Introduction
The immune status in the tumor microenvironment
appears to be an important factor in determining the progression of
cancer (1). Tumor-infiltrating
lymphocytes (TILs), effectors of cell-mediated antitumor immunity,
are closely associated with tumor growth, metastasis and
chemoresistance in colorectal cancer (CRC), affecting the prognosis
of CRC. It has been reported that a dense infiltration of TILs is
correlated with an improved survival outcome in CRC (2–4). A high
density of TILs in the metastatic tumor is also correlated with
better relapse-free and overall survival rates after resection of
the metastatic tumor (5,6). Abundant TIL infiltration is correlated
with improved efficacy of neoadjuvant chemoradiotherapy in patients
with locally advanced rectal cancer (7) and in those with a distant metastatic
tumor (8). The measurement of TILs in
the primary tumor using the method proposed by the International
TILs Working Group (https://www.tilsinbreastcancer.org) can be used as a
prognostic marker for the clinical effectiveness of palliative
chemotherapy in patients with stage IV CRC (9).
Antitumor immune activity is accelerated or
suppressed by the delicate balance between immune-stimulating and
immune-suppressive networks. T cell-mediated antitumor immunity is
regulated by the interaction between receptors and ligands of
costimulatory or coinhibitory immune checkpoint molecules (10). PD-1 is one of the pivotal coinhibitory
molecules, and the blockade of the interaction between PD-1 and its
ligand, programmed cell death ligand-1 (PD-L1), by monoclonal
antibodies leads to the activation of T cell-mediated antitumor
immunity and successful anticancer treatment (11,12).
Tumor necrosis factor receptor superfamily, member 4
(TNFRSF4) is also known as OX40 (CD 134) (13). It is a costimulatory immune checkpoint
molecule, and its expression is induced on T cells by activation
(14). OX40 ligand (OX40L) is
expressed on activated APCs (15).
The binding of OX40L to OX40 on T-cells generates an efficient
clonal expansion and the effective primary responses of
CD4+ and CD8+ T cells (14,16,17). It is
also known that the OX40/OX40L interaction blocks natural
regulatory T-cell activity and inhibits inducible regulatory T-cell
generation (18,19). It has been reported that an
artificially enhanced OX40/OX40L interaction could induce antitumor
effector CD8+ and CD4+ T cells and suppress
Treg activity, resulting in an increase of effective antitumor
immunity (20–22).
Recently, it was reported that the soluble form of
OX40 (sOX40) exists in human blood and could be quantitatively
detected by enzyme-linked immunosorbent assay (ELISA) (23). If sOX40 has a binding site to OX40L,
it has the capacity to bind to OX40L on APCs, leading to the
blockade of the natural OX40/OX40L interaction, resulting in a
suppression of T-cell activation. In fact, an administration of
soluble OX40-Ig fusion protein, which can bind to OX40L and inhibit
the OX40/OX40L interaction, suppressed the immune activity that
generates experimental autoimmune encephalomyelitis in an animal
model (24). In the present study, it
was revealed that sOX40 is detectable in the blood of patients with
CRC, indicating that high blood levels of sOX40 may affect
antitumor immunity against CRC. In the present study, the
association of blood sOX40 levels and clinical characteristics of
CRC was investigated.
Materials and methods
Patients
Blood samples and clinical information were
retrospectively collected from 22 patients who had been
histologically or cytologically diagnosed with advanced CRC or
postsurgical recurrent CRC at The Jikei University Hospital (Tokyo,
Japan). Survival was defined as the period from blood sample
collection to death or the last follow-up observation. The study
protocol was approved by the Ethics Committee of The Jikei
University School of Medicine (30–397 9418) and conducted in
accordance with the Declaration of Helsinki.
Sample collection, procedure and
restoration
Blood was collected into a cell preparation tube
with sodium citrate (BD Vacutainer® CPT™; BD
Biosciences) and centrifuged at 620 × g at room temperature for 20
min. Plasma samples were stored in 1-ml aliquots at −80°C.
Peripheral mononuclear cells (PBMCs) were collected and
frozen-stored using Cell Reservoir One (Nacalai Tesque, Inc.).
Quantification of sOX40
Blood sOX40 concentrations were measured using an
ELISA kit for sOX40 (Immuno-Biological Laboratories, Ltd.)
according to the manufacturer's protocol. The quantitative range
was 15.6–1,000 pg/ml. Blood levels of sOX40 in normal healthy
subjects were 78.7±28.5 pg/ml. Each sample was analyzed in
duplicate. The quantity of sOX40 in the culture supernatants of
Jurkat cells was determined using the same ELISA assay kit.
Cell culture
Jurkat cells (25), a
human T cell line, were obtained from the American Type Culture
Collection (ATCC) and cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml
streptomycin. Phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich;
Merck KGaA) was used for the activation of Jurkat cells.
Flow cytometry
Cell surface OX40 expression was examined by flow
cytometry. A suspension of untreated or PMA-treated Jurkat cells
was stained with phycoerythrin (PE)-conjugated anti-human OX40
(clone Ber-ACT35; BioLegend, Inc.) and the appropriate isotype
controls (BioLegend, Inc.) for 30 min at 4°C in the dark. Cells
were analyzed on a MACSQuant Analyzer (Miltenyi Biotec GmbH) using
MACSQuantify Software Version 2.0.
