Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second leading cause of cancer-associated mortality
worldwide (1). The incidence and
mortality rates of GC are the highest in East Asia, primarily in
China (1–3). Gastric carcinogenesis is a complex
process, which involves crosstalk among host, environmental and
bacterial factors, leading to different molecular alterations at
the genetic and epigenetic level; Helicobacter pylori is a
well-recognized high-risk factor (4,5).
Gastrectomy is the primary strategy for patients with early-stage
GC. However, the absence of specific symptoms in the early-stage
leads to the majority of patients with GC diagnosed in the
unresectable stage, and systemic chemotherapy is the primary option
for these patients (6).
Chemotherapy resistance frequently emerges as a cause of treatment
failure (6). At present, the
outcome for patients with advanced GC remains poor, with 5–20%
5-year survival and a median overall survival of 10 months
(3). Therefore, examining novel
biomarkers for early diagnosis and other feasible treatments based
on a better understanding of mechanisms underlying GC pathogenesis,
in addition to chemoresistance, is urgently required.
MicroRNAs (miRNAs) represent a large group of
conserved small non-coding RNAs (ncRNAs) with a length of 17–25
nucleotides, which bind to the 3′-untranslated region (UTR) of
mRNAs of their target genes, silencing expression by cleaving the
mRNA molecules or inhibiting their translation (1,5). In
this manner, ~30% human genes are modulated by miRNAs; the majority
are directly or indirectly implicated in signaling pathways
fundamental in cellular activities, including proliferation,
differentiation, apoptosis and migration (7,8). As a
result, miRNAs are crucial regulators in the initiation and
progression of various diseases, particularly cancer. In GC,
dysregulated oncogenic or tumor-suppressive miRNAs promote
malignant phenotypes, including tumor growth, metastasis,
angiogenesis and drug-resistance, by regulating downstream targets
and associated pathways (4,9,10). The
phosphatase and tensin homolog (PTEN)/phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (Akt) signaling pathway represents a
crucial one (11,12). PTEN, a dual protein and lipid
phosphatase, primarily dephosphorylates
phosphatidylinositol-3,4,5-trisphosphate (PIP3), which is the
product of PI3K and is able to recruit Akt to the membrane, where
it is phosphorylated and stimulated by other kinases dependent on
PIP3 (13). Activated Akt may
regulate multiple biological processes, including cell survival,
metabolism, cell proliferation and growth, by affecting its
downstream substrates (13–15). Genetic and epigenetic alterations
occurring in a number of components of this pathway lead to its
constitutive activation in human cancer, including GC (11,16).
Furthermore, the PTEN/PI3K/Akt pathway may be regulated by miRNAs
in GC, suggesting that this signaling serves an essential role in
mediating oncogenic effects of dysregulated miRNAs during the onset
and development of this disease (6,8). In
the present review, the dysregulation and function of miRNAs, in
addition to genetic alterations and the roles of the PTEN/PI3K/Akt
pathway in GC are summarized. Furthermore, how this signaling
serves as an important mediator of miRNAs is discussed. Based on
their involvement in the mechanism underlying gastric
carcinogenesis and progression, the clinical applications of miRNAs
and its signaling as biomarkers or therapeutic targets in GC
management are additionally discussed.
miRNAs in GC
Dysregulation and function of miRNAs
in GC
Accumulating evidence has documented the
overexpression or downregulation of specific miRNAs in GC. Due to
the extensive regulatory function of miRNAs in gene expression, the
dysregulated miRNAs may result in oncogenic activities regarding
approximately all aspects of tumorigenesis and progression,
including cell proliferation, apoptosis, invasion and migration
(1,4). Generally, oncogenic miRNAs contribute
to tumor development with their aberrantly high expression in GC,
whereas, the silenced or lost expression of tumor-suppressive
miRNAs may additionally exert positive effects during oncogenesis
(1,4). Carcinogenic effects of miRNAs have
been acknowledged to be the result of their disordered
post-transcriptional regulation of oncogenic and tumor suppressive
genes via complementary base-pairing (1,4). The
aberrant expression level of miRNAs, implicated oncogenic effects
and associated target genes in GC are summarized in Table I.
 | Table I.Dysregulated expression and target
genes of miRs in gastric cancer. |
Table I.
Dysregulated expression and target
genes of miRs in gastric cancer.
| Author, year | miRs | Studied
samples | Expression
levels | Target genes | Implicated
processes | (Refs.) |
|---|
| Zhou et al,
2018 | miR-200c | Cell lines | ↓ | ZEB1, ZEB2 | EMT,
drug-resistance, invasion, and migration | (3) |
| Petrocca et
al, 2008 | miR-106b
miR-93 | Tissues and cell
lines | ↑ | E2F1, CDKN1A
(p21) | Cell cycle | (7) |
| Petrocca et
al, 2008 | miR-25 | Tissues and cell
lines | ↑ | Bim | Cell apoptosis | (7) |
| Zhang et al,
2018 | miR-532-5p | Tissues and cell
lines | ↓ | NCF2 | Metastasis and
angiogenesis | (9) |
| Li et al,
2014 | miR-296-5p | Tissues and cell
lines | ↑ | CDX1 | Tumor cell
growth | (10) |
| Wang et al,
2017; Kang et al, 2015 | miR-15a
miR-16-1 | Tissues and cell
lines | ↓ | Twist1, YAP1 | Cell proliferation,
EMT, migration, invasion, colony formation in vitro,
tumorigenicity in vivo | (19,20) |
| Liu et al,
2014; Feng et al, 2010; Chen et al, 2014; Otsubo
et al, 2011 | miR-126 | Tissues and cell
lines | ↑/↓ | PI3KR2, VEGFA, Crk,
SOX2 | Cell growth and
colony formation, apoptosis migration and invasion, cell cycle,
angiogenesis | (22–25) |
| Kogo et al,
2011 | miR-146a | Tissues | ↓ | EGFR, IRAK1 | Cell migration and
invasion | (29) |
| Zhou et al,
2015 | miR-141 | Tissues and cell
lines | ↓ | ZEB1 | Cell proliferation,
apoptosis and invasion | (33) |
| Han et al,
2015 | miR-29c | Tissues | ↓ | ITGB1 | Cell proliferation,
adhesion, invasion and tumor growth | (34) |
| Yan et al,
2016 | miR-126 | Tissues and cell
lines | ↓ | PI3KR2, VEGFA |
drug-resistance | (75) |
| Nishida et
al, 2011 | miR-125a-5p | Tissues and cell
lines | – | HER2 | Cell
proliferation | (79) |
| Huang et al,
2016 | miR-508-3p | Cell lines | ↓ | MMP-9, NFKB1,
RELA | Cell proliferation,
growth, invasion | (96) |
| Zhou et al,
2014 | miR-141 | Tissues | STAT4 | STAT4 | Cell invasion | (97) |
| He et al,
2016 | miR-29a | Tissues | ↓ | ITGB1 | Cell invasion and
metastasis | (98) |
| Huang et al,
2015 | miR-338-3p | Tissues and cell
lines | ↓ | ZEB2 | EMT | (99) |
| Liu et al,
2014 | miR-423-5p | Tissues and cell
lines | ↑ | TFF1 | Cell proliferation,
colony formation, invasion | (100) |
| Zhang et al,
2017 | miR-424 | Tissues | ↑ | LATS1 | Cell proliferation,
invasion and colony formation | (101) |
| Han et al,
2015 | Let-7b | Tissues and cell
lines | ↓ | ING1 | Invasion and
metastasis | (102) |
Tsukamoto et al (17) detected miRNA expression in 22
surgically resected GC tissues and identified that 39 miRNAs
exhibited different expression levels between tumor and normal
tissues, among which six miRNAs were downregulated; whereas, the
other 33 miRNAs were upregulated in GC. miR-28 was demonstrated to
be upregulated in 31 GC tissues compared with the matched adjacent
non-tumor tissues (P<0.05), and the higher expression of miR-28
has been additionally observed in a series of GC cell lines
compared with the normal control (5). miR-28 contributed to GC cell
proliferation and invasion by targeting and downregulating tumor
suppressor PTEN (5). Caudal-related
homeobox 1 (CDX1), an intestinal-specific transcription factor
important in gastric intestinal metaplasia, could significantly
repress GC cell growth by inducing cell cycle arrest and apoptosis
(10). CDX1 was targeted by
oncogenic miR-296-5p, which was detected to be overexpressed in GC
tissues and abolished the suppressive effects induced by CDX1
(10). Different from the elevated
expression level of oncogenic miRNAs in GC, the expressions of
tumor-suppressor miRNAs, including miR-137, miR-34a, miR-15a and
miR-16-1, which exert negative regulations on cell proliferation,
epithelial-mesenchymal transition (EMT), migration, invasion,
colony formation in vitro, in addition to tumorigenicity and
metastasis in vivo, have been documented to be evidently
decreased in the tumor tissues and cell lines compared with normal
controls (18–21). The targets responsible for their
effects including Akt2, platelet-derived growth factor receptor
(PDGFR) and twist family bHLH transcription factor 1 (18–21).
The synergistic regulation of multiple genes, including
phosphoinositide-3-kinase regulatory subunit 2 (PI3KR2), CRK
proto-oncogene, adaptor protein, vascular cellular adhesion protein
1 and serine/threonine-protein kinase PLK2, affects the miR-126
diverse antitumor effect of restraining tumor cell growth,
migration and invasion, and inducing cell cycle arrest in
G0/G1 phase, apoptosis in vitro, in
addition to inhibiting tumorigenicity, angiogenesis and metastasis
in vivo; however, during gastric oncogenesis, the expression
of miR-126 was significantly decreased (22–24).
Otsubo et al (25) observed
that miR-126 was aberrantly upregulated in a number of GC cell
lines and tumor tissues, and miR-126 overexpression contributed to
growth and colony formation of GC cells by modulating its
tumor-suppressive target sex-determining region Y-box 2.
Possible clinical application of miRNAs
in GC
miRNAs as promising biomarkers for
GC
As mentioned, the significantly different expression
of miRNAs may be detected between tumor and normal gastric mucosa
tissues. When detecting samples from patients with GC with
different clinicopathological characteristics and prognosis, the
expression level of specific miRNAs may additionally be distinct.
The expression of miR-10b and miR-21 was markedly higher in
lymphoma node metastasis-positive tumor tissues compared with
lymphoma node metastasis-free tumor tissues (26,27).
The low expression of tumor suppressor miR-1 and miR-146a was
associated with vascular invasion, lymph node involvement and
distant metastasis, in addition to the poor prognosis of patients
with GC. Furthermore, the relative level of miR-146a expression was
an independent predictive factor for overall survival (28,29).
