Introduction
Glioblastoma multiforme (GBM) is a highly malignant
primary intracranial tumor with an average survival time after
diagnosis of only 12–14 months, and a 5-year survival rate of
merely 9% (1,2). At present, the standard treatment for
GBM is surgical resection followed by radiotherapy combined with
concurrent and/or adjuvant temozolomide (TMZ) chemotherapy
(3,4).
While there have been numerous studies on immunotherapy and gene
therapy for GBM, the effects have not been completely verified due
to inconsistencies in the treatment methods and evaluation
criteria. These, in turn, have highlighted the need for other
effective therapies, such as biological therapy, combination
therapy, interstitial brachytherapy, minimally invasive technical
and ion-channel targeted therapy. Members of the transient receptor
potential (TRP) cation channel superfamily have been observed to
perform a myriad of functions, including temperature perception,
pain transduction, vasorelaxation, male fertility and tumorigenesis
(5–7).
This emerging evidence has strongly supported the hypothesis that
transient receptor potential melastatin 8 (TRPM8), which also plays
a prominent role in thermoregulation, is one of the most promising
novel therapeutic targets in cancer treatment (8–10).
However, the functions of TRPM8 in contribution to tumorigenesis in
GBM and the precise mechanisms of TRPM8 function in glioma cells
have yet to be completely elucidated. The present study initially
evaluated TRPM8 as a promising biomarker in GBM using public
database analysis. Experiments were subsequently performed to
verify whether TRPM8 serves an important role in the apoptosis,
proliferation and invasion of human glioblastoma U251 cells.
Materials and methods
ONCOMINE analysis
ONCOMINE (http://www.oncomine.org), is an online cancer
microarray database, and was used in the present study to analyze
the transcription levels of the TRPM family in different types of
human cancers. The mRNA expression of the TRPM family in clinical
cancer specimens were compared with that in normal controls. The
present study set the thresholds as follows: The gene rank, P-value
and fold change were defined as 10%, 0.01 and 2, respectively.
Cancer Cell Line Encyclopedia (CCLE)
analysis
The CCLE (https://portals.broadinstitute.org/ccle/home) provides
public access to genomic data, analysis and visualization for
~1,000 cell lines. ‘TRPM8’ was searched in the CCLE database and
the expression data of TRPM8 from the available cancer cell lines
in a series of cancers was verified by using cell line data from
CCLE.
Kaplan-Meier plotter survival
analysis
The survival data were downloaded from CGGA
(http://www.cgga.org.cn), a newly developed
interactive web server for analyzing the gene expression data of
325 patients (203 males and 122 females). The samples were
categorized into two groups (high and low TRPM8 expression levels)
using the median gene expression value and were compared using a
Kaplan-Meier survival plot. The investigators established overall
survival (OS) time as the prognostic value of patients with
glioblastoma. Survival analysis with P<0.05 was considered to
indicate a statistically significant difference.
Cell culture and cell
transfection
The human glioblastoma U251 cells were kindly gifted
by G.F. Vande Woude, (Van Andel Research Institute, Grand Rapids,
MI, USA). The human glioblastoma U251 cells were cultured in DMEM
containing 10% fetal bovine serum and placed in a humidified cell
incubator with 5% CO2 at 37°C.
Cell monolayers (at 70% confluency) were transfected
with the pEGFP-C1-TRPM8 plasmids and the enhanced green fluorescent
protein plasmid-C1 (pEGFP-C1) vector using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. shRNA targeting TRPM8 was designed and inserted into the
pGPU6/GFP/Neo vector (Thermo Fisher Scientific, Inc.). shRNA-TRPM8
was also transfected into human glioblastoma U251 cells using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The cDNA Synthesis kit (Takara
Biotechnology Co., Ltd.) was used for the synthesis of cDNA
according to the manufacturer's protocol. RT-qPCR was performed
using The PrimeScript™ II Reverse Transcriptase kit obtained from
Takara Biotechnology Co., Ltd. The 2−ΔΔCq method was
used to calculate the expression level (defined as the fold change)
of TRPM8 compared with GAPDH expression. The primer sequences were
as follows: TRPM8 sense chain, 5′-TATCTTACTGAACACCTGTAGTCCCAG-3′
and antisense chain, 5′-TGAGTTTAGTGTATTCAAAGCTGAGAAA-3′ (256 bp);
GAPDH sense chain, 5′-AGTGAAGTCGTCGTCAAC-3′ and antisense chain,
5′-CGCTCCTGAGATGTGAT-3′ (32 bp). The experiment was repeated 3
times.