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously
described (26). TaqMan primers for
the IL-6 (Assay ID: Hs00174131_m1), IL-10 (Assay ID:
Hs00961622_m1), IL-4 (Assay ID: Hs 00174122_m1), IFN-γ (Assay ID:
Hs00989291_m1), OX40 (Assay ID: Hs00937195_g1) and 18S ribosomal
RNA (Assay ID: Hs99999901_s1) genes were purchased from Applied
Biosystems. Relative expression was calculated using the ∆∆Cq
method (27).
Statistical analysis
The software package StatFlex (version 6; Artech
Co., Ltd., Osaka, Japan) was used for statistical analysis.
Pearson's correlation coefficient was used to analyze the
association of OX40 levels with clinical characteristics.
Univariate and multivariate Cox proportional hazards models were
performed to obtain prognostic factors. Survival analysis was
performed using the Kaplan-Meier estimate. P-values were calculated
according to the log-rank test. P<0.05 was considered as
statistically significant.
Data of ELISA and flow cytometry in the experiments
using Jurkut cells are presented as the mean ± standard deviation
(SD). Comparisons between the untreated control and drug-treated
groups were performed by Dunnett's multiple comparisons test. The
software package GraphPad Prism 7 version (GraphPad Software, Inc.)
was used for statistical analysis. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Blood sOX40 levels are positively
correlated with the blood levels of tumor markers and CRP but
negatively correlated with that of albumin
Table I reveals the
characteristics of 22 patients with advanced CRC. Twelve males and
10 females were examined, and their median age was 71.5 years
(29–81). Nine patients were firstly diagnosed, and the remaining 13
patients had postsurgical recurrence. Eighteen out of 22 patients
had stage IV, 2 had stage III and 2 had stage II CRC. All patients
received chemotherapy (adjuvant, 4; 1st line, 18) based on UFT or
cisplatin.
 | Table I.Characteristics of the CRC
patients. |
Table I.
Characteristics of the CRC
patients.
| Patients
characteristics | No. of
patients | (%) |
|---|
| Sex |
|
|
|
Male | 12 | 54.5 |
|
Female | 10 | 45.5 |
| Age (years) |
|
|
|
Median | 71.5 |
|
|
Range |
29–81 |
|
| Status |
|
|
| Firstly
diagnosed | 9 | 40.9 |
|
Postsurgical recurrence | 13 | 59.1 |
| Primary site |
|
|
|
Ascending colon | 10 | 45.5 |
|
Transverse colon | 0 | 0 |
|
Descending colon | 1 | 4.5 |
| Sigmoid
colon | 4 | 18.2 |
|
Rectum | 7 | 31.8 |
| Metastatic
site |
|
|
|
Liver | 11 |
|
|
Lung | 7 |
|
|
Peritoneum | 7 |
|
| Lymph
nodes | 4 |
|
| Histologic
subtype |
|
|
|
tub1 | 8 | 36.4 |
|
tub2 | 4 | 18.2 |
|
Unknown | 10 | 45.5 |
| RAS mutation |
|
|
| Wild
type | 5 | 22.7 |
|
Mutation type | 8 | 36.4 |
|
Unknown | 9 | 40.9 |
| Stage |
|
|
| II | 2 | 9.1 |
|
III | 2 | 9.1 |
| IV | 18 | 81.8 |
| ECOG PS |
|
|
| 0 | 19 | 86.4 |
| 1 | 3 | 13.4 |
| Chemotherapy
line |
|
|
|
Adjuvant | 4 | 18.2 |
|
1st | 18 | 81.8 |
| History of
Chemotherapy |
|
|
|
Yes | 3 | 13.6 |
| No | 19 | 86.4 |
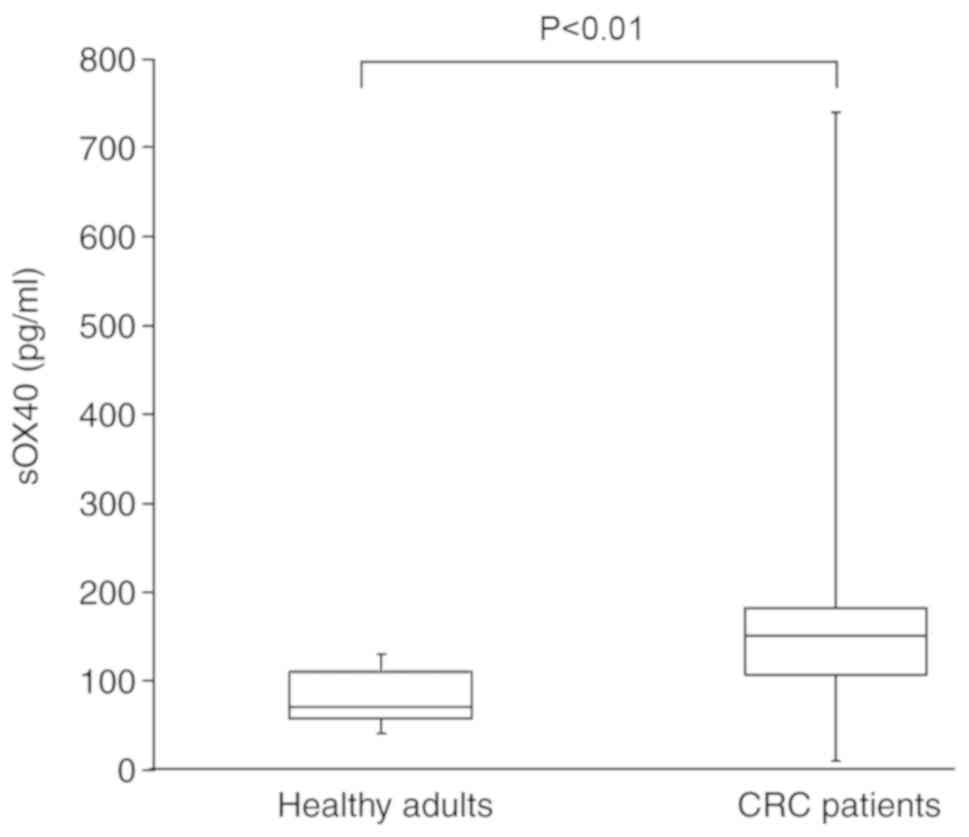
The blood levels of sOX40 in CRC patients were
significantly higher than those of healthy adults (Fig. 1). The median value of blood sOX40
level of the healthy adults was 71.0 (n=10), and that of the CRC
patients was 150.5 (n=22).