Therefore, miRNA expression levels in tumor tissues may be used to
diagnose patients with GC, in addition to distinguishing patients
with different prognosis and developing treatment strategies.
Additionally, circulating miRNAs, which were
contained in secretive exosomes, may be detected in body fluids,
including sera, plasma, urin and gastric juice (1). Due to their characteristics, including
high stability, close association with disease statuses and ease of
measurement, circulating miRNAs have been investigated in multiple
diseases, particularly cancer, regarding their promising
functionality as novel non-invasive biomarkers (1). Liu et al (30) reported that serum exosome miR-451 of
patients with GC was significantly increased compared with healthy
controls. Furthermore, the high expression level of exosome miR-451
was associated with poor differentiation, in addition to high
proliferation and metastasis potential of GC, and may further
predict poor outcomes of patients with GC post-surgery (30). Zhu et al (31) identified an miRNA signature with the
capacity to diagnose early-stage GC accurately, which consisted of
five miRNAs (miR-16, miR-25, miR-92a, miR-451 and miR-486-5p)
overexpressed in the plasma of patients with GC.
miRNAs as potential therapeutic
targets for GC
As the dysregulated miRNAs may contribute to the
pathogenesis of GC, restoring the level of downregulated
tumor-suppressive miRNAs and/or upregulated oncogenic miRNAs, they
may represent a potential treatment strategy against the tumor.
Consistently, when transfecting GC cells with an miR-590-5p
inhibitor, significantly decreased proliferative and invasive
abilities, in addition to increased sensitivity to cisplatin (DDP)
and paclitaxel (PTX) were observed (32). Whereas, ectopic expression of
miR-141 in tumor cells may lead to ~40% inhibition of proliferation
and prominent reduction in invasion (33). In addition to the in vitro
studies, miRNAs have been tested for their treatment efficacy in
vivo. Han et al (34)
identified that in a xenograft nude mouse model, tumor growth in
mice implanted with miR-29c overexpressing GC cells was
significantly slower compared with mice treated with untreated
parental cells (P<0.0001), and injecting miR-29c mimics
intratumorally additionally resulted in a marked inhibition of
tumor growth. According to the results of the bioluminescence
imaging and analysis, and the number of lung metastasis in mice,
miR-137 was demonstrated to exert anti-metastatic effects in
vivo (21).
PTEN/PI3K/Akt pathway in GC
Components of the PTEN/PI3K/Akt
pathway
PTEN is a dual lipid and protein phosphatase and a
common tumor suppressor. Since its identification, the mutational
and epigenetic silencing of PTEN have been documented in various
cancer types, owing to its extensive function in critical cellular
processes, which may be generally divided into two categories, cell
growth/survival and cell migration/adhesion, regulated through its
lipid and protein phosphatase activities, respectively (12,13).
The primary substrate of PTEN is PIP3, the product of PI3Ks, a
lipid kinase family with the typical ability to phosphorylate the
3′-OH group in inositol phospholipids on the cell membrane
(35). PI3Ks generally consist of
three classes (class I, class II and class III) (35). Class I PI3Ks, heterodimeric proteins
comprising a catalytic subunit and a regulator subunit, may be
further subdivided into subclass IA and subclass IB, which may be
activated by tyrosine kinase receptors (RTKs) and guanosine-binding
protein coupled receptors (GPCR), respectively (11). Among the three classes, only class I
PI3Ks were identified to be implicated in human cancer (35) and is the one primarily referred to
in the present review.
Akt, additionally termed PKB, is a serine-threonine
kinase downstream of PTEN/PI3K signaling. Akt includes three
isoforms transcribed from different genes, Akt1/PKBα, Akt2/PKBβ and
Akt3/PKBγ (14). Each member
comprises three conserved domains; a plekstrin homology (PH) domain
in the N-terminal, a central kinase domain and a hydrophobic
C-terminal tail (14). In the first
and the third domain, a regulatory site exists, which is necessary
for the PI3K-dependent activation of Akt (Thr308 and Ser473 in
Akt1) (36).
Activation process of the
PTEN/PI3K/Akt pathway
The stimulation of GPCRs or RTKs [including IGFR,
PDGFR, epidermal growth factor receptor (EGFR) and c-Met] by
various stimuli, including growth factors, hormones and
extracellular matrix (ECM) components, initiates the activation of
the PI3K/Akt pathway (14). The
interaction of the Src homology 2 (SH2) domains in the regulatory
subunit of PI3K with the intracellular section of the activated
cell-surface receptors or their adaptor proteins leads to the
allosteric activation of the PI3K catalytic subunit (37,38).
Alternatively, the activated Ras may stimulate PI3K by binding to a
Ras binding domain (RBD) located at p110 catalytic subunits
(39). Subsequently, activated PI3K
associates and phosphorylates lipids, which in turn phosphorylates
phosphatidylinositol-4,5-bisphosphate (PIP2) at the inner side of
the plasma membrane into phosphatidylinositol-3,4,5-trisphosphate
(PIP3), a significant second messenger in cells (14). The production of PIP3 results in the
recruitment of proteins containing a PH domain to cellular
membranes, including Akt and its activating kinase
3-phosphoinositide-dependent protein kinase 1 (PDK1), which are
critical for transducing the activity of PI3K (40). It is generally acknowledged that the
conformational alterations of Akt, which refers to the exposure of
its two primary regulatory residues, may occur following the
interaction of the PH domain with PIP3. The PH domain is
additionally accountable for the heterodimerization of Akt and
PDK1, which results in activation of Akt through the
phosphorylation of PDK1 at Thr308 of Akt (14). However, apart from the
phosphorylation of this residue, full activation of Akt still
requires phosphorylation of the other regulatory site in the
carboxyl-terminal hydrophobic motif, Ser473, which is fulfilled by
mammalian target of rapamycin (mTOR) complex 2, additionally termed
PDK2 (11,41). Subsequently, activated Akt will be
translocated into the nucleus to phosphorylate its downstream
substrates involved in multiple cellular processes, including
apoptosis, cell cycle progression, metastasis and metabolism
(14,42).
Among multiple substrates, PTEN primarily targets
and dephosphorylates lipid PIP3 at the D3 position of the inositol
ring, and thus serves as the primary negative regulator of PI3K/AKT
signaling by reducing PIP3 production and inhibiting subsequent
recruitment along with activation of Akt (14). In the same manner, other
phosphatases may additionally block the activation of PI3K/Akt
signaling, including SH2-containing inositol 5-phosphatase and
inositol polyphosphate 4-phosphatase type II (37). Carboxyl-terminal modulator protein,
a protein partner which binds to the C terminus of Akt1 at the
plasma membrane, is able to block the phosphorylation on Ser473 and
Thr308, thus reducing the activation of Akt (43).
Genetic alterations of the
PTEN/PI3K/Akt pathway in GC
Genetic alterations of different codes of
PTEN/PI3K/Akt signaling may be frequently detected in GC (Table II), which contribute to the
overactivation of this signaling (11). However, as genetic analysis is a
reliable experiment strategy for validating specific genes involved
in pathologic processes, these documented genetic alterations may
reflect the involvement of the PTEN/PI3K/Akt pathway in gastric
carcinogenesis and progression.
 | Table II.Genetic alterations of the
PTEN/PI3K/Akt pathway in GC. |
Table II.
Genetic alterations of the
PTEN/PI3K/Akt pathway in GC.
| Author, year | Altered sites | Detection
methods | Samples | Primary
results | (Refs.) |
|---|
| Soung et al,
2006 | Akt | PCR-SSCP, | Tumor
specimens | Akt2 mutation was
identified in 3 out of the direct sequencing 294 tumor samples.
Mutations were identified in 2 of 79 lung carcinomas (2.5%) and 1
of 51 GC (2.0%) cases. The GC mutation was in intron 10. There was
no mutation in Akt1 or Akt3 in these cancer tissues. |
(42) |
| Li et al,
2005 | PIK3CA | Direct
sequencing | Tumor
specimens | Mutations in PIK3CA
were identified in 4 of 94 GAC samples. In total, two cases had the
mutation A3140G (H1047R) in exon 20, and the other two cases had
mutations G1624A (E542K) and G1633A (E545K) in exon 9. |
(44) |
| Wen et al,
2010 | PTEN | Direct
sequencing | Tumor
specimens | Mutations in PTEN
were detected in 27 of 144 GC specimens, consisting of 15 cases
(55.6%) of missense mutation, nine nonsense mutations (33.3%), two
cases of 1-bp deletion (7.4%) and a mutation within intron 6
(3.7%). |
(46) |
| Samuels et
al, 2004 | PIK3CA | Direct
sequencing | Tumor
specimens | PIK3CA mutation was
detected in 3 of 12 GC cases (25%) and >75% of the mutation
occurred in two small clusters located in the helical and kinase
domains. |
(47) |
| Shi et al,
2012 | PIK3CA | Direct sequencing,
RT-qPCR | Tumor
specimens | PIK3CA mutations
and amplification (gene copy number ≥4) were detected in 8/113
(7.1%) and 88/131 (67%) GC specimens, respectively. |
(48) |
| Wang et al,
2003 | PTEN | PCR-SSCP, direct
sequencing | Tumor
specimens | Mutations in PTEN
occurred in 17 of 60 (28.3%) advanced GC specimens, consisting of
eight missense mutations (47.1%), five silent mutations (29.4%),
two nonsense mutations (11.8%), a 12-bp deletion (5.9%) and a
mutation within the splice donor site of intron 6 (5.9%). | (103) |
| Velho et al,
2005 | PIK3CA | PCR-SSCP, PCR | Tumor
specimens | Mutations in exons
9 and 20 of PIK3CA were identified in 5/47 (10.6%) of GC. The
mutation rate was significantly different between microsatellite
instable GC [19.2% (5/26)] and microsatellite stable GC [0%
(0/21)]. | (104) |
| Staal, 1987 | Akt | Southern
blotting | Tumor
specimens | In 225 human
tumors, a 20-fold amplification of Akt1 was identified in one of
the five GAC cases. | (105) |
Loss of the tumor suppressor PTEN, was considered a
common mechanism for the activation of Akt signaling and inversely,
the constitutive activation of Akt was demonstrated to be largely
responsible for PTEN-mediated carcinogenesis (14,44).
