Introduction
Pancreatic cancer (PC) has a very poor prognosis and
patients with PC often suffer severe pain. Pain affects ~80% of
patients with PC, and half require strong opioid analgesia
(1). The spread of tumor cells via
the perineural space to the retro-pancreatic region can cause pain,
increase the local recurrence rate, and decrease the likelihood of
curative-resection. Analgesic therapies have adverse effects, and
most importantly, pain relief with analgesic drugs is often
inadequate (2). Both celiac plexus
neurolysis (CPN) and celiac plexus block (CPB) are efficient on
pain relief, however there are a significant number of patients who
do not or only partially respond to these drugs and continue to
suffer refractory pain (3).
Therefore, more efficient therapies are required to treat
PC-induced refractory pain.
Implantation of iodine-125 (125I) seeds
under the guidance of endoscopic ultrasonography (EUS) has been
demonstrated to be a safe alternative therapeutic option for
advanced PC (4,5). In a previous study, it was revealed
that EUS-guided implantation of 125I around the celiac
ganglia is a safe procedure and can induce apoptosis of local
neurons in a porcine model (5). It
was also revealed that EUS-guided direct celiac ganglion
irradiation with 125I seeds reduced the visual analog
scale (VAS) score and analgesic drug consumption in patients with
unresectable PC (4). However, the
mechanisms involved in pain relief are still unclear.
Transient receptor potential vanilloid-1 (TRPV1) is
a key transducer of diverse noxious stimuli in pancreatic sensory
neurons (6). Increased TRPV1
expression and activity play a key role in pancreatic pain
(6–8). PC pain is generally transmitted
through the celiac plexus which harbors sympathetic fibers that
carry nociceptive information from the pancreas and surrounding
organs (9).
Neuroblastoma cells have many sympathetic fibers in
aerobic environment. It has been widely used in neuron related
researches (10). In the present study, using human neuroblastoma
cell lines SK-N-SH and SK-N-BE(2), the impact of 125I
administration on the expression of TRPV1 in these cells was
investigated, and the possible mechanisms of pain relief were
explored.
Materials and methods
Cell culture
SK-N-SH and SK-N-BE(2) human neuroblastoma cell
lines were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Cells were cultured in Dubecco's
modified Eagle's medium (DMEM) with 10% heat-inactivated fetal calf
serum (FCS) and 1% penicillin/streptomycin at 37°C and 5%
CO2 in a fully humidified incubator.
CCK-8 (cell viability kit)
Cell viability was determined by Cell Counting Kit-8
(CCK-8) assay kit (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, cells were seeded into plates at 2×104
cells/well in a 96-well plate overnight and treated with or without
125I.
At different time-points following treatment, cells
were incubated with 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-
5-(2,4-disulfophenyl)-2H-tetrazolium mono-sodium salt (WST-8)
according to the manufacturer's instructions. Absorbance values
were measured at 450 nm by enzyme-linked immunosorbent assay
(ELISA). All experiments were performed in triplicate.
RNA extraction and real-time reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA from cells was extracted using TRIzol
reagent (Takara Bio, Inc., Shiga, Japan). RNA was digested for 15
min with DNase followed by purification with an RNeasy kit (Qiagen
GmbH, Hilden, Germany). For mRNA detection, 1 µg of purified total
RNA was reverse-transcribed with a reverse transcription kit
(Takara Bio, Inc.) according to manufacturer's instructions. The
amount of TRPV1 in a given sample was normalized by the level of
GAPDH in that sample. Each sample was run in triplicate. Primers
used in the present study were as follows: TRPV1-F,
AATGACGCCGCTGGCTCTG, and TRPV1-R, GCCCACTCGGTGAACTTCCTG; GAPDH-F,
AATCCCATCACCATCTTCCAG, and GAPDH-R, ATCAGCAGAGGGGGCAGAGA. For
quantitative miRNA analysis, the Bulge-Loop™ miRNA qPCR
Primer Set (Guangzhou RiboBio Co., Ltd., Guangzhou, China) was used
to determine the expression levels of miR-1246 and miR-1288-5p by
qRT-PCRs with Takara SYBR Premix Ex Taq™. U6 was used as
an internal control for miRNA template normalization. The
thermocycling settings for both mRNA and miRNA were as follows:
42°C for 5 min, 95°C for 3 min, followed by 45 cycles of 95°C for 5
sec and 60°C for 40 sec. The relative expression level for each
miRNA was calculated using the ΔΔCq method (11). Primers were as follows: miR-1246,
AATGGATTTTTGGAGCAGG; miR-1288-5p, GTGGGCGGGGGCAGGTGTGTG; U6,
CTCGCTTCGGCAGCACA; universal microRNA, GCTGTCAACGATACGCTACCTA.
