Introduction
Melanoma is a malignant cancer originating from
melanocytes, which often transforms from a single deteriorated
melanocyte or a benign but dysfunctional mole (1). Melanoma is the deadliest skin
malignancy, which tends to affect younger people, showing an
alarming incidence that is increasing at ~3% annually (2). Chemotherapy is an integral part of
melanoma therapy. As a new alkylating agent that can be ingested
orally, temozolomide (TMZ) features high bioavailability, wide
tissue distribution, and can pass through the blood-brain barrier
to reach an effective concentration in the central nervous system.
TMZ is also recommended as an oral alternative to dacarbazine
(DTIC) in many countries (3,4). However, the drug resistance and
instability of TMZ limit its therapeutic efficacy in melanoma.
Thus, effective drug delivery has attracted increasing research
attention.
Advances in nanotechnology have led to developments
in the diagnosis and treatment of tumours (5). The biological structure and
physicochemical properties of nanoparticles, such as small-size
effect, surface functionality, multi-function effect and
photo-magnetic properties, have prompted researchers to explore
their biomedical applications (6,7). The
characteristics of nanoparticles, such as unique passive targeting
effect and slow release, allow them to act as carriers to deliver
drugs to the target site and achieve continuous drug release in
tumours, effectively extending the half-life of drugs, while
reducing their cytotoxicity to normal tissues (8). Although nano-delivery systems loaded
with various chemotherapeutic drugs have achieved satisfactory
results in the treatment of various cancers in recent decades, the
monotherapy mode still features numerous deficiencies, such as poor
drug release, requirement of multiple drug administration and poor
efficacy. Photothermal therapy (PTT), as a promising alternative
choice to traditional cancer therapies, uses photothermal
conversion agents to destroy tumour cells by converting light into
heat, and has attracted increasing attention (9). However, numerous problems limit the
application of individual PPTs, including the difficulty of
maintaining a constant temperature and duration, uneven heat
distribution inside the tumour and incomplete tumour ablation.
Therefore, the combination of PTT and chemotherapy in nanoparticles
has recently attracted increasing attention (10).
To date, multiple nanocarriers, such as gold and
carbon nanomaterials, have been used in combined
thermo-chemotherapy (11,12). However, given the poor degradability
and high cost of such methods, researchers have focused on the
biomedical application of near-infrared (NIR) dyes (13). For example, chemotherapeutic drugs and
NIR dyes were encapsulated in nanoparticles, and photothermal
conversion of NIR dyes was used to degrade the nanocarriers, in
order to achieve ‘controlled release’ of the drugs (14,15). The
novel indocyanine green dye IR820, as a derivative of indocyanine
green (ICG), possesses excellent stability and a longer tissue
retention time than ICG (16,17). Under NIR laser irradiation, IR820 can
produce local heat and generate cytotoxic effects when the
temperature exceeds the critical value (42.5–43°C) (18). IR820 is easily metabolized by the
liver in vivo. Thus, encapsulating IR820 in nanoparticles
could improve its stability and local concentration in tumour
sites. Li et al (19)
constructed polymeric micelles loaded with the chemotherapy drug
docetaxel (DTX) and IR820, and studied their combination treatment
for breast cancer. With the help of nanocarriers, IR820
successfully accumulated in the tumour site. NIR laser irradiation
increased the temperature of the tumour site and enhanced the
sensitivity of cancer cells to DTX. Recent years have witnessed the
rapid development of nanocarriers, such as polymeric nanoparticles,
micelles, dendrimers and lipid nanocarriers. Core-shell lipid
nanoparticles comprise a novel drug delivery system that includes a
polymeric core and a phospholipid complex shell. Different drugs,
genes or proteins can be loaded in the shell or core to achieve
programmed drug release. In addition, core-shell lipid
nanoparticles can also achieve simultaneous encapsulation of
hydrophilic and hydrophobic drugs (20). The lipid shell is usually composed of
lecithin, dipalmitoyl phosphatidylcholine (DPPC), and dioleoyl
phosphoethanolamine. Polylactide-co-glycolic acid (PLGA),
polycaprolactone, and dextran are the common biodegradable polymers
used as the core of core-shell lipid nanoparticles (21). DPPC is the main component of
thermosensitive liposomes, which can generate a gel-to-liquid phase
transition in response to local hyperthermia (41.5–41.9°C), thus
triggering the release of a large amount of drug (22,23). PLGA,
approved by the U.S. Food and Drug Administration, is a promising
biocompatible and degradable polymer that can be rapidly removed
from the body, and is an ideal nanomaterial for drug delivery
(24,25). Li et al (26) designed a delivery system consisting of
a DOX-modified PLGA core surrounded by a DPPC shell to treat breast
cancer. Compared with free DOX, the delivery system exhibited a
stronger antitumor effect at a lower concentration.
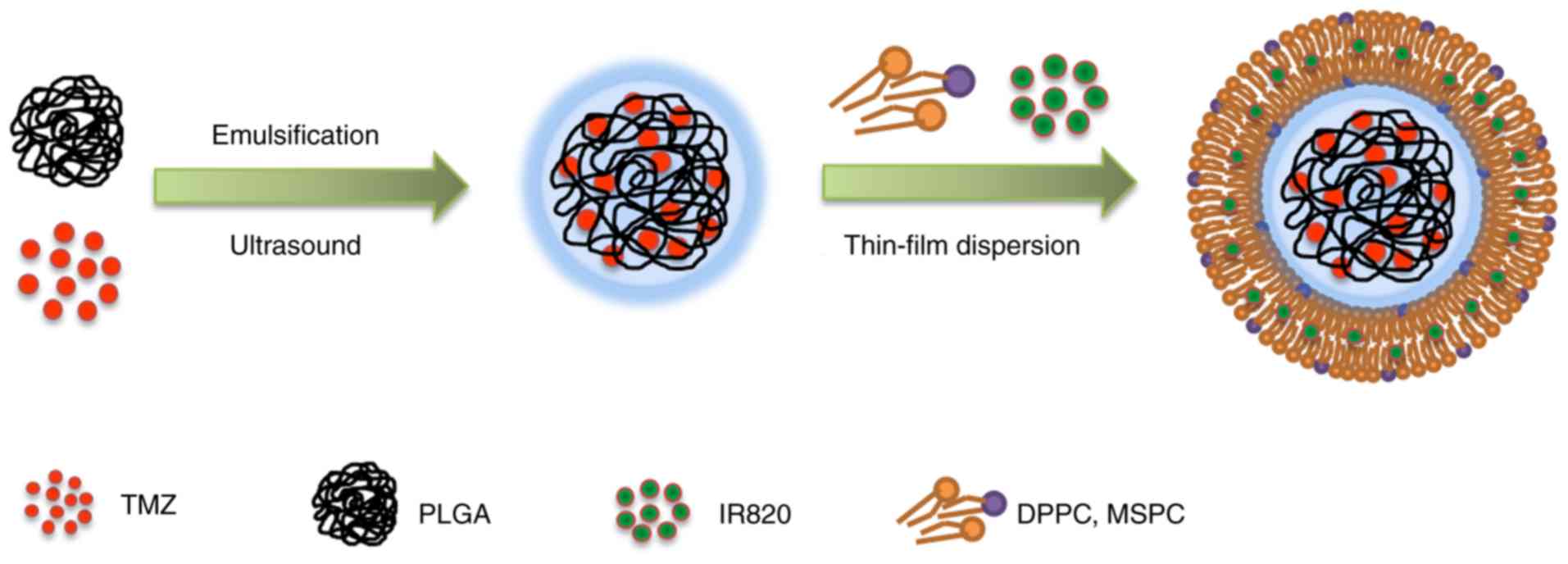
In the present study, core-shell type
thermonanoparticles (CSTNPs) co-loaded with TMZ and IR820 (termed
IT-CSTNPs) were developed as a potential chemical and photothermal
combination therapy to treat melanoma. First, TMZ and IR820 were
encapsulated in PLGA and DPPC to prepare PLGA-TMZ nanoparticles and
IR820-DPPC lipid shells, respectively. In the next step, the
IR820-DPPC lipid shells were used to coat the PLGA-TMZ
nanoparticles in order to form the IT-CSTNPs. Finally, melanoma
cells were exposed to the IT-CSTNPs and their uptake capacity and
intracellular localization were investigated in vitro.