The mutational inactivation of PTEN may be identified in numerous
carcinomas, including GC (45). Wen
et al (46) identified
mutations of PTEN in 27 of 144 patients with GC, and the mutation
rate was higher in advanced tumor, node and metastasis (TNM) stages
in addition to poorly differentiated ones, which may account for
the downregulated PTEN expression and the activation of PI3K/Akt
signaling detected in tumor tissues. Epigenetically silencing PTEN
by mesylating 5′ CpG islands in the promoter possibly accounts for
the its downregulated expression in GC (16). Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α (PIK3CA), the gene encoding PI3K
catalytic subunit p110α, has been documented to be one of the most
frequently mutated genes in human cancer (42). Samuels et al (47) identified activating mutations of
PIK3CA in numerous cancer types, among which the mutation rate in
GC was 25% (3 in 12 cases), and >75% of the mutations occurred
in two small clusters located in the helical and kinase domains,
causing the significant upregulation of lipid kinase activity and
the resultant stimulation of downstream Akt signaling. In addition
to mutations, the genomic amplification of PIK3CA was additionally
an important mechanism underlying the oncogenic activation of the
PI3K/Akt pathway in malignant diseases (14). Shi et al (48) determined that PIK3CA amplification
in 88 of 131 tested samples (67%) was closely correlated with the
elevated activation of the PI3K pathway, in addition to a poor
outcome for patients with GC. Furthermore, in the same previous
study, the PIK3CA amplification rate was significantly higher
compared with the mutation rate [8/113 (7.1%)] (48). The genetic alteration, including
mutation and amplification, may additionally occur to Akt in GC,
although the documented incidence is low.
Function of the PTEN/PI3K/Akt pathway
in GC
PI3K signaling is vital in regulating multiple
fundamental cellular activities, including cell proliferation,
apoptosis and metastasis (49).
With frequent alterations identified in GC, the PTEN/PI3K/Akt
pathway is significantly involved in gastric carcinogenesis and
progression (11,50).
The disturbed balance between cell growth and
apoptosis leads to tumorigenesis, is a result from the disorder of
complex regulation networks, comprising of pivotal proteins and
signaling cascades. The PTEN/PI3K/Akt pathway is critical due to
its fundamental role in the decision of cell death and survival
(14,42). Pro-survival effects of Akt signaling
have been identified in previous studies using antitumor factors.
Zhao et al (51) identified
that overexpression of a tumor suppressor gene, inhibitor of growth
3, in GC cells was able to reduce cell proliferation and arrest the
cell cycle at G2/M phase, in addition to induce cell
apoptosis, and the impairment of PI3K/Akt signaling was the
involved mechanism. Geridonin and paclitaxel was able to serve
synergistically to suppress Akt signaling by inhibiting Akt
phosphorylation at Thr308 and Ser473, in addition to elevating the
PTEN expression level, which led to the accumulation of p53 and
pro-apoptotic apoptosis regulator Bcl-2 (Bcl-2) family members,
together with downregulation of anti-apoptotic members (52). These molecular alterations accounted
for the enhanced apoptosis and repressed growth in vitro and
in vivo induced by this combination therapy against GC
(52).
Apart from contributing to the initiation of GC, the
aberrantly activated PI3K signaling was additionally able to
contribute to the progression of GC into highly malignant types
characterized by metastasis and resistance to chemotherapy.
Metastasis is a high potential risk and the predominant cause of
recurrence and mortality among patients with GC, and a number of
cell biological activities are controlled in this multistep
process, including adhesion, migration, invasion and angiogenesis
(1). Lectin-like oxidized
low-density lipoprotein receptor-1 was overexpressed in GC cells
and increased the migratory and invasive ability by enhancing EMT,
and activating the PI3K/Akt/glycogen synthase kinase 3β (GSK3β)
pathway (53). Vascular endothelial
growth factor-A (VEGF-A) was suggested to promote angiogenesis by
activating the downstream Akt/mTOR cascade in GC (24). Phosphatase of regenerating liver-3
(PRL-3), a protein tyrosine phosphatase, silenced PTEN by reducing
its expression and enhancing its phosphorylation, leading to the
activation of PI3K/Akt signaling and upregulation of matrix
metalloproteinase (MMP)-2/MMP-9, through which PRL-3 contributed to
the lethal peritoneal metastasis of GC (49). Chen et al (54) documented the enrichment and
activation of PI3K/Akt/mTOR signaling along with decreased PTEN
expression in GC cells resistant to PTX, a microtubule stabilizer
widely used in the front-line chemotherapy for patients with
advanced GC. The low response to other antitumor agents, including
DDP and 5-fluorouracil (5-FU), may additionally be attributed to
the stimulated PI3K signaling (6,11,32).
Anticancer drugs targeting the
PTEN/PI3K/Akt pathway
As a most frequently dysregulated pathway in cancer,
the PTEN/PI3K/Akt pathway has attracted increasing attention for
its potential in target therapies for a number of malignancies. In
this context, >40 inhibitors against different points of this
pathway, primarily PI3Ks, Akt and mTOR, reached various stages of
clinical trials; the mTOR inhibitors, temsirolimus and everolimus,
in addition to the PI3K inhibitors, idelalisib and copanlisib, have
been approved by the Food and Drug Administration for clinical
anticancer treatment (38).
Consistently, these therapies have additionally been investigated
for GC in numerous previous studies regarding treatment effects of
inhibiting tumor growth, metastasis and modifying
chemo-sensitivity, including LY294002, a commonly used PI3K
inhibitor, and the Akt inhibitor MK-2206 (6,49,55). A
number of effective and safe inhibitors, including BKM120 targeting
PI3K and everolimus against mTOR, were enrolled in the clinical
studies of different phases among patients with GC (11) Antibodies or inhibitors against the
RTKs or the ligands have additionally been examined as potential
target therapies for GC. Trastuzumab, a humanized monoclonal
antibody against human epidermal growth factor receptor 2 (HER2),
is the first molecular target drug in GC and may be combined with
chemotherapy as a standard treatment for cases with HER2
overexpression/amplification, which affect 11–20% patients with GC
(3). Furthermore, it was observed
that the combined use of trastuzumab and LY294002 may exhibit a
synergistic repression on downstream Akt signaling in GC cells
(56). Ramucirumab and apatinib,
inhibitors of vascular endothelial growth factor receptor-2
(VEGFR-2), have additionally been approved as anti-angiogenic
therapies for advanced GC (57).
Regulation of miRNAs on the PTEN/PI3K/Akt
pathway in GC
miRNAs regulate PI3K/Akt
signaling
The activated PI3K signaling contributed to the
initiation and development of GC through involvement in different
cellular activities. Multiple evidence has demonstrated that miRNAs
were highly implicated in the regulation of the PI3K/Akt pathway by
targeting this pathway (Table
III; Fig. 1), which partially
identified the mechanisms underlying the oncogene or
tumor-suppressor role of miRNAs in GC.
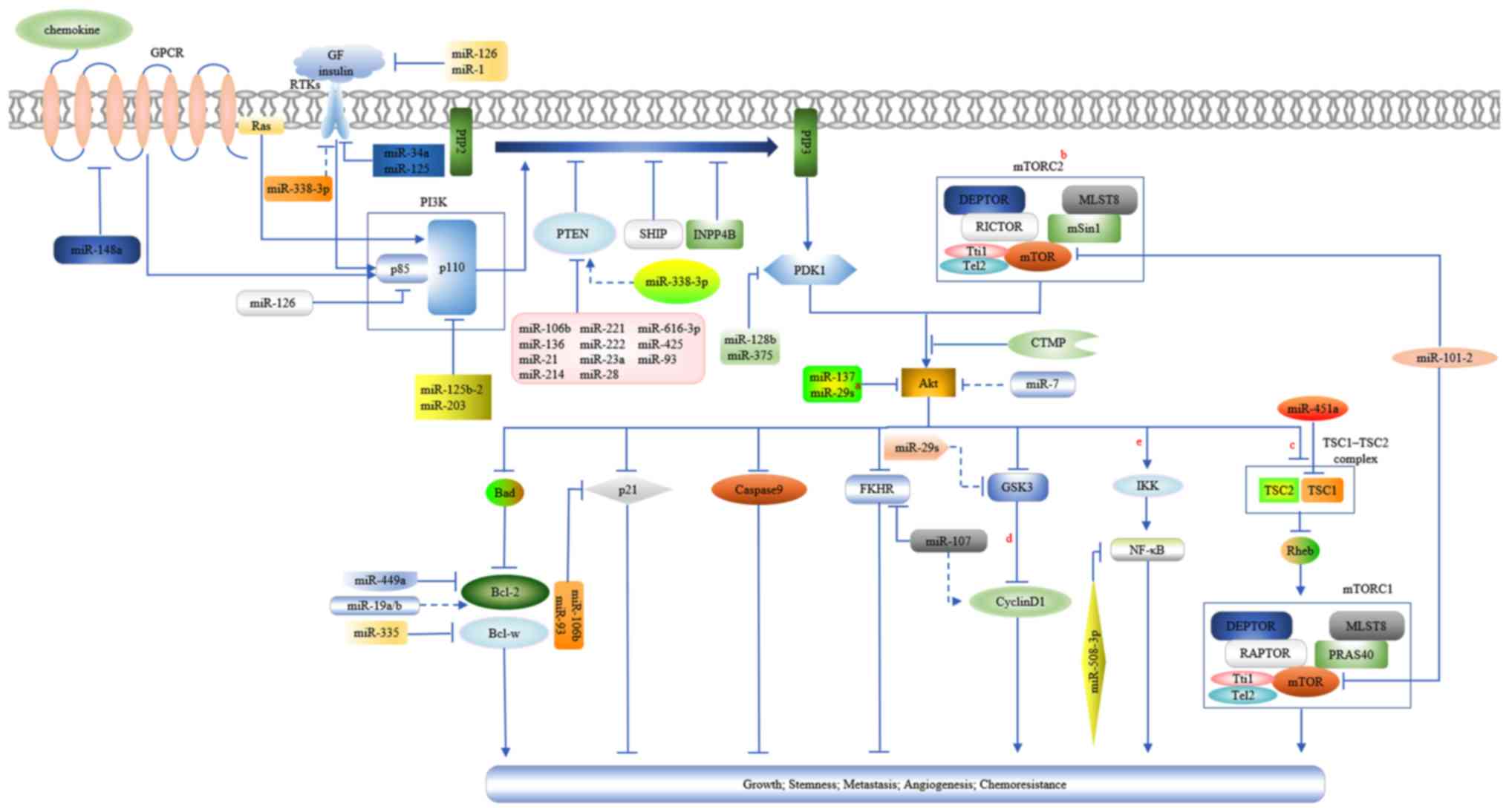 | Figure 1.A diagram presenting the activation
of the PTEN/PI3K/Akt signaling pathway and the regulations of
miRNAs on it in GC. a, miR-29s include miR-29a, miR-29b and
miR-29c. b, AKT may promote protein synthesis and cell growth by
disintegrating the TSC1/TSC2 complex by phosphorylating TSC2. c,
TSC1/TSC2 complex is a negative regulator of mTORC1 due to its
GTPase-activating protein activity for the Rheb GTPase (a member of
the Ras family), which may activate mTORC1. mTORC1 is involved in
protein and lipid synthesis and anti-autophagy, thus promoting cell
growth and proliferation. mTORC1 and mTORC2 possess the mTOR
kinase, the scaffolding protein MLST8, the mTOR regulatory subunit
DEPTOR, and the Tti1/Tel2 complex, which is critical for mTOR
complex assembly and stability. The scaffolding protein RAPTOR and
activity inhibitor PRAS40 are specific components belonging to
mTORC1. Similarly, mTORC2 has its specific negative regulators,
mSin1 and mTOR associated protein RICTOR (38,94,95).
