Introduction
Prostate cancer (PCa) is the second most common type
of male malignancy worldwide and ~1.3 million new cases of PCa are
predicted to occur in 2018. Furthermore, PCa is the fifth leading
cause of cancer-associated mortality in men, and ~359,000 cases of
PCa-associated mortality are predicted to occur worldwide in 2018
(1).
Androgens have an important role in the development
and growth of the normal prostate gland, as well as in the
proliferation of PCa cells (2,3). It
has been reported that activation of the androgen receptor (AR)
promotes the proliferation of PCa (3). Therefore, androgen deprivation therapy
(ADT) is considered the gold standard for the treatment of PCa
recurrence and metastasis. However, despite a good initial response
to ADT, the majority of patients progress to aggressive
castration-resistant PCa (CRPC) within 2–3 years (2,4).
Various mechanisms underlying resistance to ADT have been
identified, including AR hypersensitivity, mutations, amplification
and splicing variants, in addition to intratumoral steroidogenesis
(3,4). Regarding intratumoral steroidogenesis,
distinct patterns of dysregulated expression of enzymes involved in
androgen synthesis and metabolism have been reported, indicating
that tumor cells from patients with CRPC exhibit increased
expression of the steroidogenic enzymes hydroxysteroid
dehydrogenase (HSD)3β1, HSD3β2, HSD17β3, aldo-keto reductase family
1 member C3 (AKR1C3) and 5α-reductase 1 (5,6).
Notably, ADT may induce epithelial-mesenchymal
transition (EMT); activation of this transdifferentiation program
may increase tumor malignancy (7).
A previous study demonstrated the importance of zinc finger E-box
binding homeobox 1 (ZEB1), which is a canonical transcription
factor of EMT, since it is not only a key EMT factor, but also an
AR regulator (8). In the PC3 cell
line, a negative loop of regulation has been identified between
ZEB1 and the AR, resulting in decreased levels of AR in response to
high ZEB1 expression and vice versa. Furthermore, the AR is a
direct regulator of the ZEB1 gene, since it binds to two androgen
response elements located 1,000 base pairs near the site of
transcriptional initiation (8).
However, to the best of our knowledge, the alterations occurring in
androgen synthesis in relation to ZEB1 have not yet been
established. Therefore, it may be hypothesized that ZEB1 alters
androgen synthesis capacity in PCa.
The present study aimed to determine the alterations
in androgen synthesis and AR expression induced by ZEB1 knockdown
in the PCa cell line DU145. The results indicated that ZEB1
silencing significantly altered the expression of the cholesterol
transporter steroidogenic acute regulatory protein (StAR), and
enzymes involved in the synthesis and degradation of androgens,
including cytochrome P450 family 17 subfamily A member 1 (CYP17A1),
5α-reductase 1, 5α-reductase 2, aldo-keto reductase family 1 member
D1 (AKR1D1) and aldo-keto reductase family 1 member C2 (AKR1C2),
thus resulting in an increase in testosterone and
dihydrotestosterone (DHT) concentration in the cell culture medium.
These findings may provide novel information regarding the
regulation of androgen synthesis in PCa.
Materials and methods
Cell culture and transduction
The PCa cell line DU145 [cat. no. American Type
Culture Collection (ATCC) HTB-81] was obtained from the ATCC
(Manassas, VA, USA). ZEB1-knockdown cells (DU145 SH) and control
cells (DU145 SCR) were produced in our laboratory according to
protocols described in our previous study (9). Briefly, 5.5×104 cells were
transduced with 1 µg lentiviral vector containing a short hairpin
(sh)RNA against ZEB1 [pLenti-6-shRNA (h ZEB1)-Rsv (RFP-Puro)]
(GenTarget, Inc., San Diego, CA, USA). A shRNA against a random
sequence was used as a control [pLenti-U6-shRNA (neg-control)-Rsv
(RFP-Puro)] (GenTarget, Inc.). Subsequently, cells were selected
with 1.5 µg/ml puromycin and effective silencing was verified using
fluorescence microscopy, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting (9). These cell lines were cultured in
RPMI-Phenol Red-free culture medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Corning Incorporated, Corning, NY, USA) and were
incubated at 37°C in an atmosphere containing 5%
CO2.