The association between blood sOX40 levels and the
clinical characteristics of the patients was investigated. Blood
sOX40 levels were negatively associated with survival time in
clinical characteristics (Table II
and Fig. 2). In laboratory findings,
blood sOX40 levels were positively correlated with blood levels of
CA19-9, CEA, CRP and sPD-L1 but negatively correlated with blood
albumin levels (Table II and
Fig. 2).
 | Table II.Correlation between blood sOX40
levels and characteristics of CRC patients. |
Table II.
Correlation between blood sOX40
levels and characteristics of CRC patients.
| Variables | r | P-value |
|---|
| Age (years) | 0.130 | n.s. |
| Sex:
Male/female | −0.264 | n.s. |
| Survival time
(days) | −0.710 | <0.001 |
| Laboratory
findings |
|
|
| CA19-9
(U/ml) | 0.665 | <0.001 |
| CEA
(ng/ml) | 0.541 | 0.009 |
| CRP
(mg/dl) | 0.807 | <0.001 |
| LDH
(U/l) | 0.275 | n.s. |
| Albumin
(g/dl) | −0.594 | 0.004 |
| WBC
(cells/µl) | 0.170 | n.s. |
|
Lymphocyte (cells/µl) | −0.270 | n.s. |
|
Lymphocyte (%) | −0.319 | n.s. |
|
Monocyte (cells/µl) | 0.111 | n.s. |
|
Monocyte (%) | −0.032 | n.s. |
|
sPD-L1 | 0.566 | 0.006 |
Blood sOX40 levels are not associated
with the expression of cytokines or PD-1 in PBMCs
Cellular OX40 is generally expressed on activated
immune cells (14). Accordingly, the
expression of mRNAs encoding cytokines that positively or
negatively regulate T-cell immunity was examined for the
association of blood sOX40 levels. Blood sOX40 levels were not
associated with mRNA expression encoding IL-6, IL-10, IL-4 and
IFN-γ or the ratio of IL-10/IFN-γ (Table III). PD-1 expressed on T cells is a
marker for T-cell activation or exhaustion (11). Blood sOX40 levels were not associated
with the frequency of PD-1-expressing immune cells (Table III).
 | Table III.Correlation between blood sOX40
levels and the immunological markers of the CRC patients. |
Table III.
Correlation between blood sOX40
levels and the immunological markers of the CRC patients.
| Variables | r | P-value |
|---|
| Interleukin-6 | −0.056 | n.s. |
| Interleukin-10 | 0.094 | n.s. |
| Interleukin-4 | 0.155 | n.s. |
| Interferon-γ | −0.181 | n.s. |
| IL-10/IFN-γ | 0.244 | n.s. |
|
PD-1+CD4+ | −0.269 | n.s. |