Materials and methods
Materials
PLGA was purchased from Xi'an Ruixi Biotechnology
Co., Ltd. DPPC and monostearoylphosphatidylcholine (MSPC) were
purchased from Avanti Polar Lipids Co., Ltd. IR820 and polyvinyl
alcohol (PVA) were purchased from Sigma-Aldrich (Merck KGaA). TMZ
was obtained from Tokyo Chemical Industry Co., Ltd.
4–6-Diamidino-2-phenylindole (DAPI) and coumarin-6 were purchased
from Beijing Soleboard Biotechnology Co., Ltd. Lyso-Tracker Red was
purchased from Shanghai Biyuntian Biotechnology Co., Ltd. Other
solvents and reagents were purchased from Beijing Chemical
Works.
Preparation of IT-CSTNPs
PLGA-TMZ nanoparticles were synthesized using
double-emulsion solvent evaporation. First, 1 mg of TMZ was
dissolved in HCl (200 µl, 0.1 M) to form an aqueous phase. Then, 10
mg of PLGA was dissolved in 2 ml of dichloromethane (DCM) to form
an oil phase. The aqueous phase was added into the oil phase, and
the obtained mixture was emulsified for 120 sec using an ultrasonic
processor to form the initial emulsion. Then, the emulsion was
added into PVA (1%, 10 ml) solution drop by drop and emulsified
again. The homogeneous emulsion was stirred at room temperature
overnight to devolatilize the DCM. PLGA-TMZ nanoparticles was
collected by centrifugation at 16,654 × g for 20 min at 4°C and
washed three times with ultrapure water for subsequent use.
DPPC, MSPC, and IR820 (mass ratio, 14:1:1) were
placed in a 50 ml round-bottom flask with the addition of 5 ml of
chloroform/methanol (volume ratio, 4:1) solution to dissolve the
lipids, and the organic solvent was removed using rotary
evaporation under reduced pressure at 40°C to yield a thin lipid
film. The film was subsequently hydrated with phosphate-buffered
saline (PBS) solution of TMZ-NPs, followed by ultrasound for 5 min.
The spontaneously formed nanoparticles were extruded three times
through 450 and 220 nm polyethersulfone ultrafiltration membranes,
respectively. The unloaded drugs and lipids were removed by
centrifugation at 10,281 × g for 15 min at 4°C. To assess the
uptake and intracellular localization of nanoparticles, fluorescent
coumarin-6-labeled IT-CSTNPs was prepared using the same
process.
Characterization of IT-CSTNPs
The hydrodynamic size, polydispersity index (PDI),
and ζ potential of the IT-CSTNPs were measured using dynamic light
scattering (DLS) with a Zetasizer (Zetasizer Nano ZS; Malvern
Instruments, Ltd.). The nanoparticle morphology was measured using
transmission electron microscopy (TEM; FEI Tecnai G2 F20 U-TWIN;
Thermo Fisher Scientific, Inc.). Briefly, one drop of IT-CSTNP
suspension was deposited onto a carbon-coated copper grid, stained
with 1% (w/v) uranyl acetate for 1 min, dried at room temperature,
and observed by TEM.
TMZ and IR820 degradation curves
To accurately measure the TMZ content, the stability
of TMZ was first determined in PBS at pH 7.4, in 0.1 M HCl, and in
dimethylformamide (DMF). TMZ solutions at 25 µg/ml were prepared
with the corresponding solvents and then scanned in the wavelength
range of 200–400 nm to obtain the ultraviolet absorption spectrum
and determine the maximum absorption wavelength. The ultraviolet
absorption curve was monitored at intervals (0, 1, 2, 4, 6 and 24
h). The maximum absorption wavelength of IR820 was determined using
the same method.
Encapsulation efficiency (EE) of
IT-CSTNPs
Ultraspeed centrifugation at 10,281 × g for 15 min
at 4°C was used to separate the uncoated drugs, and the pellet and
supernatant after each centrifugation were collected and subjected
to demulsification with DMF. The absorbance of drugs in DMF was
determined using an ultraviolet spectrophotometer.
The EE of the IT-CSTNPs was calculated as follows:
EE (%)=(C1×V1)/(C1×V1+ C2×V2) ×100; where V1 and V2 refer to the
volume of collected pellet and supernatant, respectively; and C1
and C2 are the drug concentrations of the collected pellet and
supernatant, respectively, calculated according to the standard
curve of ultraviolet absorption value-concentration.
Temperature increase curve under NIR
laser irradiation
A NIR laser (808 nm, 6 W/cm2) was used to
irradiate the PBS solution (pH 7.4) containing IT-CSTNPs (IR820
concentrations, 0, 5, 10 and 20 µg/ml) for 5 min at an initial
temperature of 24.7°C. The temperature increase values of the
IT-CSTNP solution at different time-points were recorded using an
IR thermometer.
The PBS solution at pH 7.4 with or without 20 µg/ml
of free IR820 and IT-CSTNPs (IR820 concentration, 20 µg/ml) were
irradiated with NIR laser at 6 W/cm2 for 4.75 min.
Temperature was recorded using an IR thermometer to plot the
temperature increase curve.
In vitro TMZ release
The dialysis-bag method was used to investigate TMZ
release under different pH conditions. A total of 700 µl of
IT-CSTNPs (TMZ content, 54 µg) were added into three identical
dialysis bags (molecular weight cut-off, 8,000-14,000 Da). The two
ends were tightened with thin wires and placed in three 50 ml
centrifuge tubes. Then, PBS solutions at pH 5.0, 6.5 and 7.4 were
added into the centrifuge tubes separately. The centrifuge tubes
were placed on a constant-temperature oscillator at 100 rpm at 37°C
to simulate the release of TMZ in vitro. After removing 1 ml
of the released solution at given intervals, 1 ml of the
corresponding fresh PBS was added to the centrifuge tubes.