RT-qPCR
The cells were harvested and lysed using
TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.). RNA extraction was conducted according to
standard procedures, and RNA was quantified using a Synergy HT
Multi-Detection Microplate Reader (Biotek Instruments, Inc.,
Winooski, VT, USA). Subsequently, 100 ng total RNA was reverse
transcribed into cDNA using the Affinityscript QPCR cDNA Synthesis
commercial kit (Agilent Technologies, Inc., Santa Clara, CA, USA).
The templates were amplified in one cycle of 5 min at 25°C, 45 min
at 42°C and 5 min at 95°C. qPCR was then performed using the SYBR
green QPCR Master Mix kit (Agilent Technologies, Inc.) in an Aria
Mix Real-Time PCR system (Agilent Technologies, Inc.), according to
the manufacturer's protocol. The templates were amplified as
follows: One cycle at 95°C for 10 min, followed by 40 cycles at
95°C for 15 min, 60°C for 15 min and 72°C for 15 min, and one cycle
at 95°C for 15 min, 65°C for 15 min and 95°C for 15 min. Finally,
data were analyzed using the AriaMX 1.0 program (Agilent
Technologies, Inc.). The primers used are shown in Table I. The quantification of gene
expression was performed in triplicate using the 2−ΔΔCq
method and expression levels were normalized to those of the
housekeeping gene, pumilio RNA binding family member 1 (10).
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Marker | Forward primer | Reverse primer |
|---|
| StAR |
5′-TCCTTAGCAACCAAGAGGGC-3′ |
5′-TGACATTGGGGTTCCACTCC-3′ |
| CYP17A1 |
5′-GCCCCATCTATTCGGTTCGT-3′ |
5′-CAGAGTCAGCGAAGGCGATA-3′ |
| 5α-reductase 1 |
5′-CATGTTCCTCGTCCACTACGG-3′ |
5′-CCAACAGTGGCATAGGCTTTC-3′ |
| 5α-reductase 2 |
5′-TTCCTTCGCGGTGCCC-3′ |
5′-CCATTTCCAGTGCAGAAGGC-3′ |
| AKR1D1 |
5′-TGGTCACTTCATGCCTGTCC-3′ |
5′-CAATATGGCGGAAGCCAGC-3′ |
| AKR1C2 |
5′-ACCATTGGAATGACATACTGCATC-3′ |
5′-TGTGAGAGGAGGGACAGAGG-3′ |
| AR |
5′-TTGTGTCAAAAGCGAAATGG-3′ |
5′-AGTCAATGGGCAAAACATGG-3′ |
| PUM1 |
5′-CGGTCGTCCTGAGGATAAAA-3′ |
5′-CGTACGTGAGGCGTGAGTAA-3′ |
Western blotting
For protein extraction, cells were harvested and
were treated with radioimmunoprecipitation assay lysis buffer mixed
with protease inhibitor (Roche Diagnostics, Basel, Switzerland).
The obtained homogenate was centrifuged at 26,500 × g (Beckman
Coulter Allegra® 21R; Beckman Coulter, Inc., Brea, CA,
USA) for 15 min at 4°C. Finally, the supernatant was quantified
using the Bradford method (Bio-Rad Laboratories, Inc., Hercules,
CA, USA), according to the manufacturer's protocol. Subsequently,
50 µg protein under reducing conditions was separated by 10%
acrylamide gel electrophoresis and the proteins were
electrotransferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.) at 50 mA and 4°C overnight. Afterwards, the
membranes were blocked with 5% milk solution in TBS-Tween 0.1%
(TBST) for 2 h at room temperature, washed with TBST, and incubated
overnight at 4°C with the corresponding primary antibodies
(Table II). The membranes were
then washed and incubated with the horseradish
peroxidase-conjugated respective secondary antibodies for 1 h at
room temperature (Table II).
Finally, the membranes were developed by chemiluminescence using an
automatic system (Fusion FX5-XT; Vilber Lourmat Sté, Collégien,
France). Semi-quantification of protein expression levels was
conducted using ImageJ 1.52f software (National Institutes of
Health, Bethesda, MD, USA), with β-actin used as a loading
control.
 | Table II.Antibodies used for western
blotting. |
Table II.