|
PD-1+CD8+ | −0.227 | n.s. |
|
PD-1+CD56+ | −0.237 | n.s. |
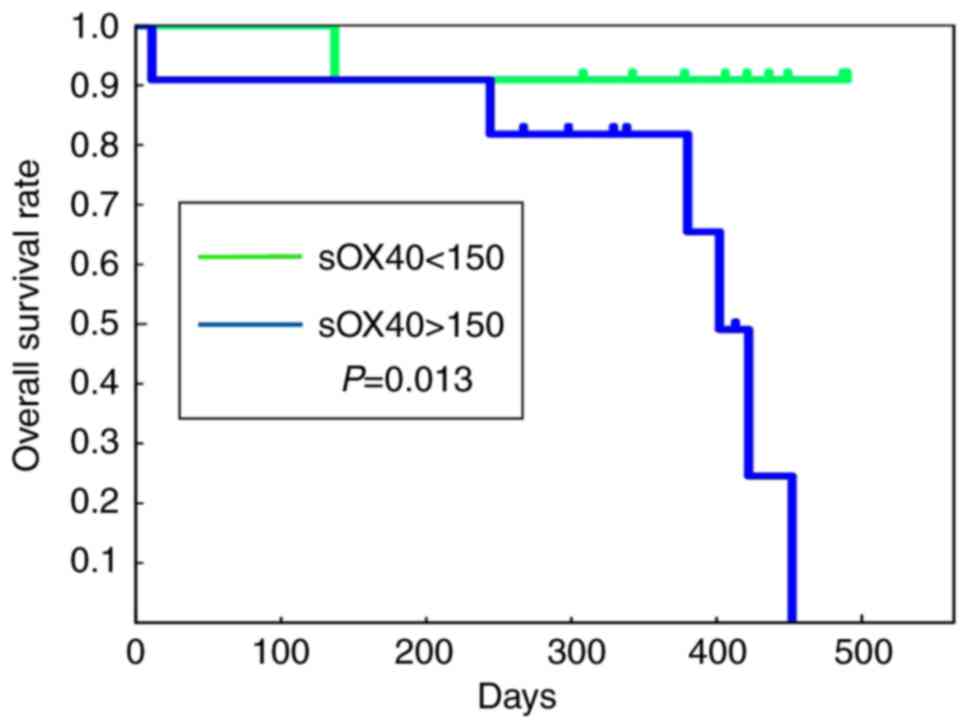
High blood sOX40 levels are
significantly correlated with reduced survival in CRC patients
The prognostic value of the clinical factors of the
CRC patients was examined. In both univariate and multivariate
analyses, blood sOX40 levels were negatively correlated with the
survival time of the patients, and high blood sOX40 levels were
significantly correlated with a reduced survival of the patients
(Table IV). No other factors were
revealed to be prognostic factors. Fig.
3 shows Kaplan-Meier curves of overall survival of the patients
based on the blood levels of sOX40. The patients with blood sOX40
levels <150 pg/ml exhibited a longer survival time, while those
with levels >150 pg/ml exhibited a significantly shorter
survival time.
 | Table IV.Prognostic value of blood sOX40 level
for the overall survival by Cox proportional hazards model. |
Table IV.
Prognostic value of blood sOX40 level
for the overall survival by Cox proportional hazards model.
| Variables | No. | HR | 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
|
| Age, years |
|
|
|
|
|
≤70 | 9 | 1.00 |
|
|
|
>70 | 13 | 1.72 | 0.32–9.11 | 0.52 |
| Sex (male vs.
female) |
|
|
|
|
|
Female | 9 | 1.00 |
|
|
|
Male | 13 | 0.59 | 0.13–2.80 | 0.59 |
| WBC (cells/µl) |
|
|
|
|
|
≤9,000 | 17 | 1.00 |
|
|
|
>9,000 | 5 | 3.14 | 0.70–14.18 | 0.14 |
| LDH (U/l) |
|
|
|
|
|
≤400 | 17 | 1.00 |
|
|
|
>400 | 5 | 1.67 | 0.32–8.66 | 0.54 |
| Albumin (g/dl) |
|
|
|
|
|
≤3.8 | 8 | 1.00 |
|
|
|
>3.8 | 14 | 0.25 | 0.05–1.39 | 0.11 |
| CRP (mg/dl) |
|
|
|
|
|
≤0.4 | 12 | 1.00 |
|
|
|
>0.4 | 10 | 5.24 | 0.99–27.60 | 0.051 |
| CEA (ng/ml) |
|
|
|
|
|
≤50.0 | 15 | 1.00 |
|
|
|
>50.0 | 7 | 3.21 | 0.70–14.77 | 0.13 |
| CA19-9 (U/ml) |
|
|
|
|
|
≤100 | 11 | 1.00 |
|
|
|
>100 | 11 | 3.64 | 0.69–19.16 | 0.13 |
| Creatinine
(mg/dl) |
|
|
|
|
|
≤1.0 | 19 | 1.00 |
|
|
|
>1.0 | 3 | 0.83 | 0.10–6.95 | 0.86 |
| sOX40 |
|
|
|
|
|
≤150 | 11 | 1.00 |
|
|
|
>150 | 11 | 9.80 | 1.15–83.41 | 0.037 |
| Multivariate
analysis |
|
|
|
|
| Age
(≤70 vs. >70) | – | 0.97 | 0.12–7.94 | 0.98 |
| Sex
(male vs. female) | – | 0.93 | 0.11–8.28 | 0.95 |
|
Creatinine (≤1.0 vs.