Absorption values of liquids at 329 nm and the concentration and
cumulative drug release rate were calculated at corresponding
time-points.
NIR-induced drug release in vitro was also
investigated. After simulating drug release on a shaker for 30 min,
the IT-CSTNPs were aspirated from the dialysis bag and transferred
to 1.5 ml Eppendorf tubes. The tubes were then irradiated using a
NIR laser (6 W/cm2) for 5 min and transferred into the
original dialysis bag. The simulated drug release was further
performed in a thermostatic oscillator. At different time-points, 1
ml of the release solution was obtained, and the absorption value
was measured at 329 nm. The concentration and cumulative drug
release rate at corresponding time-points were calculated.
Cell culture
Human melanoma cells (A375) were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and routinely grown in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% foetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc.), and 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were cultured in a 5% CO2-saturated humidity
incubator at 37°C.
Cellular uptake analysis
Uptake of IT-CSTNPs at different concentrations by
A375 cells was detected using an inverted fluorescence microscope
and flow cytometry. A375 cells, at the logarithmic growth phase,
were seeded in six-well plates at a density of 2×105
cells/well and cultured overnight. IT-CSTNPs labelled with
coumarin-6 (IR820 concentrations, 0, 5, 10 and 20 µg/ml) were added
to the culture medium. After incubation for 3 h, the cells were
washed three times with PBS and subsequently fixed with 4%
paraformaldehyde for 15 min at room temperature. Then, the nucleus
was stained with DAPI for 5 min, and the cells were observed under
an inverted fluorescence microscope. Flow cytometry was also used
to measure the cellular uptake. A375 cells were incubated with
IT-CSTNPs labelled with coumarin-6 (IR820 concentrations, 0, 5, 10
and 20 µg/ml) for 3 h and then digested with trypsin and collected
by centrifugation (300 × g, 5 min) at room temperature. The cells
were then washed with PBS and detected using a flow cytometer (BD
Biosciences), followed by analysis on FlowJo v10 software (Tree
Star, Inc.).
Intracellular localization
analysis
A375 cells at the logarithmic growth phase were
seeded in a confocal glass-bottom culture dish at a density of
1×105 cells and incubated overnight at 37°C. IT-CSTNPs
labelled with coumarin-6 (IR820, 20 µg/ml) were added to the
culture medium. After 3 h of incubation, the culture medium was
discarded, and the cells were gently washed three times with PBS.
Then, 1 ml of Lyso-Tracker Red dye (50 nM) medium was added to the
dishes and incubated for 1 h at 37°C. After washing the cells with
PBS, images were captured under a laser confocal microscope.
Statistical analysis
All values are presented as the mean ± standard
deviation. The statistical significance of the differences between
experimental groups was calculated using either unpaired Student's
t-test or one-way ANOVA followed by Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Preparation and characterization of
IT-CSTNPs
The IT-CSTNPs were prepared using a two-step
synthesis method. PLGA-TMZ nanoparticles were synthesized by the
double-emulsion solvent evaporation method. IR820 was dispersed
into DPPC to form a lipid film using thin-film dispersion. Then,
the IT-CSTNPs were synthesized through hydration, ultrasonication
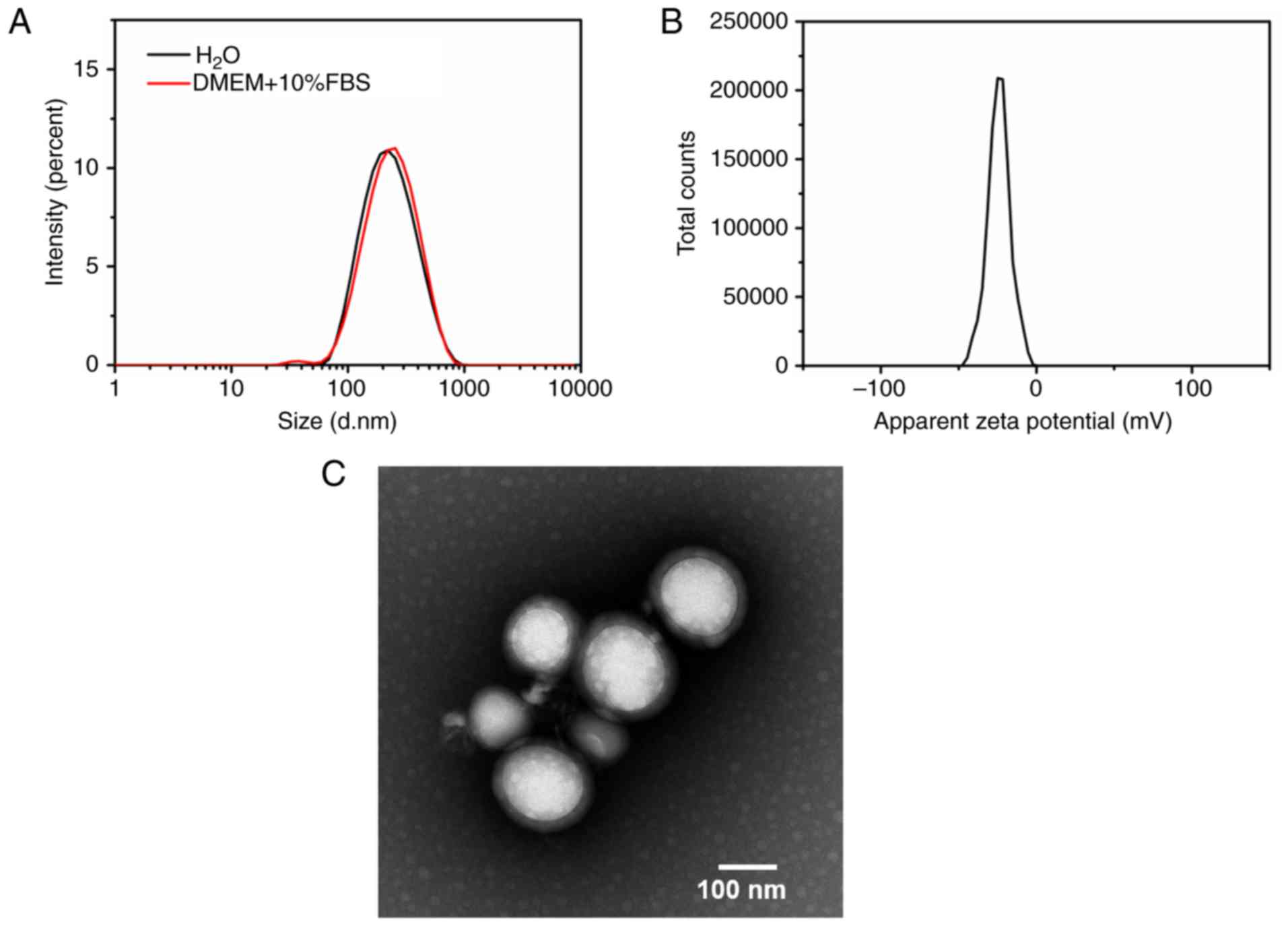
and extrusion (Fig. 1). DLS was
applied to characterize the particle size, PDI and ζ potential of
the IT-CSTNPs. The results demonstrated that the average
hydrodynamic size of an IT-CSTNP was about 196.4±3.1 nm, the PDI
was 0.181±0.03, and the ζ potential was −24.9±1.3 mV (Fig. 2A and B). In addition, the particle
size was measured in DMEM+10% FBS using DLS, and the results
demonstrated that the hydrodynamic size of the IT-CSTNPs was not
significantly altered, indicating that the nanoparticles could
maintain good integrity and stability in cell culture conditions
(Fig. 2A). The morphology of the
IT-CSTNPs was observed using TEM. The image revealed that the
IT-CSTNPs were spherical, uniform in size and exhibited no
significant agglomeration; a core-shell structure could be
observed, indicating that the PLGA core was successfully surrounded
by a lipid shell (Fig. 2C).
