Introduction
Esophageal squamous cell carcinoma (ESCC) is a
predominant type of esophageal cancer (EC) that is among the
leading causes of cancer-related death worldwide (1). China has a very high incidence of ESCC,
with more than 100 cases per 100,000 population annually. Studies
have shown that certain factors are associated with increased risk
of ESCC, such as alcohol and tobacco consumption, genetic mutation,
diet and nutrition deficiency (2,3).
Similar to other types of cancer, surgery,
chemotherapy, radiotherapy, or a combination of these methods are
the main treatments for patients with EC. However, with the
increasing incidence of esophageal cancer and the poor 5-year
survival rate, more research is needed to investigate the
underlying mechanisms underlying EC, to determine how to prevent
esophageal cancer development, and to discover more effective
treatment strategies for patients with EC.
Studies have identified many prognostic markers
related to cell proliferation, cell apoptosis, and metastasis in
regards to ESCC, e.g., epidermal growth factor receptor (EGFR) has
been reported to be associated with the clinical outcome of many
types of cancer, including ESCC. EGFR overexpression is positive in
most patients with EC (4). In
addition, the level of phosphorylated mammalian target or rapamycin
(mTOR), which has an important role in intracellular metabolic and
anabolic processes, is associated with the poor prognosis of
patients with EC (5). B-cell
CLL/Lymphoma 2 (BCL2) family proteins, which are regulators of
programmed cell death, such as Fas cell surface death receptor
(FAS), BCL2 associated X, apoptosis regulator (BAX) and,
especially, BCL2 like 1 (BCL-X) were reported to contribute to ESCC
progression (6). Octamer-binding
protein 4 (OCT4) and SRY-box 2 (SOX2) also have a high prevalence
in ESCC (7). Recently, Cheng et
al found that solute carrier family 39 member 6 (SLC39A6), a
zinc transporter, is associated with ESCC invasiveness (8). Non-coding RNAs have also been identified
as essential players in ESCC development (9,10). In most
cases, ESCC formation and progression is a complex result of
multiple factors, for example, genetic alterations and risk factors
of lifestyle. Very recently, Yokoyama et al reported that
heavy smoking and drinking substantially accelerate the remodeling
process of the esophageal epithelium via numerous driver-mutated
clones in ESCC development (11).
Overall, ESCC is a heterogeneous disease with variable outcomes.
However, there are no widely accepted biomarkers for ESCC
screening, treatment response, and recurrence prediction.
Carbohydrate sulfotransferase 15 (CHST15), is a type
II transmembrane glycoprotein that acts as a sulfotransferase and
participates in chondroitin sulfate E (CS-E) biosynthesis (12). It is widely reported that CS-E plays a
pivotal role in tumor progression (13). CHST15 is also expressed in B cells as
a membrane-integrated glycoprotein disulfide-linked dimer (14). CHST15 was previously reported to be
associated with bone marrow-derived mast cell and pulmonary cell
metastasis (15,16), as well as tissue fibrosis formation
(17–19). In addition, CHST15 correlates with
cancer clinical relevance (20–23). For
example, Nishimura et al evaluated the safety and efficacy
of a double-stranded RNA oligonucleotide that specifically
represses CHST15 for use in patients with pancreatic cancer.
The results showed that CHST15 reduction could predict tumor
progression and overall survival (20). Ito et al indicated significant
associations between CHST15 overexpression and disease-free
survival and overall survival of patients with pancreatic ductal
adenocarcinoma (21).
In the present study, we investigated the
correlation between CHST15 expression and proliferation or
apoptosis or both in esophageal cancer cells. We further performed
gene chip microarray analysis to elucidate the underlying molecular
mechanisms in the regulation of esophageal tumor formation or
progression by CHST15.
Materials and methods
Construction of a recombinant
lentiviral vector
The target sequence (ACAGCATCACAACTAGGAT) from human
CHST15 mRNA (NM_015892) was selected for the knockdown
experiment. The sequence of the control short hairpin RNA (shRNA)
was TTCTCCGAACGTGTCACGT. The CHST15 shRNA and control shRNA
oligonucleotides were designed as stem-loop structures and inserted
into vector lenti-GV115-EGFP (GeneChem, Shanghai, China) at the
AgeI/EcoRI sites. Recombinant lentiviruses were
produced by co-transfection of the shRNA vector and helper vectors
(pHelper1.0 and pHelper2.0) into 293T cells. The medium was
replaced with fresh medium 6 h post-transfection. After 48 h
post-transfection, cell debris was removed from conditioned medium
by centrifugation at 4,000 × g and 4°C for 10 min. The conditioned
medium was then filtered through a 0.45-µm pore-size filter and
centrifuged at 4°C and 100,000 × g for 2 h. Lentiviruses particles
were resuspended in fresh medium and stored at −80°C. The
lentivirus titer was calculated using a fluorescence titering
assay.
Cell culture and recombinant
lentivirus infection
Human ESCC cell lines TE-1, Eca-109, and EC9706
cells were purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and were cultured in CM2-1 medium
comprised of 90% Roswell Park Memorial Institute (RPMI)-1640
(Corning, Inc.), 10% fetal bovine serum (FBS; Ausbian) and 1%
penicillin-streptomycin (Beyotime Institute of Biotechnology) at
37°C in a 5% CO2 incubator. Cells were subjected to
mycoplasma testing before experiments. TE-1 cells were plated on
6-well plates and infected with lenti-shCtrl or lenti-shCHST15 at a
multiplicity of infection (MOI) of 10. At 72 h post-infection, the
cells were observed under a fluorescence microscope (magnification,
×200) and harvested to determine knockdown efficiency using
quantitative RT-PCR or for other purposes.
