Introduction
Breast cancer causes ~500,000 deaths worldwide each
year (1). Chemotherapy plays a
crucial role in treating this fatal disease (2). Although new therapeutics have been
developed in the last three decades, the acquisition of multiple
drug resistance (MDR) by breast cancer cells greatly impedes
effective chemotherapy (3). MDR
cancer cells have been revealed to exhibit greater
migration/invasion abilities than their parental cells (4,5).
Moreover, MDR tumors are prone to relapse and metastasis (6,7). MDR
and metastasis are two major causes of the poor prognosis of
patients with breast cancer. However, the association between MDR
and metastasis and its underlying mechanism have not been
characterized. Investigating the potential mechanisms underlying
the aberrant metastatic capacity of MDR cells is, therefore,
required for improving the efficiency of chemotherapy for advanced
breast cancer.
Epithelial-mesenchymal transition (EMT) is a
critical mechanism involved in the acquisition of metastatic
capacity by epithelial cancer cells (8). During the EMT process, epithelial
cells undergo marked morphological changes and acquire mesenchymal
properties including alterations in motility, invasion, and
cytoskeletal arrangements (9). The
primary molecular features of EMT are the downregulation of
epithelial cadherin (E-cadherin), an epithelial cell marker, and
the upregulation of mesenchymal molecules, such as neural cadherin
(N-cadherin) and vimentin (10,11).
Recent studies have revealed that EMT contributes to MDR in various
tumors including breast cancer (12,13).
However, the mechanisms of EMT-promoted MDR in breast cancer remain
poorly understood.
Cytokeratin 18 (CK18), which is known to maintain
cellular structural integrity and resist external stresses applied
to cells, has been recognized as an epithelial-specific marker of
the EMT process (14,15). CK18 also affects various cellular
processes such as cell cycle progression, apoptosis, mitosis, and
cell signaling (16).
Downregulation of CK18 has been revealed to induce EMT and promote
cancer cell migration (17,18). Reduced expression of CK18 was
revealed to promote the progression of breast tumors (19), while its increased expression,
accordingly, predicted a favorable prognosis in patients with
breast cancer (20). Additionally,
aberrant CK18 expression has been linked to apoptosis resistance
(21,22). However, whether CK18 regulates
ATP-binding cassette (ABC) transporter-mediated MDR has not been
experimentally confirmed.
We hypothesized that CK18 downregulation induces the
EMT process, enhances MDR, and is associated with poor therapeutic
outcomes in breast cancer. In the present study, we assessed CK18
expression in breast cancer tissues and determined the association
between CK18 and human breast cancer prognosis. Moreover, the
functional involvement of CK18 was examined in a mitoxantrone
(MX)-selected MCF-7/MX cell line that overexpressed breast cancer
resistant protein (BCRP) as well as in its parental human breast
cancer MCF-7 cells. Investigating and confirming the importance of
CK18 in MDR and EMT may provide a potential predictor and treatment
strategy for patients with breast cancer.
Materials and methods
Tissue samples and clinical data
collection
Sixty samples from breast cancer tissues and 15
samples from matched adjacent non-tumor tissues (ANTTs) were
collected at the Shanxi Cancer Hospital (Taiyuan, China) from
August 2012 to March 2013. The diagnosis was confirmed by two
pathologists at Shanxi Cancer Hospital. The age of the patients
ranged from 31 to 76 years (median age, 54 years). Before biopsy
sampling, none of the patients were subjected to chemotherapy or
radiotherapy. ANTT was obtained >3 cm away from the tumor
tissues. Clinicopathological characteristics including age, family
history, tumor size, lymph node metastasis, tumor-node-metastasis
(TNM) stage, histological grade, and expression of estrogen
receptor (ER), progesterone receptor (PR), HER-2, Ki-67 and
E-cadherin were obtained from hospital records. Other relevant
clinical information was also collected, including disease-free
survival (DFS), as the interval between date of diagnosis and date
of recurrence; overall survival (OS), as the interval from date of
surgery to death, and current patient status.
Ethics statement
Use of the specimens was approved by the Ethics
Committee of Shanxi Cancer Hospital. All experimental protocols
were approved by the Ethics Committee of Shanxi Medical University
(Taiyuan, China). All patients provided written informed consent
before participation in this study.
Immunohistochemical (IHC) staining and
evaluation
IHC staining was performed using a
streptavidin-peroxidase procedure as previously described (23). Briefly, tissue sections of tumor
samples (3-µm thickness) were dewaxed, rehydrated, and treated with
3% hydrogen peroxide for 10 min to inhibit the activity of
endogenous peroxidase. Non-specific binding sites were blocked with
10% normal goat serum. The tumor sections were incubated overnight
at 4°C with the anti-CK18 antibody (dilution 1:600; cat. no.
10830-1-AP; ProteinTech Group Inc.; Wuhan Sanying Biotechnology,
Wuhan, China), followed by a 30-min incubation with a biotinylated
secondary antibody (cat. no. PV-6000; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) at 37°C.
IHC results were analyzed by a semi-quantitative
method according to staining intensity and positive cell
percentage. Staining intensity was scored as: 0 (negative), 1
(weakly positive), 2 (moderately positive) and 3 (strongly
positive). The percentage of stained cells was scored as: 0
(<5%), 1 (5–25% positive), 2 (26–50% positive), 3 (51–75%
positive), 4 (>76% positive). The final IHC score was determined
by multiplying the intensity and percentage score (range 0–12). A
score ≥7 was defined as CK18 high expression, and scores <7 were
defined as CK18 low expression.
Cell culture
Human breast cancer MCF-7 and MCF-7/MX cells were
kindly provided by Dr E. Schneider (Wadsworth Center, New York, NY,
USA). Cells were grown in RPMI-1640 culture medium containing 10%
fetal bovine serum (FBS) (both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in an incubator humidified at
37°C with 5% CO2. MCF-7/MX cells were cultured in
RPMI-1640 medium containing 400 ng/ml MX (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) to maintain their MDR phenotype and
transferred to drug-free medium for at least two weeks before
experimentation.
Morphological analysis
Cells were seeded into 6-well plates at
5×105 cells/well in 2 ml of medium. The cell morphology
was observed and photographed under an inverted microscope (Leica
Microsystems GmbH, Wetzlar, Germany) at a magnification of
×200.
Cytotoxicity assay
Cell cytotoxicity was analyzed using the Cell
Counting Kit-8 (CCK-8) (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China). Cells were cultured in 96-well plates at a density
of 1.8×103 cells/well. After incubation for 24 h, the
cells were treated with various concentrations of MX, fluorouracil
(5-FU), doxorubicin (Dox), cytarabine (Ara-C), or cisplatin (DDP)
(all from Sigma-Aldrich; Merck KGaA) for another 70 h, and then
incubated with 10% CCK-8 reagent for an additional 2 h. Cell
viability was evaluated at 450 nm on an automated microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). Cells treated
without chemotherapeutic agents were used as controls. All CCK-8
tests were performed in triplicate and repeated three times. The
IC50 values were calculated using SPSS software (version
23.0; IBM Corp., Armonk, NY, USA). The cell survival rate was
quantified as the number of live cells divided by the total number
of cells and calculated as follows: Cell survival rate (%) =
optical density (OD) value of experimental well/OD value of control
well × 100%. The resistance fold (RF) was defined as the
IC50 (MCF-7/MX or MCF-7/siCK18)/IC50
(MCF-7).