EEs of TMZ and IR820
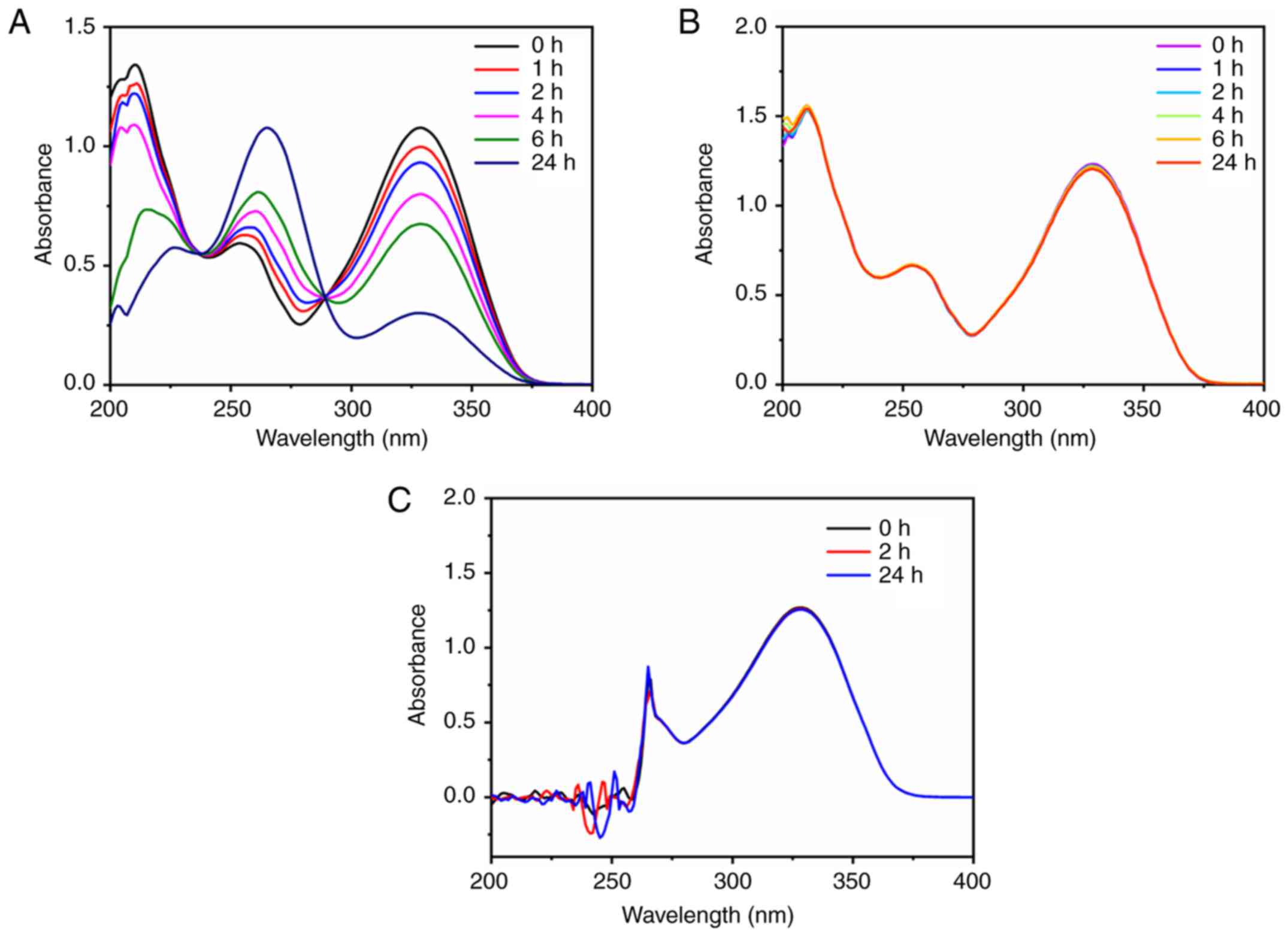
Next, the UV absorption method was used to determine
the content of TMZ and IR820. TMZ was unstable in PBS solution, and
its maximum absorption peak at 329 nm decreased with time (Fig. 3A). However, the UV absorption curve of
TMZ in 0.1 M HCl exhibited no significant change with time, and had
a maximum absorption peak at 329 nm, indicating that 0.1 M HCl
could be used as the water phase in double-emulsion solvent
evaporation to prevent TMZ degradation to a certain extent during
the synthesis process (Fig. 3B). The
maximum absorption wavelength and stability of TMZ was further
investigated in DMF. The results demonstrated that TMZ exhibited
the largest absorption peak at 329 nm and no degradation within 24
h, suggesting that TMZ was stable in DMF solution; therefore, DMF
could be used as the demulsification solvent (Fig. 3C). According to the aforementioned
formula, the EEs of TMZ and IR820 were 6.1 and 16.6%,
respectively.