>1.0) | – | 0.92 | 0.08–10.29 | 0.95 |
| sOX40
(≤150 vs. >150) | – | 9.67 | 1.07–87.59 | 0.044 |
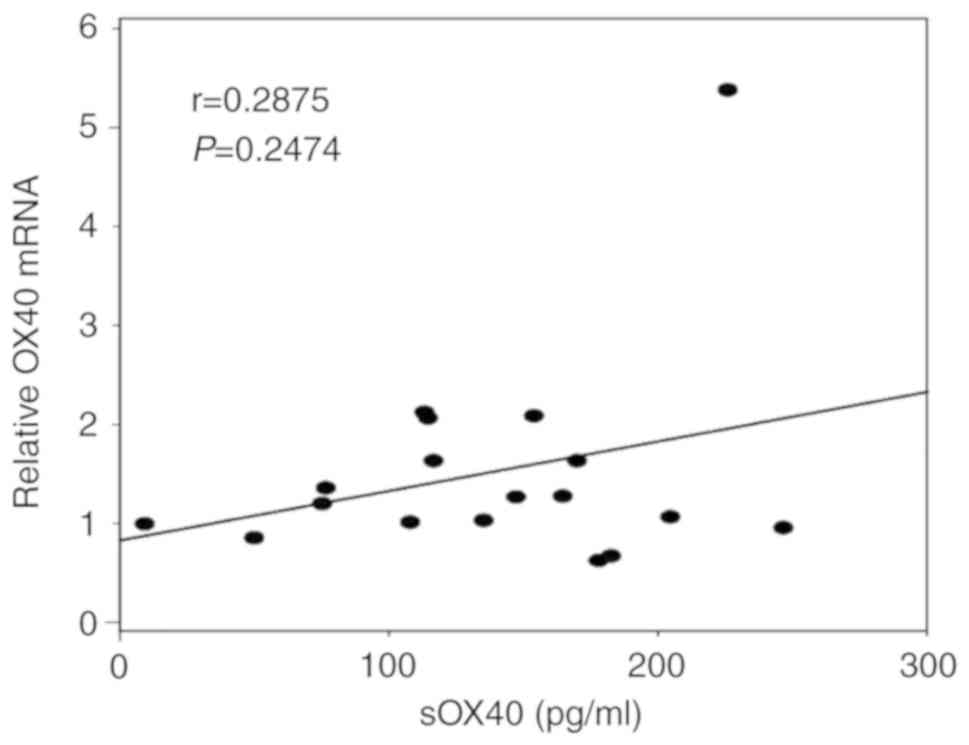
OX40 mRNA expression of the PBMCs of
patients is not associated with blood sOX40 levels
Jurkat cells activated by PMA treatment enhanced the
expression of CD69, an activation marker of T cells, and OX40
(Figs. 4 and S1) as well as the release of sOX40 into the
culture supernatants. PMA treatment also upregulated OX40 mRNA
expression in Jurkat cells (Fig. 4).
However, there was no correlation between OX40 mRNA expression in
the PBMCs of the patients and the blood sOX40 levels (P=0.2474)
(Fig. 5).
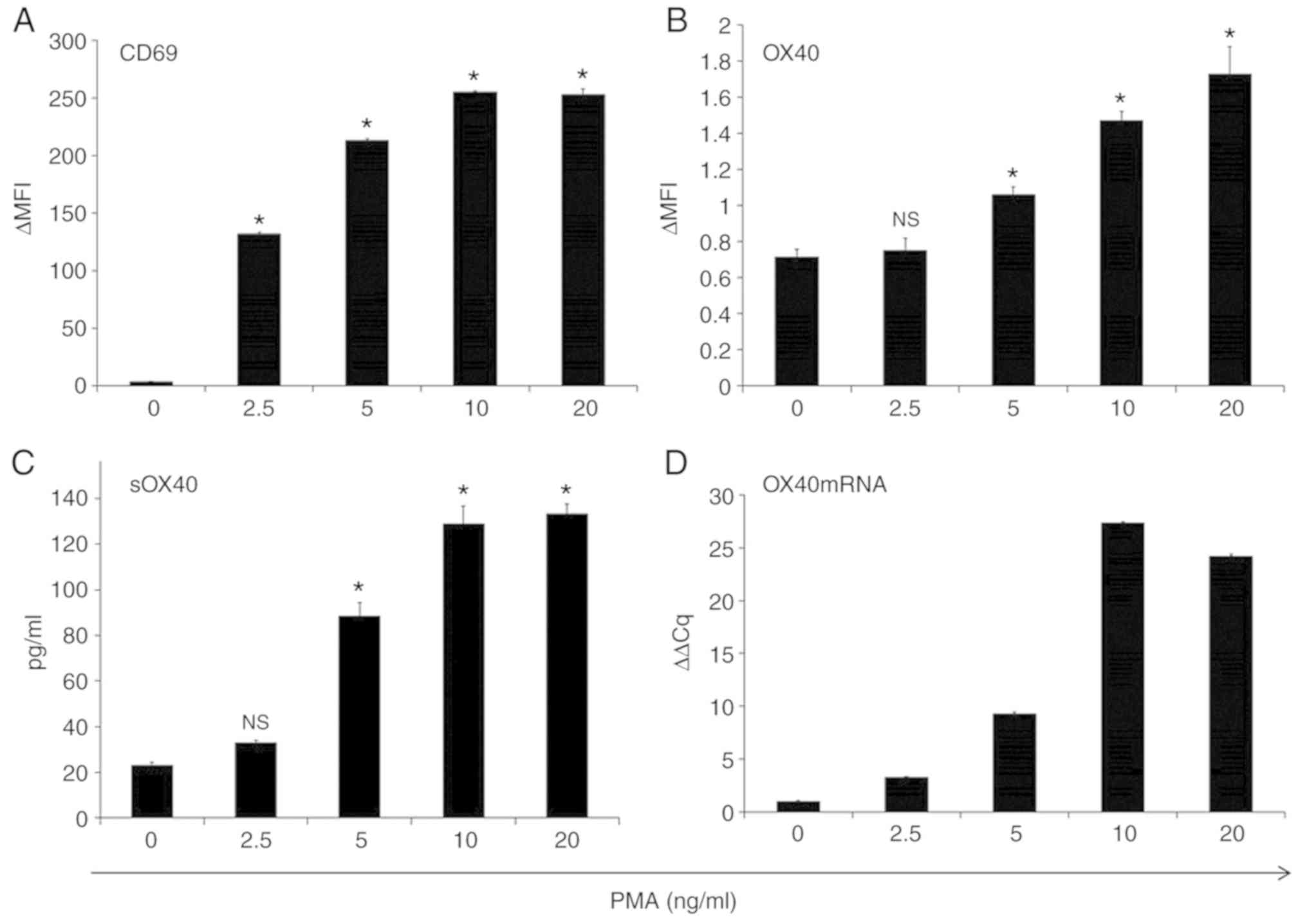 | Figure 4.PMA-activated Jurkat cells upregulate
OX40 mRNA expression and sOX40 production. Jurkat cells were seeded
in a 24-well plate (106/ml/well) and treated with PMA
(0, 2.5, 5, 10 and 20 ng/ml) for 4 h. After washing cells with PBS,
the cells were further incubated for 48 h in RPMI-1640 medium
supplemented with 10% FBS. The expression of (A) CD69 and (B) OX40
on the cell surface was analyzed by flow cytometry. The amount of
(C) sOX40 in the culture supernatants was determined by ELISA. (D)
OX40 mRNA expression was analyzed by qRT-PCR. OX40 mRNA is
expressed as relative values to OX40 mRNA at PMA 0 ng/ml, shown as
1. *P<0.01 vs. untreated samples. Error bars represent the SD.
PMA, phorbol 12-myristate 13-acetate; OX40, tumor necrosis factor
receptor superfamily, member 4; sOX40, soluble OX40; n.s., not
significant. |
Discussion
The present study demonstrated that the prognosis of
CRC patients with high blood levels of sOX40 was worse than those
with low blood levels of sOX40. The survival time of the patients
with high blood sOX40 levels was significantly shorter than those
with low blood sOX40 levels. It is conceivable that sOX40 in the
blood blocks the interaction between OX40 on T cells and OX40L on
APCs, resulting in the suppression of T cell activation and T
cell-mediated antitumor immunity. The artificially created
OX40-immunoglobulin (OX40-Ig) fusion protein revealed activity
resembling that of sOX40, preventing OX40 on T cells from reaching
OX40L on APC, thus reducing the T-cell response. Experiments in
mice have demonstrated that the administration of OX40-Ig reduces
the T cell-mediated immune response (24,28) and
that the administration of OX40L-Ig, conversely, enhances it,
including antitumor immunity (16,29). The
fact that a dense infiltration of TILs is associated with a
favorable prognosis of CRC indicates that intrinsic cellular
immunity may elicit a suppressive activity regarding the
development of CRC (2,3,5,6). The suppression of T-cell activation by
the blockade of the OX40/OX40L interaction by sOX40 may lead to the
promotion of tumor development. In fact, in the present study,
patients with higher blood levels of sOX40 exhibited higher blood
levels of CEA, CA19-9 and CRP as well as lower blood levels of