Target validation of lenti-shCHST15 in
293T cells and western blotting
293T cells were transfected with the CHST15 plasmid
(GV143-hCHST15) using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) and then infected with lenti-shCtrl or
lenti-shCHST15, respectively. Briefly, 293T cells were plated on
24-well plates and transfected with 0.5 µg of the constructs and 1
µl of Lipofectamine 2000 when cells reached 80–90% confluence.
After 36–48 h of transfection, the cells were rinsed twice with
ice-cold phosphate-buffered saline (PBS), collected by scrapping,
and lysed using Radioimmunoprecipitation assay (RIPA) buffer (cat.
no. P0013B; Beyotime Institute of Biotechnology) supplemented with
1% protease inhibitor cocktail on ice for 10–15 min. Cells were
further lysed by sonication at 200 W four times, for 5 sec each
time, with a 2-sec interval between pulses. Supernatants were
collected after centrifugation at 12,000 × g, at 4°C for 15 min.
Protein concentrations were determined using the bicinchoninic acid
assay (BCA assay). An amount of 20 µg protein of each sample were
loaded onto 10% SDS-PAGE gel. After electrophoresis, the proteins
were transferred to polyvinylidene fluoride (PVDF) membranes. The
membranes were blocked in 5% non-fat milk at room temperature for 1
h. The blots were then incubated with anti-Flag antibodies
(dilution 1:2,000, cat. no. F1804; Sigma-Aldrich;Merck KGaA) or
anti-β-GAPDH antibody (dilution 1:2,000; cat. no. sc-32233; Santa
Cruz Biotechnology, Inc.) overnight at 4°C followed by incubation
with horseradish peroxidase (HRP)-conjugated secondary antibodies
(dilution 1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. Blots were visualized using the
enhanced chemiluminescent detection method (GE Healthcare).
RNA extraction and cDNA synthesis
Cells were collected and resuspended in 1 ml TRIzol
reagent (cat. no. 3101-100; Pufei). RNA was precipitated using
isopropanol and dissolved in RNase-free water. The RNA
concentration was measured using a NanoDrop 2000 instrument (Thermo
Fisher Scientific, Inc.). cDNA for each sample was obtained via a
reverse transcription reaction using a Promega M-MLV kit (cat. no.
M1705; Promega). All the above steps were performed according to
the manufacturer's instruction.
Quantitative real-time reverse
transcription PCR (RT-qPCR)
The qPCR reaction was set up by mixing primers, SYBR
TAQ, and cDNA at the proportion according to the manufacturer's
instructions (cat. no. DRR041B, SYBR Master Mixture; Takara). The
primer sequences for GAPDH were: 5′-TGACTTCAACAGCGACACCCA-3′
(forward) and 5′-CACCCTGTTGCTGTAGCCAAA-3′ (reverse). The primer
sequences for CHST15 were: 5′-AACACCACCGACCCCTAC-3′
(forward) and 5′-TGATGGCGGAGAACTTGA-3′ (reverse); the product sizes
for GAPDH and CHST15 were 121 and 232 bp,
respectively. The qPCR reactions were performed utilizing the
Mx3000P qPCR System (Agilent) and at 95°C for 15 sec; followed by
45 cycles at 95°C for 5 sec and 60°C for 30 sec. To compare mRNA
levels between different samples, the 2−ΔΔCq method
(24) was employed to analyze the
data.
Cell growth assay
TE-1 cells infected with lenti-shCtrl or
lenti-shCHST15 were plated at 800 cells/well onto a 96-well plate
and cultured at 37°C in a 5% CO2 incubator. Cells with
enhanced green fluorescent protein (EGFP) fluorescence in each well
were counted daily using a Celigo imaging cytometer (Nexcelom) for
5 days. A cell growth curve was drawn (based on cell numbers) by
plotting the numbers of fluorescent-positive cells and time-points.
For each cell type, the cell proliferation rates were calculated by
dividing the cell number at each time-point by the cell number at
day 1.
Cell apoptosis assay
Cell apoptosis was assessed using an Annexin V
Apoptosis Detection Kit APC (cat. no. 88-8007; eBioscience). TE-1
cells were seeded on a 6-well plate and infected with lenti-shCtrl
or lenti-shCHST15. Four days later, the cells were trypsinized and
resuspended in fresh complete medium. The cells were washed with
pre-cooled D-Hanks and 1X binding buffer. The cells were suspended
in 1X binding buffer, stained with Annexin V-APC, and analyzed
using a flow cytometer (Guava easyCyte HT; Milllipore). All samples
were examined in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability after CHST15 knockdown was
measured using an MTT assay. Cells were seeded onto 96-well plates
at 2,000 cells/well. At each time point, 20 µl of 5 mg/ml MTT was
added to each well and incubated with the cells for 4 h.
Thereafter, the medium was completely removed and 100 µl of
dimethyl sulfoxide (DMSO) was added into each well. After brief
shaking, the absorbance at 570 nm of each well was obtained using a
microplate reader (M2009PR; Tecan Infinite). Blank wells without
cells were included in this experiment. All samples were examined
in quintuplicate.
Clonogenic assay
Cells infected with shCtrl or shCHST15 were seeded
into 6-well plates at 400–1,000 cells/well. After 10 days of
culture, the colonies were washed with PBS and fixed in 4%
paraformaldehyde (PFA) for 30–60 min. Colonies were stained with
0.25% crystal violet and counted for analysis. Colonies were
counted manually without an instrument. Experiments were performed
in triplicate.