Cell proliferation assay
Cells were seeded in 96-well plates at a density of
1×103 cells/well and cultured for up to three days. Cell
proliferation capacity was then assessed every 24 h by CCK-8 assay
as aforementioned. Ten duplicate wells were prepared for each
sample.
Migration and invasion assays
Cell migration and invasion potentials were assessed
using 24-well Transwell chambers (Corning Incorporated, Corning,
NY, USA) coated with or without Matrigel Matrix (Sigma-Aldrich;
Merck KGaA). Briefly, ~1×105 (migration) or
2×104 (invasion) cells were suspended in the upper well
of the chamber in RPMI-1640 medium without FBS. Medium containing
serum was placed in the lower well and served as a chemoattractant.
After 48 h, the upper chamber of the filter was scraped gently to
remove the nonmigratory cells. Migrated and invaded cells were
fixed, stained with 0.3% crystal violet, photographed, and counted
under an inverted microscope (Leica Microsystems GmbH). Each assay
was performed on triplicate filters.
Plasmids and stable transfection
MCF-7 cells were plated in 6-well plates and
incubated for 24 h; transfection was then performed with
CK18-specific (pSilencer 3.1/CK18) or non-silencing negative
control (pSilencer 3.1/NC) expression vector constructs using
Lipofectamine 2000 (Beijing SBS Genetech Co., Ltd., Beijing,
China). Stable clone cells were selected with 500 µg/ml G-418
sulfate (G418) (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China). Downregulated expression of the CK18 gene
and protein was determined by quantitative real-time polymerase
chain reaction (PCR) and western blotting, respectively.
The siRNA oligonucleotide sequences used for CK18
and NC were:
5′-gatccgAGAGGAGCTAGACAAGTACttcaagagaGTACTTGTCTAGCTCCTCTCtttttt-3′
and
5′-atccgCTTACAATCAGACTGGCGAttcaagagaTCGCCAGTCTGATTGTAAGtttttt-3′,
respectively. The validity of the inserts was verified by sequence
analysis (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Reverse transcriptase andquantitative
real-time PCR
Total RNA was isolated from cells by TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized from 1 µg of total RNA using Transcript First-Strand
cDNA Synthesis SuperMix (Beijing Transgen Biotech Co., Ltd.,
Beijing, China). The mRNA levels of Snail, N-cadherin, E-cadherin,
NF-κB (p65), vimentin, BCRP, and internal control β-actin were
detected. PCR products were separated by electrophoresis on 1–2%
agarose gels, imaged on a GelDoc™ XR (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and quantified by densitometry using Image Lab
software (version 5.2.1; Bio-Rad Laboratories, Inc.). Real-time PCR
was performed with Power SYBR-Green PCR SuperMix (Mei5
Biotechnology, Co., Ltd., Beijing, China), using a CFX96 Touch
Detection System (cat. no. 1855195; Bio-Rad Laboratories, Inc.).
The fold-change value of CK18 mRNA was normalized to β-actin using
the ΔΔCq-method (24). The
gene-specific primer pairs were as follows (5′-3′): Snail forward,
GCCTTCAACTGCAAATACTGC and reverse, CTTCTTGACATCTGAGTGGGTC;
N-cadherin forward, GATGTTGAGGTACAGAATCGT and reverse,
GGTCGGTATGGATGGCGA; E-cadherin forward, ATTCTGATTCTGCTGCTCTTG and
reverse, AGTAGTCATAGTCCTGGTCTT; NF-κB (p65) forward,
AGGCTCTGTGCGTGTCTCC and reverse, GGGTGGGCTTGGGGGCAGGT; vimentin
forward, TCGCCAACTACATCGACAAG and reverse, AAGATTGCAGGGTGTTTTCG;
BCRP forward, TGTTTGGAAGGTCCGGGTGA and reverse,
CATGATCCCATTGTAATTCG; β-actin forward, CTGGGACGACATGGAGAAAA and
reverse, AAGGAAGGATGGAAGAGTGC. PCR amplification was performed with
denaturation at 94°C for 30 sec, annealing at 55°C for 40 sec, and
extension at 72°C for 1 min in 35 cycles.
Western blot analysis
A western blot assay was carried out as previously
described (25). Cell lysates
containing 50 µg of total protein were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto nitrocellulose membranes. The membranes were
incubated with specific primary antibodies against BCRP (dilution
1:1,000; cat. no. D160018), lung resistance protein (LRP) (dilution
1:1,000; cat. no. D220930) (both from Shanghai Sangong
Pharmaceutical Co., Ltd., Shanghai, China), multidrug resistance
protein (MRP) (dilution 1:1,500; cat. no. D260613; Shanghai Sangong
Pharmaceutical Co., Ltd.), P-glycoprotein (P-gp) (dilution 1:500;
cat. no. PB0162; Wuhan Boster Biological Technology, Ltd.), CK18
(dilution 1:2,000; cat. no. 10830-1-AP; ProteinTech; Wuhan Sanying
Biotechnology, Wuhan, China), E-cadherin (dilution 1:1,000; cat.
no. 14472), Snail (dilution 1:1,000; cat. no. 3879), N-cadherin
(dilution 1:500; cat. no. 14215) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA), NF-κB p65 (dilution 1:1,000;
cat. no. D120135; Shanghai Sangong Pharmaceutical Co.), vimentin
(dilution 1:700; cat. no. ab92547; Abcam, Cambridge, UK), and
β-actin (dilution 1:5,000; cat. no. BM0627; Wuhan Boster Biological
Technology, Ltd.) overnight at 4°C. After incubation for 1 h with
corresponding horseradish peroxidase (HRP)-linked secondary
antibody (dilution 1:5,000; cat. no. ZB-2301/2305), the blots were
detected using the electrochemiluminescence (ECL) system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The band density was
quantified by densitometry using Image Lab software (version 5.2.1;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All quantitative data are presented as the means ±
standard deviations (SDs) from at least three independent
experiments. Fisher's exact test was used to evaluate associations
between CK18 expression and the clinicopathological characteristics
of breast cancer. Survival curves were estimated using the
Kaplan-Meier method and compared by a log-rank test. Differences
between two samples were analyzed by Student's t-test, and multiple
comparisons were performed by one-way analysis of variance (ANOVA)
and the Student-Newman-Keuls (SNK) test using SPSS software
(version 23.0; SPSS, Inc., Chicago, IL, USA). P<0.05
(two-tailed) was considered to indicate a statistically significant
difference.