Western blotting
Total protein from cells were extracted using the
ice-cold NP-40 lysis buffer (150 mM NaCl, 1.0% NP-40, 50 mM
Tris-HCl, pH 8.0, protease inhibitors). The concentrations of the
protein were determined by BCA method. A 50 µg (~5–10 µl) of
protein was loaded per lane and was separated by SDS-polyacrylamide
gels (8%) and transferred to polyvinylidene difluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After
blocking (5% milk, indoor temperature for 1 h), immunoblots were
incubated separately overnight at 4°C with antibodies against TRPV1
(dilution 1:1,000; cat. no. ab3487; Abcam, Cambridge, UK) and GAPDH
(dilution 1:3,000; cat. no. ab181602; Abcam) as a control. Blots
were detected with an enhanced chemiluminescence reagent (ECL;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Irradiation with 125I
seeds
Irradiation was performed with 125I
radioactive seeds (BT-125-I; Shanghai Xinke Medical Company Co.,
Shanghai, China). Each seed was 4.5 mm in length and 0.8 mm in
diameter. The seeds had a radioactive half-life of 60.1 days, with
a mean photon energy of 35.5 KeV in γ-rays. The in-house
125-I irradiation model was established based on our
previous study (12), SK-N-SH and
SK-N-BE(2) human neuroblastoma cells were seeded in a 35-mm culture
dish at a density of 5×105/plate for in-house
125-I irradiation. The radiation absorbed dose was
validated with thermoluminescent dosimetry measurement using an
empirical formula from the American Association of Physicists in
Medicine (AAPM). The delivering doses for different exposure
time-points were also assessed and ascertained. The exposure
time-points for delivering doses of 1.15, 2.13, 2.63, 3.12, 4.10,
4.27, 5.09, 5.91, 6.07 and 6.73 Gy were ~24, 48, 60, 72, 96, 100,
120, 140, 144 and 160 h, respectively.
MicroRNA array analysis and miRNA
target gene prediction
To screen miRNA expression after 125I
treatment, miRNA profiles were analyzed using the Affymetrix miRNA
4.0 (Shanghai OE Biotech, Inc., Shanghai, China) according to the
manufacturer's instructions. Briefly, miRNAs were purified from
total RNA extracted from 125I-treated cells or mocked
cells and were then labeled using an enzyme-linked oligosorbent
assay (ELOSA) and hybridized to the miRNA array. The array data
were normalized by global normalization using the miRNA QC tool
software (Affymetrix Expression Console software version 1.4.1
(Thermo Fisher Scientific, Inc.). The levels of miRNAs between the
125I-treated cells and control samples were calculated
based on the fluorescence intensities. Differential expression
levels of miRNAs between the two groups of samples were assessed
using one-way ANOVA analysis. TargetScan software analysis
(http://www.targetscan.org/vert_71/)
was used to miRNA target gene prediction.
miRNA transfection
The miR-1246 and miR-1228-5p mimics, inhibitors, and
their negative controls (NCs) were purchased from RiboBio Co., Ltd.
Cells were transfected with miR-1246 or miR-1228-5p mimics (50 nM),
inhibitors (100 nM), or their negative controls for 48 h using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The miR-1246 and
miR-1228-5p mimics (product no. B02001) and inhibitor (product no.