blood albumin. It has previously been reported that high blood
levels of sPD-L1 indicate a reduced survival time in non-small cell
lung cancer patients (30),
indicating that sPD-L1 exhibits an immune-suppressive activity that
promotes tumor development. In CRC patients, although sPD-L1 was
not associated with the prognosis of the patients, the blood levels
of sOX40 were well correlated with those of sPD-L1. These results
indicated that the soluble forms of immune checkpoint molecules may
modulate the status of antitumor immunity and affect the prognosis
of CRC patients.
The sOX40 concentration of ~150-250 pg/ml in the
blood of the CRC patients, however, appears to be too low to block
the functional OX40-OX40L interaction. It is hypothesized that the
levels of blood sOX40 would change according to the intensity of T
cell-mediated antitumor immune activity. This theory is suggested
by the result revealed in Fig. 3,
indicating that the release of sOX40 from T cells was significantly
elevated by the activation of T cells. It is possible that
activated T cells would be suppressed by sOX40-induced blockade of
OX40/OX40L, and then inactivated T cells would produce less sOX40.
Thus, it is surmised that, in the CRC patients with the sOX40
concentration of ~150-250 pg/ml, the antitumor T-cell response may
have already been suppressed under the condition of the blockade of
OX40/OX40L by sOX40. Fig. 1
demonstrates that one CRC patient exhibited a high level of blood
sOX40 with short survival. In this patient, antitumor T cells may
be activated first, however, subsequently, T-cell activation may
have been vigorously suppressed by the release of sOX40, resulting
in short survival.
Jurkat cells activated with PMA upregulated the
expression of OX40 mRNA and the release of sOX40 into the culture
supernatants. However, the blood levels of sOX40 in CRC patients
were not correlated with the expression of OX40 mRNA in the PBMCs
of patients. Two possible reasons for this discrepancy could be
considered: T cell activity was suppressed by the blockade of the
OX40/OX40L interaction by sOX40 released by activated T cells,
which is one of the autoregulatory feedback mechanisms that
regulates overactivated T cell immunity. The other is that immune
cells other than PBMCs actively release sOX40, of which activated T
cells in the tumor microenvironment are the most suspected. T cells
responding to the mutated tumor antigens in the tumor
microenvironment are activated and express various immune
checkpoint molecules that positively or negatively regulate T-cell
activity (1). It has been reported
that OX40+ T cells are infiltrated into the tumor
tissues of CRC with favorable prognosis (31). It is possible that activation status
of T cells eliciting significant surface OX40 expression but less
production of sOX40 is ideal for efficient T cell-mediated
antitumor immunity. However, further investigation is required
concerning this point.
