Introduction
Osteosarcoma, one of the most common bone tumors,
prevails particularly in children and adults (1). Worldwide, the estimated number of
newly diagnosed patients every year is ~1–14 in one million.
Surgical resection combined with chemotherapy is the principle
strategy for osteosarcoma treatment (2). Chemotherapeutic drugs including
cisplatin and methotrexate have been widely used clinically for
patients with osteosarcoma (3).
However, these drugs disrupt the DNA synthesis of normal cells in
addition to tumor cells, thereby causing severe side-effects. In
addition, once tumor cells develop chemoresistance, they are harder
to control. Therefore, novel drugs that maximize tumor cell killing
in osteosarcoma and minimize normal cell damage are urgently
required.
Isoliquiritigenin (ISL), a type of flavonoid, is
extracted from the roots of licorice and numerous other plants and
foods (4). ISL has been used as a
food additive and possesses a number of biological activities
including antioxidative stress, anti-inflammatory, anti-viral and
anti-angiogenic effects (5,6). Recent studies have focused on the
antitumor effects of ISL. A wide spectrum of tumors has been
demonstrated to be sensitive to ISL, including glioma, ovarian
cancer, hepatoma carcinoma and breast cancer (7–10).
However, it is unclear whether ISL may be used as a drug treatment
for osteosarcoma.
Accumulating evidence suggests that the PI3K/AKT
pathway is the underlying signaling pathway that mediates the
antitumor effect of ISL. It has been demonstrated that ISL inhibits
the proliferation of the prostate cell line DU145 by decreasing the
recruitment of PI3K and the phosphorylation of AKT (11). Studies investigating the effect of
ISL on breast cancer have revealed that ISL decreases cell
proliferation and induces cell apoptosis by disrupting the PI3K/AKT
pathway (12,13). It is generally accepted that the
activated PI3K/AKT signaling pathway is one of the most important
mechanisms that promotes the development of osteosarcoma (14–16).
Drugs targeting the PI3K/AKT pathway have been developed for
osteosarcoma treatment, including PI3K pan-inhibitors (16). However, due to their weak
solubility, instability and high toxicity, these inhibitors have
little potential for clinical application (16). A recent study demonstrated that ISL
significantly suppressed tumor growth in a xenograft mouse model of
endometrial cancer without apparent side-effects (17). Based on these findings, it was
proposed that ISL inhibited the progression of osteosarcoma by
deactivating the PI3K/AKT signaling pathway.
In the present study, firstly, the effect of ISL on
osteosarcoma was investigated in vitro by examining tumor
cell proliferation, cell apoptosis and cell migration. Secondly,
intracellular molecular alterations in PI3K/AKT signaling upon
treatment with ISL were assessed. Finally, the effect of ISL on
osteosarcoma growth was assessed in a xenograft mouse model. The
present study demonstrated the anti-osteosarcoma effect of ISL,
suggesting that ISL may serve as a promising agent for osteosarcoma
treatment.
Materials and methods
Cell culture and treatment
The human Saos-2 cell line and a mouse embryonic
osteoblastic cell line (MC3T3-E1) were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
maintained in RPMI-1640 containing 2 mM L-glutamine and 25 mM HEPES
supplemented with 10% fetal bovine serum, and 100 U/ml
penicillin/streptomycin at 37°C in a humidified 5% CO2
atmosphere. ISL was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany) with a purity of >98% and was dissolved in
dimethyl sulfoxide (DMSO). Cells were incubated with ISL at various
concentrations and time-points. Cells treated with DMSO were set as
the control group in the present study.
Tumor xenografts in NOD-SCID mice
A total of 10 female 5-week-old NOD-SCID mice were
purchased from the Model Animal Research Center of Nanjing
University and maintained in a pathogen-free environment with a
constant humidity and temperature at 12 h light/dark cycle with
free access to food and water. The weight of the mice ranged from
19 to 23 g. Saos-2 cells [1×106 cells in 0.1 ml
phosphate-buffered saline (PBS) for each mouse] were implanted by
subcutaneous injection into the flank of the mice. ISL was
dissolved in DMSO and further mixed with corn oil. The mice were
randomly divided into a corn oil-treated group (control group) and
an ISL-treated group (5 in each). Corn oil and ISL were
administered by oral gavage at 50 mg/kg/day for 56 consecutive
days. The tumor volume and body weight were recorded every day
until the animals were sacrificed. The mice were anaesthetized by
1% pentobarbital (i.p.) at a dose of 50 mg/kg and sacrificed by
cervical dislocation. When the mice were sacrificed, the tumors
were removed for weighting and were fixed in 4% paraformaldehyde
solution (4 g/100 ml PBS) or stored at −80°C. The max tumor size
was 920 mm3 (0.8 g). All animal experiments were
approved by the Committee on the Ethics of Animal Experiments of
Nanjing University and performed strictly in accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Bethesda, MD,
USA).
