Introduction
In 2017, 1,688,780 new cancer cases and 600,920
cancer deaths were projected to occur in the US (1). Therapy varies depending on the type of
cancer, origin and localization. Surgery is one major option for
treating malignant disease. After surgery, wound healing commences
immediately. During this process, a complex inflammatory response
is triggered, which induces the recruitment, proliferation and
activation of cells such as neutrophils, macrophages, natural
killer cells, fibroblasts and mesenchymal stem cells (2,3). The
process of wound healing is orchestrated via the interaction
between different cells, cytokines, and the extracellular matrix.
Surgical wound fluid (WF) contains blood cells, immune cells, lymph
and paracrine-released factors (4).
The composition of factors and cell components in WF differ in a
time-dependent manner, and there are ample interindividual
differences (5). From an
oncological point of view, the mechanisms of wound healing are
quite interesting, as non-resected cancer cells may be exposed to
mitogenic factors in the wound microenvironment after surgical
cancer therapy (6). In a previous
study, the presence of a variety of different cytokine and growth
factors in the WF of patients who underwent a planned neck
dissection was demonstrated (7).
The cultivation of mesenchymal stem cells (MSCs) with WF induced
enhancement of cell proliferation and cell migration. Most of the
cytokines contained in WF are known to be pro-tumorigenic, for
example, interleukin (IL)-6. IL-6 is a pleiotrophic cytokine, which
is secreted by cells from the immune system or fibroblasts. Cancer
cells, for example, from the breast, lung, or prostate also secrete
IL-6 (8). Furthermore, IL-6 is an
important pro-inflammatory cytokine and a mediator of the immune
system, and it stimulates the differentiation of B-cells.
Conversely, IL-6 serves an important role in cancer biology by
inducing tumor growth via the activation of
Ras/Raf/MEK/extracellular signal-regulated kinase 1/2 (9,10).
Increased serum levels of IL-6 seem to be associated with severity
of disease and poor outcome (11).
Suchi et al (12)
demonstrated a suppressed cisplatin-induced cytotoxicity in
esophageal cancer cells via overexpression of IL-6. Additionally,
IL-6 has been linked to enhanced cancer cell migration and
metastasis (13).
In the process of cancer progression, the activation
of signal transducers and activators of transcription (STAT)3 is
important. STAT3 transcription factors are activated by cytokines,
growth factors, and hormones (14).
The activation of STAT3 is achieved by phosphorylation of its
tyrosine and serine residues (15).
STAT3 is activated particularly by IL-6 family cytokines, which
includes IL-6, IL-8, IL-11 and Oncostatin (16). Cancer cells and cancer surrounding
stroma are able to activate STAT3 via autocrine and paracrine
production of IL-6 family cytokines (17).
The aim of the present study was to investigate the
effects of WF on the head and neck squamous carcinoma cell lines
FaDu and HLaC78 in terms of cell viability, proliferation,
migration and induction of chemoresistance. Furthermore, the
cytokine pattern of WF and possible activation of the STAT3
signaling pathway were also investigated.
Materials and methods
Culture of human carcinoma cell lines
FaDu and HLaC78
The head and neck squamous carcinoma cell lines FaDu
and HLaC78 were used (18,19). Cells were grown in RPMI-1640 medium
(Biochrom, Ltd., Cambridge, UK) with 10% fetal calf serum (FCS)
(Linaris Blue Wertheim-Bettingen, Germany), 100 U/ml penicillin,
100 µg/ml streptomycin, 1% sodium pyruvate (100 mM; Biochrom,
Ltd.), and 1% non-essential amino acids [100-fold concentration;
Biochrom AG; RPMI-expansion medium (RPMI-EM)]. Cells were cultured
in flasks at 37°C with 5% CO2. The replacement of the
medium was carried out every other day, and passaging was performed
after reaching 70–80% confluence by trypsinization (0.25% trypsin;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Experiments were performed using cells in the exponential growth
phase.
Collection of WF
The WF of 7 male patients (age, 51–88 years; the
exclusion criteria was prior administration of radiation therapy)
who underwent a planned neck dissection at the Department of
Otorhinolaryngology, Plastic, Aesthetic and Reconstructive Head and
Neck Surgery at Julius Maximilian University of Wuerzburg
(Würzburg, Germany), was collected in 2009 and 2017 from a vacuum
drain 72 h after surgery. Written, informed consent was provided by
all patients. The study was approved by the Ethics Committee of the
Medical Faculty of the University of Wuerzburg. After harvesting
WF, centrifugation at 340 × g for 10 min at 4°C was conducted
immediately in order to reduce cell debris. To remove immune cells,
a second centrifugation at 340 × g for 10 min at 4°C in Leucosep
medium (GE Healthcare, Chicago, IL, USA) followed. Next, the WF was
filtered using a 0.45-µm syringe filter (Sarstedt, Inc., Newton,
NC, USA). To avoid bacterial infection, 100 U/ml penicillin and 100
mg/ml streptomycin (1% penicillin/streptomycin) were added.