Tissue chip immunohistochemistry
The tissue chip used in this experiment included 20
paraffin-embedded sections of human esophageal squamous cell
carcinoma and four adjacent tissue sections. Briefly, the tissue
chip was incubated in a 60°C incubator for 30 min. It was then
soaked in xylene and serial gradient ethanol from 100–75% for
rehydration, incubated with 3% H2O2 for 10
min, and stored in distilled water. Antigens in the specimens were
retrieved using the sodium citrate (10 mM, pH 6.0) heat-induction
method. The tissue chip was blocked in 10% FBS and incubated with
anti-CHST15 antibodies (dilution 1:50, cat. no. SAB2701480;
Sigma-Aldrich;Merck KGaA) overnight at 4°C. A Vulcan Fast Red
Chromogen Kit 2 (Vector Laboratories) was used as the secondary
detection system. The slide was then stained with
3,3′-diaminobenzidine (DAB) and hematoxylin. Samples were then
dehydrated in gradient ethanol from 75–100% and xylene. The slide
was mounted in neutral mounting medium and observed under a
microscope (XDS-100; Carl Zeiss). CHST15 expression in each
specimen was evaluated based on the percentage of immunopositivity
and immunointensity. Each sample was scored based on a range of 0
(non-immunostaining), 1 (weak staining), 2 (moderate staining), 3
(strong staining) based on its immunointensity and scored at the
range of 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), 4 (76–100%)
based on its percentage of immunopositivity. The final score of
each sample was calculated by multiplying the immunointensity score
and the immunopositivity score. Three researchers independently
evaluated the chip result to avoid possible biased outcome.
TCGA database and bioinformatic
analysis of CHST15 expression
The CHST15 gene expression data sets for
esophageal squamous cell carcinoma and adjacent normal tissue were
obtained from The Cancer Genome Atlas (TCGA) database (http://cancergenome.nih.gov), containing a total of
185 carcinoma and 13 normal samples. Comparison of the relative
mRNA expression levels between two groups of samples was conducted
based on the ‘genomic Matrix’ file.
Gene microarray and data analysis
Total RNA was isolated from the shCHST15 and control
TE-1 cells and processed via hybridization to the GeneChip
Primeview Human gene array (901838; Affymetrix) using a
GeneChip® 3′ IVT Plus Kit (Affymetrix) following
manufacturer's protocol. Three biologically independent assays were
performed. Washing and staining were performed on the chip using a
GeneChip hybridization wash and stain kit. The array was processed
using the Affymetrix Genechip Fluidics Station 450 system, after
which they were imaged using an Affymetrix Genechip Scanner 3000 7G
for subsequent generation of cell intensity (.CEL) files. The raw
data were evaluated for quality control using signal histogram
analysis, relative signal box plot, Pearson's correlation analysis,
and principal component analysis. Qualified data were then used for
further analysis. After data cleaning, normalized probe sets were
subjected to variance analysis, Bayesian P-value computation, and
Benjamini-Hochberg false discovery rate (FDR) for multiple testing
correction. Differentially expressed genes based on the comparison
of CHST15 knockdown (KD) and control cells were selected if
|fold change|>1.5 and the FDR was <0.05.
Gene Ontology and pathway analysis of differentially
expressed genes were conducted using Ingenuity Pathway Analysis
(IPA) software (www.qiagen.com/ingenuity, Qiagen) and Databases for
Annotation, Visualization, and Integrated Discovery (https://david.ncifcrf.gov/), and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathways (https://www.genome.jp/kegg/pathway.html).
Gene Set Enrichment Analysis (GSEA) was performed
using GSEA software v2.2.1 from the Broad Institute (25) to detect if a series of pre-defined
biological processes or gene sets were enriched in the gene rank
derived from differentially expressed genes between
CHST15-KD and control cells. Gene sets were judged as
significantly enriched if P<0.05 and the false discovery rates
(FDR) was <0.25 in GSEA.
Statistical analysis
Statistical analysis was performed, and graphs were
plotted using Microsoft Excel 2013. P-values were determined using
two-tailed Student's t-test in at least three biological replicated
experiments. Significance was defined as P<0.05. The results are
presented as mean ± standard deviation (SD).
Results
CHST15 expression in ESCC cell lines
and CHST15 knockdown in TE-1 cells
We examined CHST15 expression in three
different ESCC cell lines: TE-1, Eca-109, and EC9706. Real-time PCR
was performed and the ΔCq value (CHST15-GAPDH) of each cell line
was calculated for fold change analysis. The data suggested that
CHST15 is expressed in these cells, among which TE-1 has
higher CHST15 expression than Eca-109 and EC9706 (Fig. 1). To investigate the function of
CHST15 in TE-1 cells, a lentivirus CHST15 shRNA construct was
designed and produced. CHST15-knockdown (KD) experiments
were performed in TE-1 cells. First, we conducted target validation
of lenti-CHST15 shRNA (shCHST15). 293T cells overexpressing CHST15
were infected with shCHST15. Western blot analysis showed a
significantly decreased CHST15 protein level, which confirmed the
targeting of CHST15 by lenti-shCHST15 (Fig. 2A). After 72 h of lenti-shCHST15
infection, CHST15 mRNA expression was significantly reduced
by 78% (Fig. 2B) in the shCHST15
group compared with that in the Lenti-shCtrl control cells.
Fig. 2C shows the TE-1 cells infected
with Lenti-shCHST15 and Lenti-shCtrl constructs, respectively.