Results
CK18 is downregulated in tumor tissues
and correlated with lymph node metastasis of breast cancer
We determined the expression levels of CK18 in human
breast cancer specimens and ANTT using IHC staining. Representative
images are presented in Fig. 1A.
CK18 was mainly localized at the cell membrane and the cytoplasm.
CK18 immunoreactivity was consistently weaker in primary breast
cancer tissue (P<0.01) and even less in metastatic lesions
(P<0.001) than in normal breast tissues (Fig. 1B). We classified the patients into a
CK18 low- and a high-expression group according to its IHC staining
results, and the associations between CK18 level and
clinicopathological characteristics of patients with breast cancer
were analyzed. Low expression of CK18 was associated with TNM
stage, lymph node metastasis, and E-cadherin expression (Table I). Follow-up analysis was performed
to analyze the association of CK18 expression and breast cancer
prognosis. A significant tendency of CK18 downregulation towards
unfavorable prognosis was displayed in analysis of DFS (Fig. 1C). A similar trend was observed in
OS analysis, and patients with low-level CK18 expression had
shorter OS durations than those with high-level CK18 expression,
although the trend was not statistically significant (log-rank
P=0.3844) (Fig. 1D).
 | Table I.Associations between the expression
level of CK18 and the clinicopathological characteristics of
patients with breast cancer. |
Table I.
Associations between the expression
level of CK18 and the clinicopathological characteristics of
patients with breast cancer.
|
|
| CK18
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Cases (n=60) | Low (n=37) | High (n=23) | P-value |
|---|
| Age (years) |
|
|
| 0.4313 |
|
≤50 | 33 | 22 | 11 |
|
|
>50 | 27 | 15 | 12 |
|
| Family history |
|
|
| 0.9999 |
|
Yes | 7 | 4 | 3 |
|
| No | 53 | 33 | 20 |
|
| Tumor size
(cm) |
|
|
| 0.0518 |
|
<5 | 57 | 37 | 20 |
|
| ≥5 | 3 | 0 | 3 |
|
| Lymph node
metastasis |
|
|
| 0.0088b |
|
Yes | 34 | 26 | 8 |
|
| No | 26 | 11 | 15 |
|
| AJCC TNM stage |
|
|
| 0.0205a |
|
I+II | 48 | 26 | 22 |
|
|
III | 12 | 11 | 1 |
|
| Histological
grade |
|
|
| 0.9999 |
|
I+II | 47 | 29 | 18 |
|
|
III | 13 | 8 | 5 |
|
| ER |
|
|
| 0.9999 |
| + | 45 | 28 | 17 |
|
| − | 15 | 9 | 6 |
|
| PR |
|
|
| 0.4041 |
| + | 39 | 26 | 13 |
|
| − | 21 | 11 | 10 |
|
| HER-2 |
|
|
| 0.7215 |
| + | 9 | 5 | 4 |
|
| − | 51 | 32 | 19 |
|
| Ki-67 (%) |
|
|
| 0.7650 |
|
≤14 | 16 | 9 | 7 |
|
|
>14 | 44 | 28 | 16 |
|
| E-cadherin |
|
|
| 0.0014b |
| ++ | 29 | 25 | 4 |
|
| + | 31 | 12 | 19 |
|
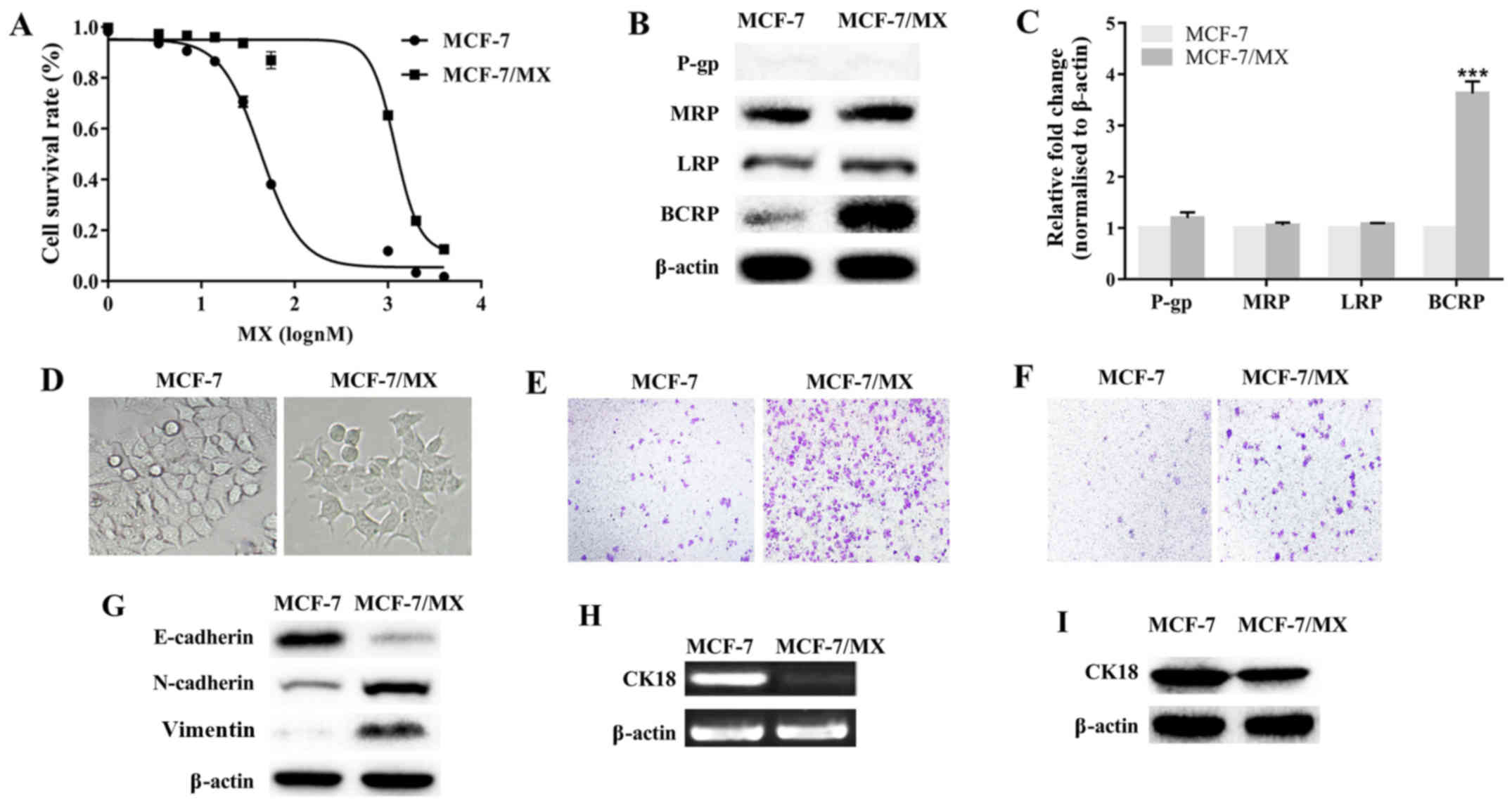
Cell migration is enhanced in MCF-7/MX
cells that overexpress BCRP
MCF-7/MX cells were ~42 times more resistant to MX
than parental cells (Fig. 2A and
Table II). MCF-7/MX was also
cross-resistant to Ara-C, Dox, DDP and 5-FU, and the
IC50 values of these drugs for MCF-7/MX cells were
significantly increased (P<0.001 in all 4 cases; Table II). The expression of four MDR
proteins (BCRP, P-gp, MRP and LRP) was investigated in these two
cell lines. BCRP levels in MCF-7/MX cells were markedly upregulated
and ~4-fold higher than those in the parental cells (P<0.001);
MRP and LRP were only slightly more upregulated in MCF-7/MX cells
than in MCF-7 cells (P>0.05); and P-gp expression was not
detected in either cell line (Fig. 2B
and C). These results confirmed that BCRP overexpression was
the primary contributor to MDR in MCF-7/MX cells.