Cytokine analysis of WF
The cytokine pattern of WF was detected with the dot
blot assay. All reagents and materials used, including the C-Series
Human Cytokine Antibody Array 3 kit (cat. no. AAH-CyT-3-4) were
supplied by RayBiotech Inc. (Norcross, GA, USA). The supplier
provided all supplements. After harvesting WF, the presence of
cytokines was investigated according to the manufacturer's
protocol. First, the WF was added to the membrane for 30 min at
room temperature. After several washing steps, incubation for 2 h
at room temperature with 1 ml biotin-conjugated antibodies
(prefabricated solution) and horseradish peroxidase
(HRP)-conjugated streptavidin (1:1,000) was conducted. The labeled
proteins were detected via chemiluminescence using detection buffer
and exposure to X-ray film. The cytokines were represented as dots
with different intensities and diameters. The quantification of the
different cytokines was achieved by densitometric methods using
ImageJ software (version 1.43u; National Institutes of Health,
Bethesda, MD, USA).
Cell viability analysis
The mitochondrial activity of HLaC78 and FaDu
cultivated with WF at different concentrations was investigated via
MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as
described previously (20). FCS was
not added to the medium containing WF. First, cells were seeded at
a density of 1×104 cells/well in a 96-well round bottom
plate. The cultivation medium consisted of RPMI containing WF at
various concentrations (10, 20, 30, 40, 50, 60, 70, 80, 90 and
100%). Cells were cultivated for 24 h. After a washing step with
PBS, all plates were incubated with 100 µl MTT solution (1 mg/ml)
for 5 h at 37°C. The MTT solution was removed and 100 µl
isopropanol was added for a further 1 h at 37°C. The multi-plate
reader (Titertek Multiskan PLUS MK II; Thermo Labsystems, Helsinki,
Finland) was used to measure the color conversion at a wavelength
of 570 nm. Further experiments were conducted with WF at a
concentration of 40%.
The enhanced proliferation activity of cancer cells
after cultivation with 40% WF was confirmed through Ki-67 staining.
First, the cells were plated on specimen slides. After cultivation
with WF for 48 h, fixation was performed using 4% paraformaldehyde
in PBS at 4°C for 30 min. Then, a further 5 min of fixation with
100% acetone at room temperature was performed. Next, cells were
incubated with 10% bovine serum albumin (BSA; Carl Roth GmbH and
Co., KG, Karlsruhe, Germany) in Tris-buffered saline [200 mM
Tris-base, pH 8; 8% NaCl; and 1% Tween-20 (TBS-T); Sigma-Aldrich;
Merck KGaA). Incubation at 4°C of cells in TBS-T containing 1% BSA
and a rabbit polyclonal antibody against Ki-67 (1:500; Abcam,
Cambridge, UK; cat. no. Ab15580) was assessed overnight. After 3
washing steps with TBS-T, the cells were treated for 1 h in 1% BSA
at room temperature with Alexa 555-conjugted goat anti-rabbit
secondary antibody (1:500; Invitrogen; Thermo Fisher Scientific,
Inc.; cat. no. A21428) and 5 mg/ml DAPI (Sigma-Aldrich; Merck
KGaA). Cancer cell lines cultivated in RPMI-EM served as a control.
A fluorescence microscope (Leica DMI 4000B Inverted Microscope;
Leica Microsystems GmbH, Wetzlar, Germany) was used for cell
examination at ×100 magnification.
Paclitaxel treatment
In order to evaluate whether WF induces resistance
toward chemotherapeutics, cells were cultivated in 40% WF and
treated with 10 nM paclitaxel (University of Wuerzburg) for 24 h at
37°C. Previously, half maximal inhibitory concentration
(IC50) was calculated (21). The MTT assay was used to determine
cell viability as described above. Cancer cells cultivated with
RPMI-EM and treated with paclitaxel served as a control.
Three-dimensional invasion assay
A possible alteration in the cell invasion activity
was investigated using the three-dimensional invasion assay. First,
a 96-multiwell plate was coated with 0.1% agar (Sigma-Aldrich;
Merck KGaA). Next, spheroids were generated from 6×103
cells (FaDu or HLaC78). After 72 h, spheroids were transferred to
well plates coated with extracellular matrix (1:80; Sigma-Aldrich;
Merck KGaA). The cells were able to spread out from the spheroids
in the well plate. To determine the migration area, the cells were
imaged directly after being transferred and after 24 h of culture
using an inverted microscope (magnification, ×50; Leica
Microsystems GmbH). The migration area was calculated using ImageJ
software (version 1.43u).
