Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignant tumor of the head and neck (1). Elucidation of the mechanisms related to
OSCC development and progression could provide candidate diagnostic
biomarkers and promising therapeutic targets for OSCC. Previous
studies have mainly focused on the tumor cell itself, as several
gene alterations have been demonstrated to be associated with OSCC
development and progression, such as inactivation of p53 (2), Notch mutation (3) and loss of NDRG2 expression (4). Actually, not tumor cells but the tumor
microenvironment is associated with tumor metastasis (5,6). However,
the mechanism of the tumor microenvironment in promoting OSCC
progression still remains not fully understood.
Cancer-associated fibroblasts (CAFs) are the main
stromal cells in the microenvironment and are distinguishable from
other cells by their spindle-shape morphology and expression of
α-smooth muscle actin (α-SMA) (7). As
abnormally reactive fibroblasts, CAFs play essential roles in
several processes of cancer biology including tumor growth, tumor
metastasis and drug resistance (8).
Furthermore, CAFs are often considered to be associated with
high-grade malignancies and poor prognosis (9,10), and
therefore are suggested to be a prognostic biomarker. CAFs promote
tumor progression mainly through actively communicating with cancer
cells (11–13), and other cells in the tumor
environment, including immunocytes (14) and vascular endothelial cells (15). The communications between CAFs and
other cells are often mediated by several growth factors, hormones
and cytokines (16,17). Actually, CAFs also modify the tumor
environment by transferring molecules to other cells by secreting
exosomes (18).
Exosomes are nano-sized vesicles (~30–150 nm)
secreted by cells (19,20), and serve as mediators of cell-to-cell
communication. They play important roles in many cell processes,
including tumor growth, invasion, metastasis and chemoresistance
(21–23). Exosomes deliver a great variety of
bioactive molecules including signal peptides, microRNAs, lipids
and DNA, from cell to cell (24).
MicroRNAs, a major class of small non-coding RNAs that mediate
post-transcriptional gene silencing of target mRNAs and participate
in various physiological and pathological processes (25), are highly enriched in exosomes and are
delivered from one cell to neighboring or distant cells (26). Compared with normal fibroblasts, one
characteristic of abnormally inactive CAFs is the dysregulation of
miRNAs, which leads to the re-modification of the tumor
microenvironment and correspondingly induces drug resistance, cell
migration and invasion, and tumor growth (18). Therefore, using miRNAs or miRNA
inhibitors to restitute the abnormal expression of miRNAs in CAFs
may be ‘a way out’ for tumor therapy.
miR-382-5p is a primary miRNA species of miR-382 and
functions as an onco-miRNA in several tumors, including breast
cancer (27), liver cancer (28), acute promyelocytic leukemia (29) and glioma (30). By regulating target gene expression,
miR-382-5p plays important roles in tumor progression. miR-382-5p
was found to target circ-DICER1 and correspondingly regulate
angiogenesis in glioma (30). In
liver cancer, miR-382-5p was reported to promote tumor metastasis
by efficiently suppressing DLC-1 expression (28). Bhome et al detected the
distinguishable miRNAs in CAFs and normal fibroblasts (NFs), and
the expression of several miRNAs, including miR-382-5p, was
significantly higher in CAFs than in NFs (31). In OSCC, it has been reported that a
newly identified circRNA, hsa-circ-0008309, could sponge miR-382-5p
and regulate ATXN1 expression (32);
however, the function of miR-382-5p in OSCC migration and invasion
remains unknown.
In the present study, the role of CAFs in mediating
OSCC cell migration and invasion was investigated, and the
participation of exosomal miR-382-5p in this process was
elucidated.
Materials and methods
Patients and tissue samples
Forty-seven OSCC patients, 27 male and 20 females,
ranged from 39 to 72 years old, who underwent tumor resection at
the Department of Oral Maxillofacial Surgery of Liaocheng People's
Hospital from 1 January 2014 to 31 December 2017 were enrolled in
this study. Patient, clinical and pathologic characteristics were
retrieved from the Medical Records Room. All patients signed
informed consent prior to participating in this study, and this
study was approved by the Ethics Committee of Shandong University
(Shandong, China).
Antibodies and reagents
Anti-α-SMA (cat. no. 19245), anti-MMP-3 (cat. no.
14351), anti-MMP-9 (cat. no. 13667), anti-β-catenin (cat. no. 8480)
and anti-N-cadherin (cat. no. 13116) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA), and anti-β-actin
(SC-70319) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). GW4869 was purchased from Selleck Chemicals
(Houston, TX, USA).
Cell culture
Tongue squamous cell carcinoma CAL-27 cells were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and incubated in Gibco DMEM (Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS) (Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator.
Isolation of fibroblasts
Fibroblasts were isolated according to a previous
study (33). Briefly, fibroblasts
were isolated from freshly resected OSCC tissue and adjacent normal
tissue from the OSCC patients treated at the Department of
Stomatology, Liaocheng People's Hospital. The adjacent normal
tissues were excised at least >3 cm distant from the margin of
the tumor, and were verified by two senior pathologists. Tissues
were minced into small pieces of about 1 mm3 and seeded
onto 10-cm dishes in DMEM with 10% FBS at 37°C in a 5%
CO2 incubator. Approximately 10 days later, homogeneous
groups of fibroblasts formatted around the pieces in the dishes.
The fibroblasts were passaged for more than 10 times and were then
used for subsequent experiments.