Several preclinical studies demonstrated that the
stimulation of OX40 using an agonistic anti-OX40 antibody or the
OX40L/Ig fusion protein revealed promising antitumor activity
(29,32–34). In
addition to these therapies targeting OX40, new therapeutic
modalities could be established by the regulation of sOX40 in
blood. The depletion or absorption of sOX40 in blood could prevent
the immune-suppressive blockade of the OX40/OX40L interaction and
maintain T cells activated. The effect of immune checkpoint
blockade therapy on CRC is limited, and only CRCs with high
microsatellite instability are sensitive to the anti-PD1 antibody
therapy (35). The regulation of
sOX40 in the blood of CRC patients may shed light on new
immunotherapies against CRC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the American Journal
Experts for significant language editing. The authors would like to
thank Immuno-Biological Laboratories, Ltd. for providing the ELISA
kit of sOX40.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, YN, TN, MN and KA made substantial contributions
to the acquisition, analysis and interpretation of data. YA made
substantial contributions to the statistical analysis. SA was
involved in drafting the manuscript and revising it critically for
important intellectual content. YS made substantial contributions
to the assay experiment for sOX40. MS made substantial
contributions to the conception and design of the work and provided
final approval of the version to be published. SH made substantial
contributions to conception and design and provided final approval
of the version to be published. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of The Jikei University School of Medicine (30–397 9418)
and conducted in accordance with the Declaration of Helsinki. The
informed consent for participation in the study was obtained from
all participants. The patient, or parent, guardian or next of kin
(in case of deceased patients) provided written informed consent
for the publication of any associated data and accompanying images.
The consent form is made available to the Editor if requested, and
will be treated confidentially.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APC
|
antigen-presenting cell
|
|
CEA
|
carcinoembryonic antigen
|
|
CRP
|
C-reactive protein
|
|
CRC
|
colorectal cancer
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IFN
|
interferon
|
|
IL
|
interleukin
|
|
OX40L
|
OX40 ligand
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PD-1
|
programmed cell death-1
|
|
PD-L1
|
programmed cell death-ligand 1
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
References
|
1
|
Anari F, Ramamurthy C and Zibelman M:
Impact of tumor microenvironment composition on therapeutic
responses and clinical outcomes in cancer. Future Oncol.
14:1409–1421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huh JW, Lee JH and Kim HR: Prognostic
significance of tumor-infiltrating lymphocytes for patients with
colorectal cancer. Arch Surg. 147:366–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teng F, Mu D, Meng X, Kong L, Zhu H, Liu
S, Zhang J and Yu J: Tumor infiltrating lymphocytes (TILs) before
and after neoadjuvant chemoradiotherapy and its clinical utility
for rectal cancer. Am J Cancer Res. 5:2064–2074. 2015.PubMed/NCBI
|
|
5
|
Lee WS, Kang M, Baek JH, Lee JI and Ha SY:
Clinical impact of tumor-infiltrating lymphocytes for survival in
curatively resected stage IV colon cancer with isolated liver or
lung metastasis. Ann Surg Oncol. 20:697–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and
CD8+ T cell densities in distant metastasis is a robust
prognostic marker for advanced colorectal cancer. Oncotarget.
7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasuda K, Nirei T, Sunami E, Nagawa H and
Kitayama J: Density of CD4(+) and CD8(+) T lymphocytes in biopsy
samples can be a predictor of pathological response to
chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 6:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halama N, Michel S, Kloor M, Zoernig I,
Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G,
Luber B, et al: Localization and density of immune cells in the
invasive margin of human colorectal cancer liver metastases are
prognostic for response to chemotherapy. Cancer Res. 71:5670–5677.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanis E, Julié C, Emile JF, Mauer M,
Nordlinger B, Aust D, Roth A, Lutz MP, Gruenberger T, Wrba F, et
al: Prognostic impact of immune response in resectable colorectal
liver metastases treated by surgery alone or surgery with
perioperative FOLFOX in the randomised EORTC study 40983. Eur J
Cancer. 51:2708–2717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foell J, Hewes B and Mittler RS: T cell
costimulatory and inhibitory receptors as therapeutic targets for
inducing anti-tumor immunity. Curr Cancer Drug Targets. 7:55–70.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J and Gu J: Efficacy and safety of PD-1
inhibitors for treating advanced melanoma: A systematic review and
meta-analysis. Immunotherapy. 10:1293–1302. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valecha GK, Vennepureddy A, Ibrahim U,
Safa F, Samra B and Atallah JP: Anti-PD-1/PD-L1 antibodies in
non-small cell lung cancer: The era of immunotherapy. Expert Rev
Anticancer Ther. 17:47–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishii N, Takahashi T, Soroosh P and
Sugamura K: OX40-OX40 ligand interaction in T-cell-mediated
immunity and immunopathology. Adv Immunol. 105:63–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Croft M, So T, Duan W and Soroosh P: The
significance of OX40 and OX40L to T-cell biology and immune
disease. Immunol Rev. 229:173–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gramaglia I, Weinberg AD, Lemon M and
Croft M: Ox-40 ligand: A potent costimulatory molecule for
sustaining primary CD4 T cell responses. J Immunol. 161:6510–6517.