Antibodies used for western
blotting.
| A, Primary
antibodies |
|---|
|
|---|
| Antibody name | Catalogue number | Type | Dilution |
|---|
| StAR | PA5-21687; Thermo
Fisher Scientific, Inc., Waltham, MA, USA | Rabbit | 1:20 |
| CYP17A1 | ABC392; EMD
Millipore, Billerica, MA, USA | Rabbit | 1:200 |
| 5α-reductase 1 | ab110123; Abcam,
Cambridge, UK | Goat | 1:500 |
| 5α-reductase 2 | ab27469; Abcam | Goat | 1:500 |
| AKR1D1 | PA5-28963; Thermo
Fisher Scientific, Inc. | Rabbit | 1:1,000 |
| AKR1C2 | PA5-36572; Thermo
Fisher Scientific, Inc. | Rabbit | 1:500 |
| AR | ab9474; Abcam | Mouse | 1:200 |
| β-actin | 69100; MP
Biomedicals, LLC, Santa Ana, CA, USA | Mouse | 1:5,000 |
|
| B, Secondary
antibodies |
|
| Antibody
name | Catalogue
number | Type |
Dilution |
|
| Anti-mouse | 115-035-003;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA. | Goat | 1:10,000 |
| Anti-rabbit | 111-035-003;
Jackson ImmunoResearch Laboratories, Inc. | Goat | 1:10,000 |
| Anti-goat | 305-035-045;
Jackson ImmunoResearch Laboratories, Inc. | Rabbit | 1:10,000 |
Determination of testosterone and DHT
levels in cell culture medium
A total of 4×105 DU145 SH and DU145 SCR
cells/plate were seeded into 100 mm plates containing 12 ml
RPMI-Phenol red-free medium, and were cultured for 3 days.
Subsequently, cells were counted using a Neubauer chamber. For
steroid extraction, 300 µl culture media was removed and
diethylether was added in a 5:1 ratio (solvent/sample). The samples
were then vortexed for 2 min and incubated at 4°C for 5 min, in
order to allow correct separation of the phases. Subsequently, the
organic phase was removed and evaporated in SpeedVac (Savant™
SC110; Thermo Fisher Scientific, Inc.). Finally, the samples were
reconstituted in 500 µl 10% ethanol or methanol, in order to
measure the levels of testosterone or DHT respectively, using an
ELISA kit for testosterone serum detection (cat. no. 11-TESHU-E01;
Alpco, Salem, MA, USA) and an ELISA kit for DHT serum detection
(cat. no. 11-DHTHU-E01; Alpco), with a modification in the
construction of the calibration curve. Briefly, since the samples
were resuspended in solution with 10% ethanol or methanol, a novel
calibration curve was constructed in these matrices using synthetic
testosterone (Sigma-Aldrich; Merck KGaA) and synthetic DHT
(Sigma-11-DHTHU-E01) at the same concentrations indicated in the
kit calibration curves. To validate the method, a control sample of
known concentration was measured and the minimum detectable dose
was calculated. Afterwards, the measurement was performed according
to the manufacturer protocols. Finally, the results were corrected
to determine the concentration obtained for 105
cells.
Immunocytochemistry
The DU145 SH and DU145 SCR cells were seeded on
coverslips at 60% confluence and were fixed with PBS, 3%
paraformaldehyde and 2% sucrose for 30 min at room temperature.
Subsequently, cells were washed with PBS-Glycine for 15 min,
permeabilized for 10 min at room temperature, with 0.1% Triton
X-100 (Sigma-Aldrich; Merck KGaA) and washed with PBS-Glycine.
Cells were then blocked in a humidified chamber using PBS-Glycine
and 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 10 min
and were incubated with the necessary primary antibodies diluted in
blocking solution. The primary antibodies used are shown in
Table III. Subsequently, cells
were incubated in the dark with the corresponding Alexa
Fluor® 488-conjugated secondary antibodies (Table III). Both incubations were
performed for 1 h at 37°C. In addition, cells were incubated in the
dark with DAPI (1:10,000; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for nuclear staining. Finally, coverslips were washed and
mounted with Fluorescence Mounting Medium (Dako; Agilent
Technologies, Inc.) for subsequent observation with a Spinning Disk
microscope (Olympus IX81; Olympus Corporation, Tokyo, Japan) at ×40
magnification. Each of the markers was analyzed in triplicate for
DU145 SH and DU145 SCR cells.
 | Table III.Antibodies used for
immunocytochemistry. |
Table III.