 | Table II.The resistance of MCF-7 and MCF-7/MX
cells to different chemotherapeutic agents. |
Table II.
The resistance of MCF-7 and MCF-7/MX
cells to different chemotherapeutic agents.
|
| IC50
(nmol/l) ± SDa |
|
|---|
|
|
|
|
|---|
| Drugs | MCF-7 | MCF-7/MX | Resistance
fold |
|---|
| MX | 35.6±0.9 |
1484.2±43.2a | 41.7 |
| Ara-C | 6.5±0.8 |
676.1±91.5a | 104.0 |
| Dox | 475.7±1.3 |
18577.0±1372.4a | 39.1 |
| DDP | 464.2±16.6 |
4544.3±220.6a | 9.8 |
| 5-FU | 271.5±6.1 |
5507.7±153.9a | 20.3 |
Whereas parental MCF-7 cells exhibited an epithelial
cobblestone phenotype, MCF-7/MX cells exhibited spindle-shaped,
fibroblastoid-like morphology (Fig.
2D), increased cell migration and invasion (Fig. 2E and F), downregulated E-cadherin,
and upregulated N-cadherin and vimentin (Fig. 2G), suggesting that MDR MCF-7/MX
cells underwent EMT and acquired a more powerful motile capacity.
Additionally, lower levels of CK18 mRNA and protein were detected
in MCF-7/MX than in MCF-7 cells (Fig.
2H and I). These results indicated that CK18 may participate in
BCRP-mediated MDR in breast cancer cells.
Downregulation of CK18 enhances the
chemoresistance of MCF-7 cells
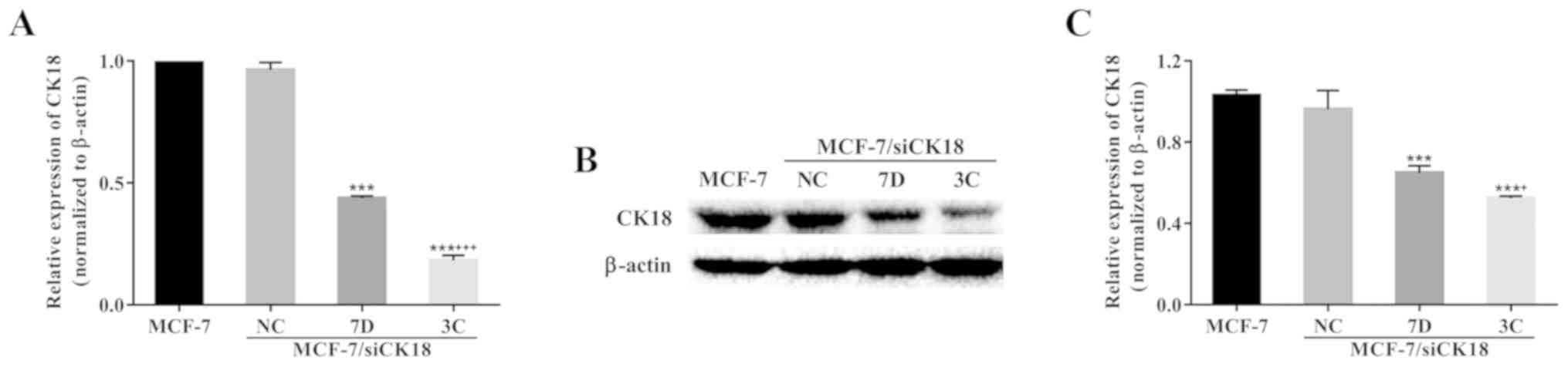
After selection, two stable clones, MCF-7/siCK18-7D
and MCF-7/siCK18-3C, were obtained. CK18 mRNA expression was 56.3%
(P<0.001) and 81.8% (P<0.001) lower in MCF-7/siCK18-7D and
−3C cells, respectively, than in MCF-7 cells (Fig. 3A). Representative western blotting
revealed a significant downregulation of CK18 expression in
MCF-7/siCK18 cells, while approximately equal amounts of CK18
protein were observed in MCF-7/siNC and MCF-7 cells (Fig. 3B). CK18 protein levels were 37.0%
(P<0.001) and 49.2% (P<0.001) lower in MCF-7/siCK18-7D and
−3C cells, respectively, than in MCF-7 cells (Fig. 3C).
Downregulation of CK18 in MCF-7 cells significantly
enhanced resistance to several chemotherapeutic agents (Table III). Compared to MCF-7 cells, the
resistance of MCF-7/siCK18-7D cells to MX, Ara-C and Dox was
increased to 4.9- (P<0.001), 6.0- and 1.5-fold (P<0.001),
respectively, while that of MCF-7/siCK18-3C cells was increased to
18.9-, 25.5- and 4.8-fold (P<0.001 in all three cases),
respectively. The IC50 values of these chemotherapeutic
agents in MCF-7/siNC were close to those in MCF-7 cells
(P>0.05).
 | Table III.Effects of CK18 downregulation on
chemosensitivity of MCF-7/MX cells to chemotherapeutic agents. |
Table III.
Effects of CK18 downregulation on
chemosensitivity of MCF-7/MX cells to chemotherapeutic agents.
|
| IC50
(nmol/l) ± SDa
(RR)b |
|---|
|
|
|
|---|
| Cells | MX | Ara-C | Dox |
|---|
| MCF-7 | 35.6±0.9 (1) | 6.5±0.8 (1) | 475.7±1.3 (1) |
| MCF-7/siNC | 42.3±2.7 (1.2) | 7.3±1.4 (1.1) | 410.2±72.5
(0.9) |
|
MCF-7/siCK18-7D | 174.7±24.7
(4.9)c | 38.8±2.2 (6.0) | 713.3±121.4
(1.5)c |
|
MCF-7/siCK18-3C | 672.0±55.0
(18.9)c,d | 165.5±54.7
(25.5)c,d | 2268.4±158.2
(4.8)c,d |
Downregulation of CK18 in MCF-7 cells
promotes BCRP expression
The BCRP gene was markedly upregulated in
MCF-7/siCK18 cells compared with that in MCF-7 cells (Fig. 4A). Quantification results revealed
that BCRP mRNA levels were 277.8% (P<0.001) and 365.6%
(P<0.001) greater in MCF-7/siCK18-7D and −3C cells,
respectively, than in MCF-7 cells (Fig.
