Y‑box‑binding protein 1 inhibits apoptosis and upregulates EGFR in colon cancer
- Authors:
- Published online on: February 28, 2019 https://doi.org/10.3892/or.2019.7038
- Pages: 2889-2896
Abstract
Introduction
The 5-year survival rate for colorectal cancer is undeniably increasing in many developed countries; for patients diagnosed with colon cancer and rectal cancer in 22 countries worldwide, it was 50% in the past (1995–1999) and has now reached over 60%, according to data from 2005 to 2009 (1). Improvements in surgical techniques for resectable cases of colorectal cancer and the development of multidisciplinary treatments, particularly the introduction of chemotherapy with molecular targeted therapeutic agents against progressive or recurrent unresectable colorectal cancer, have contributed to the improved survival rates. In recent years, efforts have been actively made in the field of colorectal cancer toward developing precision medicine to further improve the prognosis, search for and identify novel biomarkers, and develop new medicines including molecular targeted therapeutic drugs. In particular, in recent years, elucidation of the genetic mutations responsible for the development of colorectal cancer has been achieved. However, with respect to RAS (KRAS/NRAS)/RAF mutation, the positive effect of the anti-EGFR antibody could not be determined (2).
Based on these findings, it is indispensable to perform genetic analysis of RAS/RAF as a biomarker for predicting the effect of anti-EGFR antibody drugs, when performing systemic chemotherapy for unresectable and recurrent colorectal cancer.
Y-box-binding protein 1 (YB-1) is a transcription factor that contains a cold shock domain highly conserved across species, and binds to the Y-box (CCAAT) region in the promoter domain of the major histocompatibility complex II gene HLA-DRα (3) and the gene encoding the EGFR enhancer (4). It has been reported that YB-1 plays a crucial role in cell proliferation, invasion, metastasis and drug resistance of cancer cells through the actions of transcription, translational control and DNA repair (5).
Previous studies have reported that YB-1 expression is a poor prognostic factor in gastric (6), cervical (7), non-small cell lung (8) and breast cancer (9). In a previous study, we also demonstrated that the nuclear expression of YB-1 in colorectal tumor cells led to a poor prognosis (10).
A significant correlation between HER2 expression and YB-1 expression exists in estrogen receptor (ER)-positive breast cancer, with the two elements being involved in tumor growth and drug resistance. As a result, YB-1 expression in breast cancer is reported to be a prognostic factor for a worse outcome (11). Furthermore, the co-expression of EGFR and YB-1 can modulate the effect of the anti-EGFR antibody as a treatment in non-small cell lung cancer (NSCLC) (8). Even in colorectal cancer, YB-1 expression has been correlated with poor prognosis (12,13); however, the biological function and molecular correlation of YB-1 in colorectal cancer are not fully understood. The objective of the present study was to elucidate the importance and biological role of YB-1 in colorectal cancer at the molecular level.
Materials and methods
Cell culture
Five human colon cancer cell lines, T84, HT29, HCT116, CaCo2 and SW480 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Next, the expression of YB-1 and the mutational status of RAS/RAF were examined. Based on these results, three cell lines, namely CaCo2, HCT116 and HT29 were selected and used for further experiments. All cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS) (Fujifilm Wako Pure Chemical Industries, Ltd., Osaka, Japan), 100 U/ml penicillin and 100 mg/l streptomycin. The cells were maintained in a humidified incubator at 37°C with 5% CO2 (Sanyo Electric Biomedical Co., Osaka, Japan).