Identification of CHST15 as a critical
gene regulating cell proliferation and apoptosis of TE-1 cells
Three days after shCHST15 infection of TE-1 cells,
cell proliferation rates were evaluated and compared between
shCHST15 and shCtrl cells. Cells were counted and images were taken
daily from both groups for 5 days. Compared with the substantial
increase in cell numbers of the shCtrl cells, the shCHST15 KD cells
exhibited a significantly reduced proliferation rate (Fig. 3A and B), which indicated that
deprivation of CHST15 inhibited TE-1 cell proliferation. This
result was further confirmed using MTT assays, which assessed the
viability of shCHST15 KD and control cells at different time points
(Fig. 3C).
In addition, we determined whether CHST15 KD
would affect the cell colony formation capacity. As expected, the
shCHST15 group formed fewer colonies compared with the shCtrl cells
(12 vs. 140) at 10 days after plating the cells on 6-well plates
(Fig. 4). These results revealed that
CHST15 KD significantly inhibited the colony formation
capacity of the TE-1 cells.
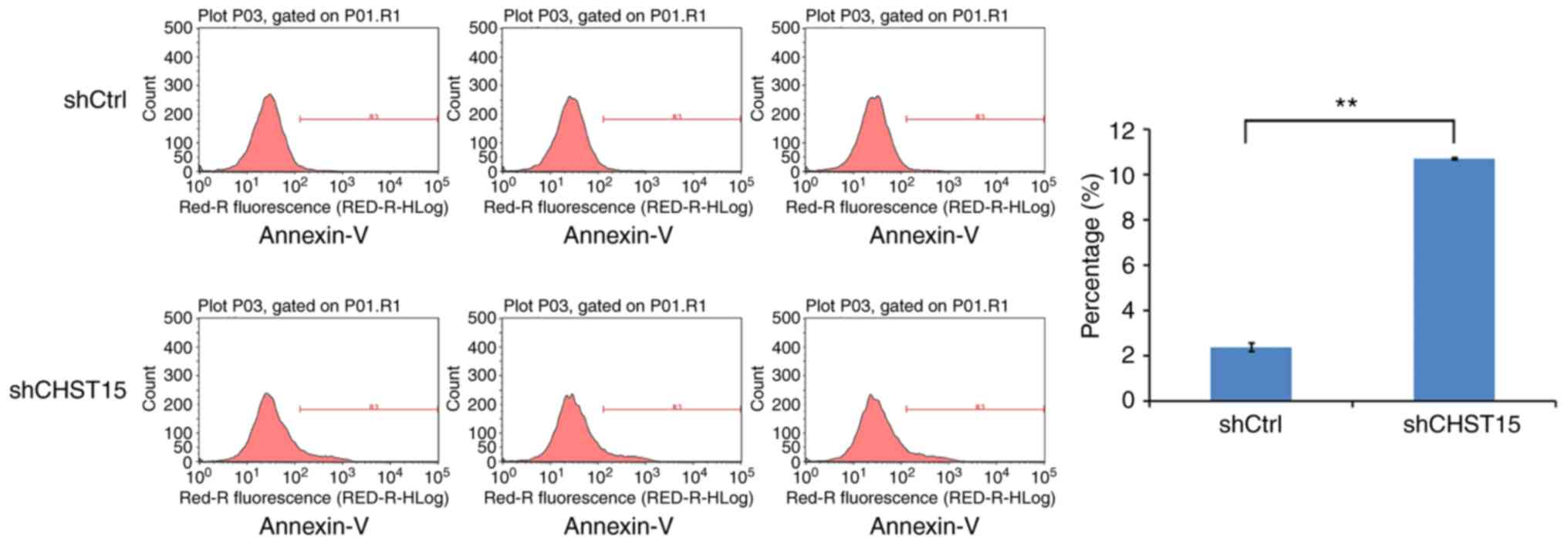
We speculated that cell apoptosis is also altered in
the CHST15 KD cells. At day 4 after infection, shCHST15 and
shCtrl cells were stained with Annexin V-APC and analyzed by
fluorescence activated cell sorting. The data revealed that the
percentage of apoptotic cells was significantly higher in the
shCHST15 infected cells (10%) compared with that in the shCtrl
cells (2%) (Fig. 5). Taken together,
the results suggested that CHST15 has a dual role of promoting
proliferation and inhibiting apoptosis in TE-1 cells.
Significant CHST15 overexpression in
esophageal squamous cell carcinoma of patients
These in vitro data prompted us to
hypothesize that CHST15 may also be expressed in esophageal
squamous cell carcinoma and play a possible role in carcinoma
formation and progression in vivo. To determine the clinical
significance of CHST15 in ESCC samples, in situ evaluation
of CHST15 was conducted using an esophageal squamous cell carcinoma
tissue chip which included 20 tissue sections from carcinoma
specimens and 4 sections from adjacent normal tissue. Patients and
sample information are shown in Table
I. Immunohistochemistry for CHST15 in this tissue array is
presented in Fig. 6A and B. The
immunostaining signal of CHST15 was found only in the cytoplasm,
but not in the cell membrane or nuclei. The CHST15 expression level
in each tumor tissue specimen was evaluated by a score based on the
percentage of immunopositivity and immunointensity. Statistical
analysis showed that esophageal squamous cell carcinoma presented a
3.5-fold higher CHST15 level than that noted in the adjacent
tissues. The relative CHST15 mRNA expression level in ESCC
samples was calculated by retrieving data sets of 185 ESCC samples
and 13 adjacent normal samples from the TCGA database (http://cancergenome.nih.gov), which showed that ESCC
samples had higher CHST15 expression (Fig. 6C). Overall, these data indicated that
CHST15 is overexpressed in esophageal squamous cell carcinoma and
may have an important role in ESCC formation or progression.
 | Table I.Characteristics of the patients with
ESCC (N=20). |
Table I.