Cell viability assay
Cell viability was assessed by a Cell Counting Kit-8
(CCK-8; Beyotime Institute of Biotechnology, Haimen, China).
Briefly, cells (3×103 cells/well) were seeded in 96-well
plates and cultured for 24 h. The cells were incubated with various
concentrations of ISL for the indicated time-points. For the cell
viability assay, cells were incubated with CCK-8 solution (10
µl/well) for 3 h, and the optical density value in each well was
determined using a microplate reader at 450 nm.
Flow cytometric analysis of the cell
cycle
The cultured cells were digested, and a single cell
suspension was prepared. Cells were fixed in cold 70% ethanol and
stored at 4°C overnight. The ethanol was removed by centrifugation
(1,000 × g for 5 min), followed by staining with propidium iodide
(PI; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min
at 4°C in the dark. The cell cycle was analyzed by flow cytometry
immediately.
Flow cytometric analysis of cell
apoptosis
The cultured cells were collected and a single cell
suspension was produced by trypsin digestion and gentle pipetting.
An Annexin V-FITC Apoptosis Detection Kit (Thermo Fisher
Scientific, Inc.) was used. The cells were incubated with Annexin
V-FITC and PI for 15 min at room temperature in the dark. The
apoptosis of the samples was analyzed using a FACSCalibur flow
cytometer (Becton-Dickinson, Heidelberg, Germany).
Scratch test
Cells were seeded into 6-well plates
(5×104 cells/well). When the cells had grown to ~80%
confluence, a sterile pipette was used to scratch a line across
each well. Following washing with PBS, cell culture was continued.
The scratch was photographed at 0 and 48 h post-scratching. The
width of the scratch was analyzed and assessed using Image-Pro Plus
6.0 software (Media Cybernetics, Rockville, MD, USA). The relative
cell migration distance was calculated as follows: (The width at 0
h in the ISL group-the width at 48 h in the ISL group)/(the width
at 0 h in the control group-the width at 48 h in the control
group). The cell migration distance in the control group was set as
1.
Transwell assay
The Transwell assay was performed using Falcon cell
culture inserts containing 8-µm pore size polyethylene
terephthalate membranes (BD Biosciences, Franklin Lakes, NJ, USA).
The Saos-2 cells were seeded into the upper chamber
(5×104 cells/well) and maintained at 37°C in a
humidified 5% CO2 atmosphere for 48 h. Non-migrating
cells remaining on the upper surfaces were removed while the
migrating cells were fixed with methanol and visualized by staining
with crystal violet solution. The images were captured by a light
microscope (Nikon, Tokyo, Japan).
Western blot analysis
Collected cells or tumor samples were homogenized in
radioimmunoprecipitation assay buffer containing
phenylmethanesulfonyl fluoride (PMSF) and a phosphatase inhibitor.
Following centrifugation (13,800 × g for 15 min), the supernatant
was collected, and the protein concentration was determined using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Quantified protein (20 µg) was separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins
were blotted onto a polyvinylidene difluoride membrane, and the
membranes were soaked in 5% milk/PBS with Tween-20 (PBST) for 1 h.
Subsequently, the membranes were incubated with primary antibodies,
including p53 (dilution 1:800; cat. no. 2524), p27 (dilution 1:800;
cat. no. 2552), apoptosis regulator Bcl-2 (Bcl-2; dilution 1:800;
cat. no. 4223), apoptosis regulator Bax (Bax; dilution 1:800; cat.
no. 2772), caspase-3 (dilution 1:800; cat. no. 9662; all from Cell
Signaling Technology, Inc., Danvers, MA, USA), p21 (dilution 1:600;
cat. no. ab109520), cyclin D1 (dilution 1:500; cat. no. ab134175),
matrix metalloproteinase (MMP)2 (dilution 1:1,000; cat. no.
ab37150), MMP9 (dilution 1:200; cat. no. ab38898), PI3K (dilution
1:1,000; cat. no. ab191606), p-PI3K (dilution 1:500; cat. no.
ab182651), AKT (dilution 1:500; cat. no. ab8805), p-AKT (dilution
1:2,000; cat. no. ab81283) and β-actin (dilution 1:2,000; cat. no.
ab8226; all from Abcam, Cambridge, UK) at 4°C overnight. Following
washing with PBST three times, the membranes were incubated with
HRP-conjugated goat anti-rabbit antibody (dilution 1:3,000; cat.
no. ab6721) and goat anti-mouse antibody (dilution 1:3,000; cat.