Western blot assay
Cells were lysed in RIPA buffer (KeyGen Biotech.
Co., Ltd., Nanjing, China) and protein in supernatant extracts was
quantified using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). A total of 50 µg per lane of total cell lysates was
resolved on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene
fluoride (PVDF) membranes (EMD Millipore). The blots were incubated
with a primary antibody overnight at 4°C. The blots were
subsequently incubated with horseradish peroxidase-linked secondary
anti-rabbit or anti-mouse antibody (dilution 1:3,000; cat. nos.
STAR71D800GA and STAR117D800GA; Bio-Rad Laboratories, Inc.).
Immunoreactivity was detected using enhanced chemiluminescence
(Amersham; GE Healthcare). Densitometric analysis was performed
using Quantity One software (Bio-Rad Laboratories, Inc.). GAPDH was
used as a loading control. TRPM8 (dilution 1:1,000; cat. no.
sc-169688), ERK (dilution 1:1,000; cat. no. sc-271270), cyclin D1
(dilution 1:1,000; cat. no. sc-8396), Bcl-2 (dilution 1:1,000; cat.
no. sc-70411), and GAPDH (dilution 1:1,000; cat. no. sc-51907)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Electrophysiology and Ca2+
measurements by fluorescence imaging of Fluo-4am
For the activity test on the TRPM8 channel,
patch-clamp recordings were performed in whole-cell configuration
at room temperature using Axonpatch 200B (Molecular Devices, LLC)
or HEKA EPC10 (HEKA Elektronik GmbH) amplifier. U251 cells were
seeded on coverslips prior to recording the TRPM8 currents via
electrophysiology with the standard extracellular solution (ECS)
containing (in mM): 130 NaCl, 5 KCl, 10 D-glucose, 10 HEPES, 1.2
MgCl2 and 1.5 CaCl2, pH 7.4. For specificity
evaluation of characteristics of the TRPM8 channel of U251 cells,
menthol (100 µM) was added in ECS to activate TRPM8, and AMTB
Hydrate (50 µM) was applied in ECS to inhibit TRPM8.
Ca2+ measurements by fluorescence imaging of Fluo-4am
were subsequently performed for further evaluation. U251 cells
grown on 10-mm glass coverslips were incubated with Fluo-4 am for
0.5 h in the dark at 37°C for the Ca2+ measurements. The
cells were then washed with HBSS containing calcium and placed on a
confocal microscope. The intracellular calcium images were recorded
using Evolve 512 EMCCD (Teledyne Photometrics) and menthol using
RSC-200 Rapid Solution Changer (Bio-Logic Science Instruments).
Then the fluorescence intensities were analyzed using ImageJ
software (version 1.8.0; National Institutes of Health). Data were
presented as the traces of average fluorescence intensities
values.
Cell proliferation assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Laboratories, Inc.).
Transfection of TRPM8 or the non-specific control was performed in
96-well plates in quadruplicate. Cell culture medium was replaced
at 24 h following transfection. CCK-8 (10 µl) was added to each
well, which also contained 100 µl medium. Following a 2-h
incubation with the CCK-8 solution, the absorbance at 450 nm was
measured at 48 h following transfection. Each experiment was
performed in triplicate.
Apoptosis assay
Apoptosis was determined using the Annexin V-PE/7AAD
apoptosis kit (Nanjing KeyGen Biotech Co., Ltd.) according to the
manufacturer's protocol. Briefly, after 72 h of culture, the cells
were collected and washed twice in PBS and re-suspended at a
density of 1×103 cells/ml. The transfected cells were
subsequently stained with Annexin V and propidium iodide in the
dark and 7-ADD for 20 min and analyzed using a FACSCalibur
instrument.
Immunofluorescence
The cells were seeded on coverslips and incubated
for 24 h under normoxic conditions. Subsequently, the cells were
fixed with 4% paraformaldehyde at room temperature and
permeabilized with 0.2% Triton X-100 for 10 min. The cells were
subsequently washed with PBS and blocked in PBS containing 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 90 min. The
cells were washed with PBS and the primary antibodies were diluted
with PBS containing 2% BSA as follows: Rabbit anti-phospho-specific
ERK (cat. no. sc-271270), Bcl-2 (cat. no. sc-70411),
phospho-specific c-Jun N-terminal protein kinase (JN; cat. no.
sc-7345), caspase-3 (cat. no. sc-271759), phospho-specific p38
mitogen-activated protein kinase (MAPK) (cat. no. sc-271759)
antibodies (dilution 1:200; Santa Cruz Biotechnology, Inc.) and
incubated overnight at 4°C. The cells were then washed with PBS and
incubated for 2 h with an anti-mouse fluorescent secondary antibody
(cat. no. A10036; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature. Finally, DAPI (Beijing ComWin Biotech Co., Ltd.)
was added to each sample for nuclear counterstaining. The
coverglass was examined and photographed to reveal representative
cells using an Olympus BX61WI-FV1200MPE confocal microscope
(Olympus Corp.).
