%) | None detected | 2/4 (50%) | 1/4 (25%) |
|
|
|
|
| 0.82±0.68 | 0.41±0.38 |
|
| 0.29±0.53 | 0.69±0.59 |
| FT821 (n=3) | Lung
metastases | 0/3 | 0/3 | 3/3 (100%) | 0/3 | 0/3 | 0/3 | 2/3 (66%) | 2/3 (66%) |
|
|
| – | – | 0.44±0.15 | – | – | – | 0.24±0.22 | 0.17±0.12 |
|
| Mediastinal lymph
nodes | None detected | 0.31±0.30 | 2/3 (66%) | None detected | 1/3 (33%) | 2/3 (66%) |
|
|
|
|
| 3/3 (100%) | 0.36±0.23 |
|
| 0.34±0.40 | 0.30±0.39 |
| PC9 (n=3) | Lung
metastases | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
|
|
| – | – | – | – | – | – | – | – |
|
| Mediastinal lymph
nodes | None detected | 3/3 (100%) | 2/3 (66%) | None detected | 3/3 (100%) | 1/3 (33%) |
|
|
|
|
| 0.59±0.37 | 0.42±0.24 |
|
| 0.67±0.54 | 0.12 |
In the histopathological analysis, the frequencies
of lung and lymph node metastases in A549-implanted mice, whose
tumors were resistant to CDDP, were slightly higher with the CDDP
treatment than in the controls (100 vs. 75%, 100 vs. 75%, for lung
and lymph nodes, respectively). There were no lung metastases in
the FT821-implanted mice, whose primary tumors had responded to
CDDP, in the CDDP group, whereas they were detected in the control
group (100%). The lymph node metastasis rate of the mediastinum in
the FT821-implanted mice was lower with the CDDP treatment than in
the control group (66 vs. 100%). The frequency of lung metastasis
in the PC-9-implanted mice, in which CDDP had been slightly
effective, was lower with the CDDP treatment than in the control
group (66 vs. 100%). In each cell line, the anticancer effects of
CDDP for implanted tumors correlated with its effects on lung and
lymph node metastases.
Regarding mice treated with or without erlotinib,
the frequency of lymph node metastasis of the mediastinum in PC-9
implants, which were responsive to erlotinib, was lower with the
erlotinib treatment than in the control group (33 vs. 100%).
Discussion
Since many patients with NSCLC have lymphadenopathy
and/or distant metastasis when diagnosed, more than 80% are
potential beneficiaries of palliative systemic therapy (23). Palliative first-line chemotherapy
was revealed to improve the quality of life and survival of
advanced NSCLC patients due to newly introduced drugs and patient
selection based on different histological subtypes and driver
mutations. These selection parameters may identify the biology of
malignancies and help predict drug efficacy (24). Recently developed molecular analyses
for NSCLC have led to proposals for many new molecularly targeted
drugs to treat NSCLC (3,4).
To provide suitable preclinical systems for
evaluating the efficacy of these drugs, we established patient-like
models of lung cancer metastasis by orthotopically implanting human
NSCLC cell lines without thoracotomy (10–15).
The models we described have the following advantages: i) The
implantation procedure is simple and reproducible; ii) many
tumor-implanted mice may be produced at once; iii) the procedure
may be applied to many NSCLC cell lines; and iv) metastatic
patterns, showing lymphatic metastases and/or distant metastases at
high frequencies, are similar to those observed clinically in human
lung cancer. However, the major disadvantage of these models is
that the tumors, including primary and metastatic, were difficult
to monitor continuously and reproducibly, and may only be assessed
at necropsy. We previously evaluated the utility of
18F-FDG PET-CT to non-invasively and repeatedly monitor
the anticancer effects of CDDP in an orthotopic lung cancer model
using Ma44-3 cells. We demonstrated the significant inhibition of
tumor growth by the CDDP treatment, and also revealed that tumor
volumes and SUVmax correlated in these mice
(r2=0.67, P=0.048) (19). In the present study, we evaluated
the utility of 18F-FDG PET-CT to monitor the anticancer
effects of CDDP in an orthotopic model using another lung cancer
cell line, A549. Tumor volumes and SUVmax were similar
in the CDDP and control groups, and tumor volume correlated with
SUVmax in this A549 model (control: r=0.76, P<0.01;
CDDP: r=0.70, P<0.01). Thus, it was demonstrated that
18F-FDG PET/CT discriminates between CDDP-responsive and
-unresponsive tumors, Ma44-3 and A549, respectively. Since
SUVmax assessed by PET correlated with tumor volumes
using CT imaging, we subsequently used CT only to estimate the
anticancer effects of CDDP in FT821- and PC-9-implanted models and
of erlotinib in models using all cell lines.