no. ab6789; both from Abcam) for 1 h at room temperature. Finally,
the protein bands were visualized via enhanced chemiluminescence
(Beyotime Institute of Biotechnology) and quantified by Image-Pro
Plus 6.0 software (Media Cybernetics).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The collected cells or tumor tissue were lysed in
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and the
total RNA was extracted. cDNA was acquired by RT using a commercial
kit (Thermo Fisher Scientific, Inc.). qPCR was performed to detect
mRNA expression levels, including MMP2 and MMP9 using a SYBR-Green
Kit (Thermo Fisher Scientific, Inc.). Thermocycling conditions for
RT-qPCR were as follows: i) 50°C for 2 min, 1 cycle; ii) 95°C for
10 min, 1 cycle; iii) 95°C for 15 sec, 60°C for 30 sec and 72°C for
30 sec, 40 cycles. The comparative ΔΔCq method was used to quantify
relative gene expression as previously descripted (18). The sequences of the primers are
listed in Table I.
 | Table I.The sequences of the primers for
RT-qPCR. |
Table I.
The sequences of the primers for
RT-qPCR.
| Gene name | Forward (from 5′ to
3′) | Reverse (from 5′ to
3′) |
|---|
| MMP2 |
CAAGTTCCCCGGCGATGTC |
CTGGACAGCCAGACACTAAAG |
| MMP9 |
TTCTGGTCAAGGTCACCTGTC |
CTCGCGGCAAGTCTTCAGAG |
Immunohistochemistry
The tumor tissues were embedded in paraffin and cut
into 5 µm sections. Following dewaxing and rehydration, the slides
were placed in boiling PBS for 10 min for antigen retrieval,
followed by incubation of the sections with 0.3% hydrogen peroxide
for 30 min at room temperature to block endogenous peroxidase
activity. The sections were blocked by 0.5% BSA/PBST containing 10%
goat serum for 60 min at room temperature. The slides were
incubated with primary antibodies, including MMP2 (dilution 1:200;
cat. no. ab37150), MMP9 (dilution 1:200; cat. no. ab38898),
proliferating cell nuclear antigen (PCNA; dilution 1:400; cat. no.
ab29; all from Abcam) and caspase-3 (dilution 1:200; cat. no. 9662;
Cell Signaling Technology, Inc.) at 4°C overnight. Following three
washes with PBS, the sections were incubated with HRP-conjugated
goat anti-rabbit antibody (dilution 1:600; cat. no. ab6721) and
goat anti-mouse antibody (dilution 1:600; cat. no. ab6789; both
from Abcam) for 1 h at room temperature. The color was developed
using the 3,5-diaminobenzidine (DAB; Vector Laboratories, Inc.,
Burlingame, CA, USA) substrate, followed by counterstaining with
hematoxylin. The images were captured by a light microscope
(Nikon).
ATP assay
The ATP content was determined in cell extracts
using a luminescent ATP detection kit (ATPlite Luminescence Assay
System; PerkinElmer Life Sciences, Waltham, MA, USA). The
experiment was performed according to the manufacturer's protocol.
The luminescence intensity was detected using a microplate
reader.
Statistical analysis
Data are expressed as the means ± standard
deviation. For the comparison of 2 groups, a Student's t-test was
used. When comparing ≥3 groups, one-way analysis of variance
(ANOVA) was used, if the ANOVA was significant, post hoc testing of
differences between groups was performed using Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Effect of ISL on Saos-2 cell
viability
To investigate whether ISL was able to specifically
inhibit the cell viability of osteosarcoma cell line Saos-2, Saos-2
and MC3T3-E1 (mouse embryonic osteoblastic cells) cells were
treated with different doses of ISL for 48 h. The results
demonstrated that when treated with 30 µM ISL, Saos-2 cell
viability began to decrease significantly, while MC3T3-E1 cells
remained unaltered (Fig. 1A).
Subsequently, 30 µM ISL was selected to treat Saos-2 cells across a
number of time-points. The growth of ISL-treated Saos-2 cells was
significantly inhibited at the indicated time-points compared with
the control group, and the optimum inhibitory effect of ISL on
Saos-2 cells was observed at 48 h (Fig.
1B).
Effect of ISL on Saos-2 cell
proliferation
To investigate whether the decrease in Saos-2 cell
viability induced by ISL was associated with cell cycle arrest, the
cell cycle distribution was analyzed. The results demonstrated that
ISL was able to significantly increase the number of cells in the
G1 phase and decrease the number of cells in the S phase (Fig. 2A and B). Western blot analysis
revealed that the expression of cyclin D1 was significantly
downregulated, while p53, p21 and p27 were significantly
upregulated in ISL-treated Saos-2 cells, compared with the control
group (Fig. 2C and D).