Mutation analysis
For the detection of KRAS, NRAS and BRAF mutations, genomic DNA was isolated from the cell lines, using an AllPrep DNA/RNA/Protein Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's protocol. The genomic DNA was subjected to polymerase chain reaction (PCR) amplification using the primers listed in Table I. The primer sets were designed to amplify exons 2, 3 and 4 of KRAS and NRAS and exon 15 of BRAF. The PCR products were treated with ExoSap-IT PCR Product Cleanup reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol to inactivate the free primers and dNTPs, and then were subjected to sequencing using the forward primers and BigDye® Terminator v3.1 (Thermo Fisher Scientific, Inc.). Each 20-µl PCR reaction consisted of 2 µl 10X LA PCR buffer II, 2 µl 10 mmol/l dNTPs, 0.1 µl AmpliTaq Gold (Thermo Fisher Scientific, Inc.), 2 µl of genomic DNA, 1 µl 100 pmol/µl forward primer, 1 µl 100 pmol/µl reverse primer and 12 µl H2O. The cycling conditions were 95°C for 12 min; 10 cycles at 94°C for 15 sec, 55°C for 15 sec, and 72°C for 30 sec; 25 cycles at 89°C for 15 sec, 55°C for 15 sec, 72°C for 30 sec; and a final extension at 72°C for 10 min. The PCR products were evaluated by electrophoresis using 2% agarose gels. Sequencing was carried out using an ABI 3100 Genetic Analyzer (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The sequences obtained were aligned to the consensus reference-sequences from the University of California, Santa Cruz (UCSC) Genome Bioinformatics website (http://genome.ucsc.edu/index.html) using the ClustalW program (https://www.genome.jp/tools-bin/clustalw) to identify nucleotide differences.
YB-1 knockdown
The small interfering RNAs (siRNAs) for YB-1 (siRNA-1: sense, 5′-GUAAAAUGGUUCAAUGUAAtt-3′ and antisense, 5′-UUACAUUGAACCAUUUUACtg-3′; and siRNA2: sense, 5′-CGAAGGUUUUGGGAACAGUtt-3′ and antisense, 5′-ACUGUUCCCAAAACCUUCGtt-3′) and the negative control siRNAs (siCtr) were purchased from Life Technologies Corp. (Thermo Fisher Scientific, Inc.). The siRNAs were transfected into the cells, using Lipofectamine RNAiMAX Transfection reagent and Opti-MEM medium (Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.) according to the manufacturer's recommendations. A total of 1×104 cells/well were seeded in 96-well plates (Iwaki Co., Ltd., Tokyo, Japan) and cultured for 24 h before transfection. The adjusted amounts of siRNAs were added to each well. Reverse transcription PCR (RT-PCR) and western blotting were performed 72 h post-transfection, and MTT and migration assays were performed 48 h post-transfection.
Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted using an AllPrep DNA/RNA/Protein Mini kit (Qiagen, Inc.) according to the manufacturer's protocol and reverse-transcribed into complementary DNA (cDNA), using SuperScript IV Reverse Transcriptase (Promega Corporation, Madison, WI, USA). RT-qPCR was performed with TaqMan Gene Expression Assays (Applied Biosystems; Thermo Fisher Scientific, Inc.) for YB-1 (Hs00358903_g1), GAPDH (Hs02758991_g1), Bax (Hs00180269_m1) and Bcl-2 (Hs00608023_m1) using the TaqMan Gene Expression Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The levels of transcripts of the indicated genes were standardized to the corresponding GAPDH transcript levels. All values reported represent the average of at least three independent experiments.
Western blotting
Total cell proteins were extracted using an AllPrep DNA/RNA/Protein Mini kit (Qiagen, Inc.) according to the manufacturer's protocol. Protein determination method is BCA. Mass of protein loaded per lane is 20 µg. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE (4–12%) and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% milk in Tris-buffered saline containing Tween-20 (TBST) buffer, the membranes were incubated at 4°C overnight with an anti-YB-1 (dilution 1:1,000; rabbit; cat. no. ab12148; Abcam, Cambridge, MA, USA) or anti-EGFR (dilution 1:1,000; rabbit; cat. no. 4267; Cell Signaling Technology, Inc., Danvers, MA, USA) primary antibody. Subsequently, the membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (dilution 1:5,000; cat. no. 7074; Cell Signaling Technology, Inc.). The immunoreactive bands were visualized using Fusion-SL7-400 (Vilber Lourmat, Marne Le-Vallée, France). Immunostaining using an anti-β-actin primary antibody (dilution 1:5,000; mouse; cat. no. ab6276; Abcam) was used as a loading control.