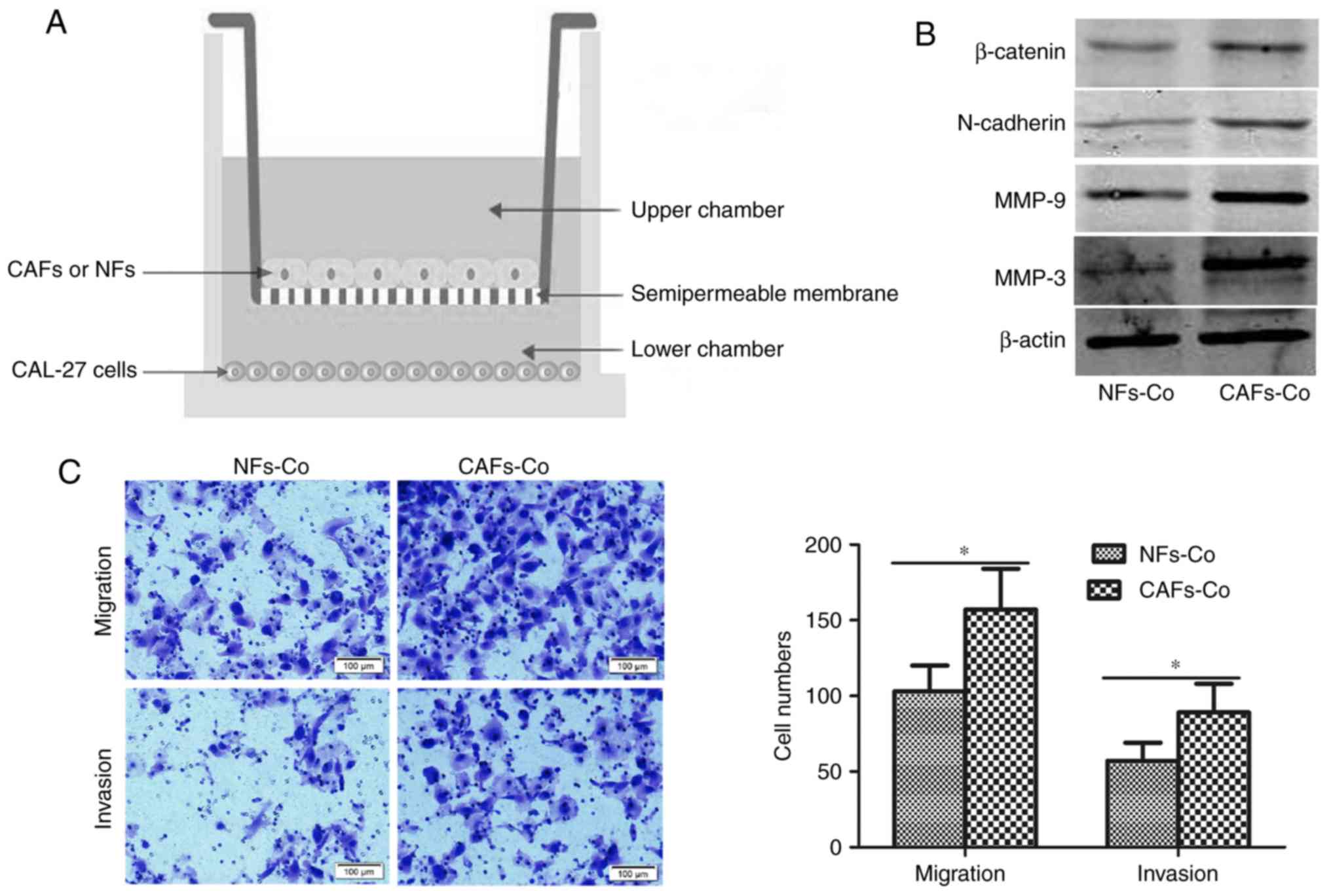
Co-culture of the OSCC cells with CAFs
or NFs
To elucidate the biological function of CAFs in OSCC
metastasis, we employed a co-culture system (Corning Inc., Corning,
NY, USA) (Fig. 3A). Briefly, CAFs or
NFs were seeded in the upper chamber of the system, and the OSCC
cells in the lower chamber. The upper chamber and lower chamber
were separated with a semipermeable membrane with pores ~0.4-µm,
which allowed the passage of exosomes and cytokines but prevented
the shuttle of cells.
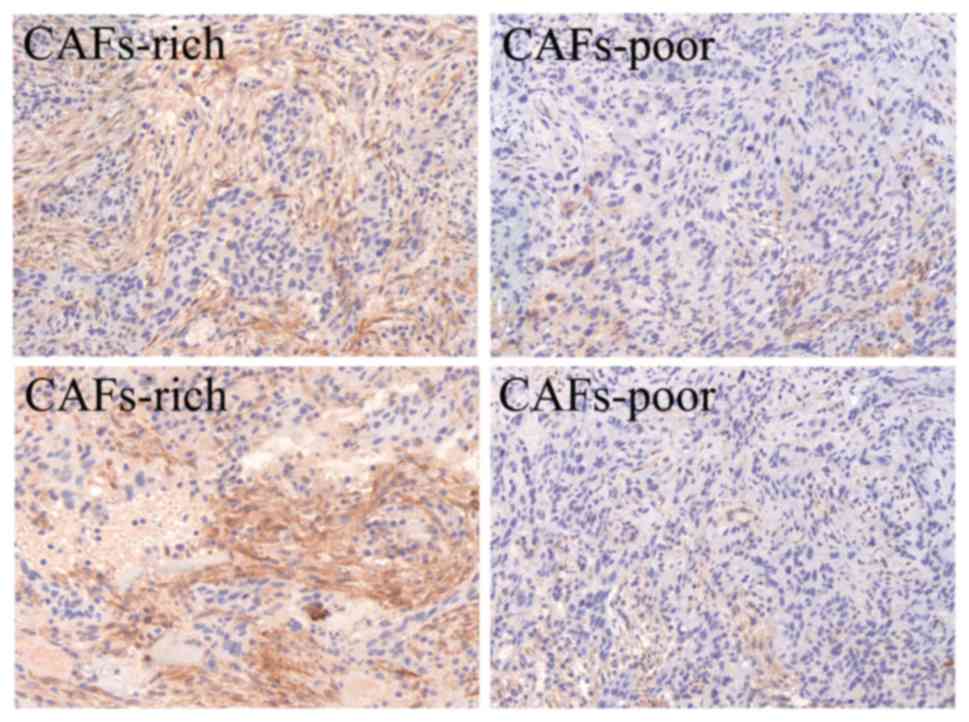
Immunohistochemistry (IHC)
OSCC tissue samples were first formalin-fixed and
paraffin-embedded, and then dewaxed in xylene, rehydrated with
gradient ethanol and treated in 0.01 mM citrate buffer (pH 6.0) for
antigen retrieval. Then samples were stained with rabbit anti-α-SMA
monoclonal antibody at 4°C overnight, followed by incubation in
secondary biotinylated anti-rabbit antibody for 30 min at 37°C, and
finally visualized with DAB solution and counterstained with
haematoxylin. Based on the density of the CAF staining, the 47 OSCC
patients were divided into two groups according to the standard
reported in a previous study (34).
Dense overlapping of CAFs distributed throughout the tumor were
grouped as CAFs-rich, correspondingly, CAFs not distributed
throughout the entire tumor were grouped as CAFs-poor (Fig. 1). Each stained sample was evaluated by
two senior pathologists unaware of the clinical information, with
conflicting cases adjudicated by a third pathologist.