Effect of ISL on Saos-2 cell
apoptosis
To investigate whether ISL was able to induce Saos-2
cell apoptosis, Annexin V and PI staining was performed. The
results demonstrated that the apoptosis rate in ISL-treated cells
was significantly higher compared with that in the control cells
(Fig. 3A and B). Further analysis
revealed that ISL was able to significantly downregulate the
expression of anti-apoptosis protein Bcl-2 and upregulate the
expression of the pro-apoptotic proteins Bax and caspase-3
(Fig. 3C and D). In addition, ATP
production was significantly inhibited by ISL (Fig. 3E).
Effect of ISL on Saos-2 cell
migration
To investigate whether ISL was able to inhibit
Saos-2 cell migration, scratch test and Transwell assays were
performed. To avoid the effects of cell viability of having an
effect on the migration ability of the cells, an ISL dose that has
no effect on cell viability is necessary and required for the cell
migration assay. Since cell viability was significantly reduced
when Saos-2 cells were treated with ISL at a dose of 30 µM, 10 µM
ISL was used to treat cells. The results demonstrated that the cell
migration distance was shorter, while the number of migratory cells
was decreased in the ISL group compared with the control group
(Fig. 4A-D). Furthermore, the
protein and mRNA expression levels of MMP2 and MMP9 in Saos-2 cells
were downregulated by treatment with ISL (Fig. 4E-G).
Effect of ISL on the PI3K/AKT pathway
in Saos-2 cells
To investigate whether ISL affected the expression
of molecules in the PI3K/AKT pathway, the expression of associated
proteins was assessed. The results demonstrated that although the
protein expression levels of total PI3K and AKT were not affected
by treatment with ISL, the expression levels of p-PI3K and p-AKT
were significantly downregulated (Fig.
5A and B).
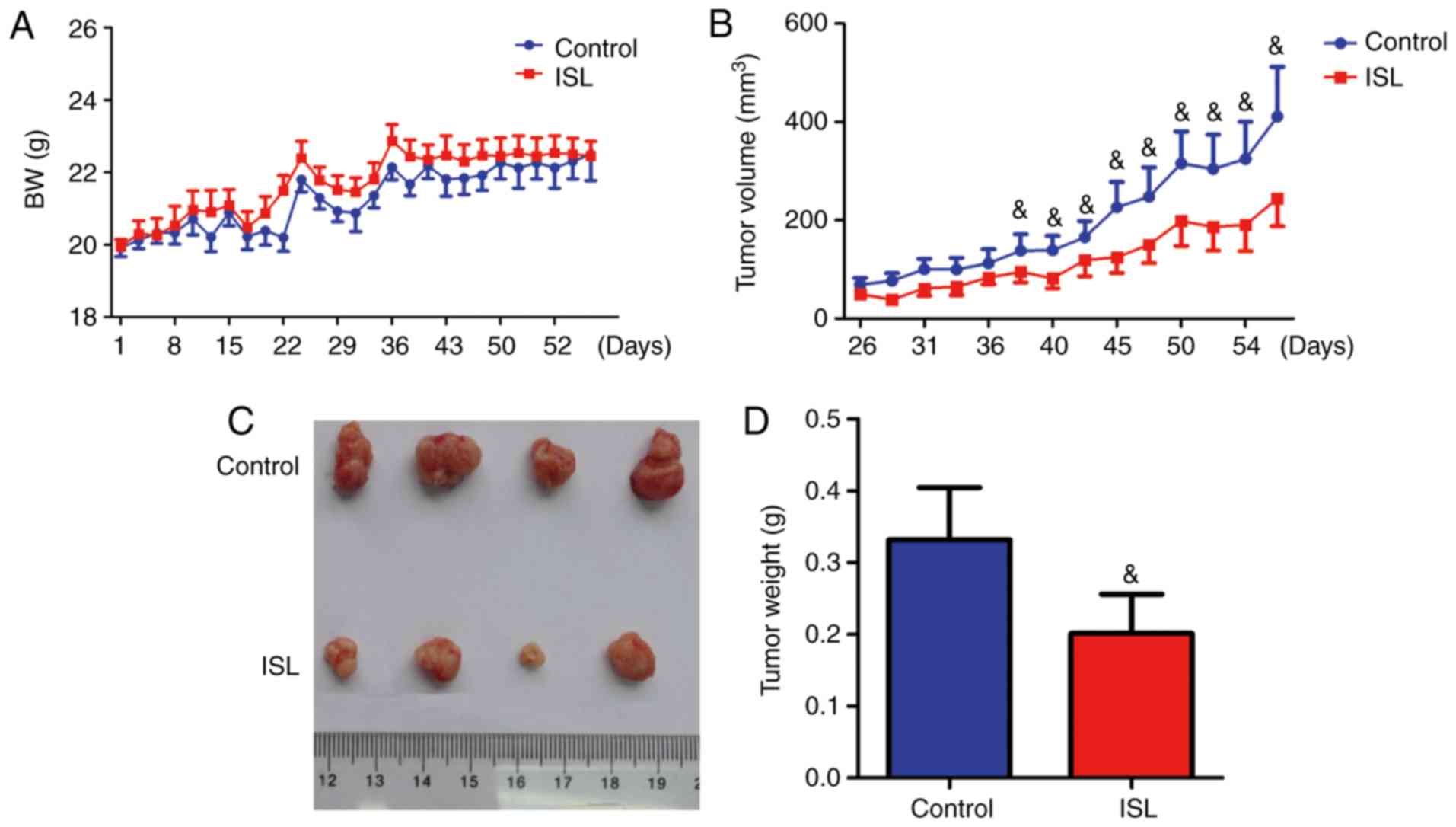
Effect of ISL on the growth of
xenograft tumors
To investigate whether ISL was able to suppress the
growth of xenograft tumors, Saos-2 cells were implanted via
subcutaneous injection into the flank of ISL-treated or control
mice. Body weight and tumor volume were monitored. The results
demonstrated that treatment with ISL had no influence on body
weight (Fig. 6A). However, the
ISL-treated tumor size began to significantly decrease at day 40
post-injection, compared with vehicle-treated tumors (Fig. 6B). Following sacrifice, the tumors
were removed, and the tumor size and weight were compared between
vehicle-treated and ISL-treated mice. The tumor size and tumor
weight were decreased in ISL-treated mice compared with control
mice (Fig. 6C and D).
Effect of ISL on cell proliferation,
apoptosis and migration in the xenograft tumor model
To investigate the effect of ISL on cell
proliferation, cell migration and cell apoptosis in the xenograft