MTT assay
For the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium (MTT) reduction assays, the three colorectal cell lines were used as either untreated (CaCo2, HT29 and HCT116), treated with siYB-1 (CaCo2-siYB-1, HT29-siYB-1 and HCT116-siYB-1), or treated with siCtr (CaCo2-siCtr, HT29-siCr and HCT116-siCtr). The cells were added to 96-well plates (1×104 cells/well) and incubated for 2 days in a humidified incubator at 37°C with 5% CO2. MTT Cell Proliferation Assay Reagent (Cell Biolabs, Inc., San Diego, CA USA) was added (10 µl) to each well, and the cells were incubated for 3 h at 37°C. Thereafter, the detergent solution provided with the assay kit was added to each well (100 µl) and the cells were incubated for 3 h at room temperature while being protected from the light. The absorbance of each sample for each well was measured at 540 nm using a microplate reader (VersaMax ELISA; Molecular Devices Japan K.K., Tokyo, Japan).
Migration assay
A total of 1×104 CaCo2, CaCo2-siYB-1, CaCo2-siCtr, HT29, HT29-siYB-1, HT29-siCtr, HCT116, HCT116-siYB-1, or HCT116-siCtr cells/well were seeded into 96-well Collagen I coated plates (Platypus Technologies, LCC, Fitchburg, WI, USA) and incubated at 37°C to allow for cell attachment. After 24 h, all silicon stoppers at the center of each well were removed, and any unattached cells were removed by washing with 100 µl of sterile phosphate-buffered saline (PBS). After 72 h, the number of migrated cells was quantified using ImageJ software (ver. 1.51, NIH; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each assay was independently performed three times. The data were analyzed using JMP software (version 13.0.0; SAS Institute Inc., Cary, NC, USA). The values were presented as the means ± standard deviation (SD). The comparisons between groups were analyzed using the Kruskal-Wallis test. P<0.05 was considered to indicate a statistically significant difference.
Results
KRAS/NRAS/BRAF mutation profiles and expression of YB-1 in human colon cancer cell lines
In colorectal cancer, the mutational status of RAS markedly affects the characteristics of the cancer cells. Therefore, in order to confirm the molecular background of each of the five colon-cancer cell lines, we determined the genetic mutation of RAS/RAF by direct sequence evaluation. A summary of the KRAS/NRAS/BRAF gene mutations is presented in Table II. The detected KRAS mutations were G12V (GGT→GTT) and G13D (GGC→GAC), and the BRAF mutation was V600E (GTG→GAG). The genotypes of the mutant KRAS and mutant BRAF are displayed in Fig. 1A.
Subsequently, western blot analysis was performed to investigate the levels of protein expression of YB-1 in the five colon cancer cell lines. As revealed in Fig. 1B, the expression of YB-1 was confirmed in all five cell lines. HCT116 demonstrated the highest level of YB-1 expression among the cell lines. Notably, the expression level of YB-1 tended to be higher in the cell lines harboring the RAS/RAF mutation compared to that in the cell line harboring wild-type RAS. To clarify the biological function of YB-1 in colorectal cancer with respect to the mutational status of the RAS, the CaCo2, HCT116 and HT29 cell lines were selected for further examination.