Transwell invasion assay
Transwell invasion was performed with 24-well
Matrigel-coated chambers (8-µM pore size) from BD Biosciences.
Briefly, cells were permitted to grow to ~75-80% confluence and
were starved for 24 h by serum. Approximately 1×105
cells resuspended in 200 µl serum-free medium were plated in the
upper Transwell chamber. Then, 600 µl of medium with 10% FBS was
added to the bottom wells of the chambers. At 48 h following
incubation, the Transwell insert was washed twice with PBS and
fixed with 5% glutaraldehyde for 5 min. The invading cells on the
bottom surface of the membrane were stained for 20 min with 0.1%
crystal violet. Then the membranes were observed at an ×100
magnification using a light microscope (Olympus Corp.). The
membrane was then dissolved by 10% acetic acid, and a
multifunctional microplate reader was employed to measure the
optical density (OD) value of each well at 570 nm. The invasion
rate = OD 570 post-crystal violet staining/OD 490 at the time of
inoculation. Each experiment was performed in triplicate.
Statistical analysis
All data in the present study are presented as the
mean ± standard deviation of at least three independent
experiments. Statistical tests were performed using SPSS version
19.0.0 software (IBM Corp.). A two-tailed Student's t-test was used
for comparisons between two groups. One-way analysis of variance
(ANOVA) test and Bonferroni post hoc test were used for evaluating
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
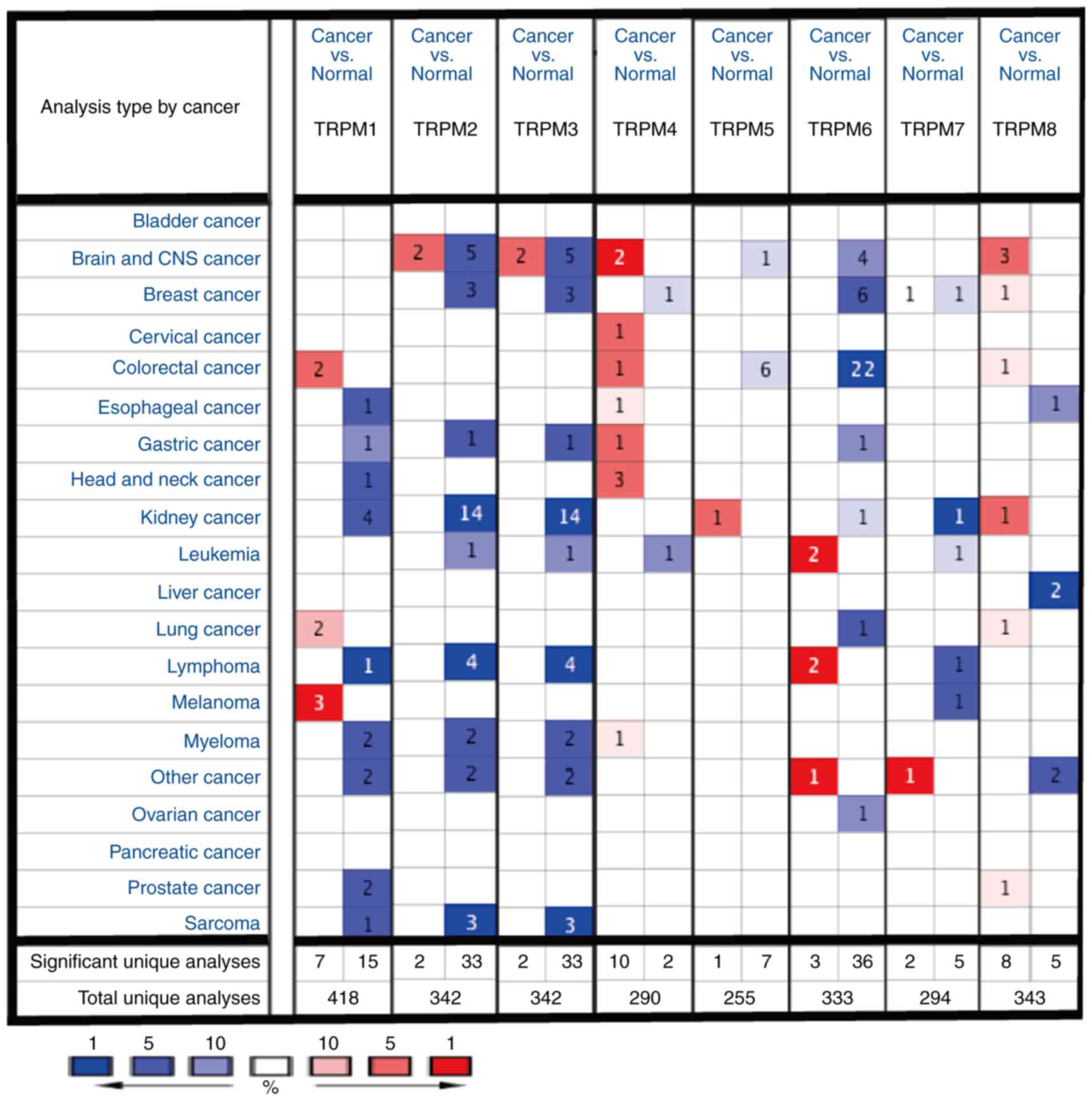
Compared with other TRPM family
members, TRPM8 is markedly overexpressed in cancer of the brain and
central nervous system (CNS)
To assess the differences in mRNA expression of TRPM
family members in human cancers and normal tissues in a variety of
cancer types, the present study performed an analysis using the
ONCOMINE database (Fig. 1). The
results of the ONCOMINE analysis indicated that TPRM8 was elevated
in cancer of the brain and CNS when compared with the normal tissue
groups.
Corresponding expression profiles of
TRPM8 in an array of cancer cell lines
By searching the CCLE (https://portals.broadinstitute.org/ccle/home), the
results revealed that TRPM8 was highly expressed in all GBM cell
lines (Fig. 2). These results
indicated that TRPM8 may serve a unique role in the development of