tumor model, PCNA, caspase-3, MMP2 and MMP9 immunohistochemistry
was performed on the tumor sections. The results demonstrated that
PCNA, MMP2 and MMP9 positive cells were significantly reduced and
caspase-3 positive cells were significantly increased in the
ISL-treated tumors compared with the control tumors (Fig. 7A-H). Western blot analysis revealed
that the expression of total PI3K and AKT in ISL-treated and
control tumors was similar, although the levels of p-PI3K and p-AKT
were significantly decreased in ISL-treated tumors (Fig. 7I and J).
 | Figure 7.Effect of ISL on cell proliferation,
apoptosis and migration in xenograft tumors of Saos-2 cells.
Immunohistochemistry on sections of tumors with antibodies against
(A) PCNA, (B) caspase-3, (C) MMP2 and (D) MMP9. Magnification is
×400 in A and B, and ×200 in C and D. Quantification of (E) PCNA
and (F) caspase-3-positive cells. Relative expression of (G) MMP2
and (H) MMP9. (I) Western blot analysis of the expression of AKT,
p-AKT, PI3K and p-PI3K. β-actin was used as a loading control. (J)
Quantification of the protein expression levels of AKT, p-AKT, PI3K
and p-PI3K. &P<0.05,
&&P<0.01,
&&&P<0.001 vs. the control group; n=5.
ISL, isoliquiritigenin; PI3K, phosphatidylinositol 3-kinase; AKT,
RAC-α serine/threonine-protein kinase; MMP, matrix
metalloproteinase; PCNA, proliferating cell nuclear antigen. |
Discussion
In the present study, the effect of ISL on the
growth of osteosarcoma was investigated. Since, compared with other
osteosarcoma cell lines including MG-63 or U2OS, Saos-2 cells
exhibit better tumorigenesis potential in vivo, which
benefits the in vivo study, Saos-2 cells were used in the
present study. It was demonstrated that ISL was able to suppress
the growth of the osteosarcoma cell line Saos-2 cells in
vitro and in vivo. This inhibitory effect of ISL was
associated with decreasing cell proliferation and increasing cell
apoptosis. Furthermore, the in vitro experiments
demonstrated that ISL was able to inhibit the migratory potential
of Saos-2 cells. The underlying mechanisms of action of ISL in
osteosarcoma may depend on the inhibitory effect of ISL on the
PI3K/AKT signaling pathway.
Rapid cell division is one of the most important
hallmarks of tumors. In cells, cell division is promoted by
cyclin-dependent kinases (CDKs) interacting with cyclins (19). This interaction may be blocked by
cell cyclin-dependent kinase inhibitors (CDKIs) (20). In the present study, it was revealed
that ISL was able to significantly upregulate the expression of
p53, p21 and p27 and downregulated cyclin D1 in cultured Saos-2
cells. Furthermore, ISL inhibited the proliferation of Saos-2 cells
in vivo. These results are consistent with previous reports
investigating the antiproliferative effect of ISL in other types of
cancer (7,21–23).