Analysis of STAT3 activation via
western blotting
Western blotting was performed as previously
described (22). Cells (FaDu and
HLaC78) were harvested by trypsinization and dissolved in
radioimmunoprecipitation assay buffer (PBS containing 1% NP40, 0.5%
sodium deoxycholate, and 0.1% SDS); then were supplemented with 10
µg/ml phenylmethanesulfonyl fluoride (PMSF). Protein concentration
was then determined according to the method detailed previously by
Lowry et al (23).
Equal amounts (20 µg/lane) of total protein lysates
were loaded onto 10% SDS-PAGE and transferred by electroblotting to
a polyvinylidene difluoride membrane. The blots were blocked for 1
h at room temperature with TBS-T (10 mM Tris, 150 mM NaCl, 0.05%
Tween-20, pH 8.0) containing 5% non-fat dry milk. Subsequently, the
membrane was incubated with primary antibody against STAT3 (1:500;
Cell Signaling Technology, Inc., Danvers, MA, USA; cat. 9145)
overnight at 4°C. Subsequently, the membrane was washed and
incubated with a species-specific secondary antibody (1:10,000;
anti-rabbit immunoglobulin G; HRP-linked antibody, Cell Signaling
Technology, Inc.; cat. no. 7074) for 1 h at room temperature to
visualize the specific bindings. Protein expression was detected
with an enhanced chemiluminescence system (GE Healthcare),
according to the manufacturer's protocol. Jurkat cells (STAT3
control extracts; Cell Signaling Technology, Inc.; cat. no. 9133)
were used as a positive control. α-tubulin (1:2,000; Sigma-Aldrich;
Merck KGaA; cat. no. T5168) was used as control. The DNA-ladder was
purchased from Thermo Fisher Scientific, Inc. (cat. no. 26616).
Statistical analysis
All data were transferred to standard spreadsheets
and analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA). First, whether the distribution was Gaussian was
analyzed. In the case of Gaussian distribution, unpaired Student's
t-test was used; otherwise, the Kruskal-Wallis test was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Culture of human carcinoma cell lines
FaDu and HLaC 87 with WF
The cultivation of FaDu and HLaC78 with WF was
possible. The cancer cells were cultivated with WF at different
concentrations. WF at a concentration of 40% induced the highest
proliferation in FaDu and HLaC78. Higher and lower concentrations
exhibited reduced cell proliferation (data not shown). Therefore,
further experiments were conducted with 40% WF. Cancer cell
proliferation was confirmed using Ki-67 staining, as the expression
of Ki-67 is associated with cell division (24). Ki-67 staining revealed an enhanced
number of Ki-67 positive cells cultured with WF compared with
RPMI-EM (Fig. 1). Microscopy also
revealed vital cells in a monolayer as well as in spheroid
configuration (Fig. 2).
Cytokine analysis of WF
The dot blot assay demonstrated that WF is comprised
of a variety of different cytokines and growth factors. A table was
used in order to assign the different dots to corresponding
cytokines (Fig. 3).