GBM, which was consistent with the results of the ONCOMINE
analysis.
Kaplan-Meier plotter survival analysis
of TRPM8 in patients with GBM
To validate the influence of TRPM8 on the prognosis
of glioma patients, an overall survival (OS) analysis was performed
using the Kaplan-Meier method via CGGA datasets. The results
indicated that the high expression of TRPM8 mRNA was associated
with a shorter overall survival (OS) time in all patients with GBM
(P<0.0001) (Fig. 3). The survival
analysis indicated that over-expression of TRPM8 was associated
with worse survival in patients with GBM, suggesting TRPM8 serves a
tumor-promoting role in GBM.
Characteristics of the TRPM8
channel
Menthol is a specific agonist against TRPM8, and
AMTB hydrate is a TRPM8 blocker (11). To evaluate the characteristics of the
TRPM8 channel, the whole cell membrane current of U251 cells was
investigated by whole-cell patch-clamp recordings. Fig. 4A and B illustrate the whole cell
membrane current of U251 cells at voltage ramps from −80 to 80 mV.
The current-time curve of the TRPM8 channel after treatment with
two consecutive 100-µM menthol activations ruled out the effects of
switching the drug delivery tube (Fig.
4A). When the transmembrane voltage was positive, the outward
current was markedly increased. The whole cell membrane currents
were significantly increased when menthol (100 µM) was added in ECS
to activate TRPM8 (Fig. 4B), and was
reversed by AMTB hydrate (50 µM), further confirming that AMTB
hydrate functions as a blocker of TRPM8. In addition, the
fluorescence imaging results revealed that the ion channel activity
of TRPM8 may be attributable to increased
[Ca2+]i following the addition of menthol.
Menthol increased U251 [Ca2+]i, and this
increase could be reversed following the removal of menthol
(Fig. 4C). The ability of menthol to
activate TRPM8 ion channels in the glioma cells was examined by
overexpressing the channel in the U251 cells. The vehicle of
pEGFP-C1 as control groups were also transfected into the U251
cells. The Ca2+ influx was monitored using Fluo-4am, and
there was a slight increase following the overexpression of TRPM8.
Following treatment with 100 µM menthol, a significant increase in
fluorescence was detected for TRPM8, whereas no fluorescence
increase was observed for the control groups (Fig. 4D).