Knockdown of YB-1 in HCT16, HT29 and CaCo2 cell lines inhibits cell proliferation and migration
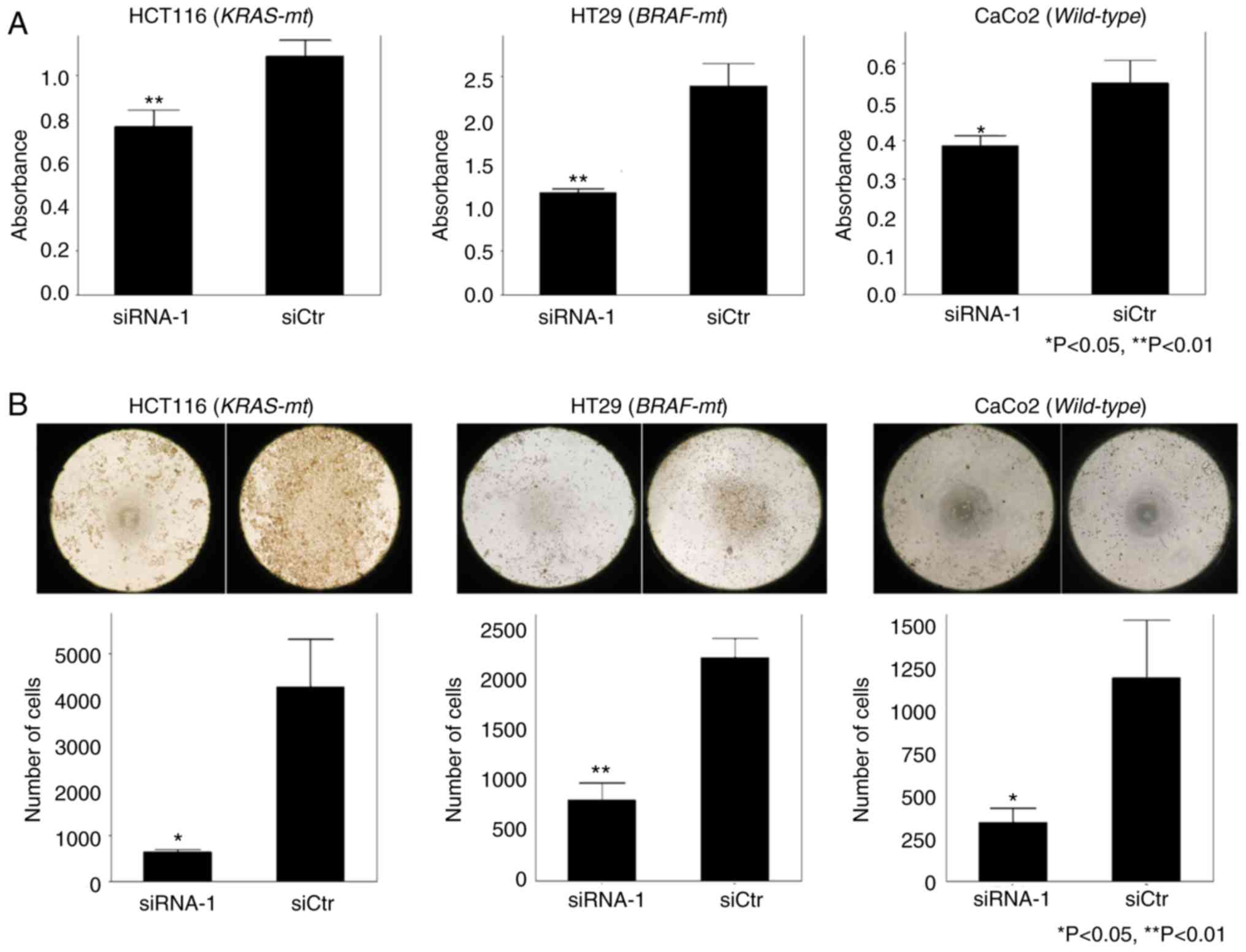
Two siRNAs (siRNA-1 and siRNA-2) were transfected into the cell lines HCT116, HT29 and CaCo2 in order to knockdown YB-1. The suppression of YB-1 mRNA levels and protein levels were confirmed by RT-qPCR and western blot analyses, respectively. As revealed in Fig. 2A, the expression level of YB-1 was significantly decreased in both siRNA-1-transfected and siRNA-2-transfected cells compared to that in siCtr-transfected cells (P<0.05). According to the RT-qPCR analysis, the reduced level of YB-1 expression was not significantly different between the siRNA-1-transfected and siRNA-2-transfected groups. However, western blot analysis revealed that the reduced level of YB-1 expression was higher in the siRNA-1-transfected group compared to that in the siRNA-2-transfected group (Fig. 2B). Based on these results, siRNA-1 was used in subsequent in vitro experiments.