1998.PubMed/NCBI
|
|
16
|
Gramaglia I, Jember A, Pippig SD, Weinberg
AD, Killeen N and Croft M: The OX40 costimulatory receptor
determines the development of CD4 memory by regulating primary
clonal expansion. J Immunol. 165:3043–3050. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bansal-Pakala P, Halteman BS, Cheng MH and
Croft M: Costimulation of CD8 T cell responses by OX40. J Immunol.
172:4821–4825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vu MD, Xiao X, Gao W, Degauque N, Chen M,
Kroemer A, Killeen N, Ishii N and Li XC: OX40 costimulation turns
off Foxp3+ Tregs. Blood. 110:2501–2510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
So T and Croft M: Cutting edge: OX40
inhibits TGF-beta- and antigen-driven conversion of naive CD4 T
cells into CD25+Foxp3+ T cells. J Immunol.
179:1427–1430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinberg AD, Rivera MM, Prell R, Morris A,
Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C and Shields J:
Engagement of the OX-40 receptor in vivo enhances antitumor
immunity. J Immunol. 164:2160–2169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piconese S, Valzasina B and Colombo MP:
OX40 triggering blocks suppression by regulatory T cells and
facilitates tumor rejection. J Exp Med. 205:825–839. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan PY, Zang Y, Weber K, Meseck ML and
Chen SH: OX40 ligation enhances primary and memory cytotoxic T
lymphocyte responses in an immunotherapy for hepatic colon
metastases. Mol Ther. 6:528–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka Y, Takahashi Y, Tanaka R, Miyagi T,
Saito M and Fukushima T: Association of high levels of plasma OX40
with acute adult T-cell leukemia. Int J Hematol. 109:319–327. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weinberg AD, Wegmann KW, Funatake C and
Whitham RH: Blocking OX-40/OX-40 ligand interaction in vitro and in
vivo leads to decreased T cell function and amelioration of
experimental allergic encephalomyelitis. J Immunol. 162:1818–1826.
1999.PubMed/NCBI
|
|
25
|
Weiss A, Wiskocil RL and Stobo JD: The
role of T3 surface molecules in the activation of human T cells: A
two-stimulus requirement for IL 2 production reflects events
occurring at a pre-translational level. J Immunol. 133:123–128.
1984.PubMed/NCBI
|
|
26
|
Hayashi K, Nagasaki E, Kan S, Ito M,
Kamata Y, Homma S and Aiba K: Gemcitabine enhances
rituximab-mediated complement-dependent cytotoxicity to B cell
lymphoma by CD20 upregulation. Cancer Sci. 107:682–689. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higgins LM, McDonald SA, Whittle N,
Crockett N, Shields JG and MacDonald TT: Regulation of T cell
activation in vitro and in vivo by targeting the OX40-OX40 ligand
interaction: Amelioration of ongoing inflammatory bowel disease
with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG
fusion protein. J Immunol. 162:486–493. 1999.PubMed/NCBI
|
|
29
|
Sadun RE, Hsu WE, Zhang N, Nien YC,
Bergfeld SA, Sabzevari H, Lutsiak ME, Khawli L, Hu P and Epstein
AL: Fc-mOX40L fusion protein produces complete remission and
enhanced survival in 2 murine tumor models. J Immunother.
31:235–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okuma Y, Hosomi Y, Nakahara Y, Watanabe K,
Sagawa Y and Homma S: High plasma levels of soluble programmed cell
death ligand 1 are prognostic for reduced survival in advanced lung
cancer. Lung Cancer. 104:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weixler B, Cremonesi E, Sorge R, Muraro
MG, Delko T, Nebiker CA, Däster S, Governa V, Amicarella F, Soysal
SD, et al: OX40 expression enhances the prognostic significance of
CD8 positive lymphocyte infiltration in colorectal cancer.
Oncotarget. 6:37588–37599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sagiv-Barfi I, Czerwinski DK, Levy S, Alam
IS, Mayer AT, Gambhir SS and Levy R: Eradication of spontaneous
malignancy by local immunotherapy. Sci Transl Med. 10(pii):
eaan44882018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Metzger TC, Long H, Potluri S, Pertel T,
Bailey-Bucktrout SL, Lin JC, Fu T, Sharma P, Allison JP and Feldman
RM: ICOS promotes the function of CD4+ effector T cells
during Anti-OX40-mediated tumor rejection. Cancer Res.
76:3684–3689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gough MJ, Ruby CE, Redmond WL, Dhungel B,
Brown A and Weinberg AD: OX40 agonist therapy enhances CD8
infiltration and decreases immune suppression in the tumor. Cancer
Res. 68:5206–5215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mehrvarz Sarshekeh A, Overman MJ and
Kopetz S: Nivolumab in the treatment of microsatellite instability
high metastatic colorectal cancer. Future Oncol. 14:1869–1874.
2018. View Article : Google Scholar : PubMed/NCBI
|