d, Inhibition of GSK3 by Akt leads to the accumulation and nuclear
translocation of cytoplasmic β-catenin, which may induce the
expression of cyclin D1 through its combination with transcription
factors in the nucleus. The increased expression of cyclin D1
facilitates cell cycle progression. Furthermore, the decreased
phosphorylation of cyclin D1 from GSK3 inhibition enhances its
stabilization (14). e, Akt
stimulates IKK by phosphorylation and subsequently, IKK activates
NF-κB by phosphorylating the inhibitor of κB, which may mask the
nuclear localization signals of NF-κB and keep it sequestered in an
inactive state in the cytoplasm. Activated NF-κB may be
translocated into the nucleus where it transcriptionally
upregulates multiple genes, including positive cell-cycle
regulators, anti-apoptotic and pro-survival factors (14,88,96).
Arrowheads and perpendicular lines indicate stimulation and
inhibition of downstream substrates, respectively; straight lines
and dotted lines indicate direct and indirect effects on downstream
substrates, respectively. Akt, protein kinase B; DEPTOR, DEP
domain-containing mTOR-interacting protein; GF, growth factor;
GSK3, glycogen synthase kinase 3; GTPase, guanosine triphosphate
hydrolase; IKK, inhibitor of nuclear factor κB kinase; miR,
microRNA; MLST8, target of rapamycin complex subunit LST8; mSin1,
mammalian stress-activated protein kinase interacting protein 1;
mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1;
mTORC2, mTOR complex 2; NF-κB, nuclear factor κB; PI3K,
phosphatidylinositol 3-kinase; PRAS40, proline-rich AKT/PKB
substrate 40 kDa; PTEN, phosphatase and tensin homolog; RAPTOR,
regulatory-associated protein of mTOR; Rheb, guanosine
triphosphate-binding protein Rheb; RICTOR, rapamycin-insensitive
companion of mTOR; Tel2, telomere maintenance 2; TSC1, tuberous
sclerosis protein 1; TSC2, tuberous sclerosis protein 2; Tti1,
Tel2-interacting protein 1. |
 | Table III.Regulation of miRs on the
PTEN/PI3K/Akt pathway in GC. |
Table III.
Regulation of miRs on the
PTEN/PI3K/Akt pathway in GC.
| Author, year | miRNAs | General role | Regulatory
sites | Pathway
activity | Results | (Refs.) |
|---|
| Li et al,
2017 | miR-28 | oncomiR | PTEN | Positive | Advancing tumor
cell proliferation and invasion | (5) |
| Yang et al,
2013 | miR-21 | oncomiR | PTEN | Positive | Conferring
drug-resistance to tumor cells | (6) |
| Chen et al,
2018 | miR-136 | oncomiR | PTEN | Positive | Enhancing tumor
cell proliferation and invasion | (12) |
| Tsukamoto et
al, 2010 | miR-375 | anti-oncomiR | PDK 1 | Negative | Inducing
apoptosis | (17) |
| Peng et al,
2014 | miR-34a | anti-oncomiR | PDGFR, MET | Negative | Inhibiting cancer
cell migration, invasion, proliferation | (18) |
| Wu et al,
2015 | miR-137 | anti-oncomiR | Akt-2 | Negative | Repressing cell
proliferation, migration and invasion in vitro, and
proliferation as well as metastasis in vivo | (21) |
| Liu et al,
2014; Chen et al, 2014 | miR-126 | anti-oncomiR | PI3KR2, VEGFA | Negative | Blocking cell
growth, clone formation and inducing apoptosis in vitro,
suppressing tumor growth and angiogenesis in vivo | (22,24) |
| Liu et al,
2012 | miR-10b | oncomiR | HOXD10 | Positive | Promoting tumor
cell invasion | (26) |
| Zhang et al,
2012 | miR-21 | oncomiR | PTEN | ND | Facilitating GC
cell growth, invasion and migration | (27) |
| Xie et al,
2018 | miR-1 | anti-oncomiR | VEGFA | ND | Inhibiting
proliferation and migration of tumor cells and endothelial cells,
repressing angiogenesis | (28) |
| Shen et al,
2016 | miR-590-5p | oncomiR | RECK | Positive | Promoting cell
proliferation, invasion and drug-resistance in vitro and
tumor growth in vivo | (32) |
| Ma et al,
2014 | miR-425 | oncomiR | PTEN | Positive | Promoting cell
survival, proliferation and drug-resistance in vitro and
tumorigenicity in vivo | (45) |
| Pan et al,
2018 | miR-7 | anti-oncomiR | PTEN, PI3K | Negative | Repressing cell
migration in vitro and tumor growth in vivo, and
inducing apoptosis | (50) |
| Qi et al,
2017 | miR-125 | anti-oncomiR | HER2 | Negative | Impairing the
migration ability of tumor cells | (56) |
| Wu et al,
2018 | miR-616-3p | oncomiR | PTEN | Positive | Facilitating EMT
and angiogenesis | (57) |
| Li et al,
2016 | miR-196b | oncomiR | ND | Positive | Positively
regulating GC cell proliferation and invasion | (58) |
| Liang et al,
2015 | miR-203 | anti-oncomiR | PIK3CA | Negative | Suppressing tumor
cell proliferation, colony formation and invasion | (59) |
| Wei et al,
2013 | miR-449a | anti-oncomiR | Bcl-2 | ND | Inducing GAC cell
apoptosis and suppressing cell growth | (60) |
| Riquelme et
al, 2016 | miR-125b-2
miR-101-2, miR-451a |
anti-oncomiR | PIK3CB, mTOR,
TSC1a | ND | Reducing cell
viability, colony formation, migration and invasion, and increasing
cell death | (61) |
| Zhang et al,
2015 |
miR-29sb | anti-oncomiR | Akt-2 | Negative | Inhibiting
invasion | (62) |
| Xu et al,
2017 | miR-379 | anti-oncomiR | FAK | Negative | Repressing EMT and
metastasis |
(63) |
| Zhang et al,
2010 | miR-221
miR-222 | oncomiR | PTEN | Positive | Facilitating tumor
cell growth, invasion and radio-resistance |
(64) |
| Yang et al,
2013 | miR-214 | oncomiR | PTEN | ND | Positively
regulating tumor cell proliferation, migration and invasion |
(65) |
| Zhang et al,
2017 | miR-106b,
miR-93 | oncomiR | PTEN | ND | ND |
(67) |
| Guo et al,
2014 | miR-338-3p | anti-oncomiR | P-Rex2a | Negative | Inhibiting tumor
cell proliferation, clonogenicity, and inducing G1/S
arrest, apoptosis in vitro and tumorigenicity in
vivo |
(68) |
| Ni et al,
2018 | miR-21 | oncomiR | PTEN | Positive | Enhancing cancer
stem cell properties, including cell self-renewal, cell migration,
and chemoresistance |
(71) |
| Wang et al,
2016 | miR-34a | anti-oncomiR | c-Myc | ND | Inhibiting cell
proliferation, microsphere formation, drug-resistance, and
migration potential |
(72) |
| Yan et al,
2016 | miR-126 | anti-oncomiR | PI3KR2, VEGFA | Negative | Modifying
drug-resistance |
(75) |
| Wang et al,
2013 | miR-19a/b | oncomiR | PTEN, Akt | Positive | Contributing to EMT
and MDR of GC cells |
(76) |
| Cao et al,
2014 | miR-34a | anti-oncomiR | ND | Negative | Mediating
drug-sensitivity |
(77) |
| Eto et al,
2014 | miR-21 | oncomiR | PTEN | Positive | Promoting
drug-resistance |
(78) |
| Yang et al,
2014 | miR-106b | oncomiR | PTEN | ND | Advancing cell
migration and invasion |
(81) |
| Liu et al,
2014 | miR-34a | anti-oncomiR | EGFR | Negative | Repressing cell
invasion |
(83) |
| Li et al,
2016 | miR-23a | oncomiR | PTEN | Positive | Inducing EMT and
tumor growth |
(86) |
| Yu et al,
2016 | miR-148a | anti-oncomiR | CCK-BR | Negative | Repressing
proliferation and migration of tumor cells in vitro, and
tumorigenicity in vivo |
(87) |
| Zhang et al,
2016 | miR-128b | anti-oncomiR | PDK 1 | Negative | Suppressing tumor
cell cycle, proliferation and invasion, and inducing apoptosis |
(89) |
| Huang et al,
2015 | miR-338-3p | anti-oncomiR | MACC1 | Negative | Inhibiting the
migration, invasion and EMT |
(99) |
| Cheng et al,
2014 | miR-137 | anti-oncomiR | Cox-2 | Negative | Suppressing cell
proliferation, impairing cell migration and invasion as well as
inducing apoptosis | (106) |
| Lu et al,
2014 | miR-19a | oncomiR | ND | Positive | Promoted cell
proliferation, migration, invasion as well as EMT | (107) |
| Li et al,
2013 | miR-107 | oncomiR | FOXO1 | ND | Advancing
proliferation and cell cycle | (108) |
| Xu et al,
2011 | miR-335 | anti-oncomiR | Bcl-w, SP1 | Negative | Repressing invasion
and metastasis in vitro and in vivo | (109) |
| Zhao et al,
2017 | miR-320 | anti-oncomiR | ND | Negative | Inhibiting invasion
and inducing apoptosis | (110) |
During gastric carcinogenesis, the oncogenic effects
caused by the overexpressed oncogenic miRNAs or downregulated
tumor-suppressive miRNAs may be attributed to the aberrantly
activated PI3K/Akt signaling. It was observed that miR-196b
promoted GC tumor growth through its promotion in the cell cycle
and cell proliferation, possibly by stimulating the PI3K/Akt/mTOR
pathway (58). Conversely, it was
identified that the tumor suppressor miR-203, inhibited GC cell
proliferation by targeting PIK3CA and consequently attenuated
activation of Akt (59). Tsukamoto
et al (17) observed that
ectopic expression of miR-375 lead to a decrease in PDK1 expression
and a subsequent decline in phosphorylation of Akt at Ser473 and
Thr308. Furthermore, the dysregulated expression level of an
anti-apoptosis substrate of Akt, E3 ubiquitin-protein ligase XIAP,
along with the increased activity of specific apoptosis
executioners, includingBcl-2 homologous antagonist/killer,
Bcl-2-like protein 11 and tumor necorosis factor ligand superfamily
member 12, was additionally documented (17). miR-137, as possible negative
component of gastric tumorigenesis, suppressed GC cell survival by
inducing early apoptosis through targeting Akt2 and affecting
downstream Bcl2-associated agonist of cell death, a pro-apoptotic
member of the Bcl-2 family, which functions by selectively
dimerizing with pro-survival members, B-cell lymphoma-extra large
and Bcl-2 (21). Bcl-2, another
substrate of Akt, may be directly regulated by miR-449a and thus
transduced its tumor-suppressive effects of inducing tumor cell
apoptosis and inhibiting cell growth in gastric adenocarcinoma
(60). Other proteins of the
PI3K/Akt/mTOR pathway, PIK3CB, downstream tuberous sclerosis 1
(TSC1) and mTOR have been demonstrated to be negatively modulated
by miR-125b-2, miR-451a and miR-101-2, respectively, which
accounted for their inhibitory effects on GC growth, including
blocking cell proliferation and colony formation, and inducing cell
death (61). Furthermore, upstream
RTKs PDGFR and hepatocyte growth factor receptor, receiving
platelet-derived growth factor and hepatocyte growth factors
respectively, were additionally targeted by miR-34a and mediated
its supression on GC cell proliferation via the PI3K/Akt pathway
(18).