To investigate the role of YB-1 in cell proliferation and migration, MTT assays and migration assays were performed, respectively. In the MTT assays, proliferation potency was significantly suppressed in all the cell lines transfected with siRNA-1 compared to that in the siCtr-transfected group (Fig. 3A). Moreover, in the migration assays, the number of cells transfected with si-RNA-1 that migrated into the center of the well was significantly less than that of the siCtr-transfected group (Fig. 3B). Similar results were obtained from siRNA knockdown experiments using the other cell lines. Overall, the effects of YB-1 on cell proliferation and cell migration were not affected by the presence or absence of mutations in KRAS or BRAF.
Knockdown of YB-1 induces apoptosis-related genes in human colon cancer
Since there are few studies regarding the relationship between YB-1 and apoptosis, the effect of YB-1 on apoptosis was analyzed by evaluating alterations in the expression of apoptosis-related genes Bax and Bcl-2 in siRNA-1-treated cells compared to that in siCtr-treated cells, by RT-qPCR analysis. There were no significant gene expression differences between the siRNA-1-treated and siCtr-treated groups for either HCT116 or HT29 cells. In contrast, Bcl-2 expression levels were significantly decreased (P=0.018) and Bax levels were significantly increased (P=0.021) in siRNA-1-treated CaCo2 cells compared to that in siCtr-treated CaCo2 cells (Fig. 4).
Knockdown of YB-1 reduces the expression of EGFR in human colon cancer
EGFR protein expression was compared between the siRNA-1-treated and siCtr-treated groups by western blot analysis. There were no significant differences in EGFR expression between the siRNA-1-treated and siCtr-treated groups for either HCT116 or HT29 cells. However, EGFR expression was significantly decreased in the siRNA-1-treated group of CaCo2 cells compared to that in siCtr-treated CaCo2 cells (Fig. 5).
Discussion
Findings from the present study suggest that Y-box-binding protein 1 (YB-1) was involved in promoting the malignancy potential of colon cancer through the enhancement of cell proliferation and -migration properties. Furthermore, YB-1 also contributed to the suppression of apoptosis and epidermal growth factor receptor (EGFR) expression in colorectal cancer cells. The alteration of apoptosis-related genes and EGFR expression by YB-1 knockdown was confirmed by our results to occur only in the cell lines expressing wild-type RAS/RAF and not in those expressing mutated RAS/RAF. These results suggest that the presence of the RAS/RAF mutation may affect the suppression of apoptosis and promotion of EGFR expression by YB-1 in colorectal cancer.
Previous studies have reported that there is a clear association between YB-1 expression and cell proliferation, and an increase in the expression levels of YB-1 was correlated with the expression of proliferation markers (14). In addition, there is a possible relationship between YB-1 expression and hyperplasia (15).
Basaki et al (16) demonstrated that knockdown of YB-1 resulted in a reduction of the number of cells in the S phase for multiple types of cancer, including breast, lung cancer and leukemia. Furthermore, they indicated that downregulation of cyclin D1 and upregulation of p21 occured as a result of YB-1 knockdown. These investigators also reported that YB-1 was associated with the cell-proliferation cycle (16). In addition, Yan et al (17) revealed that the downregulation of E-cadherin and the upregulation of vimentin and N-cadherin occured in colon cancer cell lines transfected with YB-1 and resulted in cancer cell proliferation through the enhancement of epithelial-mesenchymal transition (EMT).