Characteristics of the patients with
ESCC (N=20).
|
Characteristics | Data |
|---|
| Age (mean ± SD) in
years | 56.6±7.0 |
| Sex, n (%) |
|
Female | 10 (50) |
|
Male | 10 (50) |
| Tumor grade, n
(%) |
| G1 | 8 (40) |
| G2 | 10 (50) |
| GX | 2 (10) |
Analysis of the mRNA profiles of
CHST15 KD and control TE-1 cells
To understand the underlying molecular mechanism of
the function of CHST15 in TE-1 cell proliferation and apoptosis, as
well as its possible role in ESCC formation and proliferation, we
performed genome-wide mRNA microarray analysis to compare the mRNA
profiles of the shCHST15 and shCtrl TE-1 group cells. CHST15
KD cells were prepared by lentivirus-shCHST15 infection. Real-time
PCR was used to detect the CHST15 knockdown efficiency. The
CHST15 mRNA level was reduced by 58.3% compared with that in
the control cells. A gene microarray experiment was conducted using
shCHST15 and shCtrl cells with three biological repeats. Only
qualified RNA samples (1.7< A260/A280 <2.2, RNA Integrity
Number ≥7.0) were processed by hybridization to the GeneChip
Primeview human gene array. We evaluated the raw data from the
microarray by signal histogram analysis, relative signal box plot
analysis, Pearson's correlation analysis, and principal component
analysis. All of these analyses suggested that the microarray data
were suitable for next-step analysis. After data filtering and
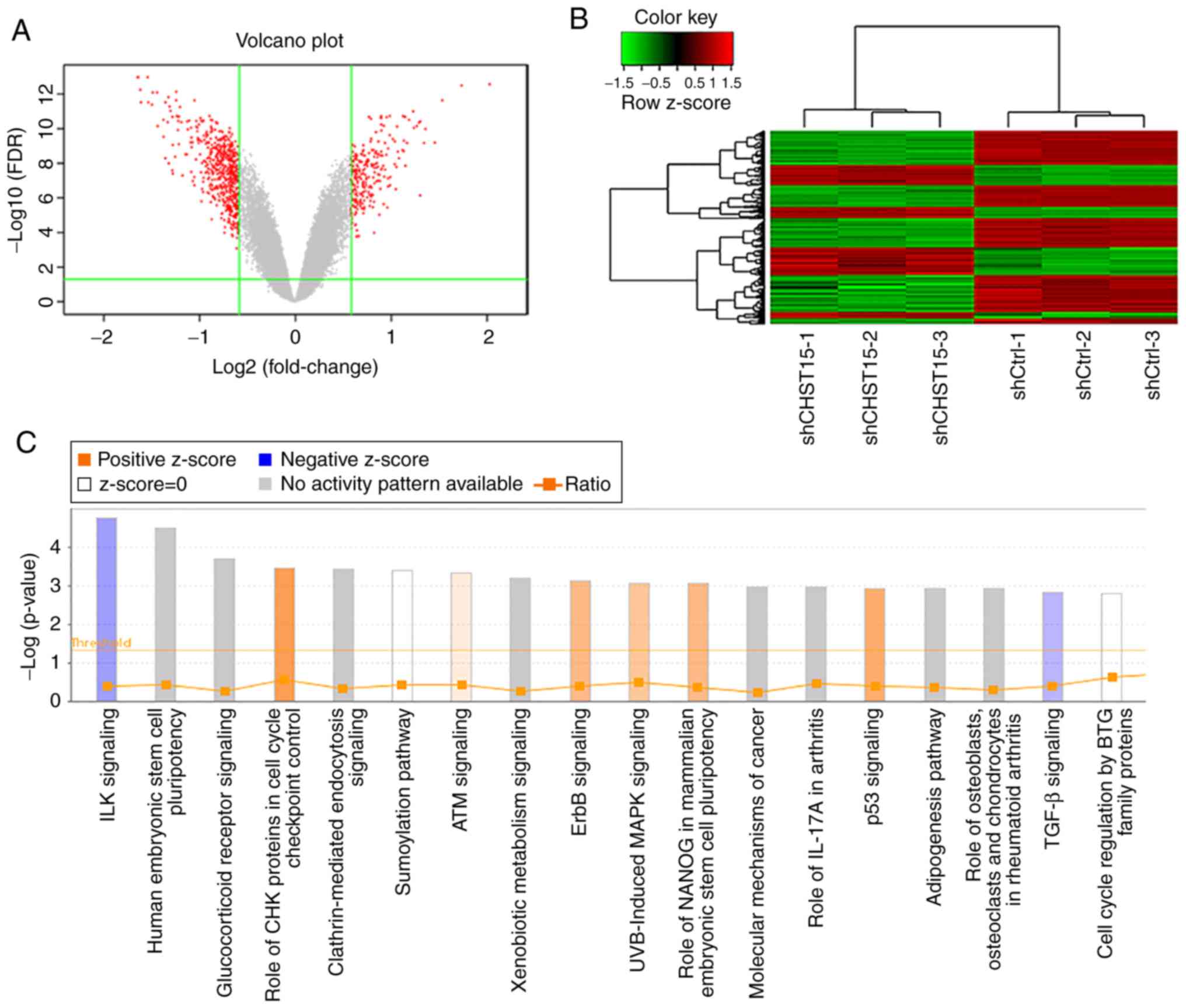
cleaning, 554 differentially expressed gene transcripts were
identified in this gene microarray, among which 188 genes were
upregulated and 366 genes were downregulated in the siCHST15 group
compared with the shCtrl control group (Fig. 7A and B).