B03001) were purchased from the Shanghai GenePharma Co., Ltd.
(Shanghai, China).
Construction of the reporter gene
system containing TRPV1 3′-untranslated region (3′UTR) and
luciferase reporter assay
To construct pGL3-REPORT vectors containing
wild-type TRPV1 3′UTR (wild) and corresponding mutant-type
(mutant), the wild sequences of 3′UTR of TRPV1 mRNA containing the
complementary sequences to the miR-1246 (ENST00000399759.3,
site:1327-1333) seed sequence were synthesized, annealed, and
ligase into the XbaI-FseI sites of the pGL3-Control
Vector (GenBank® accession no. U47296; cat. no.
selected: E1741; Promega Corp., Madison, WI, USA), while the
corresponding mutation sequences of 3′UTR cDNA sequences were
produced with a QuikChange XL Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc., North Billerica, MA, USA),
and parallelly ligased into the pGL3-Control Vector as the control.
For the luciferase assays, SK-N-SH and SK-N-BE(2) cells were
co-transfected with wild-type (WT) or mutant (Mut) 3′UTR of
pGL3-REPORT vectors and the mimics or inhibitors of miR-1246, along
with 0.01 µg of the pRL-TK vector (Promega Corp.). Luciferase
assays were performed 48 h later following treatment with the dual
luciferase assay kit (Promega Corp.) according to the
manufacturer's instructions. The luciferase activities were
normalized to the Renilla luciferase activity.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation (SD). An independent Student's t-test or one-way
analysis of variance (ANOVA) followed by Dunnett's post hoc test,
was conducted to evaluate the one-way layout data. P-values
<0.05 were considered to indicate a statistically significant
difference. All statistical analyses were performed using the SPSS
v18.0 statistics software package (SPSS, Inc., Chicago, IL,
USA).
Results
Effect of 125I radiation on
the proliferation of neuroblastoma cells
SK-N-SH and SK-N-BE(2) cells were treated with
125I radioactive seeds at a series of time-points (24,
48, 72, 96, 120 and 144 h); equivalent to 1.15, 2.13, 3.12, 4.10,
5.09 and 6.07 Gy. At each time-point, cell morphology was imaged
and the cell growth was detected by CCK-8. As revealed in Fig. 1, the lower radiation delivering
doses of <48 h (equal to 2.13 Gy) did not significantly affect
cell growth, while the radiation delivering doses of >72 h
(equal to 3.12 Gy) significantly reduced cell viability. The
radiation time-point of 60 h (equal to 2.63 Gy) was therefore
determined as the initial effective dose in the present study.
 | Figure 1.Effect of 125I radiation on
neuroblastoma cell proliferation SK-N-SH and SK-N-BE(2) cells were
treated with 125I radioactive seeds at various
time-points (24, 48, 72, 96, 120 and 144 h; equivalent to
delivering doses 1.15, 2.13, 3.12, 4.10, 5.09 and 6.07 Gy). (A)
Cell morphology images, and (B) cell growth curve detected by
CCK-8. The experiments were dependently repeated three times. Scale
bar, 5 µm. aP<0.01, SH-N-SH-125I vs.
SK-N-SH-control; bP<0.01, SK-N-BE(2)-125I
vs. SK-N-BE(2)-control. 125I, iodine-125; CCK-8, Cell
Counting Kit-8. |
Effect of 125I radiation on
TRPV1 expression
To determine the expression of TRPV1 in SK-N-SH and
SK-N-BE(2) cells, 125I radioactive seeds were
administrated to these cells. As revealed in Fig. 2, the mRNA and protein levels of
TRPV1 expression were detected at a series of time-points (60, 100,
120, 140 and 160 h; equivalent to 2.63, 4.27, 5.09, 5.91 and 6.73
Gy). The mRNA expression of TRPV1 was significantly decreased
compared with the non-radiated-cells at 60, 100, 120 and 140 h (but
not at 160 h); the absolute CT value of the TRPV1 was high (~
>40 CT). The protein levels of TRPV1 were initially extensively
reduced at 60 and 100 h, but then returned to the control level at
120 h, followed by downregulation of TRPV1 expression at 140 and
160 h again; it was surmised that the TRPV1 protein was subjected
to some type of post-translational regulation over the 140-h
irradiation treatment.