The present study revealed that the knockdown of YB-1 impaired cell proliferation and cell migration in colon cancer cell lines, regardless of the status of RAS/RAF. While additional studies are required, it is possible to conclude that YB-1 is strongly involved in cancer cell proliferation and mobility, independent of the type of cancer cell.
The knockdown of YB-1 in the colon cancer cell line expressing wild-type RAS/RAF resulted in the upregulation of Bax and downregulation of Bcl-2, which are both associated with apoptosis. Similar results were observed in breast (18), bladder cancer (19) and neuroblastoma (20). Our results may be explained by the molecular relationship between YB-1 and p53, which selectively bind with each other and cause the suppression of p53 function to induce an anti-apoptotic effect. The enhancement of apoptosis by YB-1 knockdown was not observed in the colorectal cancer cell lines HCT116 or HT29, which harbor mutated RAS/RAF.
In breast cancer, non-small cell lung and cervical cancer, phosphorylation of YB-1 via the mitogen-activated protein kinase (MAPK) pathway upregulated EGFR expression (7,21,22). Previously, we reported that there is a positive correlation between YB-1 expression and EGFR expression in colorectal cancer tissue and high expression of both YB-1 and EGFR were associated with poor prognosis (10).
In the present study, suppression of EGFR expression by YB-1 knockdown was observed in the CaCo2 cell line, which expresses wild-type RAS/RAF. It was considered that YB-1 regulated EGFR in colorectal cancer harboring wild-type RAS/RAF, similar to that confirmed in other types of cancer. However, the same phenomenon was not verified in the cell lines HCT116 and HT29, which harbor the mutated RAS/RAF.
Our results were consistent with the promotion of apoptosis and suppression of EGFR expression upon knockdown of YB-1 in colon cancer with wild-type RAS/RAF. In contrast, in colorectal cancer cells harboring mutated RAS/RAF, there were no significant differences in the promotion of apoptosis or suppression of EGFR expression, despite the knockdown of YB-1.
In colorectal cancer, the expression of EGFR and factors of the downstream MAPK pathway are involved in cancer proliferation, invasion, metastasis and cell survival. In RAS/RAF-mutated cell lines, the mutated proteins activate the MAPK pathway independent of stimulation by EGFR, which help to maintain cancer survival and proliferation (23). It has been reported that extracellular signal-regulated kinases 1 and 2 (ERK 1/2) are located downstream of the MAPK pathway and they suppress apoptosis (24). Furthermore, in several types of cancer, YB-1 functioned downstream of ERK 1/2 and promotes proliferation, invasion and metastasis of cancer cells, resulting in poor prognosis (25–27). Moreover, Chu et al (28) revealed that in colon cancer cells with KRAS mutation, the expression of YB-1 was upregulated through the MEK/Sp1/DNMT1/miR-137 pathway.
In colon cancer with wild-type RAS/RAF, it is thought that YB-1 is activated via the MAPK pathway, which then induces the suppression of apoptosis and upregulation of EGFR. On the other hand, in RAS/RAF-mutated colorectal cancer, there may be an alternative pathway not mediated by YB-1, or YB-1 may exist in abundance due to constant expression activated by the MAPK pathway. The regulation of YB-1 by siRNA may become insufficient with the induction of apoptosis and the suppression of EGFR expression not being achieved (Fig. 6). Based on these findings, YB-1 may be useful as a therapeutic target in colon cancer harboring wild-type RAS/RAF. Furthermore, there was a significant association between the high expression of YB-1 and existence of mutated RAS/RAF in colon cancer cell lines.
In conclusion, it is possible that YB-1 plays a vital role in cell proliferation, cell migration, apoptosis and EGFR expression in colon cancer. Furthermore, apoptosis and expression of EGFR mediated through the control of YB-1 may be affected by the mutational status of RAS/RAF.