To identify pathways related to transcriptome
changes, we categorized these genes by their associated canonical
signaling pathways using Ingenuity Pathway Analysis (IPA) software.
Enrichment analysis of differentially expressed genes revealed that
the integrin-linked kinase (ILK) and transforming growth factor-β
(TGF-β) signaling pathways were inhibited in the CHST15 KD
cells, whereas, check point kinase (CHK) proteins in the cell cycle
checkpoint control and p53 signaling pathways were activated in the
CHST15 KD cells (Fig. 7C). We
found that genes encoding proteins involved in the ILK signaling
network, such as integrin subunit β6 (ITGB6),
phosphatidylinositol- 4-phosphate 3-kinase catalytic subunit type
2β (PIK3C2B), protein tyrosine kinase 2 (PTK2),
fermitin family member 2 (FERMT2), and cyclin D1
(CCND1), were downregulated in the shCHST15 TE-1 cells
according to the dataset.
We also performed a GSEA analysis using the
microarray dataset to gain a further insight into the biological
processes that CHST15 may be involved in. This analysis was
performed to enrich gene sets from differentially expressed genes
between CHST15-KD cells and control cells that share common
biological function, chromosomal location, and regulation. GSEA
revealed that genes associated with cell growth, proliferation,
blood vessel morphogenesis, and tissue development were markedly
enriched in the dataset of differentially expressed genes (Fig. 8), suggesting that CHST15 may be
involved in these biological processes, which are also features of
cancer progression.
Differentially expressed genes (DEGs) identified in
the CHST15 knockdown cells were analyzed according to
several functional network criteria within IPA. We generated a
network with 22 DEGs related to cell proliferation and apoptosis
processes (Table II), showing
interactions with CHST15. This network diagram indicated a
molecular mechanism by which CHST15 regulates the
proliferation and apoptosis of TE-1 cells (Fig. 9).
 | Table II.Network of 22 differentially
expressed genes identified in the CHST15 knockdown cells
related to cell proliferation and apoptosis processes showing
interactions with CHST15. |
Table II.
Network of 22 differentially
expressed genes identified in the CHST15 knockdown cells
related to cell proliferation and apoptosis processes showing
interactions with CHST15.
| Gene symbol | Fold change | Location | Family |
|---|
| APCDD1 | −2.249056649 | Plasma
membrane | Other |
| BMP2 | 2.552303689 | Extracellular
space | Growth factor |
| BTG2 | −1.801089658 | Nucleus | Transcription
regulator |
| CCND1 | −1.574172163 | Nucleus | Transcription
regulator |
| CHST15 | −2.578621457 | Plasma
membrane | Enzyme |
| CLU | −1.726946433 | Cytoplasm | Other |
| CREM | 1.573881221 | Nucleus | Transcription
regulator |
| EEF1A1 | −1.600578387 | Cytoplasm | Translation
regulator |
| HSPA5 | −1.588890827 | Cytoplasm | Enzyme |
| LEPR | 1.966722394 | Plasma
membrane | Transmembrane
receptor |
| MIR17HG | 1.509101665 | Other | Other |
| NF1 | 1.510434197 | Cytoplasm | Other |
| PLAU | 1.906482559 | Extracellular
space | Peptidase |
| PMAIP1 | 1.714217298 | Cytoplasm | Other |
| PTHLH | 2.256082774 | Extracellular
space | Other |
| PTK2 | −1.699664502 | Cytoplasm | Kinase |
| SAE1 | −1.840426328 | Cytoplasm | Enzyme |
| TGFA | −1.540818218 | Extracellular
space | Growth factor |
| TIMP3 | −1.590628214 | Extracellular
space | Other |
| TPM1 | 1.923481561 | Cytoplasm | Other |
| USO1 | −2.010930656 | Cytoplasm | Transporter |
| WNT5A | −1.526294473 | Extracellular
space | Cytokine |
Discussion
Esophageal squamous cell carcinoma (ESCC), a common
type of esophageal cancer, is considered a serious malignancy, with
a low 5-year overall patient survival rate. Despite the current
availability of multiple treatment strategies, the survival rate
for ESCC has not significantly improved as many patients present
with local advanced disease at the time of diagnosis (3,26).
Determining the molecular mechanism underlying ESCC development
would be beneficial to identify new diagnostic approaches and
therapeutic targets.
In the present study, we showed that carbohydrate
sulfotransferase 15 (CHST15) is highly expressed in ESCC
cell lines and ESCC tissues. We designed lenti-shCHST15 virus
constructs and performed CHST15 knockdown experiments on
TE-1 cells. Silencing of CHST15 inhibited TE-1 cell
proliferation and promoted TE-1 cell apoptosis, suggesting that
CHST15 contributes to the pathogenesis of ESCC. Tissue array
immunostaining and bioinformatic analysis of TCGA data sets of ESCC
and adjacent normal tissues both showed that CHST15 is
overexpressed in ESCC samples, indicating that CHST15 may play an
essential role in mediating the tumorigenicity of ESCC cells.