These data revealed that the effect of
125I radiation on TRPV1 expression was dependent on the
125I radiation dose, and the downregulated mRNA level
was continuous at all radiation doses, while the downregulated
protein level only occurred at lower doses (2.63 and 4.27 Gy), and
then returned to normal at the intermediate dose (5.09 Gy), but
further revealed a downregulatory effect at higher doses (5.91 and
6.73 Gy).
Effect of 125I radiation on
miRNA profiling
According to the aforementioned results, the
time-point of 60 h/2.63 Gy-125I radiation was selected
for miRNA profiling in SK-N-SH cells. As revealed in Fig. 3A, 32 miRNAs such as miR-1246 and
miR-1228-5p were significantly upregulated and 22 miRNAs
downregulated based on the criterion (FC≥2, P≤0.05). As revealed in
Fig. 3B the miR-1246 and
miR-1228-5p were predicted to target the TRPV1 mRNA 3′UTR by the
TargetScan software. Therefore, both miR-1246 and miR-1228-5p
miRNAs were selected to validate their function on their regulation
of TRPV1 gene expression.
TRPV1 expression is downregulated by
miR-1246 but not miR-1228-5p
To validate the effect of miR-1246 and miR-1228-5p
on TRPV1 expression, both SK-N-SH and SK-N-BE(2) cells were
transfected with mimics or inhibitors of miR-1246 or miR-1228.
Blank groups did not undergo any treatment; mimics NC and inhibitor
NC were used as controls. As revealed in Fig. 4 for SK-N-SH cells and Fig. 5 for SK-N-BE(2) cells, the
transfection of miR-1246 mimic significantly downregulated and
miR-1246 inhibitor upregulated the expression of TRPV1
respectively, while neither the miR-1228-5p mimic nor the inhibitor
had any effect on the expression of TRPV1.