Antibodies used for
immunocytochemistry.
| A, Primary
antibodies |
|---|
|
|---|
| Antibody name | Catalogue
number | Type | Dilution |
|---|
| StAR | PA5-21687; Thermo
Fisher Scientific, Inc., Waltham, MA, USA | Rabbit | 1:100 |
| CYP17A1 | ABC392; EMD
Millipore, Billerica, MA, USA | Rabbit | 1:100 |
| 5α-reductase 1 | ab110123; Abcam,
Cambridge, UK | Goat | 1:200 |
| 5α-reductase 2 | ab27469; Abcam | Goat | 1:200 |
| AKR1D1 | PA5-28963; Thermo
Fisher Scientific, Inc. | Rabbit | 1:200 |
| AKR1C2 | PA5-36572; Thermo
Fisher Scientific, Inc. | Rabbit | 1:200 |
| AR | ab9474; Abcam | Mouse | 1:100 |
|
| B, Secondary
antibodies |
|
| Antibody
name | Catalogue
number | Type |
Dilution |
|
| Anti-mouse | A11001; Thermo
Fisher Scientific, Inc. | Goat | 1/200 |
| Anti-rabbit | A11008; Thermo
Fisher Scientific, Inc. | Goat | 1/200 |
| Anti-goat | ab150141;
Abcam | Rabbit | 1/200 |
Statistical analysis
For statistical analysis of the data, the
Mann-Whitney U test was applied using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Results were
normalized to the results from DU145 SCR cells. The samples were
processed in triplicate (n=3) and data are expressed as the means ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
mRNA and protein expression, and
cellular localization of the AR in a PCa cell line with ZEB1
silencing
Analysis of AR expression and subcellular
localization demonstrated that ZEB1 silencing resulted in an
increase in the mRNA and protein expression levels of AR (Fig. 1A and B), and AR was predominantly
located in the perinuclear region (Fig.
1C).
mRNA and protein expression, and
cellular localization of StAR, CYP17A1, 5α-reductase1,
5α-reductase2, AKR1D1 and AKR1C2 in a PCa cell line with ZEB1
silencing
The present study aimed to determine ZEB1
knockdown-induced alterations in the steroidogenic pathway
(Figs. 2 and 3). Initially, the expression levels of the
StAR transporter, which is involved in the first stage of the
pathway (11,12), were evaluated by RT-qPCR, western
blotting and immunocytochemistry. The results obtained indicated
that the mRNA expression levels of the StAR were decreased in the