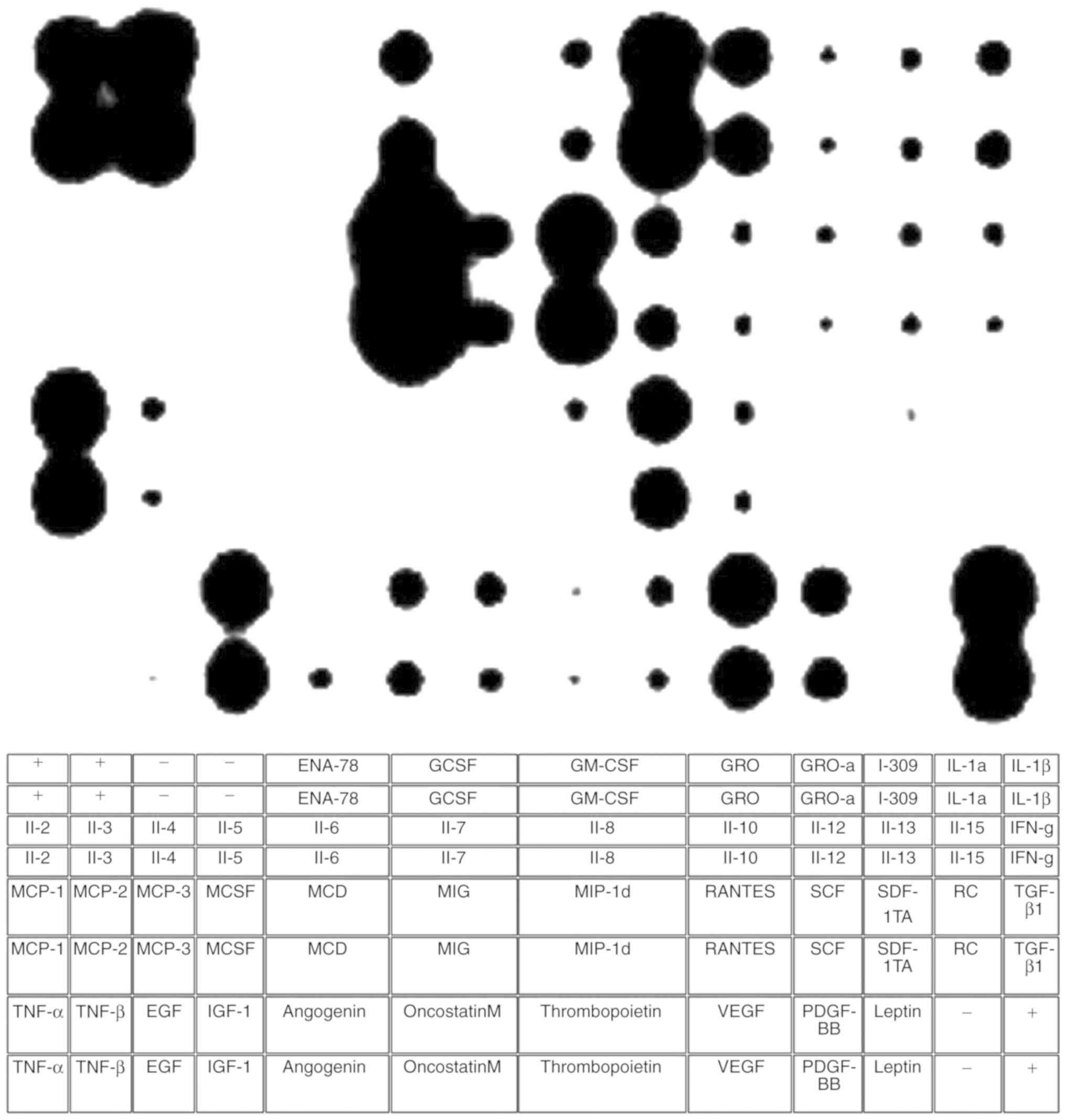 | Figure 3.Cytokine assay of WF. The dot blot
assay was used to analyze the presence of different cytokines in
WF. According to the manufacturer, a table was used to assign the
different dots to cytokines. Various types of cytokines responsible
for pro- and anti-inflammation, chemotaxis, proliferation and
angiogenesis were present in WF. WF, wound fluid; IL, interleukin;
MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor;
EGF, epidermal growth factor; MCSF, macrophage colony-stimulating
factor; IGF-1, insulin-like growth factor 1; ENA-78,
epithelial-derived neutrophil-activating protein 78; MCD, DDHDHD;
GCSF, granulocyte colony stimulating factor; MIG, monokine induced
by γ-interferon; GM-CSF, granulocyte-macrophage colony-stimulating
factor; MIP, macrophage inflammatory protein; GRO, growth related
oncogene; RANTES, regulated on activation normal t expressed and
secreted; VEGF, vascular endothelial growth factor; SCF, stem cell
factor; PDGF, platelet-derived growth factor; I-309, chemokine (C-C
motif) ligand 1; SDF, stromal cell-derived factor; TARC, thymus-
and activation-regulated chemokine; IFN, interferon; TGF,
transforming growth factor. |
Certain cytokines are responsible for inflammation,
e.g., tumor necrosis factor-α and -β. IL-6 showed the highest
density. Several anti-inflammatory cytokines were represented as
well. These cytokines are IL-6, IL-10, IL-13 and transforming
growth factor-β. Factors that induce chemotaxis (25), such as monocyte chemotactic protein
(MCP)-1, MCP-2, MCP-3 and IL-8, and factors responsible for
angiogenesis such as vascular endothelial growth factor,
angiogenin, insulin-like growth factor-1, IL-7, growth-regulated
oncogene (GRO), GRO-α and platelet-derived growth factor-BB wee
also identified (Fig. 3).