In addition to the involvement in the formation of
GC tumors, the regulation of PI3K/Akt signaling by miRNAs is
involved in oncogenic activities responsible for GC progression.
The miR-29 family, consisting of miR-29a, miR-29b and miR-29c, was
able to restrain Akt2 expression and subsequently inactivate GSK3β,
leading to the impaired invasive ability of GC cells (62). Focal adhesion kinase (FAK) is a
crucial transducer of integrin-mediated signaling to downstream
pathways, including the PI3K/Akt pathway (63). The activated FAK by integrins may
form a binding site for the SH2 domain of p85 subunit and
phosphorylate it, which may subsequently activate the p110
catalytic subunit of PI3K (63). Xu
et al (63) identified that
miR-379 inactivated Akt signaling by suppressing FAK expression,
thus leading to inhibition of cell migration, invasion and EMT
process. Cullin 4B, a scaffold protein, directly binds to the S2
region of the miR-125a promoter and transcriptionally represses
miR-125a, through which it promotes HER2 expression, thus
stimulating downstream PI3K/Akt signaling and the migration of GC
cells. In addition to EMT, the activated Akt/mTOR signaling is an
underlying mechanism of the induced angiogenesis by miR-616-3p in
GC (57). Conversely, the reduced
activation of Akt/mTOR signaling partially mediated the suppression
of miR-126 on GC tumor growth and angiogenesis (24).
miRNAs target and regulate PTEN in
GC
miRNAs are involved in PTEN
downregulation
As mentioned above, genetic and epigenetic
alteration of PTEN may lead to its downregulation in GC. A number
of overexpressed miRNAs may additionally deregulate PTEN by
directly combining to the 3′-UTR of its mRNA and thus silencing
PTEN at the post-transcriptional level, which is considered another
form of epigenetic modification. Chun-Zhi et al (64) demonstrated that miR-221 and miR-222
were upregulated in GC and possessed the ability to target PTEN.
Furthermore, ectopic expression of miR-221 and miR-222 in GC cells
led to a decreased expression level of PTEN (64). The silenced expression of PTEN in GC
may additionally be due to other miRNAs negatively regulating it,
including miR-136, miR-214 and miR-28 (5,12,65).
Furthermore, the downregulated PTEN expression level was associated
with adverse clinical parameters, including lymph node metastasis,
poor differentiation and advanced TNM stage of patients with GC
(65–67). Different from directly targeting
PTEN, the tumor suppressor miR-338-3p was able to indirectly
upregulate the activity of PTEN without altering its expression, by
suppressing phosphatidylinositol 3,4,5-trisphosphate RAC exchanger
2a, a guanine nucleotide exchange factor for the RAC guanosine
triphosphatase (GTPase), which stimulated PI3K signaling by serving
as a PTEN-interacting protein and antagonizing it (68).
PTEN mediates oncogenic activities of
miRNAs via PI3K/Akt signaling
Due to the critical negative regulator role of PTEN
in PTEN/PI3K/Akt signaling, the suppression of PTEN by oncogenic
miRNAs results in increased activity of downstream PI3K signaling,
and consequently contributed to the malignant phenotypes of GC,
including the promoted cell proliferation and survival, impaired
drug-sensitivity, and enhanced invasiveness, metastasis and
angiogenesis (Table III and
Fig. 1). Among these miRNAs, miR-21
is significant. Zhang et al (27) demonstrated that miR-21 promoted the
growth, invasion and migration of GC cells by directly binding to
the 3′-UTR of PTEN. Whereas antisense oligonucleotides against
miR-21 were able to increase PTEN expression and impair downstream
PI3K/Akt/mTOR signaling, leading to reverse effects on these
biological behaviors of GC cells (27,69).
In addition, miR-21 was involved in the regulation
of a number of Polycomb group (PcG) proteins on gastric cancer stem
cells (CSCs), a small subset of cancer cells with self-renewal and
tumor-initiating properties, which are critical in tumor
metastasis, recurrence and chemoresistance (70). PcG family proteins, including
polycomb group RING finger protein 2 (Mel-18), chromobox homolog 7
(CBX7) and enhancer of zeste homolog 2, form the multi-protein
complexes, called polycomb repressive complexes, through which they
epigenetically regulate homeotic genes expression by altering
chromosomal structure or transcriptional repression (71). In this manner, PcG proteins exert
effects in embryonic development, cell proliferation and
differentiation, stem cell properties and tumorigenesis (71). Ni et al (71) demonstrated that in addition to
inhibiting p16, CBX7 was additionally able to upregulate miR-21and
thus enhance its downstream PTEN/Akt/p53 signaling, whereby it
potentiated stem cell-like properties of GC cells, including
augmented self-renewal and cell migration, enhanced chemoresistance
and increased expression of stem cell markers (CD44 antigen and POU
domain, class 5, transcription factor 1). B cell-specific Moloney
murine leukemia virus integration site 1 (Bmi-1) is another
significant PcG family member, which positively regulates stem
cell-like phenotypes of GC, and similarly, it contributed to the
CSCs characteristics via the miR-21/PTEN/Akt axis (72). However, Bmi-1 was additionally able
to directly inhibit the expression of PTEN and thus activate Akt
signaling (72). miR-34a, which
negatively regulated CSCs phenotypes, was upregulated by Bmi-1.
Notably, miR-34a was able to conversely inhibit Bmi-1 by targeting
c-Myc, which increased the expression of Bmi-1 by binding to its
promoter, and this reciprocal modulation between Bmi-1 and miR-34a
constitute a negative feedback loop in maintaining the stem
cell-like properties of GC cells (72). Mel-18 was additionally reported to
be involved in the regulation of gastric CSCs properties; however,
inversely, it exerted negative effects by downregulating the
expression of miR-21 and therefore increasing PTEN expression
(73).
miRNAs regulate chemosensitivity via
the PTEN/PI3K/Akt pathway
miRNAs and PTEN/PI3K/Akt signaling have been
significantly implicated in the occurrence of drug-resistance in GC
(11,74). Therefore, elucidating the regulation
of PTEN/PI3K/Akt pathway by miRNAs involved in the decreased
response to chemotherapies may help to clarify the underlying
mechanisms.
There are numerous identified causes responsible for
tumor chemo-resistance, including intracellular drug efflux,
failure to undergo apoptosis, target gene alteration, drug or
metabolite detoxification and DNA repair enhancement, among which,
the increased drug efflux is a most common one, involving a family
of ATP-dependent efflux pumps, termed ATP-binding cassette (ABC)
transporters (6,74). Consistently, the enhanced resistance
of tumor cells to DDP resulted from overexpression of the ABC
transporter, and multidrug resistance-associated protein 1 was
observed in GC, which was induced by the attenuated repression of
PI3K/Akt pathway due to downregulated miR-126 expression (75). Wang et al (76) demonstrated that miR-19a/b modulated
multidrug resistance by targeting PTEN, which may be partly
attributed to the accelerated efflux through miR-19a/b induced
upregulation of permeability glycoprotein, another primary member
of the ABC transporter. The increased Bcl-2, together with the
decreased apoptosis regulator BAX and caspase-3, in GC cells
transfected with miR-19a/b represented reduced susceptibility to
drug-induced apoptosis, which is another pivotal mechanism
underlying chemoresistance as anticancer agents function by
inducing tumor cells apoptosis (76). The activated PI3K pathway frequently
transduces survival signals in GC (11,76).
Survivin, the smallest mammalian member of the inhibitor of
apoptosis gene family, inhibits apoptosis initiated by the
extrinsic or intrinsic pathways, and it engages in the positive
regulation of DDP-induced GC cell apoptosis by miR-34a through
PI3K/Akt signaling (77). In
addition to conventional chemotherapeutic drugs, suppression of
apoptosis induced by overexpressed miR-21 via the PTEN/PI3K/Akt
pathway may additionally account for the resistance to trastuzumab
of HER2-positive GC cells (78).
Consistent with the prediction that miRNAs regulate drug-resistance
by interfering with specific therapeutic targets, miR-125a, which
targets HER2 and inhibits its downstream Akt signaling, is able to
increase the efficacy of trastuzumab when used in combination with
suppressing GC cell proliferation (74,79).
CSCs are considered a cause of tumor relapse following successful
chemotherapy, owing to their quiescence, DNA repair capacity and
ABC-transporter expression (70).
miRNA has been reported to regulate resistance to epirubicin and
other stem cell-like properties through PTEN/PI3K/AKT signaling in
GC (71,72).