DU145 SH cell line compared with in control DU145 SCR cells.
Furthermore, the protein expression levels of StAR were also
reduced in DU145 SH cells (Fig. 2A and
G). The intracellular distribution of StAR was mainly
cytoplasmic (Fig. 3A).
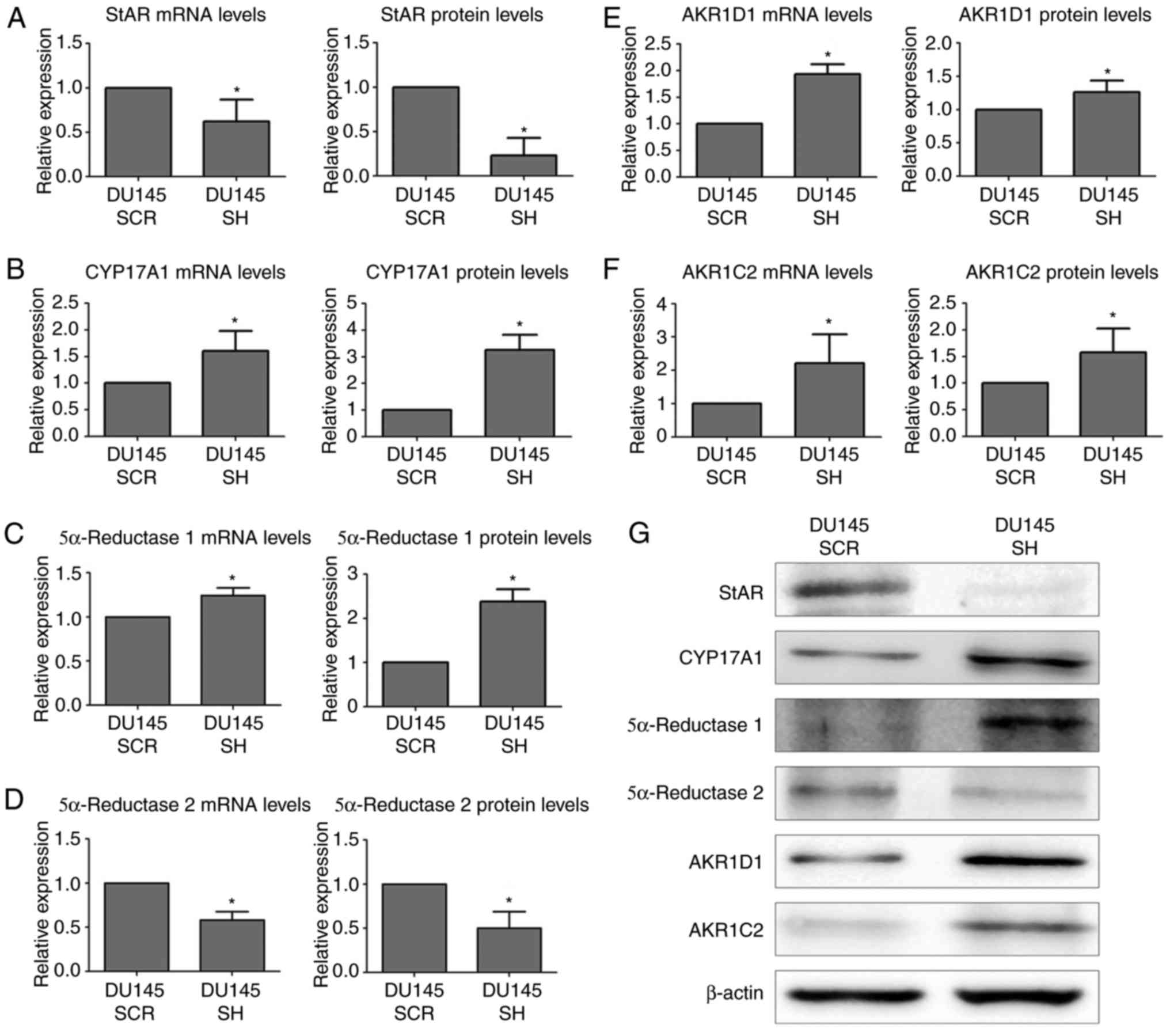 | Figure 2.mRNA and protein expression levels of
androgen synthesis and degradation markers in DU145 SCR and DU145
SH cell lines, as determined by reverse transcription-quantitative
polymerase chain reaction and western blotting. mRNA and protein
expression levels of (A) StAR, (B) CYP17A1, (C) 5α-reductase-1, (D)
5α-reductase-2, (E) AKR1D1 and (F) AKR1C2. (G) Western blot
analysis; β-actin was used as a loading control. Statistically
significant differences were determined using the Mann-Whitney U
test. n=3, *P<0.05. AKR1C2, aldo-keto reductase family 1 member
C2; AKR1D1, aldo-keto reductase family 1 member D1; CYP17A1,
cytochrome P450 family 17 subfamily A member; DU145 SCR, control
cells; DU145 SH, zinc finger E-box binding homeobox 1-knockdown
cells; StAR, steroidogenic acute regulatory protein. |
 | Figure 3.Immunocytochemistry of androgen
synthesis and degradation markers in DU145 SH and DU145 SCR cell
lines. Right panel, cell nuclei are shown in blue (DAPI); left
panel, markers are shown in green (Alexa Fluor® 488).
(A) StAR, (B) CYP17A1, (C) 5α-reductase-1, (D) 5α-reductase-2, (E)
AKR1D1 and (F) AKR1C2. magnification, ×40. n=3. AKR1C2, aldo-keto
reductase family 1 member C2; AKR1D1, aldo-keto reductase family 1
member D1; CYP17A1, cytochrome P450 family 17 subfamily A member;
DU145 SCR, control cells; DU145 SH, zinc finger E-box binding
homeobox 1-knockdown cells; StAR, steroidogenic acute regulatory
protein. |
The enzyme CYP17A1, which participates in the third
and fourth step of the steroidogenic pathway (11,12),
was also analyzed by RT-qPCR, western blotting and
immunocytochemistry. The results demonstrated that ZEB1 silencing
resulted in an increase in the mRNA and protein expression levels
of CYP17A1 compared with in the non-silenced control (Fig. 2B and G). In addition, the
intracellular distribution exhibited a cytoplasmic pattern
(Fig. 3B).