To gain a deeper insight into the molecular function
of CHST15 in ESCC cells, a gene microarray assay was conducted to
compare the mRNA profiles of CHST15-knockdown cells and
control cells. Subsequent Gene Ontology (GO) and KEGG enrichment
analysis of the identified differentially expressed genes (DEGs)
indicated that CHST15 may be involved in integrin-linked kinase
(ILK) and p53 signaling, which regulate cell proliferation and cell
apoptosis, respectively. ILKs are important regulators of
integrin-mediated signaling. The main function of ILK is to connect
integrins to the cytoskeleton. ILK recruits other adaptor molecules
into a large complex to regulate actin dynamics and integrin
function (27). Overexpression or
activation of ILK leads to increased tumor cell proliferation,
motility and invasion; thus, ILK may be a promising therapeutic
target in many types of cancer (28,29).
Downregulated ILK signaling in CHST15-knockdown cells may
contribute to the inhibition of cell proliferation. P53 is a
well-known tumor suppressor that can inhibit cancer progression by
provoking cell growth arrest, by enabling DNA repair, or by
advancing cellular death programs (30). In this study, the increased apoptosis
of TE-1 cells may be attributable to activated p53 signaling
induced by CHST15 knockdown. The results of GSEA also
suggested that CHST15 is significantly associated with cell growth
and proliferation processes.
By analyzing DEGs and mapping using IPA software, we
identified two possible signaling axes, CHST15/ILK associated
serine/threonine phosphatase (ILKAP)/CCND1 and CHST15/RAB, member
RAS oncogene family like 6
(RABL6)/phorbol-12-myristate-13-acetate-induced protein 1 (PMAIP1),
which regulate ESCC cell proliferation and apoptosis, respectively.
CHST15 is reported to interact with RABL6 (31). RABL6 is a small GTPase belonging to
the Ras superfamily, which mainly relays signals from receptors at
the cell plasma membrane and modulates many cellular signaling
pathways that regulate cell proliferation, differentiation and
survival (32). Tang et al
demonstrated that knockdown of RABL6 upregulated
retinoblastoma 1 (Rb) expression and thus downregulated Rb
inhibitory downstream targets, such as cyclin A2, cyclin D1, c-Myc,
and cyclin-dependent kinase 2 (33).
RABL6 is also a binding partner of PMAIP1, which is a pro-apoptotic
member of the BCL2 protein family, but only contains a BH3 domain.
PMAIP1 expression was found to activate the mitochondrial apoptotic
cascade and induce increased oxidative stress and calcium release,
resulting in the activation of apoptosis signal-regulating kinase 1
(ASK1) and its downstream effectors JUN N-terminal kinase (JNK) and
mitogen-activated protein kinase 14 (MAPK14, also known as p38)
(34). PMAIP1 was also found to
regulate p53-induced apoptosis (35).
According to the microarray dataset, PMAIP1 expression was
increased in the CHST15-knockdown TE-1 cells (Table II). Therefore, CHST15 may promote
TE-1 cell apoptosis by interacting with RABL6 and affecting PMAIP1
expression and their subsequent downstream molecules.
CHST15 was also indicated to interact with ILKAP
(31), which is a serine/threonine
protein phosphatase associated with ILK. It selectively inhibits
the ILK-mediated glycogen synthase kinase 3β (GSK3β) signaling
pathway and further regulates the Wnt signaling pathway by
modulating GSK3β phosphorylation. ILK-mediated inhibition of GSK3β
was found to induce the expression of cyclin D1 (36,37).
Cyclin D1 (encoded by CCND1) plays a central role in the
regulation of proliferation, linking the extracellular signaling
environment to cell cycle progression (38). In the CHST15-knockdown TE-1
cells, reduced cyclin D1 levels might be the main reason leading to
the inhibition of cell proliferation through the CHST15/ILKAP/CCND1
signaling axis. These observations and predictions provide valuable
clues for future detailed investigation of possible
CHSR15-associated intracellular signaling pathways.
In summary, the present study investigated the role
of CHST15 in cell growth and apoptosis of ESCC and demonstrated the
clinical implication of CHST15 in ESCC. CHST15 could be a promising
diagnostic marker or therapeutic target for this disease.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China Grants (21335007) and Fundamental
Research Funds for the Central Universities (3332018071).
Availability of data and materials
The microarray dataset in this study is not publicly
available since we are using this dataset for further studies.
However, it is still available from the corresponding author upon
reasonable request.
Authors' contributions
LW and NB designed the study and analyzed the data.
XW conducted the majority of experiments and wrote the manuscript.