Immunocytochemistry
Fibroblasts were fixed at room temperature for 10
min in 4% paraformaldehyde, and then cells were permeabilized in
0.5% Triton X-100 followed by a 15-min block in 5% normal goat
serum (ZSGB-BIO, Beijing, China). The cells were then incubated
with α-SMA antibody overnight at 4°C. Then the cells were washed
three times in PBS and further incubated with s secondary
biotinylated anti-rabbit antibody for 30 min at room temperature.
Cells were observed under a light microscope at ×400 magnification
(Nikon, Tokyo, Japan).
Exosome extraction
Exosomes were isolated from conditioned media
collected from CAFs using a Hieff™ Quick Exosome Isolation Kit (for
Cell Culture Media) (Yeasen, Shanghai, China) according to the
manufacturer's instructions. Briefly, the conditioned medium was
firstly cleared of cellular debris and the dead cells with two
sequential centrifugation steps at 2,500 × g for 10 min at 4°C.
Then 10 ml conditioned medium was collected into a centrifuge tube
and 2.5 ml extraction reagent was added, followed by mixing and a 2
h of standing. After that, the conditioned medium was centrifuged
at 10,000 × g at 4°C for 1 h. The precipitates were collected and
re-suspended in 100 µl PBS buffer. After re-suspension, the
solution was transferred into an EP tube and centrifuged at 12,000
× g at 4°C for 2 min. Finally, the supernatant containing the
exosomes was collected.
Western blot analysis
Cells were lysed with RIPA lysis buffer (Applygen,
Beijing, China). Protein concentrations were detected using the BCA
Protein Quantitation kit (Thermo Fisher Scientific, Inc.). Thirty
microliters of proteins were subjected to 10% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and
transferred onto a nitrocellulose membrane (EMD Millipore,
Billerica MA, USA). The membrane was blocked with 5% goat serum in
TBS-T for 1 h, and then was incubated with primary antibodies
diluted 1:1,000 in TBS-T containing 1% goat serum overnight at 4°C.
Subsequently, the membrane was washed three times in TBS-T and then
incubated with a HRP-conjugated secondary antibody for 1 h at room
temperature. Finally, the membrane was visualized with Enhanced
Chemiluminescence Plus reagents (Thermo Fisher Scientific,
Inc.).
Transfection
miR-382-5p mimics, negative control (miR-NC),
miR-382-5p inhibitor and negative control for miR-382-5p inhibitor
were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). The sequences are listed as follows: miR-382-5p mimics:
Forward, 5′-GAAGUUGUUCGUGGUGGAUUGG-3′ and reverse,
5′-AAUCCACCACGAACAACUUCUU-3′; miR-NC: Forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
miR-382-5p inhibitor, 5′-CGAAUCCACCACGAACAACUUC-3′; negative
control for inhibitor, 5′-CAGUACUUUUGUGUAGUACAA-3′. Transfection
was performed using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
Briefly, cells were seeded in a 6-well plate at 1×106
cells/well, and when the cells proliferated to a 70–90% confluence,
the cells were transfected with oligonucleotides at a final
concentration of 100 nM.
RNA extraction and quantitative
real-time PCR
RNA was extracted from the cells or exosomes using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). For
miR-382-5p detection, small RNA was transcribed into cDNA using a
RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.) with specific RT primers. Quantitative real-time
PCR (qPCR) was performed using a FastStart Universal SYBR Green
Master (Rox) kit (Roch, Basel, Switzerland) on an Applied
Biosystems ABI 7500 real-time PCR operating system (Thermo Fisher
Scientific, Inc.). The primers for qPCR were as follows: miR-382-5p
forward, 5′-ATCCGTGAAGTTGTTCGTGG-3′ and reverse,
5′-TATGGTTGTAGAGGACTCCTTGAC-3′; U6 forward,
5′-CGCTTCACGAATTTGCGT-3′ and reverse, 5′-CTCGCTTCGGCAGCACA-3′. All
primers were synthesized at Sangon Biotech (Shanghai, China).
Relative expression levels of microRNAs were calculated using the
2−ΔΔCq method (35).
Transwell migration and invasion
assays
Cell migration and invasion assays were performed in
Transwell chambers (Corning Inc., Corning, NY, USA) with a
polycarbonate membrane as described previously. For the Transwell
cell migration assay, briefly, 1×105 cells were seeded
in serum-free DMEM in the upper chamber. The lower chamber
contained the culture medium with 10% FBS. After incubation for 10
h, cells on the top surface of the membrane were wiped off. The
membrane was then stained with crystal violet at 25°C for 1 min.
Cells on the bottom surface of the membrane were examined under a
light microscope (Nikon) at ×200 magnification. Cells from 5 random
fields were counted and averaged. The same procedure was performed
for the Transwell invasion assay, except that the upper chambers
were coated with 20 µg extracellular matrix gel (Sigma-Aldrich;
Merck KGaA).
Flow cytometry
The percentage of α-SMA-positive cells was assessed
by flow cytometry. NF and CAF cell suspensions were harvested and
washed in PBS for 5 min × thrice. The cells were incubated with
anti-human α-SMA/FITC mAb (CST) in the dark for 1 h in FACS buffer
(PBS containing 1% fetal calf serum). Then the cells were subjected
to a BD flow cytometry (BD Biosciences) instrument to evaluate the
percentage of α-SMA-positive cells.
Identification of candidate targets of
miR-382-5p in Targetscan
Targetscan is a software program for searching for
predicted microRNA targets (36).
After entering the website of Targetscan (http://www.targetscan.org/vert_71/), ‘miR-382-5p’ was
input into the ‘Enter a microRNA name’ box. Then, by searching for
the presence of conserved 8mer, 7mer, and 6mer sites that match the
seed region of miR-382-5p, the predicted biological targets of
miR-382-5p were listed.
Statistical analysis
Statistical analysis was performed using SPSS 11.5
for Windows (IBM Corp., Armonk, NY, USA). Experiments were repeated
three times, and all data are presented as mean ± standard
deviation (SD). Differences between groups were analyzed by one-way
ANOVA. Tukey's post hoc test was used following ANOVA. Pearson
χ2 test was used to analyze the relationship between CAF
density and clinicopathological characteristics. P<0.05 was
considered as indicative of a statistically significant
difference.