Analysis of chemoresistance
FaDu and HLaC78 exhibited enhanced proliferation
following cultivation with 40% WF compared with RPMI-EM. Due to the
high number of cytokines and growth factors, the investigation of a
resistance induction toward chemotherapeutic substances seemed
worthwhile. Therefore, FaDu were cultivated with WF for 24 h. After
24 h, the cells were treated with 10 nM paclitaxel. Previously, the
IC50 (10 nM) of paclitaxel was investigated in FaDu
(data not shown). The MTT assay revealed no significant differences
between WF compared with control after paclitaxel treatment
(Fig. 4).
Three-dimensional invasion assay
A possible alteration in the cell invasion activity
was investigated using the three-dimensional invasion assay.
RPMI-EM served as a control. To determine the migration area,
spheroids were imaged directly after being transferred (Fig. 5A and B). In this condition, cells
were able to spread out from the spheroids. After 24 h, the cells
were photographed again (Fig. 5C and
D). WF induced an enhancement in cell motility. The invasion
area of cells cultivated with WF was significantly higher compared
with the control (Fig. 5E).
Analysis of STAT 3 activation via
western blotting
The highest IL-6 signal was the observed in the WF.
In order to investigate the activation of STAT3 by WF, a western
blot analysis was performed. The western blotting revealed an
enhanced phosphorylation of STAT3 in FaDu and HLaC78 following
cultivation with WF compared with cultivation with RPMI-EM
(Fig. 6). α-tubulin was used as
control.
Discussion
Wound healing begins directly after surgery with a
modification of the microenvironment via a well-orchestrated
interaction between cells, cytokines and growth factors. The
process of normal wound healing is dynamic and divided into 4
overlapping phases: Hemostasis, inflammation, proliferation, and
remodeling (26,27). The first phase is activated by the
endothelial vasoconstriction and clotting cascade. Furthermore, the
secretion of pro-inflammatory cytokines and growth factors is
induced (28). The inflammatory
phase starts immediately, and the migration of cells such as
neutrophil granulocytes, monocytes and MSCs is induced (28,29).
This is followed by the proliferation phase and the remodeling
phase.
Notably, growth factors involved in wound healing
can also promote cancer progression and metastasis.
Platelet-derived growth factor (PDGF), for example, has an
important role in each stage of wound healing (30). Solid cancers express PDGF-receptors,
and the stimulation of these receptors may promote carcinogenesis
(31). In cases of incomplete
tumor-removal during surgery, PDFG potentially comes into contact
with non-resected cancer cells, which may lead to the enhancement
of cancer cell proliferation (32).
Additionally, GRO has an important role in wound
healing by modulating cell migration and angiogenesis as well. In
particular, GRO-α seems to promote cancer proliferation,
angiogenesis and metastasis (33,34).
The dot blot assay revealed that wound fluid (WF) contains several
factors that have mitogenic effects on cancer cells. However, it
revealed the presence of granulocyte-macrophage colony-stimulating
factor (GM-CSF) as well. GM-CSF tends to induce apoptosis and drug
sensitization in cancer cells. A previous study demonstrated that
GM-CSF induced drug sensitization in breast cancer cells (35). Increasing GM-CSF in the cancer
milieu may be a suitable therapeutic regime in cancer
treatment.
One of the most important cytokines identified in WF
is IL-6. IL-6 is a pleiotrophic cytokine and has an important role
in inflammation, immune response hematopoesis and oncogenesis
(36). There is an association
between inflammatory diseases, e.g. Crohn's disease and malignant
neoplasia, and particularly cancer of the head and neck, and high
levels of IL-6 (37,38). The signaling cascades induced by
IL-6 depend on targeted cell receptors. One of the most important
signals activated by IL-6 is the janus kinase/STAT pathway. STAT
proteins are involved in several signaling pathways. There are 7
different STAT family members (STAT1, STAT2, STAT3, STAT4, STAT5a,
STAT5b, and STAT6) (39). IL-6 is
the most potent activator of STAT3 (40). As WF contains IL-6 in high
concentrations, it seemed worthwhile to investigate whether WF
induces enhanced activation of STAT3 in cancer cells. The western
blot assay revealed a strong activation of STAT3 by WF compared
with DMEM-EM. Several other factors such as IL-10, epidermal growth
factor (EGF) and PDGF are also potential activators of STAT3
(41–43). WF contains a variety of different
growth factors for the activation of STAT3. There may be
synergistic effects between these factors with respect to the
induction of STAT3 activation, which in turn leads to an enhanced
proliferation of cancer cells.