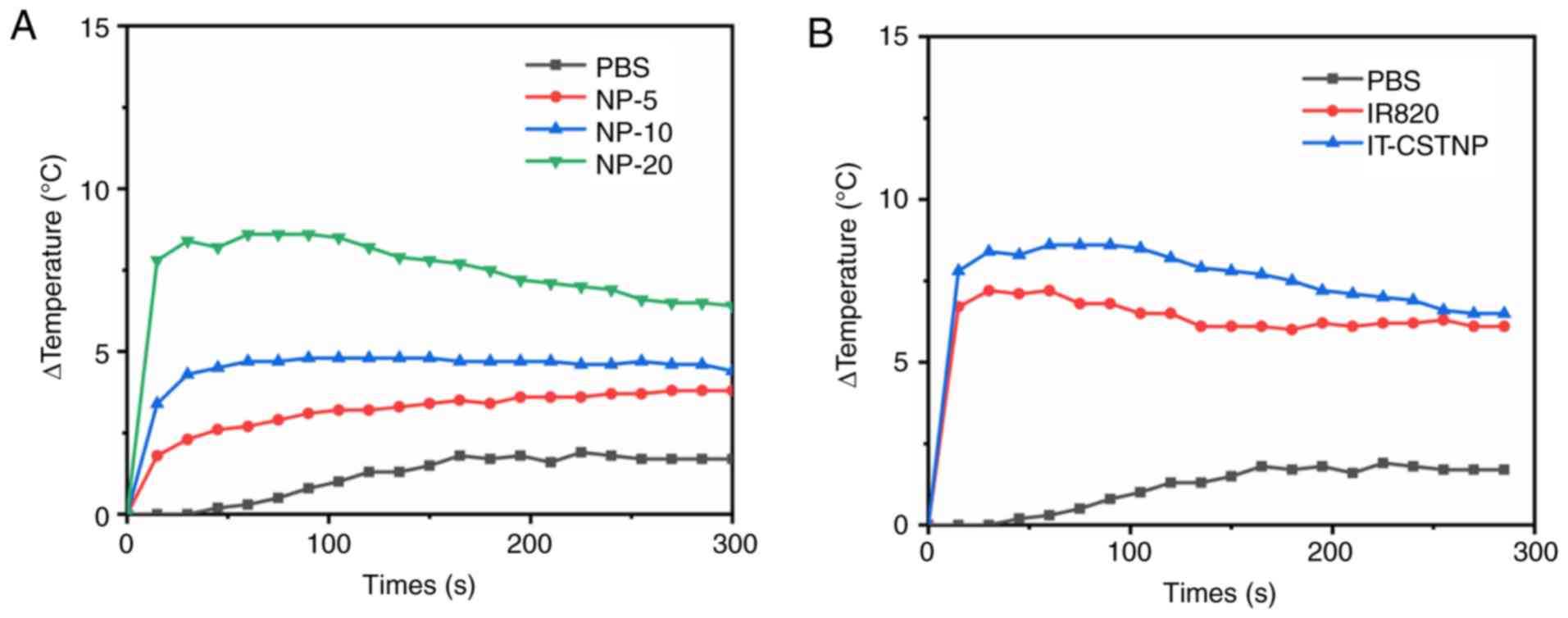
Temperature increase curve of the
IT-CSTNPs
The increasing temperature capacities of IT-CSTNPs
at different concentrations under NIR laser irradiation were
measured, in order to detect their photothermal conversion
capability. As presented in Fig. 4A,
the temperature of the IT-CSTNP solution increased as the drug
concentration increased under NIR laser irradiation. When the
concentration of IT-CSTNPs was 10 µg/ml, the maximum temperature
was 4.8°C, and at 20 µg/ml the maximum temperature was 8.6°C.
Furthermore, at the same IR820 concentration (20 µg/ml), the
increased temperature of the IT-CSTNPs was slightly higher compared
with that of free IR820 (Fig. 4B),
indicating that the IT-CSTNPs possessed a better photothermal
conversion capability than free IR820.
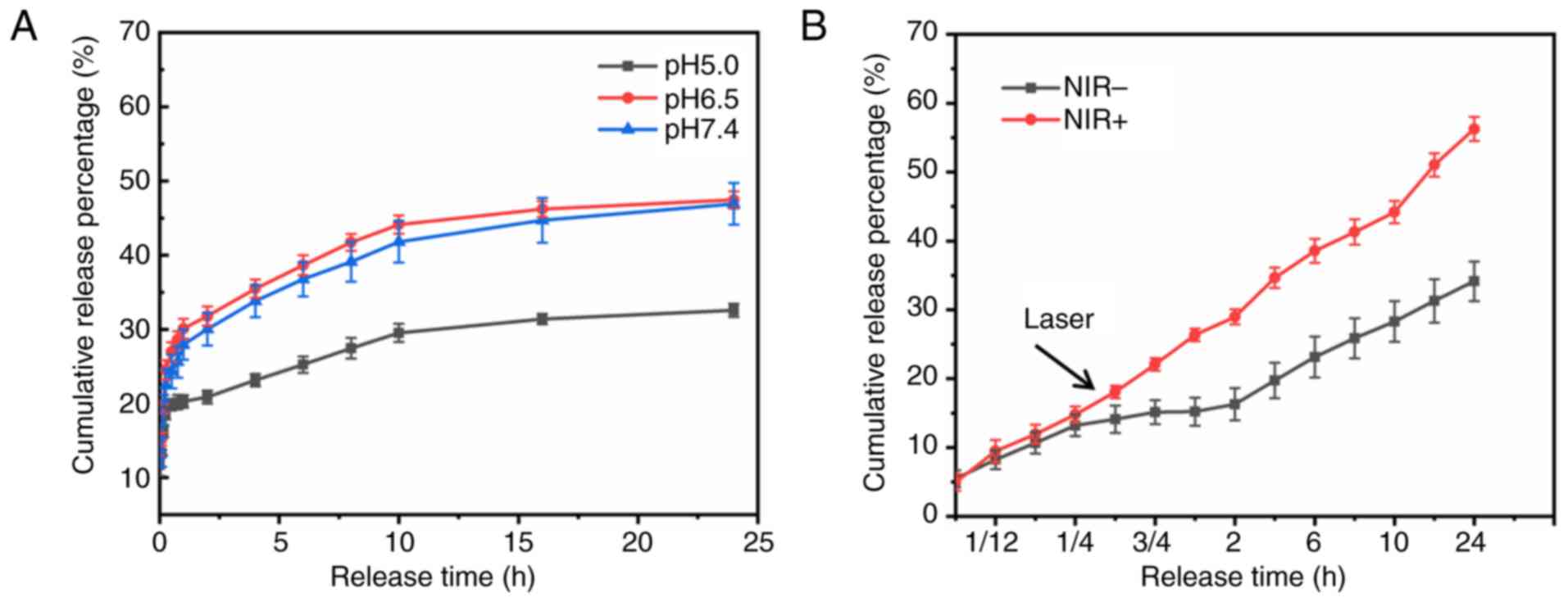
In vitro drug release profile
To examine the release properties of TMZ from
IT-CSTNPs, a simulated drug release experiment was performed.
Fig. 5A presents the drug release
profile of IT-CSTNPs in PBS at various pH values. In PBS solutions
pH 5.0, 6.5 and 7.4, the amount of TMZ released within 24 h reached
32.6, 47.4 and 46.9%, respectively.
In addition, the release of TMZ from IT-CSTNPs was
examined after NIR laser irradiation. The release curve in Fig. 5B demonstrates that drug release
increased significantly after 0.5 h of NIR laser irradiation; at 24
h, 56.3% of the TMZ was released in the NIR+ group
compared with only 34.1% in the NIR− group (Fig. 5B). These results indicated that the
high temperature generated by NIR laser irradiation promoted the
release of TMZ from the IT-CSTNPs.
Cellular uptake profile
Coumarin-6, a hydrophobic fluorescent molecule, was
encapsulated in the core-shell nanoparticles, in order to label the
whole nanoparticles and to trace their uptake and intracellular
localization. The results of inverted fluorescence microscopy
revealed that there was no green fluorescence distribution in the
control group. After coumarin-6-labeled IT-CSTNP exposure,
significant green fluorescence was observed in the A375 cells, and
the fluorescence intensity correlated positively with the
concentration of IT-CSTNPs, indicating that cellular
internalization of IT-CSTNPs by A375 cells was
concentration-dependent in the selected range.