TRPM8 enhances the sensitivity of GBM
cells to apoptosis
Firstly, by detecting the gene and protein
expression levels of TRPM8 via RT-qPCR and western blot assay, the
present study confirmed that transfection with shRNA-TRPM8 could
significantly inhibit the mRNA and protein expression of TRPM8
(P<0.01). Conversely, the overexpression of TRPM8 was
significantly increased following transfection with the
pEGFP-C1-TRPM8 plasmid (P<0.05). These results indicated that
the downregulated and upregulated TRPM8 U251 cell lines were
successfully constructed (named U251/shRNA and U251/TRPM8,
respectively) (Fig. 5). As revealed
in Fig. 6A, inactivation of TRPM8
expression could significantly increase the apoptosis rate
(P<0.01), indicating that the modulation of cell apoptosis was
significantly affected by the expression of TRPM8. The expression
levels of p-ERK, Bcl-2 were significantly decreased when TRPM8 was
upregulated and increased when TRPM8 was downregulated in human
glioblastoma U251 cells as revealed in Fig. 6B. The present study also confirmed
these results via cellular immunofluorescence (Fig. 7). In addition, cellular
immunofluorescence also revealed that the expression of
phospho-specific JNK, caspase-3 and phospho-specific p38 MAPK were
increased when TRPM8 was downregulated (Fig. 7).
TRPM8 regulates the proliferation and
invasion abilities of human glioblastoma U251 cells
The results of the CCK-8 assay indicated that the
proliferative ability of U251 cells in the U251/TRPM8 group was
higher than that in the U251 and U251/Con groups (P<0.05);
however, there was no significant difference between the U251 and
U251/Con groups (P>0.05). The details of the proliferation
capacity are presented in Table I. In
addition, the expression level of cyclin D1 was markedly altered
when TRPM8 was regulated in human glioblastoma U251 cells (Fig. 6B). Collectively, the results
demonstrated that the proliferation ability of U251 cells was
significantly affected by the expression of TRPM8.
 | Table I.Comparison of the proliferation
capacity (OD value) and invasion rate of human glioma U251 cells in
each group (x±S). |
Table I.
Comparison of the proliferation
capacity (OD value) and invasion rate of human glioma U251 cells in
each group (x±S).
| Groups | Proliferation
capacity | Invasion rate
(%) |
|---|
| U251 group |
0.147±0.012a |
1.363±0.035a |
| U251/Con group |
0.151±0.017a |
1.425±0.042a |
| U251/TRPM8
group | 0.361±0.024 | 3.283±0.051 |
The invasion rate is a crucial cancer cell property
that accurately reflects the invasive ability of human glioblastoma
U251 cells. As illustrated in Fig. 8,
the Transwell invasion assay revealed that the U251 cells in the
U251/TRPM8 group had an increased number of invasive cells when
compared with the U251 and U251/Con groups. According to the OD
value, the transfer rate indicated that the U251/TRPM8 group had a
higher invasion rate than the U251 and the U251/Con groups
(P<0.05); however, the difference between the U251 and U251/Con
groups was not statistically significant (P>0.05) (Table I). Collectively, these results
indicated that the invasion of human glioblastoma U251 cells was
positively associated with the expression level of TRPM8.
Discussion
Glioblastoma is a Grade IV glioma, and is the most
common and malignant tumors in glioma (12). Due to the limited efficacy of
conventional treatments such as surgery, chemotherapy, and
radiotherapy, there is an urgent need to identify novel and
effective treatments. Over the past decade, numerous studies have
made great progress in investigating the genetics and epigenetics
of GBM (13,14). Despite advances in early diagnosis and
treatment, GBM has strong invasion cababilities and is
characterized by its significantly shorter patient survival among
intracranial tumors (15–18). There is a large volume of published
studies describing the role of Ca2+ homeostasis
(19–21). Multiple factors including
Ca2+ signaling have been identified to influence the
processes of tumorigenesis, proliferation and invasion (22). An increasing amount of evidence has
recognized the importance of TRP cation channels, and the pivotal
roles they serve in controlling these processes (23–25). TRP
channels have been identified to exert pivotal functions in
cellular calcium homeostasis and Ca2+ signaling
(19). As the subclass of the TRP
channels, TRPMs have varying permeability to Ca2+ and
Mg2+ in various types of cancer (5,26,27). Therefore, TRPM protein family members
may serve attractive targets for anticancer therapeutic strategies
or prognostic biomarkers to cure specific types of cancers
(26,28). It is common knowledge that TRPM8 is
involved in the initiation and progression of tumors (29,30).
Although these findings undoubtledly require scrutinization, they
suggested that TRPM8 may be involved in the transformation of
normal cells to tumorigenic cells, which culminates in biological
changes, such as tumor proliferation, apoptosis, gene transcription
and angiogenesis (31). Morever, few
studies have been performed where the gene expression data is
correlated with the survival of patients (8,9,11,28).
Combined, TRPM8 may serve as an appropriate GBM biomarker, and this
biomarker is urgently required for the early diagnosis and
validation of interventions. The present study subsequently focused
on describing the role of TRPM8 in GBM.