4B). CK18 downregulation also induced an increase in BCRP
protein levels, as determined by western blot analysis (Fig. 4C). The expression of BCRP protein
was 37.8% (P<0.01) and 72.3% (P<0.001) greater in
MCF-7/siCK18-7D and −3C cells, respectively, than in MCF-7 cells,
while no significant increase in BCRP levels was observed in
MCF-7/siNC cells (P>0.05) (Fig.
4D).
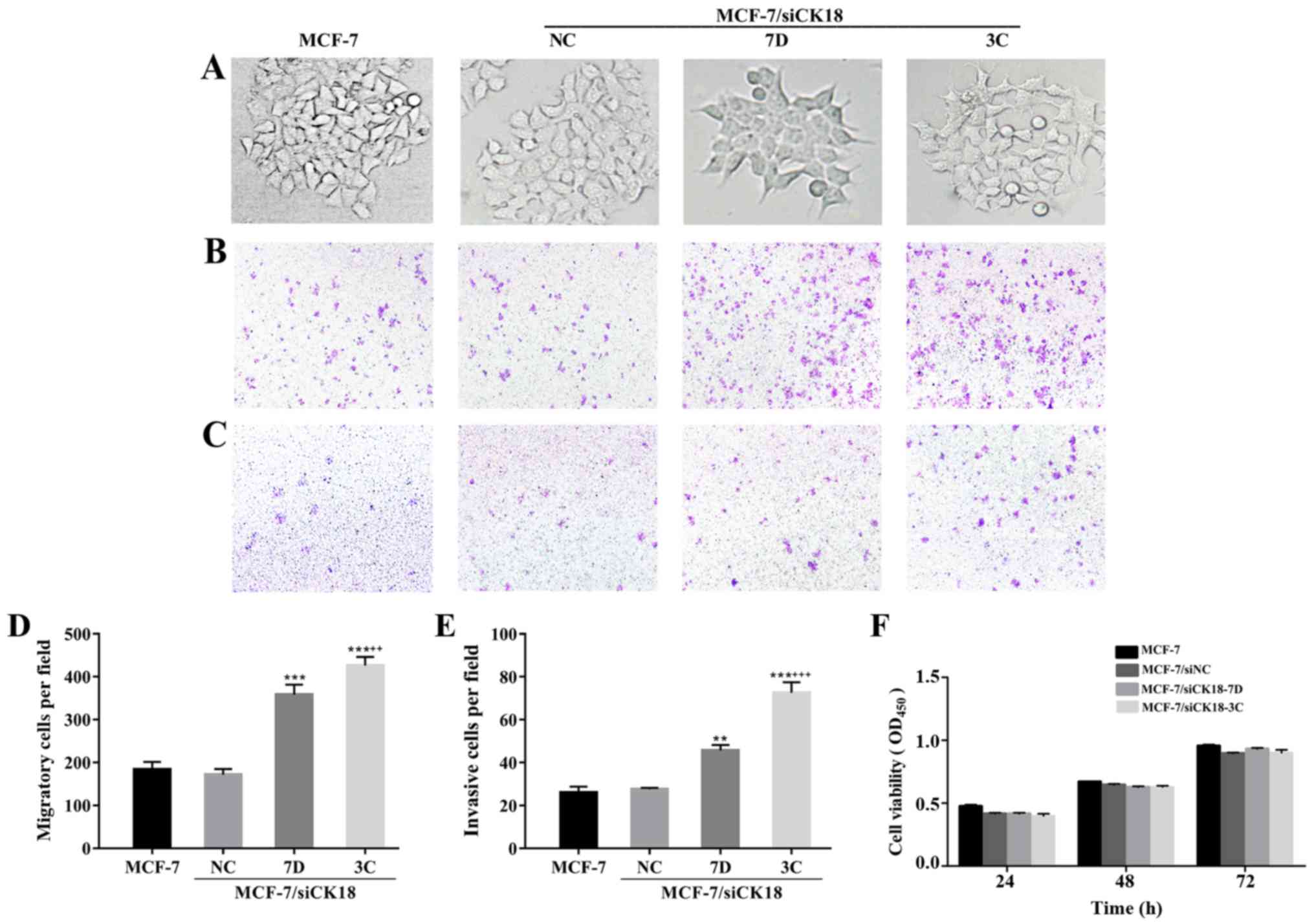
CK18 downregulation increases cell
motility and invasion capacity
In contrast to the epithelial cobblestone phenotype
of MCF-7 and MCF-7/siNC cells, MCF-7/siCK18 cells exhibited
elongated, fibroblastic morphology which was similar to that of
MCF-7/MX cells (Fig. 5A). As
revealed in Fig. 5B-E, the number
of MCF-7/siNC cells that migrated through the permeable membrane or
invaded through the Matrigel-coated membrane was approximately
equal to that of MCF-7 cells (P>0.05), while MCF-7/siCK18-7D and
−3C cells demonstrated greater migration (P<0.001) and invasion
abilities (P<0.01 and P<0.001, respectively), than MCF-7
cells. There were no significant differences in cell proliferation
ability between the four cell lines (P>0.05; Fig. 5F), suggesting that the increase in
cell migration and invasion was not due to enhanced
proliferation.
CK18 downregulation modulates the
expression of EMT markers
To further determine the effects of CK18
downregulation on EMT, the expression of EMT-related molecules was
assessed. As revealed in Fig. 6A and
B, mRNA expression of E-cadherin was 15.1% (P<0.01) and
36.5% (P<0.001) lower in MCF-7/siCK18-7D and −3C cells,
respectively, than in MCF-7 cells. The expression of N-cadherin and
vimentin genes was 188.0% (P<0.01) and 387.0% (P<0.001), and
108.9 and 291.9% (P<0.01), greater in MCF-7/siCK18-7D and −3C
cells, respectively, than in MCF-7 cells. Representative western
blotting revealed downregulation of E-cadherin and upregulation of
N-cadherin and vimentin in MCF-7/siCK18 cells (Fig. 6C). Densitometric analysis
revealed1.2- (P<0.001) and 2.2-fold (P<0.001) lower
E-cadherin expression, 1.3- and 2.2-fold (P<0.001) greater
N-cadherin expression, and 1.4- (P<0.01) and 2.2-fold
(P<0.001) greater vimentin expression in MCF-7/siCK18-7D and −3C
cells, respectively, than in MCF-7 cells, while differences in the
expression of these EMT-related markers were not statistically
significant between MCF-7/siNC and MCF-7 cells (Fig. 6D). These results indicated that CK18
participated in BCRP-mediated MDR by modulating the expression of
EMT-related factors in breast cancer cells.
 | Figure 6.CK18 downregulation regulates the
expression of EMT markers. (A) Western blotting revealed the
expression levels of epithelial and mesenchymal protein markers in
MCF-7, MCF-7/siNC, and MCF-7/siCK18 cells. (B) The protein
expression of E-cadherin, N-cadherin, and vimentin was normalized
to the β-actin levels. Bar graphs represent the mean ± standard
deviation (SD) of three independent experiments. **P<0.01 and
***P<0.001 vs. MCF-7 cells; +P<0.05 and
+++P<0.001 vs. MCF-7/siCK18-7D cells. (C) The mRNA
level of E-cadherin, N-cadherin, and vimentin in MCF-7, MCF-7/siNC,
and MCF-7/siCK18 cells was determined by quantitative RT-PCR. (D) A
bar diagram revealing densitometric quantified data for the ratios
of E-cadherin, N-cadherin, and vimentin to β-actin mRNA. The graph
displays the means ± SD of three independent experiments.