The uptake of IT-CSTNPs was further quantified using
flow cytometry. With the increase in drug concentration, the
fluorescence signal of cells increased, exhibiting concentration
dependence (Fig. 6B; Table I). The result was consistent with the
results of inverted fluorescence microscopy.
 | Table I.Uptake of IT-CSTNPs by A375
cells. |
Table I.
Uptake of IT-CSTNPs by A375
cells.
| Experimental
groups | Average
fluorescence intensity (mean ± SD) |
|---|
| NP-0 (control) | 2746±79.9 |
| NP-5 |
10733.33±1011.789a |
| NP-10 |
21122±3372.866a,b |
| NP-20 |
28858.67±1825.35a–c |
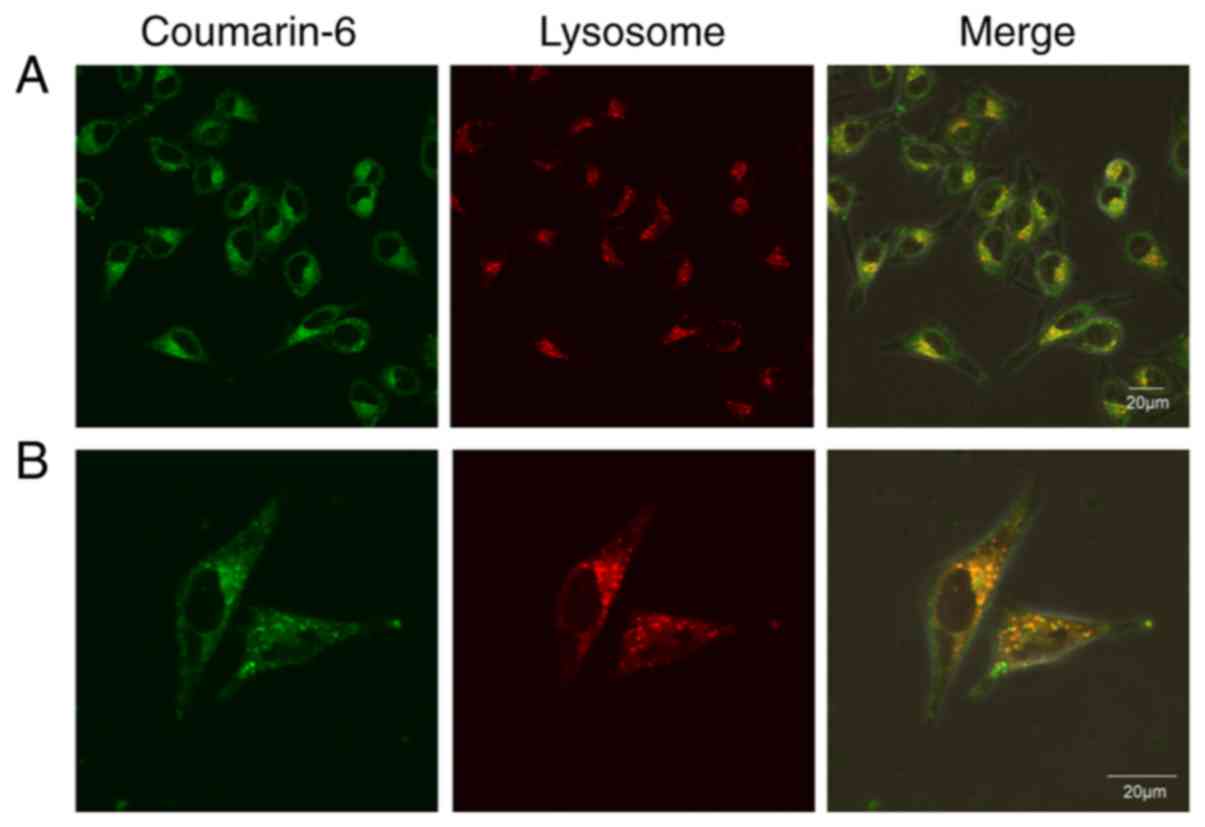
Intracellular localization
profile
Laser confocal microscopy images (Fig. 7) revealed the presence of substantial
amounts of green fluorescence in A375 cells, which was consistent
with the aforementioned results of inverted fluorescence microscopy
and flow cytometry (Fig. 6). After
staining the lysosomes with the specific Lyso-Tracker Red dye, it
was observed that the IT-CSTNP signal (green) coincided with that
of the lysosomes (red), indicating the entry of IT-CSTNPs into the
lysosomes. Therefore, it was concluded that IT-CSTNPs entered the
cells via endocytosis and were transported to lysosomes by
endocytic vesicles.
Discussion
Melanoma is a malignant cancer with insidious onset
and high invasiveness. In recent years, the incidence of melanoma
has increased continually globally and is showing a trend of
affecting a younger population, although its incidence in Asia is
lower than that in Europe and the United States (2). Melanoma is one of the main causes of
skin cancer-associated mortality. The median survival time of
patients is 6–10 months, and the five-year survival rate is <20%
(27,28). Early diagnosis and surgical resection
can improve the prognosis. Advanced melanoma progresses rapidly and
is prone to metastasis. Radiotherapy, chemotherapy, immunotherapy
and targeted therapy can be used to treat melanoma. However,
conventional treatments, such as radiotherapy and chemotherapy,
have very limited effects. TMZ, as a new second-generation oral
alkylating agent, has advantages, such as high bioavailability,
wide tissue distribution, the capability to pass the blood-brain
barrier and a favourable therapeutic effect to treat brain
metastatic melanoma. TMZ is recommended in numerous countries as an
oral replacement for DTIC. However, several problems such as drug
resistance and side effects still limit TMZ's application and
development. Therefore, there is an urgent need to develop
effective, safe and non-toxic anti-melanoma drugs.
In recent years, the rapid development of
nanomedicine has provided new insights into the challenges faced by
melanoma treatment. Nanoparticles have been used widely in
biomedical fields as highly effective and targeted drug carriers.
The increased vascular permeability of the tumour tissue and
dysfunction of lymphatic system reflux lead to an enhanced
permeation retention (EPR) effect, causing nanoparticles in the
range of 10–500 nm to easily aggregate at the tumour site,
representing the ‘passive targeting effect’ of nanoparticles. Thus,
drugs encapsulated in nanoparticles can be retained at the tumour
site by the EPR effect, and an effective concentration can be
reached to yield the optimal therapeutic effect while reducing
systemic toxicity (27,29). With continued research progress, the
design focus of nanoparticles is gradually moving toward more
complex core-shell structures using a single nano-delivery system
to combine multiple functions of different nanoparticles. Lipid
polymer hybrid nanoparticles are favoured by researchers because
they combine the structural stability of polymers with the biofilm
compatibility of lipid shells (21).