Survival analysis of TRPM8 in the present study
demonstrated that overexpression of TRPM8 was significantly
associated with OS of patients with GBM, which suggests that TRPM8
serves a tumor-promoting role in GBM. Furthermore, the results of
the present study suggested that Ca2+-permeable TRPM8
nonselective cation channels were functional in human glioblastoma
U251 cells. In addition, TRPM8 has the characteristic of outward
rectification and may play an important role in regulating the
biological behavior of glioblastoma cells. When menthol was used to
activate the TRPM8 channel or overexpressed TRPM8, the
[Ca2+]i was significantly increased.
The MAPK signaling pathway has been confirmed to be
closely associated with the occurrence and development of tumors
(32,33). ERK, as an important regulator of the
MAPK pathway, serves an important role in the proliferation and
apoptosis of tumor cells (33,34). ERK
is an important subtribe of the family of mitogen-activated protein
kinases (MAPKs). ERK can be activated by various growth factors and
other mitogens (33). The activation
of ERK is considered to be closely associated with cell
proliferation, differentiation, migration, invasion, apoptosis and
malignant transformation (35,36). In
addition, ERK has a direct regulatory effect on cyclin D1, which
can accelerate cell mitosis and promote cell proliferation
following activation; however, overexpression of ERK can activate
nuclear factor-κB (NF-κB), thereby inducing the expression of
apoptosis-related proteins, such as Bcl-2 and Bcl-xL (37,38). In
addition, ERK can also exert anti-apoptotic effects by inhibiting
the phosphorylation of pro-apoptotic proteins Bad and Bim (37). The present study revealed that the
expression levels of ERK, Bcl-2 and cyclin D1 in human glioma cells
were significantly lower than those in the U251/Con group when
TRPM8 was downregulated. This indicated that part of the mechanism
of TRPM8 in regulating the biological behaviors of human glioma
cells may be via the regulation of ERK factors, thereby affecting
the expression of apoptosis and proliferation-related factors. The
activated caspase can hydrolyze important proteins including cell
regulation, cell signal transduction and DNA repair, thus making
the cells appear to wither (39).
Apoptosis is characterized by specific morphological and
biochemical features of death, in which caspase-3 is the ultimate
performer of apoptotic death (40).
Is TRPM8-inhibiting tumor cell apoptosis also caspase-3 dependent?
The results of the present study demonstrated that TRPM8 can
inhibit the activity of JNK/MAPK signal transduction pathway. In
addition, the results of the present study also suggested that part
of the mechanism by which TRPM8 regulates the biological behavior
of human glioma cells may be via the regulation of p38/MAPK signal
transduction pathway, thereby affecting the expression level of
apoptosis-related factors. However, it is worth noting that the
regulation of the biological behavior of tumors involves multiple
signaling pathways, and interactions between these signaling
pathways are likely. In the future, a more in-depth study of the
mechanism of action of TRPM8 will be conducted. It was hypothesized
that the proliferation and apoptosis of human glioma cells
regulated by TRPM8 may be associated with the MAPK pathway.
One of the most striking observations from data
comparisons was the significant promotion of proliferative capacity
of human glioblastoma U251 cells following the overexpression of
TRPM8. Previous research revealed that the hepatocyte growth
factor/scatter factor (HGF/SF) exhibited a modulatory effect on
TRPM8 expression during oncogenesis (8,41). In
addition, the TPRM8 channel contributes to glioma invasion by
inducing Ca2+ signaling, cytoskeleton changes and
invasion (41). Activation of TRPM8
channel by its agonist, menthol, has been previously reported to
stimulate the invasion of glioblastoma cells by increasing the
intracellular Ca2+ concentration (41,42), which
is necessary for cell migration, and presumably tumor invasion
(8,41). Using human glioblastoma U251 cells,
the present study investigated whether cell invasion and chemotaxis
were dependent on TRPM8 channel activity, with the results
indicating that TRPM8 significantly accelerated the invasion speed
of U251 cells.
In summary, the present study indicated that the
Ca2+-permeable channel of TRPM8 may have functional
implications for glioma survival, proliferation, apoptosis and
local tumor invasion. Therefore, TRPM8 may be utilized as an
appealing anticancer target as well as a useful biomarker for
cancer prognosis and treatment of GBM.
Acknowledgements
We sincerely express our thanks to Dr Wei Yang and
Dr Yafei Yu for the advice and writing assistance on this
experiment. At the same time, we appreciate Dr Tianfu Sun and Dr
Kangli Xu for their help in analyzing and organizing the data.