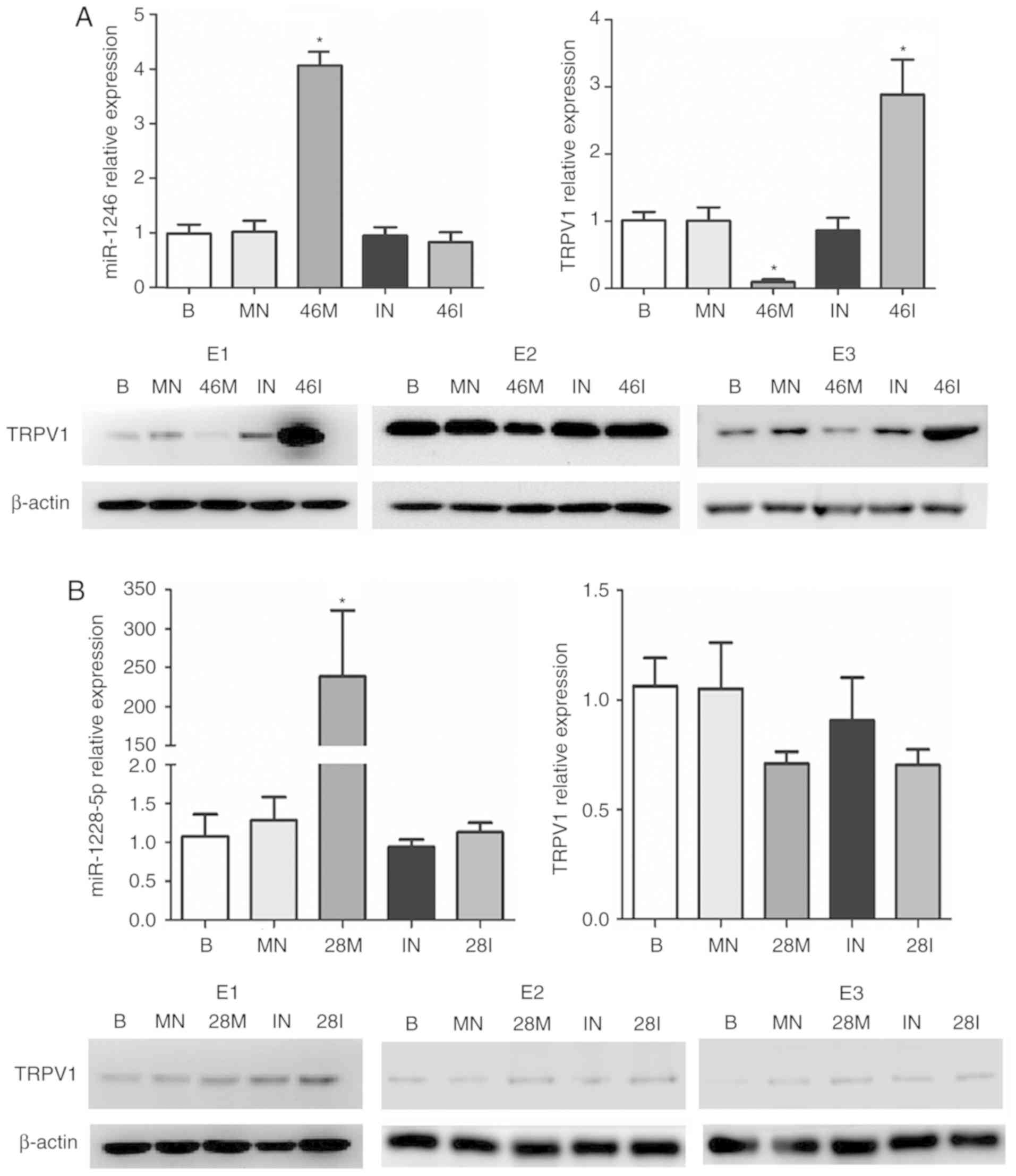 | Figure 4.miR-1246, but not miR-1228-5p,
downregulates the expression of TRPV1. SK-SY-SH cells were
transfected with mimics or inhibitors of (A) miR-1246 or (B)
miR-1228-5p. The transfection efficiencies were detected by
miR-1246 or miR-1228 expression. Both mRNA and protein levels of
TRPV1 were determined. The experiments were dependently repeated
three times (E1, E2 and E3). B, blank; MN, mimics negative; 46M,
miR-1246 mimics; IN, inhibitor negative; 46I, miR-1246 inhibitor;
28M, miR-1228-5p mimics; 28I, miR-1228-5p inhibitor; *P<0.01,
compared with the corresponding negative group. TRPV1, transient
receptor potential vanilloid-1. |
 | Figure 5.miR-1246, but not miR-1228-5p,
downregulates the expression of TRPV1. SK-N-BE(2) cells were
transfected with mimics or inhibitors of (A) miR-1246 or (B)
miR-1228-5p. The transfection efficiencies were detected by
miR-1246 or miR-1228 expression. Both mRNA and protein levels of
TRPV1 were determined. The experiments were dependently repeated
three times (E1, E2 and E3). B, blank; MN, mimics negative; 46M,
miR-1246 mimics; IN, inhibitor negative; 46I, miR-1246 inhibitor;
28M, miR-1228-5p mimics; 28I, miR-1228-5p inhibitor; *P<0.01,
compared with the corresponding negative group. TRPV1, transient
receptor potential vanilloid-1. |
miR-1246 regulates TRPV1 expression by
targeting TRPV1 3′UTR
To explore the mechanism of miR-1246 on the
regulation of TRPV1 expression, pGL3-REPORT vectors containing
wild-type TRPV1 3′UTR (wild) and corresponding mutant-type (mutant)
were constructed, and then transfected into both SK-N-SH and
SK-N-BE(2) cells. As revealed in Fig.