Therefore, the suppressive effect of ISL on osteosarcoma is partly
mediated by inhibition of cell division.
Increasing evidence suggests that ISL is a potent
apoptosis inducer in tumor cells (24–27).
The present study demonstrated that ISL was able to induce the
apoptosis of Saos-2 cells in vitro and in xenograft tumors.
Previous studies have demonstrated that ISL-triggered cell
apoptosis is associated with disruption of mitochondrial function
(28–30). It is generally acknowledged that
mitochondria serve a critical role in mediating cell apoptosis. A
cluster of proteins belonging to the Bcl-2 family localize to the
outer membrane of mitochondria to regulate cell apoptosis (31). The Bcl-2 family proteins may be
classified into two groups: One is the anti-apoptotic proteins,
including Bcl-2, and the other is the pro-apoptotic proteins
including Bax. The balance of these two proteins determines whether
a cell undergoes apoptosis (32,33).
In the present study, it was observed that ISL downregulated the
expression of the anti-apoptotic protein Bcl-2 and upregulated the
expression of the pro-apoptotic protein Bax in cultured Saos-2
cells. Consistent with this, ATP synthesis which occurs primarily
in mitochondria was significantly inhibited by ISL. Therefore, ISL
induced osteosarcoma apoptosis via mitochondrial signaling. A
previous study demonstrated that ISL induced the apoptosis of
Hep-G2 cells in a p53-dependent manner (34). In the present study, the
upregulation of p53 may have been involved in promoting
osteosarcoma apoptosis. In conclusion, ISL-induced cell apoptosis
partly accounted for the osteosarcoma growth-inhibitory effect of
ISL.
It has been noted that ISL may inhibit the
metastasis of various tumors (13,35).
In the present study, it was demonstrated that ISL inhibited the
migratory potential of cultured Saos-2 cells. Breakdown of the
extracellular matrix (ECM) is a crucial event in tumor cell
invasion. This process is regulated by a number of molecules,
particularly MMP2, MMP9 and tissue inhibitors of metalloproteinases
(TIMPs) (36–38). It has been demonstrated that MMP2
and MMP9 are required for osteosarcoma metastasis (39,40). A
previous study demonstrated that ISL inhibits the metastasis of
breast cancer by downregulating the expression of MMP2 and MMP9
(13). Consistent with this
finding, it was observed that ISL downregulated the expression of
MMP2 and MMP9 in cultured Saos-2 cells and in xenograft tumors.
One critical underlying mechanism of the biological
function of ISL is its ability to modify the intracellular PI3K/AKT
pathway. It has been demonstrated that ISL induces melanin
degradation in human epidermal keratinocytes and inhibits the
proliferation of human arterial smooth muscle cells by suppressing
PI3K/AKT signaling (41,42). In addition, numerous studies have
demonstrated that ISL may inhibit the phosphorylation of PI3K and
AKT in tumor cells, thereby suppressing the growth of the tumor
(11–13). In the present study, it was
demonstrated that ISL was able to significantly reduce the levels
of p-AKT and p-PI3K in Saos-2 cells in vitro and in
xenograft tumors. It has previously been demonstrated that the
PI3K/AKT pathway is vital for the initiation and progression of
osteosarcoma (16). A broad
spectrum of molecules involved in cell proliferation, survival and
invasion are directly or indirectly regulated by PI3K/AKT signaling
(16). Drugs targeting PI3K/AKT
signaling have been developed against osteosarcoma, including PI3K
pan inhibitors. However, the safety of these drugs is undergoing
evaluation and remains controversial. In the present study, it was
revealed that treatment with ISL was associated with low
cytotoxicity in MC3T3-E1 cells and had no influence on the body
weight of mice, indicating that ISL may be a safe drug for
osteosarcoma treatment. This result is consistent with a previous
study reporting that ISL may significantly decrease the tumor size
of mice without evident weight loss (17).
In conclusion, it was demonstrated that ISL was able
to inhibit cell proliferation and induce the cell apoptosis of
osteosarcoma in vitro and in vivo, possibly by
deactivating the PI3K/AKT signaling pathway. In addition, it was
also demonstrated that ISL attenuated the migration of cultured
Saos-2 cells. The present study indicated that ISL may serve as a
potential drug for osteosarcoma treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the China Postdoctoral
Science Foundation Funded Project (grant no. 2016M593039).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
SW and XS conceived and designed the study. CL, XZ,
CS and XL performed the experiments and analyzed the data. CL and
XZ wrote the paper. SW and XS reviewed and edited the manuscript.