Within the enzymes involved in androgen synthesis,
two isoforms were analyzed, 5α-reductase 1 and 5α-reductase 2,
which convert testosterone to DHT (12). The mRNA and protein expression
levels of 5α-reductase 1 were increased in the ZEB1-silenced cells
(Fig. 2C and G). With regards to
its subcellular localization, 5α-reductase 1 was predominantly
detected in the cytoplasm of DU145 SH and DU145 SCR cells (Fig. 3C). Conversely, ZEB1 silencing
induced a decrease in the mRNA and protein expression levels of
5α-reductase 2 compared with the corresponding control group
(Fig. 2D and G). Furthermore,
cytoplasmic intracellular distribution was detected in both cell
lines (Fig. 3D).
The enzyme AKR1D1, which is responsible for
inactivation of progesterone, 17OH-progesterone, androstenedione
and testosterone, thus converting the substrates into
5β-metabolites (13), was analyzed.
The results indicated that knockdown of ZEB1 resulted in an
increase in the mRNA and protein expression levels of AKR1D1
compared with in non-silenced control cells (Fig. 2E and G). In addition, the results of
immunocytochemistry revealed that AKR1D1 exhibited nuclear location
in DU145 SH cells (Fig. 3E).
Finally, the enzyme AKR1C2, which participates in
the inactivation of DHT, was analyzed (14). The results revealed that ZEB1
silencing resulted in an increase in the mRNA and protein
expression levels of AKR1C2 compared with in the corresponding
controls (Fig. 2F and G).
Furthermore, AKR1C2 was strictly located in the nuclei of DU145 SH
cells and was weakly detected in DU145 SCR cells (Fig. 3F).
Testosterone and DHT concentrations in
the culture medium of ZEB1-silenced PCa cells
The results revealed that ZEB1 silencing resulted in
an increase in testosterone and DHT concentrations in DU145 SH
cells compared with in the DU145 SCR control cells (Fig. 4).
A summary of the steroidogenic pathway markers,
including those associated with androgen synthesis and degradation,
in DU145 SH and DU145 SCR cell lines is presented in Fig. 5.
Discussion
Our recent study revealed that DU145 cells express
high levels of ZEB1; therefore, this cell line was selected to
perform stable silencing in the present study. The results of our
previous study demonstrated that cells with ZEB1 silencing undergo
alterations in the expression levels of canonical markers of EMT,
including E-cadherin and Vimentin; in particular, E-cadherin is
increased and Vimentin is decreased in response to ZEB1 knockdown.
In addition, it has been revealed that these cells exhibit a
decrease in proliferation, migration and invasion (9). Taken together these results indicate
that DU145 SH cells may acquire marked epithelial
characteristics.
In the prostate, it has been widely described that
testosterone synthesis begins with the transport of cholesterol to
the inner mitochondrial membrane by StAR, followed by reactions
catalyzed by the enzymes CYP11A1, CYP17A1, HSD3β and AKR1C3
(11). In the present study, some
of these markers were analyzed, and the results suggested that an
increase in CYP17A1 may contribute to an increase in testosterone
synthesis in cells with ZEB1 silencing. Therefore, CYP17A1 may be
considered the key enzyme in the production of testosterone in
these cells. This finding is in accordance with the findings of
Montgomery et al (15),
which revealed that, in samples from patients with CRPC, an
increase in the expression of CYP17A1 and testosterone
concentration was detected compared with in primary prostate tumors
samples, thus suggesting intratumoral steroidogenesis in advanced
stages and progression to CRPC. The production of DHT may be
determined by detecting the enzymes 5α-reductase 1 and 5α-reductase
2, and testosterone concentration (16). The expression levels of the
5α-reductase isoforms were detected in the present study and
suggested that the main factor affecting DHT production may be the
initial concentration of testosterone, since the decrease in one
isoform was compensated with an increase in the other.
Testosterone and DHT concentrations are also
determined by their inactivation and subsequent degradation
(13,14,16).
Analysis of the enzyme AKR1D1, which is responsible for converting
progesterone, 17OH-progesterone, androstenedione and testosterone
into inactive metabolites (13),
revealed that its expression was increased in ZEB1-silenced cells,
which may result in a greater inactivation of these hormone
metabolites; however, the concentration of testosterone was
increased in ZEB1-silenced cells. These findings suggested that the
inactivation performed by AKR1D1 was not sufficient to prevent the
high levels of testosterone in these cells. Similarly, the AKR1C2
enzyme, which is responsible for the inactivation of DHT, was also
increased in cells with ZEB1 knockdown.