6A for SK-N-SH cells and Fig.
6B for SK-N-BE(2) cells, miR-1246 mimics significantly
downregulated and miR-1246 inhibitor upregulated luciferase
activity, respectively.
Discussion
TRPV1 is a non-selective cation channel activated by
capsaicin (13). It is expressed in
human dorsal root ganglia (DRGs), brain, kidney, pancreas, and many
other crucial organs (14). TRPV1
expression is also widely distributed in visceral innervation of
all organs, and the upregulated expression of TRPV1 is closely
correlated with the degree of visceral pain (6,15). The
importance of TRPV1 in visceral innervation is also supported by
the pain-inducing effects of capsaicin application in several
animal models and human studies (16).
Researchers have demonstrated that pancreatic pain
has a complicated relationship with TRPV1 (6,7,17), and
thus the present study focused on the mechanism of pain in PC.
miRNAs are universally involved in the development of tumors,
including PC. Previous studies have revealed that miR-1246 aberrant
expression is widely involved in many types of cancers (18–24).
In PC, the plasma exosome miR-1246 was revealed to be significantly
elevated in patients with intraductal papillary mucinous neoplasms
(IPMN) (25); increased expression
of miR-1246 was detected in pancreatic stellate cells (26), and aberrantly expressed in
serum-exosomes (27).
In the present study, it was revealed that
125I treatment could enhance miR-1246 expression, thus
downregulating the expression of TRPV1, which plays a key role in
pancreatic pain. Concurrently, it was also demonstrated that
miR-1246 regulated TRPV1 expression by binding to its 3′UTR. Thus,
by targeting miR-1246, an effective treatment for pain in PC
patients may have potentially been revealed. In contrast, it was
surmised that the downregulated expression of miR-1246 in neurons
around pancreatic tissues may be involved in the mechanism causing
sustained pain in PC patients.
In the present study, a different effect of
125I radiation on TRPV1 expression was observed between
the mRNA and protein. The downregulated mRNA level was continuous
at all radiation doses, while the downregulated protein level
occurred at lower and then higher doses. It was thus proposed that
the undulation of TRPV1 expression was dependent on the radiation
dose; in particular, the return to a normal level at an
intermediate dose was due to radiation hormesis (28), and was subjected to some type of
post-translational regulation over the 140-h irradiation
treatment.
It should be noted that there were several
limitations in the present study. Although the human neuroblastoma
cell lines SK-N-SH and SK-N-BE(2) are closely correlated to the
sympathetic nervous system, they are not the same as nerves in PC
tissues. In addition, further tests should validate that the TRPV1
abundance regulated by miR-1246 may be sufficient to induce the
change of the cation channel activity and the pain in cells and an
animal model. With regard to the translational outcomes of these
results to clinical experiments in the future, although some
studies revealed that miR-1246 promoted angiogenesis in colorectal
cancer (29), enhanced cell
migration and invasion in hepatocellular carcinoma (22), and promoted tumor progression in
cervical (30) and lung cancer
(31), the effect of miR-1246 on
pancreatic ductal adenocarcinoma has not been intensively studied.
Administration of miR-1246 should weigh the advantages of pain
release and disadvantages of cancer progression.