**P<0.01 and ***P<0.001 vs. MCF-7 cells;
+P<0.05 and +++P<0.001 vs.
MCF-7/siCK18-7D cells. EMT, epithelial-mesenchymal transition. |
CK18 downregulation activates
NF-κB/Snail signaling in MCF-7 cells
The transcription factor Snail has been revealed to
directly inhibit the expression of E-cadherin to regulate EMT
(26,27). Snail mRNA levels were 145.7%
(P<0.001) and 335.2% (P<0.001) greater, and protein levels
were 48.2% (P<0.01) and 81.2% (P<0.001) greater, in
MCF-7/siCK18-7D and −3C cells, respectively, than in MCF-7 cells,
while MCF-7/siNC cells contained levels of Snail that were
approximately equal to those of MCF-7 (P>0.05) (Fig. 7A-D).
 | Figure 7.CK18 downregulation activates
NF-κB/Snail signaling pathway in MCF-7 cells. Representative PCR
images of (A) Snail and (E) NF-κB in MCF-7, MCF-7/siNC, and
MCF-7/siCK18 cells. The relative expression of (B) Snail and (F)
NF-κB was further quantified. Representative western blot images of
(C) Snail and (G) NF-κB in MCF-7, MCF-7/siNC and MCF-7/siCK18
cells. The protein expression of (D) Snail and (H) NF-κB was
normalized to the β-actin levels. (I) Western blotting revealed the
expression levels of NF-κB, Snail, E-cadherin, N-cadherin, and
vimentin in MCF-7/MX cells in the absence or presence of PDTC (50
and 100 µM, a specific NF-κB inhibitor) for 24 h. (J) The protein
expression of NF-κB, Snail, E-cadherin, N-cadherin and vimentin was
normalized to the β-actin levels. The graph displays the means ±
standard deviation (SD) of three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 vs. MCF-7 cells;
+P<0.05, ++P<0.01 and
+++P<0.001 vs. MCF-7/siCK18-7D cells. PDTC,
pyrrolidinedithiocarbamate. |
NF-κB p65 has been reported to induce the activity
of the Snail promoter (28,29). CK18 knockdown led to a significant
upregulation of NF-κB p65 mRNA and protein levels (Fig. 7E and F). Band intensity results
revealed that NF-κB p65 mRNA expression was 107.2% (P<0.01) and
355.2% (P<0.001) greater, and protein was 291.5% (P<0.001)
and 459.8% (P<0.001) greater, in MCF-7/siCK18-7D and −3C cells,
respectively, than in MCF-7 cells, while MCF-7/siNC cells showed
non-significant increases in NF-κB p65 levels (P>0.05) (Fig. 7G and H).
To further determine whether the NF-κB/Snail
signaling pathway acts upstream of the EMT process, we observed the
effects of pyrrolidinedithiocarbamate (PDTC), a specific NF-κB
inhibitor, in MCF-7/MX cells. As revealed in Fig. 7I and J, after incubation with PDTC
(50 and 100 µM) for 24 h, the expression level of NF-κB in MCF-7/MX
cells was significantly decreased to 77.2% (P<0.05) and 39.4%
(P<0.01), respectively. Treatment of MCF-7/MX cells with PDTC
(50 and 100 µM) also resulted in significant reductions in the
protein levels of Snail (P<0.05), which was accompanied by
upregulation of E-cadherin (P<0.05 and P<0.01, respectively)
and downregulation of N-cadherin (P<0.05 and P<0.01,
respectively) and vimentin (P<0.05 and P<0.01,
respectively).
Discussion
Overexpression of BCRP, a newly discovered ABC
transporter, may confer MDR to various types of human malignancies
including breast cancer (30–32).
In addition to chemotherapy resistance, high BCRP expression
promotes cellular resistance to radiation therapy (33). BCRP has also been identified as a
cancer stem cell marker in diverse malignancies (34,35).
The correlations between high BCRP activity and decreased survival
rate have been observed in clinical studies (36,37).
Attention has been focused on the contribution of BCRP to the
promotion of MDR during cancer therapy, however, the mechanisms
involved in BCRP upregulation in drug-resistant cancer cells remain
largely unexplored. Therefore, overcoming BCRP-mediated MDR would
contribute to cancer chemotherapy.
EMT-mediated MDR has been confirmed in various types
of cancers, including breast cancer (38). Tumors with positive EMT markers can
develop a subpopulation with a resistant phenotype, which can
become a major obstacle to treatment (39). Overexpression of transcription
factors that mediate EMT was reported to lead to ABC transporter
upregulation and subsequent MDR in breast cancer cells (40). Various EMT-related factors have been
revealed to be used to indicate the prognosis of chemotherapy in
breast cancer patients (41,42). A
previous study demonstrated that BCRP expression in breast cancer
cells could be regulated during EMT (43). In the present study, we observed
that BCRP-overexpressing MCF-7/MX cells exhibited a typical
mesenchymal phenotype, more aggressive and invasive behavior,
downregulation of E-cadherin, and upregulation of N-cadherin and
vimentin, compared with parental MCF-7 cells. These observations
strongly indicated that EMT was closely associated with
BCRP-mediated chemoresistance in MCF-7/MX cells.
Reduction or loss of epithelial keratins has been
considered a hallmark of EMT (44).
CK18 has been revealed to be necessary for initiation of EMT in
breast epithelial cells and is frequently used as an epithelial EMT
marker (15). Accordingly, reducing
CK18 expression increased the aggressiveness of established breast
cancer cell lines (45). Consistent
with these findings, our current study revealed that reduction of
CK18 expression in MCF-7 cells promoted the EMT process as
evidenced by mesenchymal morphology, enhanced migration and
invasion, and altered expression of EMT markers. Furthermore, CK18
downregulation resulted in reduced sensitivity of MCF-7 human
breast cancer cells to chemotherapeutic agents and increased BCRP
expression, suggesting that CK18 downregulation is a candidate
promoter of BCRP-mediated MDR. Several studies have revealed that
high CK18 expression was correlated with a good prognosis (19,20).