Funding
The present study was supported by the Traditional
Chinese Medicine Science and Technology Plan of Zhejiang province
(grant no. 2019ZB030) and the Key Research & Development
(R&D) Plan of Zhejiang Province (grant no. 2019C03095).
Availability of data and materials
The datasets used in this study are available from
the corresponding author upon reasonable request.
Authors' contributions
JZ, YW and LQ performed the experiments and wrote
the original draft. SZ performed the experiments. SH analyzed and
interpreted the data. YZ was a guarantor and interpreted the data.
JP conducted the bioinformatics analysis. RM polished the
manuscript and was responsible for data curation. RZ conceived,
designed, revised the study critically for important intellectual
content. All authors read, reviewed and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN: Molecular pathology of malignant
gliomas. Annu Rev Pathol. 1:97–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang
X, Jiang C, Kang C, Li X, Chen L, et al: CGCG clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 375:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li MY, Yang P, Liu YW, Zhang CB, Wang KY,
Wang YY, Yao K, Zhang W, Qiu XG, Li WB, et al: Low c-Met expression
levels are prognostic for and predict the benefits of temozolomide
chemotherapy in malignant gliomas. Sci Rep. 6:211412016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Dietrich PY, Ostermann Kraljevic
S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G,
Miralbell R, et al: Promising survival for patients with newly
diagnosed glioblastoma multiforme treated with concomitant
radiation plus temozolomide followed by adjuvant temozolomide. J
Clin Oncol. 20:1375–1382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Luan Y, Yu R, Zhang Z, Zhang J and
Wang W: Transient receptor potential (TRP) channels, promising
potential diagnostic and therapeutic tools for cancer. Biosci
Trends. 8:1–10. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wetsel WC: Sensing hot and cold with TRP
channels. Int J Hyperthermia. 27:388–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YR, Chun JN, So I, Kim HJ, Baek S,
Jeon JH and Shin SY: Data-driven analysis of TRP channels in
cancer: Linking variation in gene expression to clinical
significance. Cancer Genomics Proteomics. 13:83–90. 2016.PubMed/NCBI
|
|
8
|
Liu Z, Wu H, Wei Z, Wang X, Shen P, Wang
S, Wang A, Chen W and Lu Y: TRPM8: A potential target for cancer
treatment. J Cancer Res Clin Oncol. 142:1871–1881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alptekin M, Eroglu S, Tutar E, Sencan S,
Geyik MA, Ulasli M, Demiryurek AT and Camci C: Gene expressions of
TRP channels in glioblastoma multiforme and relation with survival.
Tumour Biol. 36:9209–9213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L and Barritt GJ: TRPM8 in prostate
cancer cells: A potential diagnostic and prognostic marker with a
secretory function? Endocr Relat Cancer. 13:27–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burke RC, Bardet SM, Carr L, Romanenko S,
Arnaud-Cormos D, Leveque P and O'Connor RP: Nanosecond pulsed
electric fields depolarize transmembrane potential via
voltage-gated K+, Ca2+ and TRPM8 channels in
U87 glioblastoma cells. Biochim Biophys Acta Biomembr.
1859:2040–2050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang M, Li Y, Chilukuri K, Brady OA,
Boulos MI, Kappes JC and Galileo DS: L1 stimulation of human glioma
cell motility correlates with FAK activation. J Neurooncol.
105:27–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polivka J Jr, Polivka J, Holubec L,
Kubikova T, Priban V, Hes O, Pivovarcikova K and Treskova I:
Advances in experimental targeted therapy and immunotherapy for
patients with glioblastoma multiforme. Anticancer Res. 37:21–33.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stoyanov GS and Dzhenkov DL: On the
concepts and history of glioblastoma Multiforme-Morphology,
genetics and epigenetics. Folia Med (Plovdiv). 60:48–66. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jhaveri N, Chen TC and Hofman FM: Tumor
vasculature and glioma stem cells: Contributions to glioma
progression. Cancer Lett. 380:545–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hardee ME and Zagzag D: Mechanisms of
glioma-associated neovascularization. Am J Pathol. 181:1126–1141.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. New Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sturm D, Bender S, Jones DT, Lichter P,
Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, et
al: Paediatric and adult glioblastoma: Multiform (epi)genomic
culprits emerge. Nat Rev Cancer. 14:92–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roderick HL and Cook SJ: Ca2+
signalling checkpoints in cancer: Remodelling Ca2+ for
cancer cell proliferation and survival. Nat Rev Cancer. 8:361–375.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Mu H, Dai K and Yi L: Calreticulin:
A potential anti-cancer therapeutic target. Pharmazie. 72:503–510.