CL, CS and XL performed the experiments required for revision. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the
Committee on the Ethics of Animal Experiments of Nanjing University
and were performed strictly in accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (Bethesda, MD, USA).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ISL
|
isoliquiritigenin
|
|
CDKs
|
cyclin-dependent kinases
|
|
CDKIs
|
cyclin-dependent kinase inhibitors
|
References
|
1
|
Zambo I and Vesely K: WHO classification
of tumours of soft tissue and bone 2013: The main changes compared
to the 3rd edition. Cesk Patol. 50:64–70. 2014.(In Czech).
PubMed/NCBI
|
|
2
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eilber FR and Rosen G: Adjuvant
chemotherapy for osteosarcoma. Semin Oncol. 16:312–322.
1989.PubMed/NCBI
|
|
4
|
Chin YW, Jung HA, Liu Y, Su BN, Castoro
JA, Keller WJ, Pereira MA and Kinghorn AD: Anti-oxidant
constituents of the roots and stolons of licorice (Glycyrrhiza
glabra). J Agric Food Chem. 55:4691–4697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng F, Du Q, Peng C, Wang N, Tang H, Xie
X, Shen J and Chen J: A review: The pharmacology of
isoliquiritigenin. Phytother Res. 29:969–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asl MN and Hosseinzadeh H: Review of
pharmacological effects of Glycyrrhiza sp. and its bioactive
compounds. Phytother Res. 22:709–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin Y, Sun H, Dang Y and Li Z:
Isoliquiritigenin inhibits the proliferation and induces the
differentiation of human glioma stem cells. Oncol Rep. 39:687–694.
2018.PubMed/NCBI
|
|
8
|
Chen HY, Huang TC, Shieh TM, Wu CH, Lin LC
and Hsia SM: Isoliquiritigenin induces autophagy and inhibits
ovarian cancer cell growth. Int J Mol Sci. 18(pii): E20252017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu YL, Kuo PL, Lin LT and Lin CC:
Isoliquiritigenin inhibits cell proliferation and induces apoptosis
in human hepatoma cells. Planta Med. 71:130–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Wang N, Han S, Wang D, Mo S, Yu L,
Huang H, Tsui K, Shen J and Chen J: Dietary compound
isoliquiritigenin inhibits breast cancer neoangiogenesis via
VEGF/VEGFR-2 signaling pathway. PLoS One. 8:e685662013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung JI, Chung E, Seon MR, Shin HK, Kim
EJ, Lim SS, Chung WY, Park KK and Park JH: Isoliquiritigenin (ISL)
inhibits ErbB3 signaling in prostate cancer cells. Biofactors.
28:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai
H, Zhang J, Falck JR, Guo AM, Yue J, et al: Isoliquiritigenin
induces growth inhibition and apoptosis through downregulating
arachidonic acid metabolic network and the deactivation of PI3K/Akt
in human breast cancer. Toxicol Appl Pharmacol. 272:37–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KL, Hsia SM, Chan CJ, Chang FY, Huang
CY, Bau DT and Wang PS: Inhibitory effects of isoliquiritigenin on
the migration and invasion of human breast cancer cells. Expert
Opin Ther Targets. 17:337–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Sun Y, Lu Y, Jiang X, Ma B, Yu L,
Zhang J, Dong X and Zhang Q: Glaucocalyxin A exerts anticancer
effect on osteosarcoma by inhibiting GLI1 nuclear translocation via
regulating PI3K/Akt pathway. Cell Death Dis. 9:7082018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Angulo P, Kaushik G, Subramaniam D,
Dandawate P, Neville K, Chastain K and Anant S: Natural compounds
targeting major cell signaling pathways: A novel paradigm for
osteosarcoma therapy. J Hematol Oncol. 10:102017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu CH, Chen HY, Wang CW, Shieh TM, Huang
TC, Lin LC, Wang KL and Hsia SM: Isoliquiritigenin induces
apoptosis and autophagy and inhibits endometrial cancer growth in
mice. Oncotarget. 7:73432–73447. 2016.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ingham M and Schwartz GK: Cell-cycle
therapeutics come of age. J Clin Oncol. 35:2949–2959. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akin S, Babacan T, Sarici F and Altundag
K: A novel targeted therapy in breast cancer: Cyclin dependent
kinase inhibitors. J Buon. 19:42–46. 2014.PubMed/NCBI
|
|
21
|
Wang Y, Ma J, Yan X, Chen X, Si L, Liu Y,
Han J, Hao W and Zheng Q: Isoliquiritigenin inhibits proliferation
and induces apoptosis via alleviating hypoxia and reducing
glycolysis in mouse melanoma B16F10 cells. Recent Pat Anticancer
Drug Discov. 11:215–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Yeung ED, Wang J, Panzhinskiy EE,
Tong C, Li W and Li J: Isoliquiritigenin, a natural anti-oxidant,
selectively inhibits the proliferation of prostate cancer cells.