In the present study, the cancer cells exhibited
enhanced motility following cultivation with WF. The reason for
this enhancement may be the presence of chemokines such as
chemokine (C-C motif) ligand 5 (CCL5) in WF. Chemokines are
produced and secreted by the majority of cell types and induce cell
migration and various physiological and pathological processes
(44). A variety of chemokines are
produced during wound healing. In an experiment conducted by
Karnoub et al, breast cancer cell motility was enhanced and
promoted via secretion of CCL5 from MSC. By adding anti-CCL5, the
enhancement of cell motility was counteracted (45). The dot blot assay revealed a strong
secretion of CCL5. The contact between residual cancer cells and
CCL5 may support cancer cell motility and metastasis. Besides the
cytokines and growth factors, lipid acids serve an important role
in cancer progression. Surgery may result in microenvironmental
stress due to acidosis. The reason for acidosis after surgery are
hypovolemia, hypoperfusion and lactic acidosis (46). According to Corbet and Feron
(35) and Menard et al
(47,48), acidosis and hypoxia result from an
accumulation of lipoproteins. This is associated with increased
spheroid-formation capacity in vitro and enhanced metastatic
potential of cancer cells in vivo.
Additionally, other groups have conducted the
cultivation of breast cancer cells and WF as well. Wang et
al (49) recently demonstrated
an enhanced proliferation and migration capacity of breast cancer
cells following cultivation with WF. They revealed the presence of
several cytokines and growth factors. However, the evaluation of
signaling cascades was not conducted. Licitra et al
(50) previously investigated the
stimulation of EGF receptor (EGFR)-positive residual cancer cells
after surgery in the head and neck cancer. They demonstrated an
enhanced cell proliferation in EGFR-positive cancer cells, which
was inhibited by adding anti-EGFR reagents. The present study
demonstrated an enhanced secretion of IL-6 in the WF. The
activation of STAT3 via IL-6 may be one of the main reasons for the
enhancement of cell proliferation.
The interval between surgery and postoperative
radiation therapy is usually 4–6 weeks. During this period,
residual cancer cells may recover and form novel tumor
manifestations and early metastases. The delayed adjuvant therapy
may not target these metastatic cells, which attenuates their
survival prognosis significantly. Most cancer cells in the head and
neck express epidermal EGFR (51).
The EGFR pathway modulates cancer proliferation and metastasis and
cancer survival. Sano et al (52) previously postulated that the reason
for local-regional failure of oral squamous cell carcinoma may be
due to the activation of the EGFR pathway in residual cancer cells
during wound healing. Hence, the administration of an anti-EGFR
monoclonal antibody such as cetuximab may be valuable. Other
monoclonal antibodies such as bevacizumab may result in the
inhibition of early vasculogenesis. However, bevacizumab is also
associated with multiple complications involved in wound healing
and wound infection (53), which
may delay the administration of planned adjuvant therapy and
counteract the survival prognosis.
In conclusion, enhanced cancer cell proliferation
after cultivation with WF was demonstrated in the present study;
this was achieved via activation of the STAT3 signaling pathway.
Furthermore, WF supported cancer cell motility. However, enhanced
resistance to paclitaxel, was not observed. Overall, the present
findings emphasize the importance of WF in cancer cell
proliferation and motility during wound healing. Future studies
should focus on the inhibition of mitogenic factors after cancer
surgery in order to prevent early metastasis and cancer
recurrence.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analysed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AS and SH designed and conceived the study. AS, RE,
SH, TM, PI and MB performed the experiments. The interpretation of
the data was made by AS, SH and TG. RH and NK contributed to the
writing of the manuscript and were involved in data interpretation.
TG, AS and RE collected the samples. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Informed consent was provided by all patients. The
study was approved by the Ethics Committee of the Medical Faculty
of the University of Wuerzburg (Würzburg, Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valeta-Magara A, Hatami R, Axelrod D,
Roses DF, Guth A, Formenti SC and Schneider RJ: Pro-oncogenic
cytokines and growth factors are differentially expressed in the
post-surgical wound fluid from malignant compared to benign breast
lesions. SpringerPlus. 4:4832015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cutting KF: Wound exudate: Composition and
functions. Br J Community Nurs. 8 (Suppl 9):S4–S9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abramovitch R, Marikovsky M, Meir G and
Neeman M: Stimulation of tumour growth by wound-derived growth
factors. Br J Cancer. 79:1392–1398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scherzed A, Hackenberg S, Froelich K,
Radeloff A, Technau A, Kessler M, Hagen R, Rak K, Koehler C and
Kleinsasser N: The effect of wound fluid on adipose-derived stem
cells in vitro: A study in human cell materials. Tissue Eng Part C
Methods. 17:809–817. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Ma Q, Liu T, Guan G, Zhang K,
Chen J, Jia N, Yan S, Chen G, Liu S, et al: Interleukin-6
suppression reduces tumour self-seeding by circulating tumour cells
in a human osteosarcoma nude mouse model. Oncotarget. 7:446–458.