The preferential nuclear localization of AKR1D1 and
AKR1C2 suggested that after being translocated from the cytoplasm,
androgen receptor-testosterone and androgen receptor-DHT complexes
(17) may be inactivated in the
nucleus. Taylor et al (18)
analyzed the genomic profile of samples from patients with PCa, and
revealed that AKR1C2 expression is increased in patients with
advanced disease, thus suggesting that the increase in AKR1C2
expression determined in this study may be associated with more
advanced stages of PCa. In addition, the expression levels of the
enzyme AKR1C2 are correlated with the expression of AR (19). Huang et al (20) revealed that LNCaP PCa cells, which
express AR, have a higher expression of AKR1C2 compared with in
cell lines that do not express AR. It was also demonstrated that
silencing AR in LNCaP cells produces a decrease in AKR1C2; however,
the regulatory mechanism remains unknown. Furthermore, Ji et
al (21) reported that DHT
treatment of LNCaP and DU145 cells results in induction of AKR1C2
expression, indicating that this possibly occurs to counteract the
increase in DHT concentration. These findings are in accordance
with the present results, suggesting a possible regulation of
androgen availability by the AR as a mechanism of control.
Notably, the relevance of increased concentration of
androgens in cells with ZEB1 knockdown may be associated with
activation of AR during EMT (6).
Therefore, an increase in the expression of steroidogenic enzymes
has been suggested as a mechanism underlying resistance to ADT
(22,23). According to the present results, it
may be hypothesized that an increase in androgen synthesis could
lead to an increase in the activation of AR due to its increased
availability.
The present results revealed that the isoform
5α-reductase 1 was increased whereas 5α-reductase 2 was decreased
in response to ZEB1 knockdown. An expression switch between these
isoforms has been suggested, since both enzymes act at different pH
levels, and in the tumor environment, the activity of 5α-reductase
1 may be favored allowing an increase in the production of DHT
(12). In addition Audet-Walsh
et al (24) revealed that
the inverse regulation of these enzymes is conducted directly by
the AR, which binds to the promoter sequences of the genes
increasing the expression of 5α-reductase 1 and suppressing the
expression of 5α-reductase 2.
Locke et al (25) reported an increase in StAR
expression, and testosterone and DHT concentrations, in a
castration-resistant xenotransplant model. Conversely, the present
results suggested that an inverse relationship may exist between
the concentration of androgens and StAR. It may be hypothesized
that a reduction in StAR could act as a compensatory mechanism
against the increase in androgens induced by ZEB1-independent
mechanisms.
In conclusion, the silencing of transcription factor
ZEB1 produced an alteration in the steroidogenic pathway and AR
expression, specifically increasing the expression levels of
CYP17A1, 5α-reductase 1, AKR1D1 and AKR1C2 enzymes, and decreasing
the expression levels of StAR and 5α-reductase 2. In addition, ZEB1
knockdown increased testosterone and DHT concentrations. An
increase in androgen concentration in tumor cells is a
characteristic of patients with ADT resistance; therefore,
alterations in ZEB1 expression may be associated with the mechanism
underlying treatment failure in patients undergoing ADT and
subsequent disease progression to CRPC.
Acknowledgements
The authors would like to thank Ms. Graciela Caroca
(Department of Basic and Clinic Oncology, Faculty of Medicine,
University of Chile) for her technical assistance.
Funding
The present study was supported by grants from the
Fondo Nacional de Ciencia y Tecnología, FONDECYT [grant nos.
1151214 (HRC) and 1140417 (EAC)]. U-REDES, University of Chile
[grant no. URC-007/17 (HRC)], and the CONICYT scholarship [grant
no. 21140772 (OOS) and 21160703 (MJT)].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH performed the gene expression studies,
immunocytochemistry, ELISA and the statistical analyses. OOS
transduced the cell line, and contributed to the gene expression
and immunocytochemistry experiments. PV and MJT contributed to the
gene expression studies and protein analyses. HRC, RP and EAC
participated in the study design and interpreted the results. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anantharaman A and Frieddlaner TW:
Targeting the androgen receptor in metastatic castrate-resistant
prostate cancer: A review. Urol Oncol. 34:356–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lonergan PE and Tindall DJ: Androgen
receptor signaling in prostate cancer development and progression.