Lipid polymer hybrid nanoparticles contain at least two components:
A polymer and a lipid. PLGA is often used as the polymeric core
because of its good biodegradability and biocompatibility. DPPC is
used as the lipid shell to encapsulate PLGA to form a cell-like
structure. Simple PLGA nanoparticles are easily removed from blood
circulation via opsonization, and the exposed PLGA encounters
difficulty in entering cells. The lipid layer can serve as a
molecular fence to protect the PLGA core and improve nanoparticle
absorption by tumour cells (30).
In the present study, the TEM results revealed that
IT-CSTNPs comprising DPPC lipids and PLGA polymers were
successfully synthesized. The DLS results indicated that the
IT-CSTNPs were 196.4±3.1 nm in size, uniform, and monodispersed,
benefitting the passive targeting of tumour sites via the EPR
effect.
Although TMZ is listed as a first-line treatment for
malignant tumours in many countries, it is easily degradable under
physiological pH conditions, resulting in changes in the
ultraviolet absorption wavelength and thereby increasing the
difficulty of characterization. TMZ is more stable in an acidic
environment; therefore, 0.1 M HCl was used as the aqueous phase.
The present study used a lipid shell and polymer core to
encapsulate IR820 and TMZ and to synthesize IT-CSTNPs, and explored
their photothermal conversion capability under NIR laser
irradiation. The EEs of TMZ and IR820 measured via the ultraviolet
absorption method were 6.1 and 16.6%, respectively. The EE of TMZ
was consistent with the results of Ananta et al (31), whereas that of IR820 was slightly
lower than the 19.8% reported by Wu et al (16). Such results can be possibly attributed
to the poor solubility of TMZ in water, which made it difficult for
TMZ to embed in PLGA, whereas excess lipids could adsorb the drug
to form vesicles and be lost during hydration and extrusion. In
addition, the ‘two-step synthesis’ preparation method caused the
IR820 to release the lipid shell on the nanoparticle surface, and
this condition might be accompanied by the release of the drug from
the film, thereby affecting their EE values (32).
PTT is a safe and effective non-invasive treatment
that has been widely used in cancer treatment. Under irradiation by
an external light source, PTT uses the thermal effect of
photothermal conversion agents to convert light energy into heat;
thus, the temperature of the tumour site is raised to an effective
treatment temperature, thereby achieving the effect of directly or
indirectly killing the tumour cells (33,34). The
temperature increase curve generated in the present study revealed
that under NIR irradiation, and when the IT-CSTNPs concentration
was 20 µg/ml, the highest temperature rise could reach 8.6°C. If
the initial temperature was 37.5°C, the maximum temperature could
thus reach 46.1°C, and at a concentration of 10 µg/ml, the maximum
temperature that could be reached would be 42.3°C, which is within
the moderate thermal therapy range (41–43°C). This temperature not
only inhibits the growth of tumour cells, but also facilitates drug
release. In addition, the present results demonstrated that the
ability of IT-CSTNPs to increase the temperature at the same IR820
concentration was better compared with that of free IR820, which
might be caused by the accumulation of nanoparticles.
The results of NIR laser-induced drug release
experiments showed that, compared with the control group, the
NIR+ group released significantly more of the drug,
indicating that when the temperature increased to the phase
transition temperature, DPPC generated a gel-to-liquid phase
transition, thereby realizing the ‘burst release’ of the drug. In
addition, the pH gradient release curve showed no significant
difference in the percentage of release from IT-CSTNPs in PBS
solution at pH 6.5 and 7.4 within 24 h (47.4 and 46.9%,
respectively), with both values being greater than that observed at
pH 5.0 (32.6%). Possibly because the nanoparticles are negatively
charged, a large amount of H+ present in the acidic
environment wraps around them, resulting in the slow release of
TMZ.
The main challenge of traditional chemotherapy for
cancer is multidrug resistance; a predominant mechanism for this is
thought to occur through the activity of the transmembrane protein
P-glycoprotein (P-gp), which can transfer drugs out of tumour cells
(35). Drug-loaded nanoparticles can
enter tumour cells by endocytosis and be transported by endocytic
vesicles to lysosomes, thereby avoiding being pumped out by P-gp
(36). The results of flow cytometry
and inverted fluorescence microscopy revealed obvious green
fluorescence in the cells after incubation with IT-CSTNPs for 3 h;
the uptake of IT-CSTNPs in the different concentration groups was
significantly higher compared with that in the control group
(P<0.05; Table I) and increased
with increasing concentration (P<0.05; Table I). The results of laser confocal
microscopy showed that the signals for IT-CSTNPs (green) and for
the lysosomes (red) were fused, indicating that the nanoparticles
colocalized with the lysosomes, suggesting that IT-CSTNPs entered
cells via endocytosis and concentrated in the lysosomes.
In conclusion, the present study successfully
synthesized IT-CSTNPs with simultaneous loading of the chemotherapy
drugs TMZ and NIR dye IR820, and explored their photothermal
conversion capability, cellular uptake and intracellular
localization. The current findings are expected to provide new
directions for melanoma treatment.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (grant nos. 81572976, 81872493 and
81803151), the China Postdoctoral Science Foundation (grant nos.
2016M590505 and 2017T100407), the Jiangsu Provincial Medical Talent
Foundation, the ‘Six Talent Peaks’ Project of Jiangsu Province
(grant nos. WSW-074 and WSN-254), the Science and Technology
Project of Huai'an city (grant no. HAB201812), and the Innovation
of Graduate Student Training Projects in Jiangsu Province of China
(grant no. KYCX18_2187).
Availability of data and materials
The datasets supporting the conclusions of this
article are included within this article.
Authors' contributions
YL and XH designed the study. XH, YP, XL, CY and WL
performed the experiments and analysed the data. XH, YP and XL
wrote the manuscript. YL and GJ helped to revise the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Damsky WE and Bosenberg M: Melanocytic
nevi and melanoma: Unraveling a complex relationship. Oncogene.