Clin Exp Pharmacol Physiol. 37:841–847. 2010.PubMed/NCBI
|
|
23
|
Maggiolini M, Statti G, Vivacqua A,
Gabriele S, Rago V, Loizzo M, Menichini F and Amdò S: Estrogenic
and antiproliferative activities of isoliquiritigenin in MCF7
breast cancer cells. J Steroid Biochem Mol Biol. 82:315–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZX, Li J, Li Y, You K, Xu H and Wang J:
Novel insights into the apoptosis mechanism of DNA topoisomerase I
inhibitor isoliquiritigenin on HCC tumor cell. Biochem Biophys Res
Commun. 464:548–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung SK, Lee MH, Lim DY, Kim JE, Singh P,
Lee SY, Jeong CH, Lim TG, Chen H, Chi YI, et al: Isoliquiritigenin
induces apoptosis and inhibits xenograft tumor growth of human lung
cancer cells by targeting both wild type and L858R/T790M mutant
EGFR. J Biol Chem. 289:35839–35848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirchaud F, Hermetet F, Ablise M,
Fauconnet S, Vuitton DA, Prétet JL and Mougin C: Isoliquiritigenin
induces caspase-dependent apoptosis via downregulation of HPV16 E6
expression in cervical cancer Ca Ski cells. Planta Med.
79:1628–1635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G, Hu X, Zhang W, Xu N, Wang FQ, Jia
J, Zhang WF, Sun ZJ and Zhao YF: Mammalian target of rapamycin
regulates isoliquiritigenin-induced autophagic and apoptotic cell
death in adenoid cystic carcinoma cells. Apoptosis. 17:90–101.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan X, Zhang B, Gan L, Wang ZH, Yu BC,
Liu LL, Zheng QS and Wang ZP: Involvement of the
mitochondrion-dependent and the endoplasmic reticulum
stress-signaling pathways in isoliquiritigenin-induced apoptosis of
HeLa cell. Biomed Environ Sci. 26:268–276. 2013.PubMed/NCBI
|
|
29
|
Jung JI, Lim SS, Choi HJ, Cho HJ, Shin HK,
Kim EJ, Chung WY, Park KK and Park JH: Isoliquiritigenin induces
apoptosis by depolarizing mitochondrial membranes in prostate
cancer cells. J Nutr Biochem. 17:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang HH, Zhang C, Lai SH, Zeng CC, Liu YJ
and Wang XZ: Isoliquiritigenin induces cytotoxicity in PC-12 cells
in vitro. Appl Biochem Biotechnol. 183:1173–1190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maes ME, Schlamp CL and Nickells RW: BAX
to basics: How the BCL2 gene family controls the death of
retinal ganglion cells. Prog Retin Eye Res. 57:1–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strasser A and Vaux DL: Viewing BCL2 and
cell death control from an evolutionary perspective. Cell Death
Differ. 25:13–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu YL, Kuo PL and Lin CC:
Isoliquiritigenin induces apoptosis and cell cycle arrest through
p53-dependent pathway in Hep G2 cells. Life Sci. 77:279–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwon GT, Cho HJ, Chung WY, Park KK, Moon A
and Park JH: Isoliquiritigenin inhibits migration and invasion of
prostate cancer cells: Possible mediation by decreased JNK/AP-1
signaling. J Nutr Biochem. 20:663–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chien MH, Lin CW, Cheng CW, Wen YC and
Yang SF: Matrix metalloproteinase-2 as a target for head and neck
cancer therapy. Expert Opin Ther Targets. 17:203–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang JS, Lin CW, Su SC and Yang SF:
Pharmacodynamic considerations in the use of matrix
metalloproteinase inhibitors in cancer treatment. Expert Opin Drug
Metab Toxicol. 12:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lan H, Hong W, Fan P, Qian D, Zhu J and
Bai B: Quercetin inhibits cell migration and invasion in human
osteosarcoma cells. Cell Physiol Biochem. 43:553–567. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kunz P, Sahr H, Lehner B, Fischer C,
Seebach E and Fellenberg J: Elevated ratio of MMP2/MMP9 activity is
associated with poor response to chemotherapy in osteosarcoma. BMC
Cancer. 16:2232016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Z, Zeng B, Pan Y, Huang P and Wang C:
Autophagy participates in isoliquiritigenin-induced melanin
degradation in human epidermal keratinocytes through PI3K/AKT/mTOR
signaling. Biomed Pharmacother. 97:248–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen T, Deng S and Lin R: The inhibitory
effect of Isoliquiritigenin on the proliferation of human arterial
smooth muscle cell. BMC Pharmacol Toxicol. 18:572017. View Article : Google Scholar : PubMed/NCBI
|