In previous studies examining the involvement of
PI3K/Akt signaling in GC drug-resistance, inhibitors against this
pathway have been frequently employed. Yang et al (6) demonstrated that LY294002 inhibited
miR-21-activated PI3K/Akt signaling and consequently promoted cell
survival and DDP resistance. Similarly, compared with controls,
lower activity of PI3K/Akt signaling while higher percentage of
apoptosis cells were documented in DDP incubated GC cells following
treatment with miR-34a mimics and wortmannin (77). These results not only suggested the
association of this pathway in sensitivity to anticancer drugs
modulated by miRNAs; however, additionally suggest the potential
clinical usage of these small molecule inhibitors in modifying
chemotherapy efficacy for patients with GC.
miRNA/PTEN axis is involved in gastric
tumor microenvironment
During cancer progression, a co-evolving and
supportive environment termed the tumor microenvironment is
important, which consists of ECM adjacent to cancer cells and
non-malignant stromal cells, including fibroblasts, endothelial
cells and hematopoietic cells, primarily macrophages and
lymphocytes (2). The crosstalk
between cancer cells and the environment may have substantial
impacts on tumor development, including tumor growth, metastasis
and refractoriness to therapies, wherein the regulation of
PTEN/PI3K/Akt by miRNAs may be involved (2,80).
Yang et al (81) identified
four upregulated miRNAs and seven downregulated miRNAs in gastric
cancer associated fibroblasts (CAFs), the most abundant cells in
the tumor microenvironment, compared with normal fibroblasts. CAFs
promoted the growth and metastasis of tumor cells, and silencing
miR-106b in CAFs abolished the contributive effect on cell motility
due to PTEN upregulation (81).
Similarly, when cultured in conditioned medium derived from
miR-1-transfected GC cells, microvascular endothelial cells were
prevented from migrating and forming novel vessels, which was
associated with the decreased VEGF-A and endothelin 1 (28). Zheng et al (2) observed that miR-21 in exosomes
secreted from tumor-associated macrophages (TAMs) may be ingested
by GC cells, and led to impaired sensitivity to DDP via the
PTEN/PI3K/Akt pathway. Under low glucose condition, the
energy-responding miR-451 may be transferred from GC cells to
infiltrated T cells through exosomes, thus enhancing the
differentiation of T-helper 17, wherein the upregulated mTOR
signaling induced by miR-451 was involved (30).
The bidirectional impact between cancer cells and
tumor microenvironment may additionally be fulfilled by the
secreted soluble factors, including cytokines, chemokines and
growth factors in autocrine or paracrine manner (80). Ma et al (45) demonstrated that when exposing GC
cells to pro-inflammatory cytokine interleukin-1β, miR-425 was
transcriptionally induced, and thus the PTEN expression level was
decreased, leading to promoted cell survival and proliferation
in vitro in addition to tumor growth in vivo. EMT of
GC cells induced by TGF-β1 may be stimulated by miR-21 through its
negative regulation of PTEN and the subsequent activation of
PI3K/Akt signaling (66). MMPs, a
family of zinc-dependent proteases released by tumor cells and
TAMs, were implicated in numerous oncogenic processes, including
ECM degradation, tumor cell migration and the release of growth
factors sequestered within the ECM (82). A previous study demonstrated that
MMP-7 was regulated by miR-34a through EGFR/PI3K/Akt signaling
during GC progression (83).
Other ncRNAs in co-regulation of the
PTEN/PI3K/Akt pathway
ncRNAs, refer to a type of RNA, which do not encode
proteins, primarily including miRNAs, long non-coding RNAs
(lncRNAs), in addition to pseudogenes and circular RNAs (circRNAs)
(84). In the competitive
endogenous RNA (ceRNA) hypothesis depicting a post-transcriptional
regulation network mediated by miRNAs, any RNA molecules sharing
one or more miRNA response elements, including protein-coding and
ncRNAs, are able to compete for binding to miRNAs and subsequently
co-regulate expression of one another (9). Due to their complexity, diversity and
frequent dysregulation in cancer, ncRNAs as ceRNAs have attracted
increasing attention in oncogenesis and development, regarding the
novel layer of post-transcriptional regulation on miRNA-targeted
mRNAs provided by them (84).
HOX transcript antisense intergenic RNA (HOTAIR), a
lncRNA associated with Polycomb repressive complex 2, was
demonstrated to be capable of functioning as a ceRNA of miR-126 to
promote expression of miR-126 target genes VEGF-A and PI3KR2,
resulting in the activation of PI3K/Akt signaling, which was
underlying the DDP-resistance induced by overexpressed HOTAIR in GC
(75). Pseudogenes are segments of
DNA associated with real genes, which are frequently derived from
accumulated mutations in a gene and thus commonly lose specific
functionality compared with the complete gene (85). Phosphatase and tensin homolog,
pseudogene 1, was reported to serve as a tumor suppressor of GC by
inhibiting tumor growth and metastasis, whereby it upregulated the
expression of its cognate gene PTEN, by sponging miR-106b and
miR-93 (67,84). circRNAs are another class of
non-coding RNAs, with the 3′ and 5′ ends of RNA molecules joined
together to form a covalently closed continuous loop. Pan et
al (50) identified that the
circRNA sponge for miR-7, was able to antagonize the effects of
miR-7 on the PTEN/PI3K/Akt pathway, and reverse its repression of
tumor cell migration and colony formation in vitro and GC
genesis in vivo.
miRNAs synergistically modulate the
PI3K cascade with other pathways
The complex crosstalk and pathway convergence among
different signaling pathways ensure that no pathway operates in
isolation; however, miRNAs exert extensive regulation and a single
miRNA may regulate multiple targets due to its imperfect
complementary pairing with mRNAs. It is therefore rational to
address that miRNAs may regulate PTEN/PI3K/Akt signaling with other
crucial pathways co-operatively. Indeed, this has been validated in
the pathologic process of GC.
The complex associations between the PI3K/Akt
pathway and the Ras/Raf proto-oncogene serine/threonine-protein
kinase (Raf)/mitogen-activated protein kinase (MAPK) kinase/MAPK
pathway have been well documented, including the inhibition of the
extracellular-regulated kinase (ERK) pathway by Akt via its direct
phosphorylation of c-Raf on T259, and similarly, the
phosphorylation of Akt on amino acid residue S83 of apoptosis
signal-regulated kinase 1, which leads to the impaired activation
of the c-Jun N-terminal kinase and p38 pathways (4,15). Ras
was additionally able to function upstream of PI3K by interacting
with the RBD of p110 catalytic subunit and thus activating PI3K
signaling (39). In GC, Li et
al (86) demonstrated that the
activation of Akt in addition to the ERK pathways accounted for
miR-23a-induced EMT. It was observed that miR-126 repressed
angiogenesis by simultaneously inhibiting downstream Akt/mTOR and
ERK signaling (24). In addition to
Akt and ERK signaling, the activation of the signal transducer and
activator of transcription 3 (STAT3) pathway was involved in the
oncogenic activities induced by miR-590-5p, including the promotion
of cell proliferation, invasion, drug-resistance and tumor growth
(32). Conversely, the negative
regulation of Akt and STAT3 signaling by targeting the GPCR for
gastrin and cholecystokinin may be the underlying mechanism for the
tumor-suppressive role of miR-148a in GC (87).
In GC cells, it has been suggested that the
activation of PI3K or loss of PTEN was able to protect
integrin-linked kinase (ILK) from proteasome-mediated degradation
(88). Enhanced ILK may
subsequently activate Ras and promote the formation of the IQ
motif-containing GTPase-activating protein 1-Ras complex, which
stimulated the c-Raf/MEK1/2/ERK1/2/ribosomal S6 kinase/inhibitor of
κBα/nuclear factor (NF)-κB signaling, leading to the increased cell
growth, migration and decreased sensitivity of 5-FU and DDP
(88). Consistently, miR-128b was
reported to repress GC cell growth, invasion and promote apoptosis
through Akt/NF-κB signaling due to its negative
post-transcriptional regulation of PDK1 (89). Furthermore, PI3K/Akt/NF-κB signaling
inactivation was a possible mechanism of the pro-apoptosis effect
of celastrol, an antitumor plant triterpene with the ability to
inhibit miR-21 expression (90).
Apart from serving roles downstream of the PI3K/Akt pathway, NF-κB
was additionally able to affect the expression of PTEN and
downstream Akt signaling, mediated by its transcriptional
regulation of miRNAs, including miR-425 and miR-21 (45,71).
Notably, the increased NF-κB transcriptional activity may be due to
the upregulated Akt activation (45,71).
Conclusion
In the last decade or two, the close association
between miRNA dysregulation and GC has been well documented. In
previous studies investigating the mechanisms by which miRNAs are
involved in gastric tumorigenicity and progression, the results
demonstrated that dysregulated miRNAs exerted their promotive
effects by post-transcriptionally regulating oncogenes and
tumor-suppressors, and thus affecting associated canonical
pathways, among which PTEN/PI3K/Akt was a critical one due to its
decisive role in GC tumor growth, metastasis, angiogenesis,
stemness and chemoresistance.
Identifying molecular causes of GC is of great
importance in pattern recognition and therapeutic strategies. The
poor prognosis of patients with GC primarily results from late
detection, aggressive progression, poor response to available
therapies and relapse (6,8,49).
Therefore, in the present review, it was discussed how miRNAs are
involved in the regulation of gastric CSCs and tumor environment
through PTEN/PI3K/Akt signaling, which bears resemblance to the
‘seed’ and ‘soil’ in GC pathogenesis (91), respectively. Similar investigations
have additionally been summarized regarding sensitivity to
conventional chemotherapies in addition to target therapies against
this tumor. Based on these molecular mechanisms, the potential
applications of miRNAs as novel biomarkers for diagnosis and
prognosis-prediction were discussed. As drugs (including miRNA
mimics and inhibitors) have been tested in preclinical and clinical
trials of a number of diseases (92), the role of miRNAs as promising
therapeutic targets for GC was additionally discussed. Besides,
inhibitors or monoclonal antibodies against the PTEN/PI3K/Akt
pathway and its up/down stream molecules have been in different
phases of clinical trial or in applications for GC. Furthermore, it
has been reported that ectopic expression of miRNA in combination
with a PI3K/Akt pathway inhibitor may acquire synergistic treatment
efficacy (93). Therefore, further
investigations into miRNAs and PTEN/PI3K/Akt signaling in addition
to their interactions are necessary and rewarding for improving the
clinical management and outcome of patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from
National Science Foundation Grants of China (grant nos. 81160307
and 81560395), the Jiangxi Science & Technology Pillar Program
and the Science Foundation for Young Scholars of Jiangxi Province
(grant no. 2007GQY1167).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
MH and SZ wrote the manuscript. SX and XX
constructed the tables and diagrams. MH, SZ, SX, XX and XZ checked
and revised the manuscript and were involved in the conception of
the study. Additionally, XZ was responsible for the organization,
revision and submission of this manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan HW, Li SC and Tsai KW: MicroRNA
dysregulation in gastric cancer. Curr Pharm Des. 19:1273–1284.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou X, Men X, Zhao R, Han J, Fan Z, Wang
Y, Lv Y, Zuo J, Zhao L, Sang M, et al: miR-200c inhibits
TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting
ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 25:68–76. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Li Z, Li Y and Zang A: MicroRNA
and signaling pathways in gastric cancer. Cancer Gene Ther.