Results
CAFs are associated with metastasis
and the clinical stage of OSCC
Forty-seven OSCC patients were included in this
study. CAFs were stained with α-SMA. According to the CAF density,
tissues of OSCC patients were divided into two groups, CAFs-rich
and CAFs-poor group (Fig. 1). We
analyzed the relevance of CAF density and the clinicopathological
characteristics. As documented in Table
I, lymph node metastasis, as well as TNM stage between the
CAFs-rich and CAFs-poor groups exhibited significant
differences.
 | Table I.Association of CAF density and
clinicopathologic features of the OSCC patients. |
Table I.
Association of CAF density and
clinicopathologic features of the OSCC patients.
|
Characteristics | Total (n=47) | CAFs-rich
(n=19) | CAFs-poor
(n=28) | P-value |
|---|
| Sex |
|
|
| 0.959 |
|
Male | 27 | 11 | 16 |
|
|
Female | 20 | 8 | 12 |
|
| Age (years) |
|
|
| 0.759 |
|
<60 | 17 | 6 | 11 |
|
|
≥60 | 30 | 13 | 17 |
|
| Lymph node
metastasis |
|
|
| 0.036a |
|
Yes | 21 | 12 | 9 |
|
| No | 26 | 7 | 19 |
|
| TNM stage |
|
|
| 0.029a |
|
I/II | 17 | 3 | 14 |
|
|
III/IV | 30 | 16 | 14 |
|
| Differentiation
grade |
|
|
| 0.240 |
|
Well | 12 | 5 | 7 |
|
|
Moderate | 21 | 6 | 15 |
|
|
Poor | 14 | 8 | 6 |
|
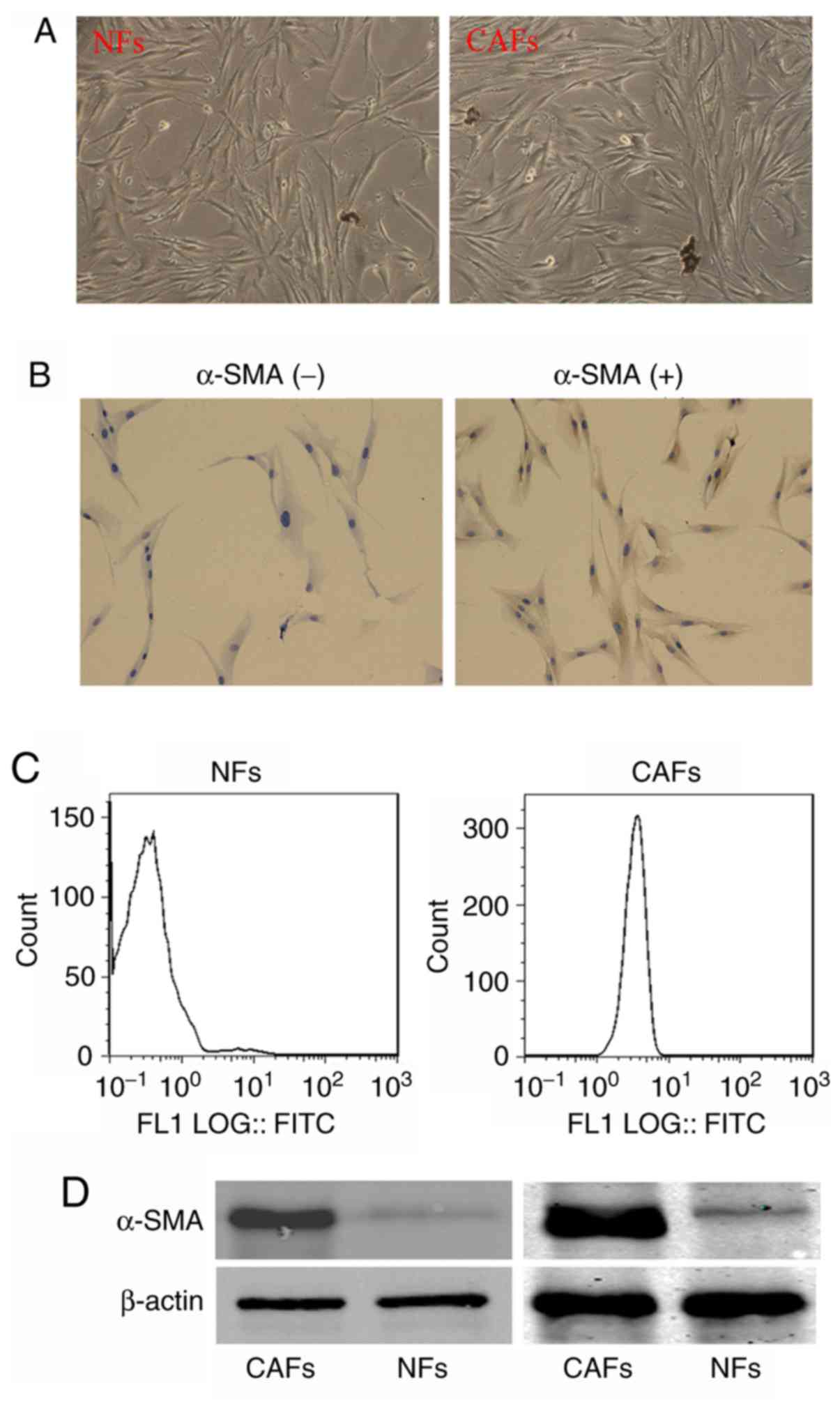
Isolation of CAFs
To detect the functions and underlying mechanism of
CAFs in OSCC metastasis, we isolated the fibroblasts in OSCC
tissues and corresponding para-tumor normal tissues. The
fibroblasts in OSCC and adjacent normal tissues presented no
difference in morphology (Fig. 2A).
However, when the cells were labeled with α-SMA antibodies; it was
found that the fibroblasts in OSCC were α-SMA-positive, while the
fibroblasts in the corresponding adjacent normal tissues were
α-SMA-negative (Fig. 2B). The
percentage of α-SMA-positive cells in isolated fibroblasts was also
analyzed. As shown in Fig. 2C, the
percentage of α-SMA-positive cells in fibroblasts isolated from
adjacent normal tissues was ~1.91%, however, that in the
fibroblasts isolated from OSCC tissues was ~98.92%. Furthermore,
western blot analysis showed that α-SMA was overexpressed in the
fibroblasts of OSCC compared with the trace expression of α-SMA in
fibroblast of the corresponding adjacent normal tissue (Fig. 2D). These results indicate that the
fibroblasts we separated from OSCC are CAFs.
CAFs promote OSCC cell migration and
invasion
The present study was designed to reveal the
mechanism involved in the CAF-induced OSCC migration and invasion.