Our histological results correlated with anticancer
effects. FT821 and PC-9, but not A549 tumors exhibited necrotic
lesions with CDDP. Therefore, CT imaging detected the anticancer
effects of CDDP, a major cytotoxic anticancer drug that is
intravenously administered. We then used erlotinib, a molecularly
targeted drug that is orally administered. PC-9 tumors were very
responsive to erlotinib, while A549 and FT821 tumors were not.
Histological results correlated with these anticancer effects; PC-9
exhibited necrotic lesions, whereas A549 and FT821 did not. PC-9
cells have an EGFR mutation. The CT monitoring system may detect
the anticancer effects of molecularly targeted drugs or orally
administered drugs such as erlotinib. Moreover, anticancer effects
correlated with the frequencies of lung and lymph node metastases.
Our orthotopic models of lung cancer revealed frequent metastases
to the lymph nodes, similar to the metastatic patterns of lung
cancer observed in clinical settings. We found that PET/CT imaging
detected lymph node metastases of the mediastinum, while CT imaging
detected lung metastases. In the present study, all cell lines,
A549, FT821, and PC-9, exhibited lymph node metastasis of the
mediastinum, as identified histopathologically. CT imaging did not
detect lymph node metastasis of the mediastinum due to poor
contrast between the normal mediastinum tissue and lymph node
metastatic tumors. However, 18F-FDG PET/CT imaging
detected FDG uptake in lymph node metastases of the mediastinum.
Therefore, the latter method may be used to non-invasively and
repeatedly monitor the anticancer effects of agents in lymph node
metastases of the mediastinum. In patients, 18F-FDG
PET/CT is useful for the diagnosis of mediastinal lymph node
metastasis and estimating the responses of lymph node metastases to
anticancer drugs (16). The present
results demonstrated that CT imaging may be used to non-invasively
and repeatedly monitor the anticancer effects of less specific
drugs, such as CDDP, and molecularly targeted drugs, including
erlotinib, on several different lung cancers.
In conclusion, we herein demonstrated that PET/CT
and CT imaging of our orthotopic SCID mouse models of lung cancer
enabled the non-invasive and repeated monitoring of the anticancer
efficacies of CDDP and erlotinib. These effects were detected not
only in implanted tumors, but also in mediastinal lymph node and
lung metastases. These methods may be applied to a number of NSCLC
cell lines and anticancer drugs. Therefore, our models have
potential as fundamental tools for the design and development of
new therapies for cancer.
Acknowledgements
We would like to thank Professor Seiji Yano
(Professor and Chairman, Division of Medical Oncology, Cancer
Research Institute, Kanazawa University) for providing the PC-9
cell line.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology (24659634), Japan.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KKo conceived and designed the study. TO, HT and HF
acquired the data. TO, HT, KKa, HO and HM analyzed the data. TO and
KKo drafted the manuscript and were involved in the conception of
the study. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were reviewed and
approved by the Animal Research Committee of The University of
Tokushima, Japan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stinchcombe TE, Bogart J, Veeramachaneni
NK, Kratzke R and Govindan R: Annual review of advances in
non-small cell lung cancer research: A report for the year 2010. J
Thorac Oncol. 6:1443–1450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horner MJ, Ries LAG, Krapcho M, Neyman N,
Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A,
et al: National Cancer Institute SEER cancer statistics review.
1975-2006, http://seer.cancer.gov/csr/1975_2006November.