2016.PubMed/NCBI
|
|
9
|
Ara T, Song L, Shimada H, Keshelava N,
Russell HV, Metelitsa LS, Groshen SG, Seeger RC and DeClerck YA:
Interleukin-6 in the bone marrow microenvironment promotes the
growth and survival of neuroblastoma cells. Cancer Res. 69:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ogata A, Chauhan D, Teoh G, Treon SP,
Urashima M, Schlossman RL and Anderson KC: IL-6 triggers cell
growth via the Ras-dependent mitogen-activated protein kinase
cascade. J Immunol. 159:2212–2221. 1997.PubMed/NCBI
|
|
11
|
Ataie-Kachoie P, Pourgholami MH and Morris
DL: Inhibition of the IL-6 signaling pathway: A strategy to combat
chronic inflammatory diseases and cancer. Cytokine Growth Factor
Rev. 24:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suchi K, Fujiwara H, Okamura S, Okamura H,
Umehara S, Todo M, Furutani A, Yoneda M, Shiozaki A, Kubota T, et
al: Overexpression of Interleukin-6 suppresses cisplatin-induced
cytotoxicity in esophageal squamous cell carcinoma cells.
Anticancer Res. 31:67–75. 2011.PubMed/NCBI
|
|
13
|
Sierra A: Metastases and their
microenvironments: Linking pathogenesis and therapy. Drug Resist
Updat. 8:247–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abroun S, Saki N, Ahmadvand M, Asghari F,
Salari F and Rahim F: STATs: An οld σtory, υet μesmerizing. Cell J.
17:395–411. 2015.PubMed/NCBI
|
|
15
|
Klemm JD, Schreiber SL and Crabtree GR:
Dimerization as a regulatory mechanism in signal transduction. Annu
Rev Immunol. 16:569–592. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berishaj M, Gao SP, Ahmed S, Leslie K,
Al-Ahmadie H, Gerald WL, Bornmann W and Bromberg JF: Stat3 is
tyrosine-phosphorylated through the interleukin-6/glycoprotein
130/janus kinase pathway in breast cancer. Breast Cancer Res.
9:R322007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zenner HP, Lehner W and Herrmann IF:
Establishment of carcinoma cell lines from larynx and submandibular
gland. Arch Otorhinolaryngol. 225:269–277. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scherzed A, Hackenberg S, Froelich K,
Kessler M, Koehler C, Hagen R, Radeloff A, Friehs G and Kleinsasser
N: BMSC enhance the survival of paclitaxel treated squamous cell
carcinoma cells in vitro. Cancer Biol Ther. 11:349–357. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scherzad A, Steber M, Gehrke T, Rak K,
Froelich K, Schendzielorz P, Hagen R, Kleinsasser N and Hackenberg
S: Human mesenchymal stem cells enhance cancer cell proliferation
via IL-6 secretion and activation of ERK1/2. Int J Oncol.
47:391–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
24
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loetscher P, Seitz M, Clark-Lewis I,
Baggiolini M and Moser B: Monocyte chemotactic proteins MCP-1,
MCP-2, and MCP-3 are major attractants for human CD4+
and CD8+ T lymphocytes. FASEB J. 8:1055–1060. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gonzalez AC, Costa TF, Andrade ZA and
Medrado AR: Wound healing - A literature review. An Bras Dermatol.
91:614–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo S and Dipietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ho J, Walsh C, Yue D, Dardik A and Cheema
U: Current advancements and strategies in tissue engineering for
wound healing: A comprehensive review. Adv Wound Care. 6:191–209.
2017. View Article : Google Scholar
|
|
29
|
Lee DE, Ayoub N and Agrawal DK:
Mesenchymal stem cells and cutaneous wound healing: Novel methods
to increase cell delivery and therapeutic efficacy. Stem Cell Res
Ther. 7:372016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barrientos S, Brem H, Stojadinovic O and
Tomic-Canic M: Clinical application of growth factors and cytokines
in wound healing. Wound Repair Regen. 22:569–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heldin CH: Targeting the PDGF signaling
pathway in tumor treatment. Cell Commun Signal. 11:972013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alieva M, van Rheenen J and Broekman ML:
Potential impact of invasive surgical procedures on primary tumor
growth and metastasis. Clin Exp Metastasis. 35:319–331. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang B, Hendricks DT, Wamunyokoli F and
Parker MI: A growth-related oncogene/CXC chemokine receptor 2
autocrine loop contributes to cellular proliferation in esophageal
cancer. Cancer Res. 66:3071–3077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lian S, Zhai X, Wang X, Zhu H, Zhang S,
Wang W, Wang Z and Huang J: Elevated expression of growth-regulated
oncogene-alpha in tumor and stromal cells predicts unfavorable
prognosis in pancreatic cancer. Medicine. 95:e43282016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chaubey N and Ghosh SS: Overexpression of
granulocyte macrophage colony stimulating factor in breast cancer
cells leads towards drug sensitization. Appl Biochem Biotechnol.