36:5771–5792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tripp MK, Watson M, Balk SJ, Swetter SM
and Gershenwald JE: State of the science on prevention and
screening to reduce melanoma incidence and mortality: The time is
now. CA Cancer J Clin. 66:460–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiarion-Sileni V, Guida M, Ridolfi L,
Romanini A, Del Bianco P, Pigozzo J, Brugnara S, Colucci G, Ridolfi
R and De Salvo GL; Italian Melanoma Intergroup (IMI), : Central
nervous system failure in melanoma patients: Results of a
randomised, multicentre phase 3 study of temozolomide- and
dacarbazine-based regimens. Br J Cancer. 104:1816–1821. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim C, Lee CW, Kovacic L, Shah A, Klasa R
and Savage KJ: Long-term survival in patients with metastatic
melanoma treated with DTIC or temozolomide. Oncologist. 15:765–771.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jahangirian H, Kalantari K, Izadiyan Z,
Rafiee-Moghaddam R, Shameli K and Webster TJ: A review of small
molecules and drug delivery applications using gold and iron
nanoparticles. Int J Nanomedicine. 14:1633–1657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jindal AB: The effect of particle shape on
cellular interaction and drug delivery applications of micro- and
nanoparticles. Int J Pharm. 532:450–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshyar N, Gray S, Han H and Bao G: The
effect of nanoparticle size on in vivo pharmacokinetics and
cellular interaction. Nanomedicine (Lond). 11:673–692. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samanta D, Meiser JL and Zare RN:
Polypyrrole nanoparticles for tunable, pH-sensitive and sustained
drug release. Nanoscale. 7:9497–9504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheng W, He S, Seare WJ and Almutairi A:
Review of the progress toward achieving heat confinement-the holy
grail of photothermal therapy. J Biom Opt. 22:809012017. View Article : Google Scholar
|
|
10
|
Zhang X, Du J, Guo Z, Yu J, Gao Q, Yin W,
Zhu S, Gu Z and Zhao Y: Efficient near infrared light triggered
nitric oxide release nanocomposites for sensitizing mild
photothermal therapy. Adv Sci (Weinh). 6:18011222018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Acunto M: Detection of intracellular
gold nanoparticles: An overview. Materials (Basel). 11:E8822018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doughty ACV, Hoover AR, Layton E, Murray
CK, Howard EW and Chen WR: Nanomaterial applications in
photothermal therapy for cancer. Materials (Basel). 12:E7792019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Chung K, Lee S, Kim DH and Lee H:
Near-infrared light-responsive nanomaterials for cancer
theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol.
8:23–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou B, Li Y, Niu G, Lan M, Jia Q and
Liang Q: Near-infrared organic dye-based nanoagent for the
photothermal therapy of cancer. ACS Appl Mater Interfaces.
8:29899–29905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Chen Q, Zhu Q, Liu J, Li Y, Gao X,
Chen D and Zhu X: Small-molecular theranostic assemblies
functionalized by doxorubicin-hyaluronic acid-methotrexate prodrug
for multiple tumor targeting and imaging-guided combined
chemo-photothermal therapy. Mol Pharm. 16:2470–2480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu B, Wan B, Lu ST, Deng K, Li XQ, Wu BL,
Li YS, Liao RF, Huang SW and Xu HB: Near-infrared light-triggered
theranostics for tumor-specific enhanced multimodal imaging and
photothermal therapy. Int J Nanomed. 12:4467–4478. 2017. View Article : Google Scholar
|
|
17
|
Hu X, Tian H, Jiang W, Song A, Li Z and
Luan Y: Rational design of IR820- and ce6-based versatile micelle
for single NIR laser-induced imaging and dual-modal Phototherapy.
Small. 14:e18029942018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Peng J, Tan L, Wu J, Shi K, Qu Y,
Wei X and Qian Z: Mild photothermal therapy/photodynamic
therapy/chemotherapy of breast cancer by Lyp-1 modified
Docetaxel/IR820 Co-loaded micelles. Biomaterials. 106:119–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mukherjee A, Waters AK, Kalyan P, Achrol
AS, Kesari S and Yenugonda VM: Lipid-polymer hybrid nanoparticles
as a next-generation drug delivery platform: State of the art,
emerging technologies, and perspectives. Int J Nanomedicine.
14:1937–1952. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mandal B, Bhattacharjee H, Mittal N, Sah
H, Balabathula P, Thoma LA and Wood GC: Core-shell-type
lipid-polymer hybrid nanoparticles as a drug delivery platform.
Nanomedicine. 9:474–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Matos MBC, Beztsinna N, Heyder C, Fens
MHAM, Mastrobattista E, Schiffelers RM, Leneweit G and Kok RJ:
Thermosensitive liposomes for triggered release of cytotoxic
proteins. Eur J Pharm Biopharm. 132:211–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turner DC, Moshkelani D, Shemesh CS, Luc D
and Zhang H: Near-infrared image-guided delivery and controlled
release using optimized thermosensitive liposomes. Pharm Res.
29:2092–2103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong L, Liao Q, Zhao Y, Huang H, Gao A,
Zhang W, Gao X, Wei W, Guan M, Chu PK and Wang H: Near-infrared
light control of bone regeneration with biodegradable photothermal
osteoimplant. Biomaterials. 193:1–11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albert C, Huang N, Tsapis N, Geiger S,
Rosilio V, Mekhloufi G, Chapron D, Robin B, Beladjine M, Nicolas V,
et al: Bare and sterically stabilized PLGA nanoparticles for the
stabilization of pickering emulsions. Langmuir. 34:13935–13945.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Xu H, Li Z, Yao M, Xie M, Shen H,
Shen S, Wang X and Jin Y: Bypassing multidrug resistance in human
breast cancer cells with lipid/polymer particle assemblies. Int J
Nanomedicine. 7:187–197. 2012.PubMed/NCBI
|
|
27
|
Chen J, Shao R, Zhang XD and Chen C:
Applications of nanotechnology for melanoma treatment, diagnosis,
and theranostics. Int J Nanomedicine. 8:2677–2688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Silva IP and Long GV: Systemic therapy in
advanced melanoma: Integrating targeted therapy and immunotherapy
into clinical practice. Curr Opin Oncol. 29:484–492. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yingchoncharoen P, Kalinowski DS and
Richardson DR: Lipid-Based drug delivery systems in cancer therapy:
What is available and what is yet to come. Pharmacol Rev.
68:701–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sah H, Thoma LA, Desu HR, Sah E and Wood
GC: Concepts and practices used to develop functional PLGA-based
nanoparticulate systems. Int J Nanomedicine. 8:747–765. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ananta JS, Paulmurugan R and Massoud TF:
Temozolomide- loaded PLGA nanoparticles to treat glioblastoma
cells: A biophysical and cell culture evaluation. Neurol Res.
38:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheow WS and Hadinoto K: Factors affecting
drug encapsulation and stability of lipid-polymer hybrid
nanoparticles. Colloids Surf B Biointerfaces. 85:214–220. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen WR, Singhal AK, Liu H and Nordquist
RE: Antitumor immunity induced by laser immunotherapy and its
adoptive transfer. Cancer Res. 61:459–461. 2001.PubMed/NCBI
|
|
34
|
Hu Y, Chi C, Wang S, Wang L, Liang P, Liu
F, Shang W, Wang W, Zhang F, Li S, et al: A comparative study of
clinical intervention and interventional photothermal therapy for
pancreatic cancer. Adv Mater. 29:2017. View Article : Google Scholar
|
|
35
|
Hunyadi A, Csabi J, Martins A, Molnar J,
Balazs A and Toth G: Backstabbing P-gp: Side-chain cleaved
ecdysteroid 2,3-dioxolanes hyper-sensitize MDR cancer cells to
doxorubicin without efflux inhibition. 22:E1992017.PubMed/NCBI
|
|
36
|
Chen HH, Lu IL, Liu TI, Tsai YC, Chiang
WH, Lin SC and Chiu HC: Indocyanine green/doxorubicin-encapsulated
functionalized nanoparticles for effective combination therapy
against human MDR breast cancer. Colloids Surf B Biointerfaces.
177:294–305. 2019. View Article : Google Scholar : PubMed/NCBI
|