Therefore, we choose the NFs and CAFs isolated from the patients
with node metastasis and with CAFs-rich. NFs and CAFs from these
patients were used in the following experiments. We co-cultured the
CAL-27 cells with CAFs or NFs for 24 h (Fig. 3A), and then detected several
metastasis-related proteins, including MMP-3, MMP-9, N-cadherin and
β-catenin. As shown in Fig. 3B,
compared with CAL-27 cells co-cultured with NFs, CAL-27 cells
co-cultured with CAFs showed increased expression of MMP-3, MMP-9,
N-cadherin and β-catenin, indicating that CAFs elevated the
migration and invasion capacity of the CAL-27 cells. To manifest
this, we performed Transwell assays. CAL-27 cells were co-cultured
with CAFs or NFs for 24 h, and then CAL-27 cells were subjected to
Transwell migration and invasion assays. As shown in Fig. 3C, a higher number of migrated and
invaded cells were observed in the CAL-27 cells co-cultured with
CAFs than those cells co-cultured with NFs.
CAF-derived exosomes promote OSCC cell
migration and invasion
As it has been reported that exosomes play essential
roles in the cell-cell interaction in the tumor microenvironment,
we aimed to ascertain whether this is the mechanism involved in the
CAF-induced OSCC cell migration and invasion. We extracted exosomes
in NF or CAF cultured medium, and then added the exosomes into the
CAL-27 cell medium. Compared with the NF-derived exosomes, the
CAF-derived exosomes exerted stronger effects on upregulating
MMP-3, MMP-9, N-cadherin and β-catenin in CAL-27 cells (Fig. 4A), and on the migration and invasion
abilities of the CAL-27 cells (Fig.
4B).
miR-382-5p can be transferred from
CAFs to OSCC cells through exosomes, inducing cell migration and
invasion
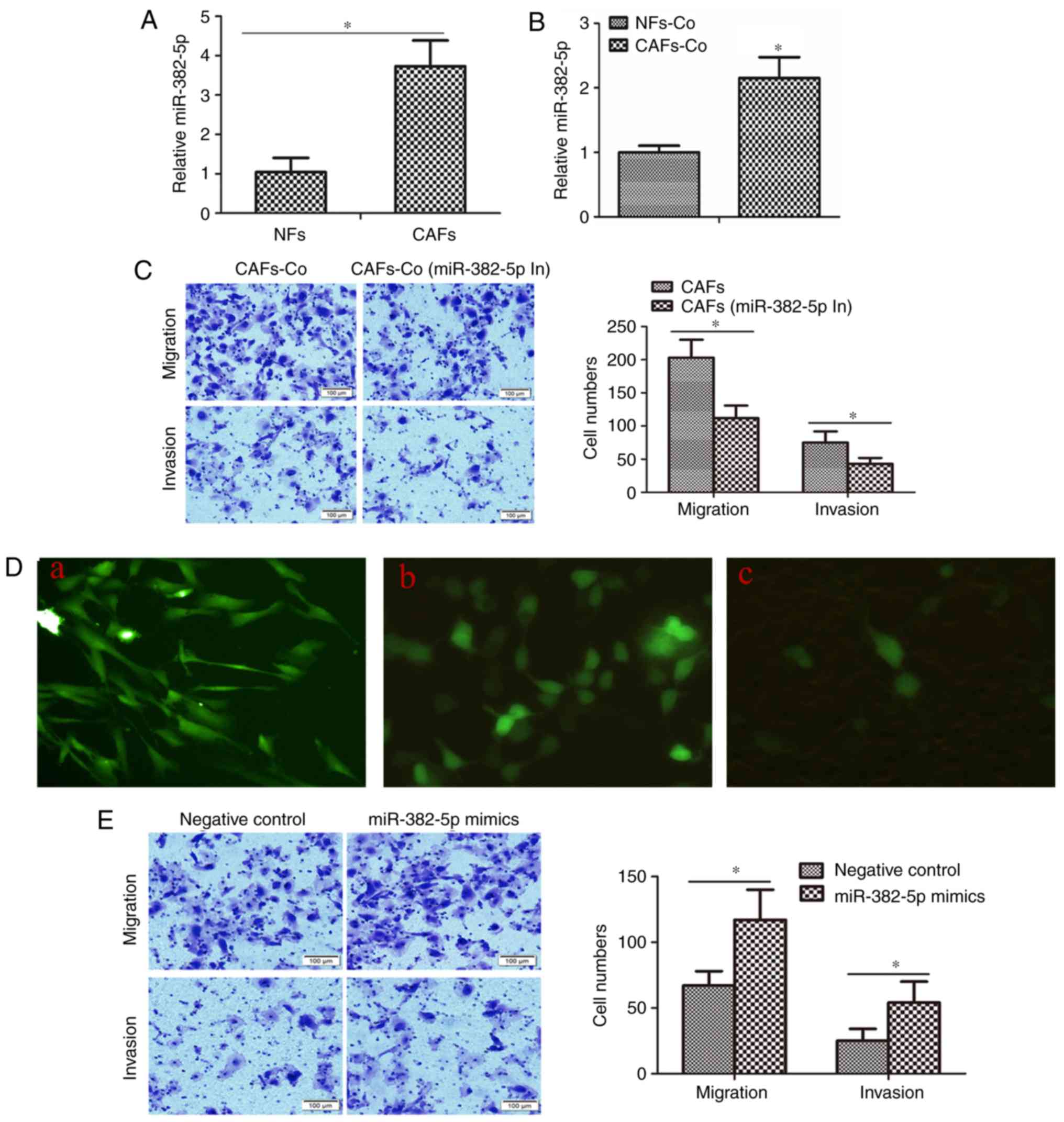
We further aimed to ascertain whether miR-382-5p
participates in the CAF-induced CAL-27 cell migration and invasion.
We compared the miR-382-5p expression in CAFs and NFs. As shown in
Fig. 5A, the expression of miR-382-5p
in CAFs was significantly elevated ~3.83-fold compared with that in
NFs. We also co-cultured CAFs or NFs with CAL-27 cells, and
measured the miR-382-5p in CAL-27 cells. As shown in Fig. 5B, miR-382-5p in CAL-27 cells
co-cultured with CAFs was ~2.15-fold increased compared with that
in CAL-27 cells co-cultured with NFs, indicating that even if the
increasing fold was not in accordance, the increasing miR-382-5p in
CAL-27 cells was relevant to the increasing miR-382-5p in CAFs.