In the present study, we observed that CK18 expression was
significantly decreased in breast cancer tissues, and low CK18
expression was found to be associated with TNM stage and lymph node
metastasis in breast cancer. Kaplan-Meier analysis revealed that
patients with CK18 low expression presented poorer prognosis
compared with those with CK18 high expression. Based on these
results, it was concluded that downregulation of CK18 conferred
BCRP-mediated resistance to breast cancer MCF-7 cells partly via
EMT induction, thereby enhancing tumor invasion and metastasis and
promoting MDR; this may provide an explanation for the poor
prognostic outcomes in breast cancer patients with CK18-low
tumors.
The mechanisms by which CK18 downregulation induces
EMT and confers BCRP-mediated MDR in breast cancer MCF-7 cells are
worthy of investigation. Numerous studies have indicated that the
NF-κB/Snail signaling pathway promotes metastasis/invasion through
EMT (29,46). The expression of Snail can be
regulated by NF-κB signaling through both transcriptional and
post-translational mechanisms (29,47).
The activation of NF-κB may induce significantly increased
expression of Snail, which directly suppresses transcription of
E-cadherin (48) and upregulates
N-cadherin and vimentin expression (49), subsequently inducing EMT and
metastasis/invasion in cancer cells. In the present study,
knockdown of CK18 activated NF-κB/Snail signaling accompanied by
attenuation of E-cadherin and upregulation of N-cadherin and
vimentin expression, which clearly promoted the malignant phenotype
of MCF-7 cells. These results indicated that CK18 downregulation
may induce EMT through the NF-κB/Snail signaling pathway in breast
cancer MCF-7 cells.
EMT-mediated therapeutic resistance has been
observed in several types of cancers. Although the mechanisms by
which EMT regulates MDR are not clearly understood, accumulating
evidence suggests that EMT may result in overexpression of ABC
transporters, thereby conferring MDR to tumor cells (8,50).
Several ABC transporters have been demonstrated to contain binding
sites for EMT-related transcription factors, including Snail
(40). Overexpression of Snail has
been revealed to be highly correlated with BCRP-mediated MDR
(51). Snail has been frequently
reported to promote tumor progression and to predict prognosis
(52,53). NF-κB/Snail signaling has been
demonstrated to be required for the EMT process in human breast
cancer cells (54). Therefore, we
surmised that downregulation of CK18 in MCF-7 cells activated the
NF-κB/Snail signaling pathway and induced the EMT process,
consequently enhancing the expression and activity of BCRP.
In conclusion, our study demonstrated that CK18 was
significantly downregulated during the progression of human breast
cancer as well as during invasion, metastasis, and acquisition of
drug resistance. Downregulation of CK18 was revealed to play
crucial roles in the regulation of metastasis and MDR of breast
cancer, which is helpful for prognosis assessment in breast cancer.
Further pre-clinical and clinical studies may be required to
evaluate the role of CK18 as a potential predictor of breast
cancer.
Acknowledgements
We would like to thank the patients with breast
cancer and their families for participation. We would also like to
thank Dr Liwu Xie (Department of Pathology, Shanxi Cancer Hospital)
and Wenjing Du (Department of Radiation, Shanxi Cancer Hospital)
for their contributions to the collection of breast cancer
cases.
Funding
The present study was funded by grants from the
National Natural of Science Foundation of China (no. 81502641), the
Fund for Shanxi Key Subjects Construction (FSKSC) and the Fund for
Shanxi ‘1331 Project’ Key Subjects Construction (1331KSC).
Availability of data and materials
The datasets of IHC and/or analyzed during the study
are available from the corresponding author on reasonable request.
Other datasets generated or analyzed during this study are included
in this published article.
Authors' contributions
RS was responsible for the design of the experiment
and the writing of the manuscript. CW and ZW performed the IHC of
the breast cancer tissue. NF and CW analyzed the data of breast
cancer patient. CW, DZ, NF and LL performed the western blot
analysis and PCR analysis of the experiment. LL and DZ were
contributed to the migration and invasion of cells. RS and CW
participated in the determination of cell mechanics. RS and HZ
performed the statistical analysis. JX and YW performed the
technical and material support. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Use of the specimens was approved by the Ethics
Committee of Shanxi Cancer Hospital. All experimental protocols
were approved by the Ethics Committee of Shanxi Medical University
(Taiyuan, China), and in line with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards. All
samples were obtained before treatment according to the guidelines
of the local ethics committees. Written informed consent was
received from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Königsberg R, Obermayr E, Bises G, Pfeiler
G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M and
Dittrich C: Detection of EpCAM positive and negative circulating
tumor cells in metastatic breast cancer patients. Acta Oncol.
50:700–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart CD, Migliaccio I, Malorni L,
Guarducci C, Biganzoli L and Di Leo A: Challenges in the management
of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin
Oncol. 12:541–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saeki T, Tsuruo T, Sato W and Nishikawsa
K: Drug resistance in chemotherapy for breast cancer. Cancer
Chemother Pharmacol. 56 (Suppl 1):84–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin Y, Zhang W, Wang H, Zhang Z, Chu C,
Liu X and Zou Q: EGFR/HER2 inhibitors effectively reduce the
malignant potential of MDR breast cancer evoked by P-gp substrates
in vitro and in vivo. Oncol Rep. 35:771–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Zou L, Zhou D, Zhou Z, Tang F, Xu
Z and Liu X: Overexpression of ubiquitin carboxyl terminal
hydrolase-L1 enhances multidrug resistance and invasion/metastasis
in breast cancer by activating the MAPK/Erk signaling pathway. Mol
Carcinog. 55:1329–1342. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu LS, Chen L, Ding WX, Li K and Wu JJ:
Elevated expression of both MDR1 and MMP-2 genes in metastasized
lymph node of invasive ductal breast cancer. Eur Rev Med Pharmacol
Sci. 16:2037–2043. 2012.PubMed/NCBI
|
|
7
|
Matsuo K, Eno ML, Ahn EH, Shahzad MM, Im
DD, Rosenshein NB and Sood AK: Multidrug resistance gene (MDR-1)
and risk of brain metastasis in epithelial ovarian, fallopian tube,
and peritoneal cancer. Am J Clin Oncol. 34:488–493. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dave B, Mittal V, Tan NM and Chang JC:
Epithelial-mesenchymal transition, cancer stem cells and treatment
resistance. Breast Cancer Res. 14:2022012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition - A Hallmark of breast cancer metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Makino T, Yamasaki M, Takeno A, Shirakawa
M, Miyata H, Takiguchi S, Nakajima K, Fujiwara Y, Nishida T,
Matsuura N, et al: Cytokeratins 18 and 8 are poor prognostic
markers in patients with squamous cell carcinoma of the oesophagus.