175:1948–1959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitsuyama K, Sata M and Rose-John S:
Interleukin-6 trans-signaling in inflammatory bowel disease.
Cytokine Growth Factor Rev. 17:451–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gallo O, Gori AM, Attanasio M, Martini F,
Paola G, Storchi OF and Abbate R: Acute-phase proteins and
interleukin 6 serum level in head and neck cancer. Arch Otolaryngol
Head Neck Surg. 118:1366–1367. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Malhotra PS, Thomas GR, Ondrey FG,
Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, et al:
Expression of proinflammatory and proangiogenic cytokines in
patients with head and neck cancer. Clin Cancer Res. 5:1369–1379.
1999.PubMed/NCBI
|
|
39
|
Ihle JN: The stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Muller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, van Boxel-Dezaire AH, Cheon H,
Yang J and Stark GR: STAT3 activation in response to IL-6 is
prolonged by the binding of IL-6 receptor to EGF receptor. Proc
Natl Acad Sci USA. 110:16975–16980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan JF, Huang WJ, Zhao JF, Fu HY, Zhang
GY, Huang XJ and Lv BD: The platelet-derived growth factor
receptor/STAT3 signaling pathway regulates the phenotypic
transition of corpus cavernosum smooth muscle in rats. PLoS One.
12:e01721912017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Simon AR, Takahashi S, Severgnini M,
Fanburg BL and Cochran BH: Role of the JAK-STAT pathway in
PDGF-stimulated proliferation of human airway smooth muscle cells.
Am J Physiol Lung Cell Mol Physiol. 282:L1296–L1304. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ishida Y, Kimura A, Kuninaka Y, Inui M,
Matsushima K, Mukaida N and Kondo T: Pivotal role of the CCL5/CCR5
interaction for recruitment of endothelial progenitor cells in
mouse wound healing. J Clin Invest. 122:711–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Waters JH, Miller LR, Clack S and Kim JV:
Cause of metabolic acidosis in prolonged surgery. Crit Care Med.
27:2142–2146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Corbet C and Feron O: Emerging roles of
lipid metabolism in cancer progression. Curr Opin Clin Nutr Metab
Care. 20:254–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Menard JA, Christianson HC, Kucharzewska
P, Bourseau- Guilmain E, Svensson KJ, Lindqvist E, Indira Chandran
V, Kjellén L, Welinder C, Bengzon J, et al: Metastasis stimulation
by hypoxia and acidosis-induced extracellular lipid uptake is
mediated by proteoglycan-dependent endocytosis. Cancer Res.
76:4828–4840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang D, Hu K, Gao N, Zhang H, Jiang Y, Liu
C, Wang S and Zhao Z: High throughput screening of cytokines,
chemokines and matrix metalloproteinases in wound fluid induced by
mammary surgery. Oncotarget. 6:29296–29310. 2015.PubMed/NCBI
|
|
50
|
Licitra L, Perrone F, Tamborini E, Bertola
L, Ghirelli C, Negri T, Orsenigo M, Filipazzi P, Pastore E,
Pompilio M, et al: Role of EGFR family receptors in proliferation
of squamous carcinoma cells induced by wound healing fluids of head
and neck cancer patients. Ann Oncol. 22:1886–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zimmermann M, Zouhair A, Azria D and
Ozsahin M: The epidermal growth factor receptor (EGFR) in head and
neck cancer: Its role and treatment implications. Radiat Oncol.
1:112006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sano D, Gule MK, Rosenthal DI, Bell D,
Yates J, El-Naggar AK and Myers JN: Early postoperative epidermal
growth factor receptor inhibition: Safety and effectiveness in
inhibiting microscopic residual of oral squamous cell carcinoma in
vivo. Head Neck. 35:321–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gordon CR, Rojavin Y, Patel M, Zins JE,
Grana G, Kann B, Simons R and Atabek U: A review on bevacizumab and
surgical wound healing: An important warning to all surgeons. Ann
Plastic Surg. 62:707–709. 2009. View Article : Google Scholar
|




