Furthermore, after transfection with miR-382-5p inhibitors, CAFs
were co-cultured with CAL-27 cells. CAFs without miR-382-5p
inhibitor transfection showed a higher potential in promoting
CAL-27 cell migration and invasion than CAFs with miR-382-5p
inhibitor transfection (Fig. 5C).
Then we aimed to ascertain whether iR-382-5p could be transported
from CAFs to CAL-27 cells through exosomes. We marked miR382-5p
mimics with FAM, and transfected the mimics in CAFs (Fig. 5D), and then cultured the CAFs with
CAL-27 cells; 24 h later, miR-382-5p mimics labeled with FAM were
transferred into CAL-27 cells. Exosome inhibitor, GW4869, cut off
the transportation of miR-382-5p (Fig.
5D). Finally, to identify the role of miR-382-5p in modulating
OSCC cell migration and invasion, we directly transfected
miR-382-5p mimics into CAL-27 cells. As shown in Fig. 5E, miR-382-5p mimics promoted migration
and invasion capacity of the CAL-27 cells.
miR-382-5p is overexpressed and is
associated with metastasis and clinical stage of OSCC
As miR-382-5p is transferred from CAFs to OSCC cells
and regulates cell migration and invasion, we further confirmed the
overexpression of miR-382-5p in patient samples and analyzed the
correlation between miR-382-5p overexpression and the metastasis,
clinical stage and the density of CAFs. We extracted RNA from OSCC
samples and the corresponding adjacent normal tissues and detected
the expression of miR-382-5p. We compared the expression of
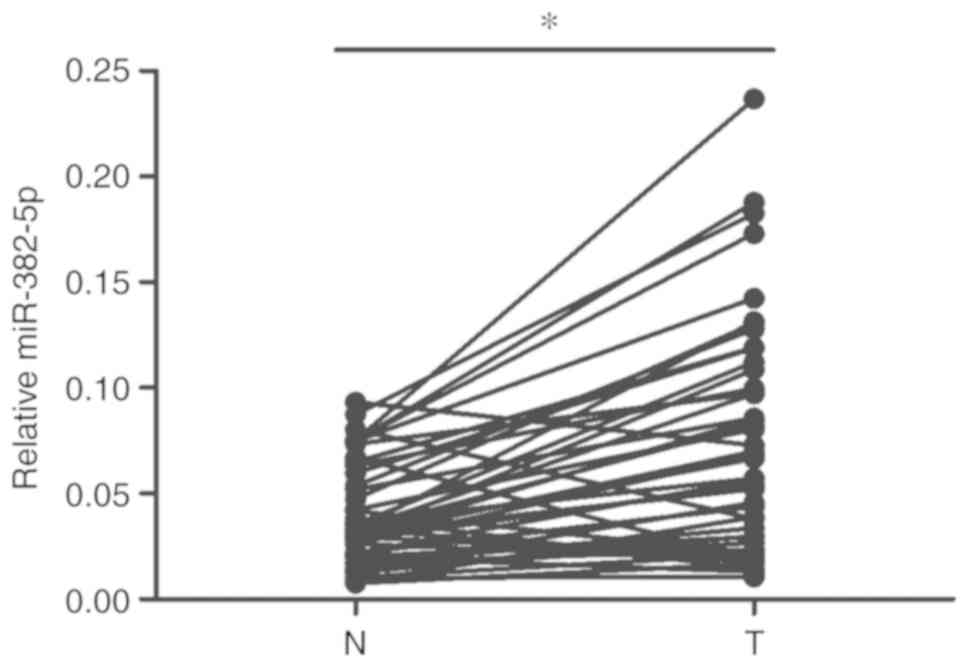
miR-382-5p. As shown in Fig. 6,
miR-382-5p was higher in the OSCC samples than that in the
corresponding adjacent normal tissues. We further analyzed the
relationship of miR-382-5p with the metastasis, clinical stage and
the density of CAFs. As shown in Table
II, miR-382-5p overexpression showed a positive association
with OSCC metastasis, clinical stage, but shows no relevance with
CAFs density. To further confirm whether there is relationship of
miR-382-5p expression with CAFs density, we transfected miR-382-5p
mimics in CAFs and measured the cell proliferation of CAFs. As
shown in Fig. S1, miR-382-5p mimic
transfection did not alter the proliferation of CAFs, indicating
that miR-382-5p may not be a regulator of CAF cell proliferation
and therefore is not correlated with CAF density.
 | Table II.Association of miR-382-5p
overexpression and clinicopathologic features of the OSCC
patients. |
Table II.
Association of miR-382-5p
overexpression and clinicopathologic features of the OSCC
patients.
|
|
| miR-382-5p
overexpression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total (n=47) | + (n=31) | - (n=16) | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 27 | 18 | 9 | 0.574 |
|
Female | 20 | 13 | 7 |
|
| Age (years) |
|
|
|
|
|
<60 | 17 | 11 | 6 | 0.569 |
|
≥60 | 30 | 20 | 10 |
|
| Lymph node
metastasis |
|
|
|
|
|
Yes | 21 | 18 | 3 | 0.011a |
| No | 26 | 13 | 13 |
|
| TNM stage |
|
|
|
|
|
I/II | 17 | 8 | 9 | 0.042a |
|
III/IV | 30 | 23 | 7 |
|
| Differentiation
grade |
|
|
|
|
|
Well | 12 | 6 | 6 | 0.139 |
|
Moderate | 21 | 13 | 8 |
|
|
Poor | 14 | 12 | 2 |
|
| CAF density |
|
|
|
|
|
Rich | 19 | 14 | 5 | 0.274 |
|
Poor | 28 | 17 | 11 |
|
Discussion
In the present study, we showed that exosomes from
CAFs can transfer miR-382-5p to OSCC cells and are correspondingly
associated with OSCC cell migration and invasion. We firstly
analyzed the relevance of CAF density in OSCC to the
clinicopathological characteristics of patients. Next, we
determined that CAF-conditioned media boost OSCC cell migration and
invasion compared with NF-conditioned media. More importantly, we
demonstrated that CAF prolifically expressed miR-382-5p can be
transported from CAFs to OSCC cells through exosomes.