21:305–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Zhu X, Shou T, Yang L, Cheng X, Wang
J, Deng L and Zheng Y: MicroRNA-28 promotes cell proliferation and
invasion in gastric cancer via the PTEN/PI3K/AKT signalling
pathway. Mol Med Rep. 17:4003–4010. 2017.PubMed/NCBI
|
|
6
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrocca F, Visone R, Onelli MR, Shah MH,
Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini
M, et al: E2F1-regulated MicroRNAs impair TGFbeta-dependent
cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell.
13:272–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Qu Y, Jingjing D, Deng T, Liu R,
Zhang L, Bai M, Li J, Zhou L, Ning T, et al: Integrated analysis of
the miRNA, gene and pathway regulatory network in gastric cancer.
Oncol Rep. 35:1135–1146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JX, Chen ZH, Chen DL, Tian XP, Wang
CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS, et al: LINC01410-
miR-532-NCF2-NF-κB feedback loop promotes gastric cancer
angiogenesis and metastasis. Oncogene. 37:2660–2675. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Lu YY, Zhao XD, Guo HQ, Liu CH, Li
H, Zhou L, Han YN, Wu KC, Nie YZ, et al: MicroRNA-296-5p increases
proliferation in gastric cancer through repression of
Caudal-related homeobox 1. Oncogene. 33:783–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuoka T and Yashiro M: The role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers (Basel).
6:1441–1463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Huang Z and Chen R: Microrna-136
promotes proliferation and invasion ingastric cancer cells through
Pten/Akt/P-Akt signaling pathway. Oncol Lett. 15:4683–4689.
2018.PubMed/NCBI
|
|
13
|
Dahia PL: PTEN, a unique tumor suppressor
gene. Endocr Relat Cancer. 7:115–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang YH, Lee HS and Kim WH: Promoter
methylation and silencing of PTEN in gastric carcinoma. Lab Invest.
82:285–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M and
Moriyama M: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Guo JJ, Liu YM and Wu XL:
MicroRNA-34A inhibits the growth, invasion and metastasis of
gastric cancer by targeting PDGFR and MET expression. Bioscience
Reports. 34:e001122014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Hou J, Li Z, Zheng Z, Wei J, Song
D, Hu T, Wu Q, Yang JY and Cai JC: miR-15a-3p and miR-16-1-3p
negatively regulate twist1 to repress gastric cancer cell invasion
and metastasis. Int J Biol Sci. 13:122–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang W, Tong JHM, Lung RWM, Dong Y, Zhao
J, Liang Q, Zhang L, Pan Y, Yang W, Pang JCS, et al: Targeting of
YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor
function in gastric adenocarcinoma. Mol Cancer. 14:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Chen J, Ding C, Wei S, Zhu Y, Yang
W, Zhang X, Wei X and Han D: MicroRNA-137 contributes to dampened
tumorigenesis in human gastric cancer by Targeting AKT2. PLoS One.
10:e01301242015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu LY, Wang W, Zhao LY, Guo B, Yang J,
Zhao XG, Hou N, Ni L, Wang AY, Song TS, et al: miR-126 inhibits
growth of SGC-7901 cells by synergistically targeting the oncogenes
PI3KR2 and Crk, and the tumor suppressor PLK2. Int J Oncol.
45:1257–1265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Li L, Wang S, Lei Y, Ge Q, Lv N,
Zhou X and Chen C: Reduced miR-126 expression facilitates
angiogenesis of gastric cancer through its regulation on VEGF-A.
Oncotarget. 5:11873–11885. 2014.PubMed/NCBI
|
|
25
|
Otsubo T, Akiyama Y, Hashimoto Y, Shimada
S, Goto K and Yuasa Y: MicroRNA-126 inhibits SOX2 expression and
contributes to gastric carcinogenesis. PLoS One. 6:e166172011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
27
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan MIN: MicroRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie M, Dart DA, Guo T, Xing XF, Cheng XJ,
Du H, Jiang WG, Wen XZ and Ji JF: MicroRNA-1 acts as a tumor
suppressor microRNA by inhibiting angiogenesis-related growth
factors in human gastric cancer. Gastric Cancer. 21:41–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: Clinical significance of miR-146a in gastric cancer cases.
Clin Cancer Res. 17:4277–4284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu F, Bu Z, Zhao F and Xiao D: Increased
T-helper 17 cell differentiation mediated by exosome-mediated
microRNA-451 redistribution in gastric cancer infiltrated T cells.
Cancer Sci. 109:65–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu C, Ren C, Han J, Ding Y, Du J, Dai N,
Dai J, Ma H, Hu Z, Shen H, et al: A five-microRNA panel in plasma
was identified as potential biomarker for early detection of
gastric cancer. Br J Cancer. 110:2291–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong
J and Feng J: miR-590-5p regulates gastric cancer cell growth and
chemosensitivity through RECK and the AKT/ERK pathway. OncoTargets
Ther. 9:6009–6019. 2016. View Article : Google Scholar
|
|
33
|
Zhou X, Ye F, Yin C, Zhuang Y, Yue G and
Zhang G: The interaction between miR-141 and lncRNA-H19 in
regulating cell proliferation and migration in gastric cancer. Cell
Physiol Biochem. 36:1440–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han TS, Hur K, Xu G, Choi B, Okugawa Y,
Toiyama Y, Oshima H, Oshima M, Lee HJ, Kim VN, et al: MicroRNA-29c
mediates initiation of gastric carcinogenesis by directly targeting
ITGB1. Gut. 64:203–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burgering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Janku F, Yap TA and Meric-Bernstam F:
Targeting the PI3K pathway in cancer: Are we making headway? Nat
Rev Clin Oncol. 15:273–291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang HW, Shin MG, Lee S, Kim JR, Park WS,
Cho KH, Meyer T and Heo WD: Cooperative activation of PI3K by Ras
and Rho family small GTPases. Mol Cell. 47:281–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
Rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soung YH, Lee JW, Nam SW, Lee JY, Yoo NJ
and Lee SH: Mutational analysis of AKT1, AKT2 and AKT3 genes in
common human carcinomas. Oncology. 70:285–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maira SM, Galetic I, Brazil DP, Kaech S,
Ingley E, Thelen M and Hemmings BA: Carboxyl-terminal modulator
protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the
plasma membrane. Science. 294:374–380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li VS, Wong CW, Chan TL, Chan AS, Zhao W,
Chu KM, So S, Chen X, Yuen ST and Leung SY: Mutations of PIK3CA in
gastric adenocarcinoma. BMC Cancer. 5:292005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma J, Liu J, Wang Z, Gu X, Fan Y, Zhang W,
Xu L, Zhang J and Cai D: NF-kappaB-dependent MicroRNA-425
upregulation promotes gastric cancer cell growth by targeting PTEN
upon IL-1β induction. Mol Cancer. 13:402014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wen YG, Wang Q, Zhou CZ, Qiu GQ, Peng ZH
and Tang HM: Mutation analysis of tumor suppressor gene PTEN in
patients with gastric carcinomas and its impact on PI3K/AKT
pathway. Oncol Rep. 24:89–95. 2010.PubMed/NCBI
|
|
47
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shi J, Yao D, Liu W, Wang N, Lv H, Zhang
G, Ji M, Xu L, He N, Shi B and Hou P: Highly frequent PIK3CA
amplification is associated with poor prognosis in gastric cancer.
BMC Cancer. 12:502012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiong J, Li Z, Zhang Y, Li D, Zhang G, Luo
X, Jie Z, Liu Y, Cao Y, Le Z, et al: PRL-3 promotes the peritoneal
metastasis of gastric cancer through the PI3K/Akt signaling pathway
by regulating PTEN. Oncol Rep. 36:1819–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pan H, Li T, Jiang Y, Pan C, Ding Y, Huang
Z, Yu H and Kong D: Overexpression of circular RNA ciRS-7 abrogates
the tumor suppressive effect of miR-7 on gastric cancer via
PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 119:440–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao S, Wang L, Zhang C, Deng Y, Zhao B,
Ren Y, Fu Y and Meng X: Inhibitor of growth 3 induces cell death by
regulating cell proliferation, apoptosis and cell cycle arrest by
blocking the PI3K/AKT pathway. Cancer Gene Ther. 25:240–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang SQ, Wang C, Chang LM, Zhou KR, Wang
JW, Ke Y, Yang DX, Shi HG, Wang R, Shi XL, et al: Geridonin and
paclitaxel act synergistically to inhibit the proliferation of
gastric cancer cells through ROS-mediated regulation of the
PTEN/PI3K/Akt pathway. Oncotarget. 7:72990–73002. 2016.PubMed/NCBI
|
|
53
|
Li C, Zhang J, Wu H, Li L, Yang C, Song S,
Peng P, Shao M, Zhang M, Zhao J, et al: Lectin-like oxidized
low-density lipoprotein receptor-1 facilitates metastasis of
gastric cancer through driving epithelial-mesenchymal transition
and PI3K/Akt/GSK3β activation. Sci Rep. 7:452752017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li
Z, Wang J, Li B, Hu Y, Dong B, et al: Dual PI3K/mTOR inhibitor
BEZ235 as a promising therapeutic strategy against
paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR
pathway. Cell Death Dis. 9:1232018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jin P and Sun L: Tu1968 the effect of a
novel Akt inhibitor MK-2206 on proliferation and chemosensitivity
of gastric cancer. Gastroenterology. 148 Suppl 1:S948–S949. 2015.
View Article : Google Scholar
|
|
56
|
Qi M, Jiao M, Li X, Hu J, Wang L, Zou Y,
Zhao M, Zhang R, Liu H, Mi J, et al: CUL4B promotes gastric cancer
invasion and metastasis-involvement of upregulation of HER2.