In conclusion, 125I radiation upregulated
miR-1246, which downregulated the expression of TRPV1, a key
molecule involved in PC pain. The knowledge of this novel mechanism
promises a new strategy for pain release in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81372482 to KW;
nos. 81472279 and 81272663 to JG), the Natural Science Foundation
of Shanghai (no. 13ZR1409300 to KW), and three engineering training
funds in Shenzhen (no. SYJY201714 to DZ).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DZ, HX and YW collaboratively performed almost all
the experiments and the acquisition, analysis, or interpretation of
the data, and they were equally contributed to this study. YW and
JZ participated in the experiments of the detection of the cell
viability and the expression of TRPV1 and miRNAs. LP and BW
participated in the experiments of the vector constructions and
luciferase reporter assay. Both of KW and JG designed the study,
and KW drafted the work and integrated any part of the work
appropriately, and JG revised the paper critically for important
intellectual content. ZL contributed to the design of the study and
was in charge of the agreement to be accountable for all aspects of
the work in ensuring that questions related to the accuracy and
gave the final approval of the version to be published. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caraceni A and Portenoy RK: Pain
management in patients with pancreatic carcinoma. Cancer. 78 (Suppl
3):S639–S653. 1996. View Article : Google Scholar
|
|
2
|
de Oliveira R, dos Reis MP and Prado WA:
The effects of early or late neurolytic sympathetic plexus block on
the management of abdominal or pelvic cancer pain. Pain.
110:400–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mekaroonkamol P, Willingham FF and Chawla
S: Endoscopic management of pain in pancreatic cancer. JOP.
16:33–40. 2015.PubMed/NCBI
|
|
4
|
Wang KX, Jin ZD, Du YQ, Zhan XB, Zou DW,
Liu Y, Wang D, Chen J, Xu C and Li ZS: EUS-guided celiac ganglion
irradiation with iodine-125 seeds for pain control in pancreatic
carcinoma: A prospective pilot study. Gastrointest Endosc.
76:945–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang K, Jin Z, Du Y, Chen J, Zhan X, Wang
L, Li Z, Zou D and Liu Y: Evaluation of
endoscopic-ultrasound-guided celiac ganglion irradiation with
iodine-125 seeds: A pilot study in a porcine model. Endoscopy.
41:346–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li
C, Mehta K and Pasricha PJ: Nerve growth factor modulates TRPV1
expression and function and mediates pain in chronic pancreatitis.
Gastroenterology. 141:370–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwartz ES, Christianson JA, Chen X, La
JH, Davis BM, Albers KM and Gebhart GF: Synergistic role of TRPV1
and TRPA1 in pancreatic pain and inflammation. Gastroenterology.
140:1283–1291.e1-e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceyhan GO, Michalski CW, Demir IE, Muller
MW and Friess H: Pancreatic pain. Best Prac Res Clin Gastroenterol.
22:31–44. 2008. View Article : Google Scholar
|
|
9
|
Uomo I: Pain in pancreatic cancer: Does
drug treatment still play a role? JOP. 12:435–437. 2011.PubMed/NCBI
|
|
10
|
Rosenthal LM, Tong G, Walker C, Wowro SJ,
Krech J, Pfitzer C, Justus G, Berger F and Schmitt KRL:
Neuroprotection via RNA-binding protein RBM3 expression is
regulated by hypothermia but not by hypoxia in human SK-N-SH
neurons. Hypoxia (Auckl). 5:33–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma JX, Jin ZD, Si PR, Liu Y, Lu Z, Wu HY,
Pan X, Wang LW, Gong YF, Gao J and Zhao-shen L: Continuous and
low-energy 125I seed irradiation changes DNA
methyltransferases expression patterns and inhibits pancreatic
cancer tumor growth. J Exp Clin Cancer Res. 30:352011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: A
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayes P, Meadows HJ, Gunthorpe MJ, Harries
MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K,
Prinjha RK, et al: Cloning and functional expression of a human
orthologue of rat vanilloid receptor-1. Pain. 88:205–215. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Q, Yang L, Larson S, Basra S, Merwat
S, Tan A, Croce C and Verne GN: Decreased miR-199 augments visceral
pain in patients with IBS through translational upregulation of
TRPV1. Gut. 65:797–805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frias B and Merighi A: Capsaicin,
nociception and pain. Molecules. 21:E7972016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terada Y, Tsubota M, Sugo H, Wakitani K,
Sekiguchi F, Wada K, Takada M, Oita A and Kawabata A: Tacrolimus
triggers transient receptor potential vanilloid-1-dependent relapse
of pancreatitis-related pain in mice. Pharmacology. 99:281–285.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao L, Wang J, Ouyang S, Zhang P, Wang J
and Zhang M: Expression and clinical significance of microRNA-1246
in human oral squamous cell carcinoma. Med Sci Monit. 21:776–781.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Xie YJ, Xu Q, Chen JX, Shan NC and
Zhang Y: Down-regulation of miR-1246 in cervical cancer tissues and
its clinical significance. Gynecol Oncol. 138:683–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao JM, Zhou X, Zhang Y and Lu H:
miR-1246: A new link of the p53 family with cancer and Down
syndrome. Cell Cycle. 11:2624–2630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hasegawa S, Eguchi H, Nagano H, Konno M,
Tomimaru Y, Wada H, Hama N, Kawamoto K, Kobayashi S, Nishida N, et
al: MicroRNA-1246 expression associated with CCNG2-mediated
chemoresistance and stemness in pancreatic cancer. Br J Cancer.