Recently, multiple studies have shown that CAFs
enhance the migration and invasion in several tumor types (37), through either re-modifying the tumor
microenvironment or regulating the gene expression of the tumor
cell itself. Here, we manifested that CAF density is related to
OSCC metastasis, and furthermore, demonstrated that CAFs can
promote OSCC cell migration and invasion, indicating that CAFs are
one of the motivators for OSCC metastasis. Our finding of CAFs
being responsible for OSCC metastasis agrees with several previous
reports. Wu et al reported that knockdown of galectin-1 in
CAFs inhibits oral squamous cell carcinoma metastasis by
downregulating MCP-1/CCL2 expression (38). Ohata et al showed that
activated fibroblast-secreted leukemia inhibitory factor
participates in the invasiveness of OSCC (39). Therefore, CAFs may be a promising
target for OSCC therapy, as well as a candidate biomarker for OSCC
prognosis.
Dysregulation of miRNAs in cancer cells has been
widely reported to be involved in cancer growth and progression
(40,41); however, the dysregulation and
dysfunction of miRNAs in CAFs is ambiguous. As the most abundant
stromal cells in the tumor microenvironment, CAFs play essential
roles in cell-cell communication and in the facilitation of more
aggressive behaviors of tumor cells (42). Dysregulation of miRNAs in CAFs is
increasingly recognized to be important elements for tumor
proliferation, invasion, and metastasis in many types of solid
tumors (42). The present study
showed that miR-382-5p is upregulated in CAFs of OSCC compared with
NFs. miR-382-5p aggravates ther progression of several types of
cancer via multiple mechanisms. It has been found that miR-382-5p
promoted breast cancer cell proliferation, migration and invasion
through the RERG/Ras/ERK signaling axis (27). Through targeting ZIC4, miR-382-5p was
found to promote angiogenesis in glioma (29). As distinguished from other studies of
miR-382-5p functioning in tumor cells, our study focused on the
function of miR-382-5p in assisting CAFs to regulate OSCC,
indicating that targeting miR-382-5p or blocking its transport from
CAFs to OSCC cells could be a possible strategy for OSCC treatment.
However, there is one ambiguous point that we have not elucidated.
Although we verified that miR-382-5p plays important roles in the
regulation of OSCC by CAFs, whether miR-382-5p is the only or the
most upregulated miRNA in CAFs in OSCC remains unknown. Huge
individual difference between OSCC patients must be taken into
account. Multicenter, large-scale trials may be needed to shed
light on this point.
miR-382-5p may be a driver of CAF function but does
not determine the CAF density in OSCC. miR-382-5p is overexpressed
in CAFs and can be transferred to OSCC cells to induce cell
migration and invasion, indicating that miR-382-5p at least is one
of the mediators participating in CAF-OSCC cell communications.
Yet, miR-382-5p overexpression shows no correlation with CAF
density, indicating that even if miR-382-5p regulates CAF function,
it does not determine the amount of CAFs in OSCC tissues. The in
vitro result showing that miR-382-5p does not alter CAF
proliferation can be an explanation of why miR-382-5p is not
correlate with CAF density.
Actually, there are still several ambiguous points
requiring further study, including whether downregulation of
miR-382-5p could reverse CAFs to NFs and how miR-382-5p induces
cell migration and invasion when transported to OSCC cells.
Although we verified that miR-382-5p can be transported from CAFs
to OSCC cells, the target genes or signaling pathways participating
in the miR-382-5p-mediated regulation of cell migration and
invasion remain unknown. It has been reported that DLC-1 and
RERG/Ras/ERK signaling axis are downstream targets of miR-382-5p
and are responsible for miR-382-5p-induced breast cancer or liver
cancer migration and invasion (27,28). In
addition, Targetscan (http://www.targetscan.org/vert_71/), which is a
software program for searching for predicted microRNA targets,
predicted that PTEN, YBX1, RUNX1, STC1, JAM2 and
MMP16 were candidate target genes of miR-382-5p. The targets
and downstream pathways involved in the mediation of OSCC migration
and invasion by miR-382-5p warrant further investigation.
In conclusion, we elucidated a new mechanism of
CAF-induced OSCC progression. CAF-derived exosomes transport
miR-382-5p to OSCC cells and contributed to OSCC cell migration and
invasion. Our finding may be beneficial for discovering novel
cancer therapeutic targets.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (81602374) and by the Natural
Science Foundation of Shandong Province (ZR2016HQ40).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BiZ and ZM designed this research project. LPS
performed the western blot experiment and isolated the CAFs. KX
collected the tissue samples. BoZ performed the cell culture
experiments. JC performed the IHC and immunocytochemistry. DYY
carried out the exosome extraction. Transfection RNA extraction and
qPCR were performed by JL and JLL. Transwell assays were performed
by KYL. All authors participated in data analysis and the writing
of the manuscript for the relevant sections. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shandong University, and the reference number was #201635. All
patients signed informed consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards ZC, Trotter EW, Torres-Ayuso P,
Chapman P, Wood HM, Nyswaner K and Brognard J: Survival of head and
neck cancer cells relies upon LZK kinase-mediated stabilization of
mutant p53. Cancer Res. 77:4961–4972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song X, Xia R, Li J, Long Z, Ren H, Chen W
and Mao L: Common and complex Notch1 mutations in Chinese oral
squamous cell carcinoma. Clin Cancer Res. 20:701–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamura T, Ichikawa T, Nakahata S, Kondo Y,
Tagawa Y, Yamamoto K, Nagai K, Baba T, Yamaguchi R, Futakuchi M, et
al: Loss of NDRG2 expression confers oral squamous cell carcinoma
with enhanced metastatic potential. Cancer Res. 77:2363–2374. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lorusso G and Rüegg C: The tumor
microenvironment and its contribution to tumor evolution toward
metastasis. Histochem Cell Biol. 130:1091–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Augsten M: Cancer-associated fibroblasts
as another polarized cell type of the tumor microenvironment. Front
Oncol. 4:622014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Udagawa T and Wood M: Tumor-stromal cell
interactions and opportunities for therapeutic intervention. Curr
Opin Pharmacol. 10:369–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimoda M, Mellody KT and Orimo A:
Carcinoma-associated fibroblasts are a rate-limiting determinant
for tumour progression. Semin Cell Dev Biol. 21:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saigusa S, Toiyama Y, Tanaka K, Yokoe T,
Okugawa Y, Fujikawa H, Matsusita K, Kawamura M, Inoue Y, Miki C and
Kusunoki M: Cancer-associated fibroblasts correlate with poor
prognosis in rectal cancer after chemoradiotherapy. Int J Oncol.
38:655–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leca J, Martinez S, Lac S, Nigri J, Secq
V, Rubis M, Bressy C, Sergé A, Lavaut MN, Dusetti N, et al:
Cancer-associated fibroblast-derived annexin A6+ extracellular
vesicles support pancreatic cancer aggressiveness. J Clin Invest.