Acknowledgments
We would like to thank Ms. Eriko Matsuo (Research assistant at the Molecular Targeting Therapeutics Division, Kurume University, Kurume, Japan) for research assistance in the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
SN designed the study and wrote the initial draft of the manuscript. TSu contributed to the analysis and interpretation of the data and assisted in the preparation of the manuscript. TK, TY, KF, TSh and YA contributed to the collection and interpretation of the data and critically reviewed the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in an effort to ensure that any questions related to the accuracy or integrity of any portion of the work are appropriately addressed and resolved.
Ethics approval and consent to participate
Since the present study used only commercially-available human-derived cells, approval by the institutional ethics committee was not necessary.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, et al: Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 385:977–1010. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kawazoe A, Shitara K, Fukuoka S, Kuboki Y, Bando H, Okamoto W, Kojima T, Fuse N, Yamanaka T, Doi T, et al: A retrospective observational study of clinicopathological features of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with metastatic colorectal cancer. BMC Cancer. 15:2582015. View Article : Google Scholar : PubMed/NCBI | |
|
Didier DK, Schiffenbauer J, Woulfe SL, Zacheis M and Schwartz BD: Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci USA. 85:7322–7326. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Sakura H, Maekawa T, Imamoto F, Yasuda K and Ishii S: Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene. 73:499–507. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Lasham A, Print CG, Woolley AG, Dunn SE and Braithwaite AW: YB-1: Oncoprotein, prognostic marker and therapeutic target? Biochem J. 449:11–23. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Guo T, Yu Y, Yip GW, Baeg GH, Thike AA, Lim TK, Tan PH, Matsumoto K and Bay BH: Y-box binding protein 1 is correlated with lymph node metastasis in intestinal-type gastric cancer. Histopathology. 66:491–499. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Nishio S, Ushijima K, Yamaguchi T, Sasajima Y, Tsuda H, Kasamatsu T, Kage M, Ono M, Kuwano M and Kamura T: Nuclear Y-box-binding protein-1 is a poor prognostic marker and related to epidermal growth factor receptor in uterine cervical cancer. Gynecol Oncol. 132:703–708. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kashihara M, Azuma K, Kawahara A, Basaki Y, Hattori S, Yanagawa T, Terazaki Y, Takamori S, Shirouzu K, Aizawa H, et al: Nuclear Y-box binding protein-1, a predictive marker of prognosis, is correlated with expression of HER2/ErbB2 and HER3/ErbB3 in non-small cell lung cancer. J Thorac Oncol. 4:1066–1074. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Janz M, Harbeck N, Dettmar P, Berger U, Schmidt A, Jürchott K, Schmitt M and Royer HD: Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int J Cancer. 97:278–282. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Shiraiwa S, Kinugasa T, Kawahara A, Mizobe T, Ohchi T, Yuge K, Fujino S, Katagiri M, Shimomura S, Tajiri K, et al: Nuclear Y-box-binding protein-1 expression predicts poor clinical outcome in stage III colorectal cancer. Anticancer Res. 36:3781–3788. 2016.PubMed/NCBI | |
|
Fujii T, Kawahara A, Basaki Y, Hattori S, Nakashima K, Nakano K, Shirouzu K, Kohno K, Yanagawa T, Yamana H, et al: Expression of HER2 and estrogen receptor alpha depends upon nuclear localization of Y-box binding protein-1 in human breast cancers. Cancer Res. 68:1504–1512. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Yan X, Yan L, Zhou J, Liu S, Shan Z, Jiang C, Tian Y and Jin Z: High expression of Y-box-binding protein 1 is associated with local recurrence and predicts poor outcome in patients with colorectal cancer. Int J Clin Exp Pathol. 7:8715–8723. 2014.PubMed/NCBI | |
|