Oncogene. 37:1075–1085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ,
Liu X and Guo WJ: miR-616-3p promotes angiogenesis and EMT in
gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res
Commun. 501:1068–1073. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li NA, Wang W, Xu B and Gong H: miR-196b
regulates gastric cancer cell proliferation and invasion via
PI3K/AKT/mTOR signaling pathway. Oncol Lett. 11:1745–1749. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liang M, Shi B, Liu J, He L, Yi G, Zhou L,
Yu G and Zhou X: Downregulation of miR203 induces overexpression of
PIK3CA and predicts poor prognosis of gastric cancer patients. Drug
Des Devel Ther. 9:3607–3616. 2015.PubMed/NCBI
|
|
60
|
Wei B, Song Y, Zhang Y and Hu M:
microRNA-449a functions as a tumor-suppressor in gastric
adenocarcinoma by targeting Bcl-2. Oncol Lett. 6:1713–1718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Riquelme I, Tapia O, Leal P, Sandoval A,
Varga MG, Letelier P, Buchegger K, Bizama C, Espinoza JA, Peek RM,
et al: miR-101-2, miR-125b-2 and miR-451a act as potential tumor
suppressors in gastric cancer through regulation of the
PI3K/AKT/mTOR pathway. Cell Oncol (Dordr). 39:23–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang H, Cheng Y, Jia C, Yu S, Xiao Y and
Chen J: MicroRNA-29s could target AKT2 to inhibit gastric cancer
cells invasion ability. Med Oncol. 32:3422015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu M, Qin S, Cao F, Ding S and Li M:
MicroRNA-379 inhibits metastasis and epithelial-mesenchymal
transition via targeting FAK/AKT signaling in gastric cancer. Int J
Oncol. 51:867–876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC Cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: miR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li C, Song L, Zhang Z, Bai XX, Cui MF and
Ma LJ: MicroRNA-21 promotes TGF-β1-induced epithelial-mesenchymal
transition in gastric cancer through up-regulating PTEN expression.
Oncotarget. 7:66989–67003. 2016.PubMed/NCBI
|
|
67
|
Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F
and Liu L: Long non-coding RNA PTENP1 functions as a ceRNA to
modulate PTEN level by decoying miR-106b and miR-93 in gastric
cancer. Oncotarget. 8:26079–26089. 2017.PubMed/NCBI
|
|
68
|
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z,
Song T and Huang C: miR-338-3p suppresses gastric cancer
progression through a PTEN-AKT axis by targeting P-REX2a. Mol
Cancer Res. 12:313–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang P, Guan Q, Zhou D, Yu Z, Song Y and
Qiu W: miR-21 inhibitors modulate biological functions of gastric
cancer cells via PTEN/PI3K/mTOR pathway. DNA Cell Biol. 37:38–45.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ni SJ, Zhao LQ, Wang XF, Wu ZH, Hua RX,
Wan CH, Zhang JY, Zhang XW, Huang MZ, Gan L, et al: CBX7 regulates
stem cell-like properties of gastric cancer cells via p16 and
AKT-NF-κB-miR-21 pathways. J Hematol Oncol. 11:172018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang X, Wang C, Zhang X, Hua R, Gan L,
Huang M, Zhao L, Ni S and Guo W: Bmi-1 regulates stem cell-like
properties of gastric cancer cells via modulating miRNAs. J Hematol
Oncol. 9:902016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang XF, Zhang XW, Hua RX, Du YQ, Huang
MZ, Liu Y, Cheng YF and Guo WJ: Mel-18 negatively regulates stem
cell-like properties through downregulation of miR-21 in gastric
cancer. Oncotarget. 7:63352–63361. 2016.PubMed/NCBI
|
|
74
|
Li H and Yang BB: Friend or foe: The role
of microRNA in chemotherapy resistance. Acta Pharmacol Sin.
34:870–879. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yan J, Dang Y, Liu S, Zhang Y and Zhang G:
lncRNA HOTAIR promotes cisplatin resistance in gastric cancer by
targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol.
Tumour Biol. Nov 30–2016.(Epub ahead of print). View Article : Google Scholar :
|
|
76
|
Wang F, Li T, Zhang B, Li H, Wu Q, Yang L,
Nie Y, Wu K, Shi Y and Fan D: MicroRNA-19a/b regulates multidrug
resistance in human gastric cancer cells by targeting PTEN. Biochem
Biophys Res Commun. 434:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cao W, Yang W, Fan R, Li H, Jiang J, Geng
M, Jin Y and Wu Y: miR-34a regulates cisplatin-induce gastric
cancer cell death by modulating PI3K/AKT/survivin pathway. Tumor
Biol. 35:1287–1295. 2014. View Article : Google Scholar
|
|
78
|
Eto K, Iwatsuki M, Watanabe M, Ida S,
Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N
and Baba H: The microRNA-21/PTEN pathway regulates the sensitivity
of HER2-positive gastric cancer cells to trastuzumab. Ann Surg
Oncol. 21:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nishida N, Mimori K, Fabbri M, Yokobori T,
Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y and Mori M:
MicroRNA-125a-5p is an independent prognostic factor in gastric
cancer and inhibits the proliferation of human gastric cancer cells
in combination with trastuzumab. Clin Cancer Res. 17:2725–2733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chou J, Shahi P and Werb Z:
microRNA-mediated regulation of the tumor microenvironment. Cell
Cycle. 12:3262–3271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang TS, Yang XH, Chen X, Wang XD, Hua J,
Zhou DL, Zhou B and Song ZS: MicroRNA-106b in cancer-associated
fibroblasts from gastric cancer promotes cell migration and
invasion by targeting PTEN. FEBS Lett. 588:2162–2169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu G, Jiang C, Li D, Wang R and Wang W:
miRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in
gastric cancer. Tumor Biol. 35:9801–9806. 2014. View Article : Google Scholar
|
|
84
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Balakirev ES and Ayala FJ: Pseudogenes:
Are they ‘junk’ or functional DNA? Annu Rev Genet. 37:123–151.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li L, Zhang J, Pan Q and Lei C:
MicroRNA-23a regulates cell migration and invasion by target PTEN
in gastric cancer. Int J Clin Exp Pathol. 9:877–887. 2016.
|
|
87
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z and Liu B: miR-148a functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and Akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tseng PC, Chen CL, Shan YS, Chang WT, Liu
HS, Hong TM, Hsieh CY, Lin SH and Lin CF: An increase in
integrin-linked kinase non-canonically confers NF-κB-mediated
growth advantages to gastric cancer cells by activating ERK1/2.
Cell Commun Signal. 12:692014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang L, Lei J, Fang ZL and Xiong JP:
miR-128b is down-regulated in gastric cancer and negatively
regulates tumour cell viability by targeting PDK1/Akt/NF-κB axis. J
Biosci. 41:77–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sha M, Ye J, Zhang LX, Luan ZY, Chen YB
and Huang JX: Celastrol induces apoptosis of gastric cancer cells
by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway.
Pharmacology. 93:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
92
|
Lauschke VM, Mkrtchian S and
Ingelman-Sundberg M: The role of microRNAs in liver injury at the
crossroad between hepatic cell death and regeneration. Biochem
Biophys Res Commun. 482:399–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nagaraja AK, Creighton CJ, Yu Z, Zhu H,
Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM,
et al: A link between mir-100 and FRAP1/mTOR in clear cell ovarian
cancer. Mol Endocrinol. 24:447–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kwiatkowski DJ: Rhebbing up mTOR: New
insights on TSC1 and TSC2, and the pathogenesis of tuberous
sclerosis. Cancer Biol Ther. 2:471–476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kim LC, Cook RS and Chen J: mTORC1 and
mTORC2 in cancer and the tumor microenvironment. Oncogene.
36:2191–2201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang T, Kang W, Zhang B, Wu F, Dong Y,
Tong JH, Yang W, Zhou Y, Zhang L, Cheng AS, et al: miR-508-3p
concordantly silences NFKB1 and RELA to inactivate canonical NF-κB
signaling in gastric carcinogenesis. Mol Cancer. 15:92016.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhou X, Xia Y, Su J and Zhang G:
Down-regulation of miR-141 induced by Helicobacter pylori
promotes the invasion of gastric cancer by targeting STAT4. Cell
Physiol Biochem. 33:1003–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
He B, Xiao YF, Tang B, Wu YY, Hu CJ, Xie
R, Yang X, Yu ST, Dong H, Zhao XY, et al: hTERT mediates gastric
cancer metastasis partially through the indirect targeting of ITGB1
by microRNA-29a. Sci Rep. 6:219552016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma
H, Xia J, Bin J, Liao Y and Liao W: miR-338-3p inhibits
epithelial-mesenchymal transition in gastric cancer cells by
targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget.
6:15222–15234. 2015.PubMed/NCBI
|
|
100
|
Liu J, Wang X, Yang X, Liu Y, Shi Y, Ren J
and Guleng B: miRNA423-5p regulates cell proliferation and invasion
by targeting trefoil factor 1 in gastric cancer cells. Cancer Lett.
347:98–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Han X, Chen Y, Yao N, Liu H and Wang Z:
MicroRNA let-7b suppresses human gastric cancer malignancy by
targeting ING1. Cancer Gene Ther. 22:122–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wang JY, Huang TJ, Chen FM, Hsieh MC, Lin
SR, Hou MF and Hsieh JS: Mutation analysis of the putative tumor
suppressor gene PTEN/MMAC1 in advanced gastric carcinomas. Virchows
Arch. 442:437–443. 2003.PubMed/NCBI
|
|
104
|
Velho S, Oliveira C, Ferreira A, Ferreira
AC, Suriano G, Schwartz S Jr, Duval A, Carneiro F, Machado JC,
Hamelin R and Seruca R: The prevalence of PIK3CA mutations in
gastric and colon cancer. Eur J Cancer. 41:1649–1654. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Staal SP: Molecular cloning of the akt
oncogene and its human homologues AKT1 and AKT2: Amplification of
AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci
USA. 84:5034–5037. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cheng Y, Li Y, Liu D, Zhang R and Zhang J:
miR-137 effects on gastric carcinogenesis are mediated by targeting
Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett.
588:3274–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lu W, Xu Z, Zhang M and Zuo Y: miR-19a
promotes epithelial-mesenchymal transition through PI3K/AKT pathway
in gastric cancer. Int J Clin Exp Pathol. 7:7286–7296.
2014.PubMed/NCBI
|
|
108
|
Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W
and Zhang A: Upregulation of MicroRNA-107 induces proliferation in
human gastric cancer cells by targeting the transcription factor
FOXO1. FEBS Lett. 588:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhao Y, Dong Q and Wang E: MicroRNA-320
inhibits invasion and induces apoptosis by targeting CRKL and
inhibiting ERK and AKT signaling in gastric cancer cells. Onco
Targets Ther. 10:1049–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|