126:4140–4156. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Curtis M, Kenny HA, Ashcroft B, Mukherjee
A, Johnson A, Zhang Y, Helou Y, Batlle R, Liu X, Gutierrez N, et
al: Fibroblasts mobilize tumor cell glycogen to promote
proliferation and metastasis. Cell Metab. 29:141–155.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdogan B, Ao M, White LM, Means AL,
Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, et
al: Cancer-associated fibroblasts promote directional cancer cell
migration by aligning fibronectin. J Cell Biol. 216:3799–3816.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allaoui R, Bergenfelz C, Mohlin S,
Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K,
Påhlman S, et al: Cancer-associated fibroblast-secreted CXCL16
attracts monocytes to promote stroma activation in triple-negative
breast cancers. Nat Commun. 7:130502016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang D, Gao J, Wang S, Ye N, Chong Y,
Huang Y, Wang J, Li B, Yin W and Wang D: Cancer-associated
fibroblasts promote angiogenesis in gastric cancer through
galectin-1 expression. Tumour Biol. 37:1889–1899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Fan X, Zhang Q, Shi X, Xu G and Zou
C: Cancer- associated fibroblasts secrete FGF-1 to promote ovarian
proliferation, migration, and invasion through the activation of
FGF-1/FGFR4 signaling. Tumour Biol. 39:10104283177125922017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y
and Shen H: Exosomal miRNAs and miRNA dysregulation in cancer-
associated fibroblasts. Mol Cancer. 16:1482017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ramirez MI, Amorim MG, Gadelha C, Milic I,
Welsh JA, Freitas VM, Nawaz M, Akbar N, Couch Y, Makin L, et al:
Technical challenges of working with extracellular vesicles.
Nanoscale. 10:881–906. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
21
|
Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen
S, Wang Y, Wang T and Hou Y: Carcinoma-associated fibroblasts
promote the stemness and chemoresistance of colorectal cancer by
transferring exosomal lncRNA H19. Theranostics. 8:3932–3948. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miranda AM, Lasiecka ZM, Xu Y, Neufeld J,
Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA and Di Paolo
G: Neuronal lysosomal dysfunction releases exosomes harboring APP
C-terminal fragments and unique lipid signatures. Nat Commun.
9:2912018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ambros V: microRNAs: Tiny regulators with
great potential. Cell. 107:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bronisz A, Godlewski J and Chiocca EA:
Extracellular vesicles and MicroRNAs: Their role in tumorigenicity
and therapy for brain tumors. Cell Mol Neurobiol. 36:361–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho JY, Hsu RJ, Liu JM, Chen SC, Liao GS,
Gao HW and Yu CP: MicroRNA-382-5p aggravates breast cancer
progression by regulating the RERG/Ras/ERK signaling axis.
Oncotarget. 8:22443–22459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du J, Bai F, Zhao P, Li X, Li X, Gao L, Ma
C and Liang X: Hepatitis B core protein promotes liver cancer
metastasis through miR-382-5p/DLC-1 axis. Biochim Biophys Acta Mol
Cell Res. 1865:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu D, Zhong L, Yuan Z, Yao J, Zhong P,
Liu J, Yao S, Zhao Y, Liu L, Chen M, et al: miR-382-5p modulates
the ATRA-induced differentiation of acute promyelocytic leukemia by
targeting tumor suppressor PTEN. Cell Signal. 54:1–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Q, Zhao L, Liu X, Zheng J, Liu Y, Liu
L, Ma J, Cai H, Li Z and Xue Y: MOV10 binding circ-DICER1 regulates
the angiogenesis of glioma via miR-103a-3p/miR-382-5p mediated ZIC4
expression change. J Exp Clin Cancer Res. 38:92019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bhome R, Goh RW, Bullock MD, Pillar N,
Thirdborough SM, Mellone M, Mirnezami R, Galea D, Veselkov K, Gu Q,
et al: Exosomal microRNAs derived from colorectal cancer-associated
fibroblasts: Role in driving cancer progression. Aging.
9:2666–2694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li B, Wang F, Li X, Sun S, Shen Y and Yang
H: Hsa_circ_0008309 May be a potential biomarker for oral squamous
cell carcinoma. Dis Markers. 2018:74968902018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang J, Lu Q, Shen B, Huang X, Shen L,
Zheng X, Huang R, Yan J and Guo H: TGFβ1 secreted by
cancer-associated fibroblasts induces epithelial-mesenchymal
transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep.
5:119242015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Y, Wang K, Ma W, Zhang X, Song Y,
Wang J, Wang N, Song Q, Cao F, Tan B and Yu J: Cancer-associated
fibroblasts are associated with poor prognosis in esophageal
squamous cell carcinoma after surgery. Int J Clin Exp Med.
8:1896–1903. 2015.PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 42015.doi: 10.7554/eLife.05005.
|
|
37
|
Tang X, Hou Y, Yang G, Wang X, Tang S, Du
YE, Yang L, Yu T, Zhang H, Zhou M, et al: Stromal miR-200s
contribute to breast cancer cell invasion through CAF activation
and ECM remodeling. Cell Death Differ. 23:132–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT
and Chen YL: Targeting galectin-1 in carcinoma-associated
fibroblasts inhibits oral squamous cell carcinoma metastasis by
downregulating MCP-1/CCL2 expression. Clin Cancer Res.
17:1306–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohata Y, Tsuchiya M, Hirai H, Yamaguchi S,
Akashi T, Sakamoto K, Yamaguchi A, Ikeda T and Kayamori K: Leukemia
inhibitory factor produced by fibroblasts within tumor stroma
participates in invasion of oral squamous cell carcinoma. PLoS One.
13:e01918652018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu XW, Wang J, Zhu MX, Wang YF, Yang SY
and Ke XY: MicroRNA-506 inhibits the proliferation and invasion of
mantle cell lymphoma cells by targeting B7H3. Biochem Biophys Res
Commun. 508:1067–1073. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian W, Wu W, Li X, Rui X and Wu Y:
MiRNA-139-3p inhibits the proliferation, invasion, and migration of
human glioma cells by targeting MDA-9/syntenin. Biochem Biophys Res
Commun. 508:295–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He Z, You C and Zhao D: Long non-coding
RNA UCA1/miR-182/PFKFB2 axis modulates glioblastoma- associated
stromal cells-mediated glycolysis and invasion of glioma cells.
Biochem Biophys Res Commun. 500:569–576. 2018. View Article : Google Scholar : PubMed/NCBI
|