J Carcinog. 10:202011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chandrasekar T, Yang JC, Gao AC and Evans
CP: Mechanisms of resistance in castration-resistant prostate
cancer (CRPC). Transl Androl Urol. 4:365–380. 2015.PubMed/NCBI
|
|
5
|
Mitsiades N, Sung C, Schultz N, Danila DC,
He B, Eedunuri VK, Fleisher M, Sander C, Sawyers CL and Scher HI:
Distinct patterns of dysregulated expression of enzymes involved in
androgen synthesis and metabolism in metastatic prostate cancer
tumors. Cancer Res. 72:6142–6152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banerjee P, Banerjee S, Brown T and Zirkin
B: Androgen action in prostate function and disease. Am J Clin Exp
Urol. 6:62–77. 2018.PubMed/NCBI
|
|
7
|
Sun Y, Wang BE, Leong KG, Yue P, Li L,
Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, et al: Androgen
deprivation causes epithelial-mesenchymal transition in the
prostate: Implications for androgen-deprivation therapy. Cancer
Res. 72:527–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anose BM and Sanders MM: Androgen receptor
regulates transcription of the ZEB1 transcription factor. Int J
Endocrinol. 2011:9039182011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orellana-Serradell O, Herrera D, Castellon
E and Contreras HR: The transcription factor ZEB1 promotes an
aggressive phenotype in prostate cancer cell lines. Asian J Androl.
20:294–299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mostaghel EA: Steroid hormone synthetic
pathways in prostate cancer. Transl Androl Urol. 2:212–227.
2013.PubMed/NCBI
|
|
13
|
Chen M and Penning TM: 5β-Reduced steroids
and human Δ4-3-ketosteroid 5β-reductase (AKR1D1). Steroids.
83:17–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng CM, Chang LL, Ying MD, Cao J, He QJ,
Zhu H and Yang B: Aldo-keto reductase AKR1C1-AKR1C4: Functions,
regulation, and intervention for anti-cancer therapy. Front
Pharmacol. 8:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, Higano CS, True LD and Nelson PS: Maintenance
of intratumoral androgens in metastatic prostate cancer: A
mechanism for castration-resistant tumor growth. Cancer Res.
68:4447–4454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizner TL, Lin HK, Peehl DM, Steckelbroeck
S, Bauman DR and Penning TM: Human type 3 3alpha-hydroxysteroid
dehydrogenase (aldo-keto reductase 1 C2) and androgen metabolism in
prostate cells. Endocrinology. 144:2922–2932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basu S and Tindall DJ: Androgen action in
prostate cancer. Horm Cancer. 1:223–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang A, Zhang J, Plymate S and Mostaghel
EA: Classical and non-classical roles for pre-receptor control of
DHT metabolism in prostate cancer progression. Horm Cancer.
7:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang KH, Chiou SH, Chow KC, Lin TY, Chang
HW, Chiang IP and Lee MC: Overexpression of aldo-keto reductase 1C2
is associated with disease progression in patients with prostatic
cancer. Histopathology. 57:384–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji Q, Chang L, VanDenBerg D, Stanczyk FZ
and Stolz A: Selective reduction of AKR1C2 in prostate cancer and
its role in DHT metabolism. Prostate. 54:275–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi T, Inoue T, Kamba T and Ogawa O:
Experimental evidence of persistent androgen-receptor-dependency in
castration-resistant prostate cancer. Int J Mol Sci.
14:15615–15635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lubik A, Nouri M, Truong S, Ghaffari M,
Adomat HH, Corey E, Cox ME, Li N, Guns ES, Yenki P, et al:
Paracrine sonic hedgehog signaling contributes significantly to
acquired steroidogenesis in the prostate tumor microenvironment.
Int J Cancer. 140:358–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Audet-Walsh É, Yee T, Tam IS and Giguère
V: Inverse regulation of DHT synthesis enzymes 5α-reductase types 1
and 2 by the androgen receptor in prostate cancer. Endocrinology.
158:1015–1021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locke JA, Guns ES, Lubik AA, Adomat HH,
Hendy SC, Wood CA, Ettinger SL, Gleave ME and Nelson CC: Androgen
levels increase by intratumoral de novo steroidogenesis during
progression of castration-resistant prostate cancer. Cancer Res.
68:6407–6415. 2008. View Article : Google Scholar : PubMed/NCBI
|



