Zhang Y, Zhao PW, Feng G, Xie G, Wang AQ, Yang YH, Wang D and Du XB: The expression level and prognostic value of Y-box binding protein-1 in rectal cancer. PLoS One. 10:e01193852015. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Q, Huang S, Zhang A, Chen Q, Guo X, Chen R and Yang T: Y-box protein 1 stimulates mesangial cell proliferation via activation of ERK1/2. Nephron Exp Nephrol. 113:e16–e25. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Bergmann S, Royer-Pokora B, Fietze E, Jürchott K, Hildebrandt B, Trost D, Leenders F, Claude JC, Theuring F, Bargou R, et al: YB-1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 65:4078–4087. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Basaki Y, Taguchi K, Izumi H, Murakami Y, Kubo T, Hosoi F, Watari K, Nakano K, Kawaguchi H, Ohno S, et al: Y-box binding protein-1 (YB-1) promotes cell cycle progression through CDC6-dependent pathway in human cancer cells. Eur J Cancer. 46:954–965. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yan XB, Zhu QC, Chen HQ, Peng JY, Chao HL, Du HX, Wang ZG and Jin ZM: Knockdown of Y-box-binding protein-1 inhibits the malignant progression of HT-29 colorectal adenocarcinoma cells by reversing epithelial-mesenchymal transition. Mol Med Rep. 10:2720–2728. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lee C, Dhillon J, Wang MY, Gao Y, Hu K, Park E, Astanehe A, Hung MC, Eirew P, Eaves CJ and Dunn SE: Targeting YB-1 in HER-2 overexpressing breast cancer cells induces apoptosis via the mTOR/STAT3 pathway and suppresses tumor growth in mice. Cancer Res. 68:8661–8666. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Shiota M, Yokomizo A, Itsumi M, Uchiumi T, Tada Y, Song Y, Kashiwagi E, Masubuchi D and Naito S: Twist1 and Y-box-binding protein-1 promote malignant potential in bladder cancer cells. BJU Int. 108:E142–E149. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wang H, Sun R, Gu M, Li S, Zhang B, Chi Z and Hao L: shRNA-mediated silencing of Y-box binding protein-1 (YB-1) suppresses growth of neuroblastoma cell SH-SY5Y in vitro and in vivo. PLoS One. 10:e01272242015. View Article : Google Scholar : PubMed/NCBI | |
|
Hyogotani A, Ito K, Yoshida K, Izumi H, Kohno K and Amano J: Association of nuclear YB-1 localization with lung resistance-related protein and epidermal growth factor receptor expression in lung cancer. Clin Lung Cancer. 13:375–384. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wu J, Lee C, Yokom D, Jiang H, Cheang MC, Yorida E, Turbin D, Berquin IM, Mertens PR, Iftner T, et al: Disruption of the Y-box binding protein-1 results in suppression of the epidermal growth factor receptor and HER-2. Cancer Res. 66:4872–4879. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hynes NE and Lane HA: ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 5:341–354. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Bonni A, Brunet A, West AE, Datta SR, Takasu MA and Greenberg ME: Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 286:1358–1362. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Sutherland BW, Kucab J, Wu J, Lee C, Cheang MC, Yorida E, Turbin D, Dedhar S, Nelson C, Pollak M, et al: Akt phosphorylates the Y-box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage-independent growth of breast cancer cells. Oncogene. 24:4281–4292. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Basaki Y, Hosoi F, Oda Y, Fotovati A, Maruyama Y, Oie S, Ono M, Izumi H, Kohno K, Sakai K, et al: Akt-dependent nuclear localization of Y-box-binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene. 26:2736–2746. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Sinnberg T, Sauer B, Holm P, Spangler B, Kuphal S, Bosserhoff A and Schittek B: MAPK and PI3K/AKT mediated YB-1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp Dermatol. 21:265–270. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chu PC, Lin PC, Wu HY, Lin KT, Wu C, Bekaii-Saab T, Lin YJ, Lee CT, Lee JC and Chen CS: Mutant KRAS promotes liver metastasis of colorectal cancer, in part, by upregulating the MEK-Sp1-DNMT1-miR-137-YB-1-IGF-IR signaling pathway. Oncogene. 37:3440–3455. 2018. View Article : Google Scholar : PubMed/NCBI |