2017.PubMed/NCBI
|
|
21
|
Ohshima Y, Takata N, Suzuki-Karasaki M,
Yoshida Y, Tokuhashi Y and Suzuki-Karasaki Y: Disrupting
mitochondrial Ca2+ homeostasis causes tumor-selective
TRAIL sensitization through mitochondrial network abnormalities.
Int J Oncol. 51:1146–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizzuto R, Pinton P, Ferrari D, Chami M,
Szabadkai G, Magalhães PJ, Di Virgilio F and Pozzan T: Calcium and
apoptosis: Facts and hypotheses. Oncogene. 22:8619–8627. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fiorio Pla A and Gkika D: Emerging role of
TRP channels in cell migration: From tumor vascularization to
metastasis. Front Physiol. 4:3112013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hecquet CM, Zhang M, Mittal M, Vogel SM,
Di A, Gao X, Bonini MG and Malik AB: Cooperative interaction of trp
Melastatin channel transient receptor potential (TRPM2) with its
splice variant TRPM2 Short variant is essential for endothelial
cell apoptosis. Circ Res. 114:469–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Landsberg JW and Yuan JX: Calcium and TRP
channels in pulmonary vascular smooth muscle cell proliferation.
News Physiol Sci. 19:44–50. 2004.PubMed/NCBI
|
|
26
|
Hantute-Ghesquier A, Haustrate A,
Prevarskaya N and Lehen'kyi V: TRPM family channels in cancer.
Pharmaceuticals (Basel). 11(pii): E582018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaunt HJ, Vasudev NS and Beech DJ:
Transient receptor potential canonical 4 and 5 proteins as targets
in cancer therapeutics. Eur Biophys J. 45:611–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong KK, Banham AH, Yaacob NS and Nur
Husna SM: The oncogenic roles of TRPM ion channels in cancer. J
Cell Physiol. Feb 2–2019.doi: 10.1002/jcp.28168 (Epub ahead of
print).
|
|
29
|
Okamoto Y, Ohkubo T, Ikebe T and Yamazaki
J: Blockade of TRPM8 activity reduces the invasion potential of
oral squamous carcinoma cell lines. Int J Oncol. 40:1431–1440.
2012.PubMed/NCBI
|
|
30
|
Kijpornyongpan T, Sereemaspun A and
Chanchao C: Dose-dependent cytotoxic effects of menthol on human
malignant melanoma A-375 cells: Correlation with TRPM8 transcript
expression. Asian Pac J Cancer Prev. 15:1551–1556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gkika D and Prevarskaya N: Molecular
mechanisms of TRP regulation in tumor growth and metastasis.
Biochim Biophys Acta. 1793:953–958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sideris M, Emin EI, Abdullah Z, Hanrahan
J, Stefatou KM, Sevas V, Emin E, Hollingworth T, Odejinmi F,
Papagrigoriadis S, et al: The role of KRAS in endometrial cancer: A
mini-review. Anticancer Res. 39:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu F, Yang X, Geng M and Huang M:
Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer
therapy. Acta Pharm Sin B. 8:552–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugden PH and Clerk A: Regulation of the
ERK subgroup of MAP kinase cascades through G protein-coupled
receptors. Cell Signal. 9:337–351. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krueger JS, Keshamouni VG, Atanaskova N
and Reddy KB: Temporal and quantitative regulation of
mitogen-activated protein kinase (MAPK) modulates cell motility and
invasion. Oncogene. 20:4209–4218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samatar AA and Poulikakos PI: Targeting
RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug
Discov. 13:928–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cobb MH, Hepler JE, Cheng M and Robbins D:
The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer
Biol. 5:261–268. 1994.PubMed/NCBI
|
|
38
|
Chen WT, Hsu FT, Liu YC, Chen CH, Hsu LC
and Lin SS: Fluoxetine induces apoptosis through
extrinsic/intrinsic pathways and inhibits ERK/NF-κB-modulated
anti-apoptotic and invasive potential in hepatocellular carcinoma
cells in vitro. Int J Mol Sci. 20(pii): E7572019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wondergem R, Ecay TW, Mahieu F, Owsianik G
and Nilius B: HGF/SF and menthol increase human glioblastoma cell
calcium and migration. Biochem Biophys Res Commun. 372:210–215.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wondergem R and Bartley JW: Menthol
increases human glioblastoma intracellular Ca2+, BK
channel activity and cell migration. J Biomed Sci. 16:902009.
View Article : Google Scholar : PubMed/NCBI
|