2009
|
|
3
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francia G, Cruz-Munoz W, Man S, Xu P and
Kerbel RS: Mouse models of advanced spontaneous metastasis for
experimental therapeutics. Nat Rev Cancer. 11:135–141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
7
|
Wilmanns C, Fan D, O'Brian CA, Bucana CD
and Fidler IJ: Orthotopic and ectopic organ environments
differentially influence the sensitivity of murine colon carcinoma
cells to doxorubicin and 5-fluorouracil. Int J Cancer. 52:98–104.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fidler IJ: Models for spontaneous
metastasis. Cancer Res. 66:97872006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peterson JK and Houghton PJ: Integrating
pharmacology and in vivo cancer models in preclinical and clinical
drug development. Eur J Cancer. 40:837–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyoshi T, Kondo K, Ishikura H, Kinoshita
H, Matsumori Y and Monden Y: SCID mouse lymphogenous metastatic
model of human lung cancer constructed using orthotopic inoculation
of cancer cells. Anticancer Res. 20:161–163. 2000.PubMed/NCBI
|
|
11
|
Ishikura H, Kondo K, Miyoshi T, Kinoshita
H, Hirose T and Monden Y: Artificial lymphogenous metastatic model
using orthotopic implantation of human lung cancer. Ann Thorac
Surg. 69:1691–1695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikura H, Kondo K, Miyoshi T, Kinoshita
H, Takahashi Y, Fujino H and Monden Y: Suppression of mediastinal
metastasis by uracil-tegafur or cis-diamminedichloroplatinum(II)
using a lymphogenous metastatic model in a human lung cancer cell
line. Clin Cancer Res. 7:4202–4208. 2001.PubMed/NCBI
|
|
13
|
Fujino H, Kondo K, Miyoshi T, Ishikura H,
Takahashi Y, Sawada N, Hirose Y, Takizawa H, Nagao T, Sakiyama S,
et al: Establishment of patient-like SCID mouse model by
orthotopically implanting primary cultured cells from
surgically-resected lung cancer tissues. Oncol Rep. 10:1709–1715.
2003.PubMed/NCBI
|
|
14
|
Fujino H, Kondo K, Ishikura H, Maki H,
Kinoshita H, Miyoshi T, Takahashi Y, Sawada N, Takizawa H, Nagao T,
et al: Matrix metalloproteinase inhibitor MMI-166 inhibits
lymphogenous metastasis in an orthotopically implanted model of
lung cancer. Mol Cancer Ther. 4:1409–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takizawa H, Kondo K, Toba H, Kenzaki K,
Sakiyama S and Tangoku A: Fluorescence diagnosis of lymph node
metastasis of lung cancer in a mouse model. Oncol Rep. 22:17–21.
2009.PubMed/NCBI
|
|
16
|
Sauter AW, Spira D, Schulze M, Pfannenberg
C, Hetzel J, Reimold M, Klotz E, Claussen CD and Horger MS:
Correlation between [18F]FDG PET/CT and volume perfusion
CT in primary tumours and mediastinal lymph nodes of non-small-cell
lung cancer. Eur J Nucl Med Mol Imaging. 40:677–684. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walker CM, Chung JH, Abbott GF, Little BP,
El-Sherief AH, Shepard JA and Lanuti M: Mediastinal lymph node
staging: From noninvasive to surgical. AJR Am J Roentgenol.
199:W54–W64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silvestri GA, Gould MK, Margolis ML,
Tanoue LT, McCrory D, Toloza E and Detterbeck F: American College
of Chest Physicians: Noninvasive staging of non-small cell lung
cancer: ACCP evidenced-based clinical practice guidelines (2nd
edition). Chest. 132((3 Suppl)): 178S–201S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mokhtar M, Kondo K, Takizawa H, Ohtani T,
Otsuka H, Kubo H, Kajiura K, Nakagawa Y, Kawakami Y, Yoshida M, et
al: Non-invasive monitoring of anticancer effects of cisplatin on
lung cancer in an orthotopic SCID mouse model using
[18F] FDG PET-CT. Oncol Rep. 31:2007–2014. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Li Q, Yamada T, Matsumoto K,
Matsumoto I, Oda M, Watanabe G, Kayano Y, Nishioka Y, Sone S and
Yano S: Crosstalk to stromal fibroblasts induces resistance of lung
cancer to epidermal growth factor receptor tyrosine kinase
inhibitors. Clin Cancer Res. 15:6630–6638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishikawa D, Takeuchi S, Nakagawa T, Sano
T, Nakade J, Nanjo S, Yamada T, Ebi H, Zhao L, Yasumoto K, et al:
mTOR inhibitors control the growth of EGFR mutant lung cancer even
after acquiring resistance by HGF. PLoS One. 8:e621042013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nanjo S, Ebi H, Arai S, Takeuchi S, Yamada
T, Mochizuki S, Okada Y, Nakada M, Murakami T and Yano S: High
efficacy of third generation EGFR inhibitor AZD9291 in a
leptomeningeal carcinomatosis model with EGFR-mutant lung cancer
cells. Oncotarget. 7:3847–3856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson DH, Schiller JH and Bunn PA Jr:
Recent clinical advances in lung cancer management. J Clin Oncol.
32:973–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noonan KL, Ho C, Laskin J and Murray N:
The influence of the evolution of first-line chemotherapy on
steadily improving survival in advanced non-small-cell lung cancer
clinical trials. J Thorac Oncol. 10:1523–1531. 2015. View Article : Google Scholar : PubMed/NCBI
|