Br J Cancer. 101:1298–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung H, Kim B, Moon BI and Oh ES:
Cytokeratin 18 is necessary for initiation of TGF-β1-induced
epithelial-mesenchymal transition in breast epithelial cells. Mol
Cell Biochem. 423:21–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oshima RG, Baribault H and Caulín C:
Oncogenic regulation and function of keratins 8 and 18. Cancer
Metastasis Rev. 15:445–471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schaafsma HE, Van Der Velden LA, Manni JJ,
Peters H, Link M, Rutter DJ and Ramaekers FC: Increased expression
of cytokeratins 8, 18 and vimentin in the invasion front of mucosal
squamous cell carcinoma. J Pathol. 170:77–86. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luan Y, Chao S, Ju ZY, Wang J, Xue X, Qi
TG, Cheng GH and Kong F: Therapeutic effects of baicalin on
monocrotaline-induced pulmonary arterial hypertension by inhibiting
inflammatory response. Int Immunopharmacol. 26:188–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woelfle U, Sauter G, Santjer S, Brakenhoff
R and Pantel K: Down-regulated expression of cytokeratin 18
promotes progression of human breast cancer. Clin Cancer Res.
10:2670–2674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schaller G, Fuchs I, Pritze W, Ebert A,
Herbst H, Pantel K, Weitzel H and Lengyel E: Elevated keratin 18
protein expression indicates a favorable prognosis in patients with
breast cancer. Clin Cancer Res. 2:1879–1885. 1996.PubMed/NCBI
|
|
21
|
Yoon HN, Yoon SY, Hong JH and Ku NO: A
mutation in keratin 18 that causes caspase-digestion resistance
protects homozygous transgenic mice from hepatic apoptosis and
injury. J Cell Sci. 130:2541–2550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leifeld L, Kothe S, Söhl G, Hesse M,
Sauerbruch T, Magin TM and Spengler U: Keratin 18 provides
resistance to Fas-mediated liver failure in mice. Eur J Clin
Invest. 39:481–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi R, Zhu D, Wei Z, Fu N, Wang C, Liu L,
Zhang H, Liang Y, Xing J, Wang X, et al: Baicalein attenuates
monocrotaline-induced pulmonary arterial hypertension by inhibiting
endothelial-to-mesenchymal transition. Life Sci. 207:442–450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi R, Wei Z, Zhu D, Fu N, Wang C, Yin S,
Liang Y, Xing J, Wang X and Wang Y: Baicalein attenuates
monocrotaline-induced pulmonary arterial hypertension by inhibiting
vascular remodeling in rats. Pulm Pharmacol Ther. 48:124–135. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song R, Song H, Liang Y, Yin D, Zhang H,
Zheng T, Wang J, Lu Z, Song X, Pei T, et al: Reciprocal activation
between ATPase inhibitory factor 1 and NF-κB drives hepatocellular
carcinoma angiogenesis and metastasis. Hepatology. 60:1659–1673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barberà MJ, Puig I, Domínguez D,
Julien-Grille S, Guaita-Esteruelas S, Peiró S, Baulida J, Francí C,
Dedhar S, Larue L, et al: Regulation of Snail transcription during
epithelial to mesenchymal transition of tumor cells. Oncogene.
23:7345–7354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allen JD, Brinkhuis RF, Wijnholds J and
Schinkel AH: The mouse Bcrp1/Mxr/Abcp gene: Amplification and
overexpression in cell lines selected for resistance to topotecan,
mitoxantrone, or doxorubicin. Cancer Res. 59:4237–4241.
1999.PubMed/NCBI
|
|
31
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volk EL, Farley KM, Wu Y, Li F, Robey RW
and Schneider E: Overexpression of wild-type breast cancer
resistance protein mediates methotrexate resistance. Cancer Res.
62:5035–5040. 2002.PubMed/NCBI
|
|
33
|
Ingram WJ, Crowther LM, Little EB, Freeman
R, Harliwong I, Veleva D, Hassall TE, Remke M, Taylor MD and
Hallahan AR: ABC transporter activity linked to radiation
resistance and molecular subtype in pediatric medulloblastoma. Exp
Hematol Oncol. 2:262013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Golebiewska A, Brons NH, Bjerkvig R and
Niclou SP: Critical appraisal of the side population assay in stem
cell and cancer stem cell research. Cell Stem Cell. 8:136–147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benderra Z, Faussat AM, Sayada L, Perrot
JY, Chaoui D, Marie JP and Legrand O: Breast cancer resistance
protein and P-glycoprotein in 149 adult acute myeloid leukemias.
Clin Cancer Res. 10:7896–7902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uggla B, Ståhl E, Wågsäter D, Paul C,
Karlsson MG, Sirsjö A and Tidefelt U: BCRP mRNA expression v.
clinical outcome in 40 adult AML patients. Leuk Res. 29:141–146.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nantajit D, Lin D and Li JJ: The network
of epithelial-mesenchymal transition: Potential new targets for
tumor resistance. J Cancer Res Clin Oncol. 141:1697–1713. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shintani Y, Okimura A, Sato K, Nakagiri T,
Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K, et
al: Epithelial to mesenchymal transition is a determinant of
sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann
Thorac Surg. 92:1794–1804; discussion 1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporters. Cell Death Dis. 2:e1792011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fanelli MA, Montt-Guevara M, Diblasi AM,
Gago FE, Tello O, Cuello-Carrión FD, Callegari E, Bausero MA and
Ciocca DR: P-cadherin and beta-catenin are useful prognostic
markers in breast cancer patients; beta-catenin interacts with heat
shock protein Hsp27. Cell Stress Chaperones. 13:207–220. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taube JH, Herschkowitz JI, Komurov K, Zhou
AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et
al: Core epithelial-to-mesenchymal transition interactome
gene-expression signature is associated with claudin-low and
metaplastic breast cancer subtypes. Proc Natl Acad Sci USA.
107:15449–15454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin L, Castagnino P and Assoian RK: ABCG2
expression and side population abundance regulated by a
transforming growth factor beta-directed epithelial-mesenchymal
transition. Cancer Res. 68:800–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bühler H and Schaller G: Transfection of
keratin 18 gene in human breast cancer cells causes induction of
adhesion proteins and dramatic regression of malignancy in vitro
and in vivo. Mol Cancer Res. 3:365–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takano M, Yamamoto C, Yamaguchi K, Kawami
M and Yumoto R: Analysis of TGF-β1- and drug-induced
epithelial-mesenchymal transition in cultured alveolar epithelial
cell line RLE/Abca3. Drug Metab Pharmacokinet. 30:111–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen WJ, Wang H, Tang Y, Liu CL, Li HL and
Li WT: Multidrug resistance in breast cancer cells during
epithelial-mesenchymal transition is modulated by breast cancer
resistant protein. Chin J Cancer. 29:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kwon CH, Park HJ, Choi JH, Lee JR, Kim HK,
Jo HJ, Kim HS, Oh N, Song GA and Park DY: Snail and serpinA1
promote tumor progression and predict prognosis in colorectal
cancer. Oncotarget. 6:20312–20326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Z, Zhang B, Liu B, Xie Y and Cao X:
Combined Runx2 and Snail overexpression is associated with a poor
prognosis in breast cancer. Tumour Biol. 36:4565–4573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|