111:1572–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Meng C, Wang S, Zhou N, Guan M, Bai
C, Lu S, Han Q and Zhao RC: MicroRNA-1246 enhances migration and
invasion through CADM1 in hepatocellular carcinoma. BMC Cancer.
14:6162014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Machida T, Tomofuji T, Maruyama T, Yoneda
T, Ekuni D, Azuma T, Miyai H, Mizuno H, Kato H, Tsutsumi K, et al:
miR1246 and miR4644 in salivary exosome as potential biomarkers for
pancreatobiliary tract cancer. Oncol Rep. 36:2375–2381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Cao L, Zhang Y, Lian H, Sun Z and
Cui Y: MicroRNA-1246 inhibits cell invasion and epithelial
mesenchymal transition process by targeting CXCR4 in lung cancer
cells. Cancer Biomark. 21:251–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu YF, Hannafon BN, Zhao YD, Postier RG
and Ding WQ: Plasma exosome miR-196a and miR-1246 are potential
indicators of localized pancreatic cancer. Oncotarget.
8:77028–77040. 2017.PubMed/NCBI
|
|
26
|
Masamune A, Yoshida N, Hamada S, Takikawa
T, Nabeshima T and Shimosegawa T: Exosomes derived from pancreatic
cancer cells induce activation and profibrogenic activities in
pancreatic stellate cells. Biochem Biophys Res Commun. 495:71–77.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madhavan B, Yue S, Galli U, Rana S, Gross
W, Müller M, Giese NA, Kalthoff H, Becker T, Büchler MW and Zöller
M: Combined evaluation of a panel of protein and miRNA
serum-exosome biomarkers for pancreatic cancer diagnosis increases
sensitivity and specificity. Int J Cancer. 136:2616–2627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baldwin J and Grantham V: Radiation
hormesis: Historical and current perspectives. J Nucl Med Technol.
43:242–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada N, Tsujimura N, Kumazaki M,
Shinohara H, Taniguchi K, Nakagawa Y, Naoe T and Akao Y: Colorectal
cancer cell-derived microvesicles containing microRNA-1246 promote
angiogenesis by activating Smad 1/5/8 signaling elicited by PML
down-regulation in endothelial cells. Biochim Biophys Acta.
1839:1256–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Yao D, Zhao S, He C, Ding N, Li L
and Long F: miR-1246 promotes SiHa cervical cancer cell
proliferation, invasion, and migration through suppression of its
target gene thrombospondin 2. Arch Gynecol Obstet. 290:725–732.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang WC, Chin TM, Yang H, Nga ME, Lunny
DP, Lim EK, Sun LL, Pang YH, Leow YN, Malusay SR, et al:
Tumour-initiating cell-specific miR-1246 and miR-1290 expression
converge to promote non-small cell lung cancer progression. Nat
Commun. 7:117022016. View Article : Google Scholar : PubMed/NCBI
|




