QF, DC and ZZ were involved in construction of the recombinant
lentiviral vector. WW and LD conducted the cell growth and
apoptosis assays and collected the data. XW, KX, XX, and GC
conducted the gene chip microarray experiment and bioinformatic
analyses. TZ and XW carried out the tissue chip
immunohistochemistry experiment. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The use of human tissue specimen was approved by the
Ethics Committee of National Cancer Center/National Clinical
Research Center for Cancer/Cancer Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College. The reference
number is 19/140-1924. Twenty patients signed consent forms prior
to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Domper Arnal MJ, Ferrández Arenas Á and
Lanas Arbeloa Á: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Yu JM, Jing SW, Guo Y, Wu YJ, Li
N, Jiao WP, Wang L and Zhang YJ: Relationship between EGFR
over-expression and clinicopathologic characteristics in squamous
cell carcinoma of the esophagus: A meta-analysis. Asian Pac J
Cancer Prev. 15:5889–5893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirashima K, Baba Y, Watanabe M, Karashima
R, Sato N, Imamura Y, Hiyoshi Y, Nagai Y, Hayashi N, Iyama K and
Baba H: Phosphorylated mTOR expression is associated with poor
prognosis for patients with esophageal squamous cell carcinoma. Ann
Surg Oncol. 17:2486–2493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayama T, Nagao M, Sawada H, Yamada Y,
Emoto K, Fujimoto H, Ueno M, Hirao S and Nakajima Y: Bcl-X
expression in esophageal squamous cell carcinoma: Association with
tumor progression and prognosis. J Surg Oncol. 78:116–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaraja V and Eslick GD: Forthcoming
prognostic markers for esophageal cancer: A systematic review and
meta-analysis. J Gastrointest Oncol. 5:67–76. 2014.PubMed/NCBI
|
|
8
|
Cheng X, Wei L, Huang X, Zheng J, Shao M,
Feng T, Li J, Han Y, Tan W, Tan W, et al: Solute carrier family 39
member 6 gene promotes aggressiveness of esophageal carcinoma cells
by increasing intracellular levels of zinc, activating
phosphatidylinositol 3-kinase signaling, and up-regulating genes
that regulate metastasis. Gastroenterology. 152:1985–1997.e1912.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang HJ, Lee HS, Burt BM, Lee GK, Yoon KA,
Park YY, Sohn BH, Kim SB, Kim MS, Lee JM, et al: Integrated genomic
analysis of recurrence-associated small non-coding RNAs in
oesophageal cancer. Gut. 66:215–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Yu X, Zhang Z, Pang L, Xu J, Jiang
J, Liang W, Chai Y, Hou J and Li F: Linc-ROR promotes esophageal
squamous cell carcinoma progression through the derepression of
SOX9. J Exp Clin Cancer Res. 36:1822017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokoyama A, Kakiuchi N, Yoshizato T,
Nannya Y, Suzuki H, Takeuchi Y, Shiozawa Y, Sato Y, Aoki K, Kim SK,
et al: Age-related remodelling of oesophageal epithelia by mutated
cancer drivers. Nature. 565:312–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohtake S, Ito Y, Fukuta M and Habuchi O:
Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is
related to human B cell recombination activating gene-associated
gene. J Biol Chem. 276:43894–43900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takakura K, Shibazaki Y, Yoneyama H, Fujii
M, Hashiguchi T, Ito Z, Kajihara M, Misawa T, Homma S, Ohkusa T and
Koido S: Inhibition of cell proliferation and growth of pancreatic
cancer by silencing of carbohydrate sulfotransferase 15 in vitro
and in a xenograft model. PLoS One. 10:e01429812015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verkoczy LK, Guinn Ba and Berinstein NL:
Characterization of the human B cell RAG-associated gene, hBRAG, as
a B cell receptor signal-enhancing glycoprotein dimer that
associates with phosphorylated proteins in resting B cells. J Biol
Chem. 275:20967–20979. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohtake-Niimi S, Kondo S, Ito T, Kakehi S,
Ohta T, Habuchi H, Kimata K and Habuchi O: Mice deficient in
N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase are unable to
synthesize chondroitin/dermatan sulfate containing
N-acetylgalactosamine 4,6-bissulfate residues and exhibit decreased
protease activity in bone marrow-derived mast cells. J Biol Chem.
285:20793–20805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizumoto S, Watanabe M, Yamada S and
Sugahara K: Expression of N-acetylgalactosamine 4-sulfate
6-O-sulfotransferase involved in chondroitin sulfate synthesis is
responsible for pulmonary metastasis. Biomed Res Int.
2013:6563192013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato H, Sagara S, Nakajima N, Akimoto T,
Suzuki K, Yoneyama H, Terai S and Yahagi N: Prevention of
esophageal stricture after endoscopic submucosal dissection using
RNA-based silencing of carbohydrate sulfotransferase 15 in a
porcine model. Endoscopy. 49:491–497. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki K, Arumugam S, Yokoyama J, Kawauchi
Y, Honda Y, Sato H, Aoyagi Y, Terai S, Okazaki K, Suzuki Y, et al:
Pivotal role of carbohydrate sulfotransferase 15 in fibrosis and
mucosal healing in mouse colitis. PLoS One. 11:e01589672016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kai Y, Tomoda K, Yoneyama H, Kitabatake M,
Nakamura A, Ito T, Yoshikawa M and Kimura H: Silencing of
carbohydrate sulfotransferase 15 hinders murine pulmonary fibrosis
development. Mol Ther Nucleic Acids. 6:163–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishimura M, Matsukawa M, Fujii Y, Matsuda
Y, Arai T, Ochiai Y, Itoi T and Yahagi N: Effects of EUS-guided
intratumoral injection of oligonucleotide STNM01 on tumor growth,
histology, and overall survival in patients with unresectable
pancreatic cancer. Gastrointest Endosc. 87:1126–1131. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito Z, Takakura K, Suka M, Kanai T, Saito
R, Fujioka S, Kajihara M, Yanagisawa H, Misawa T, Akiba T, et al:
Prognostic impact of carbohydrate sulfotransferase 15 in patients
with pancreatic ductal adenocarcinoma. Oncol Lett. 13:4799–4805.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Steen SC, van Tilborg AA, Vallen
MJ, Bulten J, van Kuppevelt TH and Massuger LF: Prognostic
significance of highly sulfated chondroitin sulfates in ovarian
cancer defined by the single chain antibody GD3A11. Gynecol Oncol.
140:527–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ten Dam GB, van de Westerlo EM,
Purushothaman A, Stan RV, Bulten J, Sweep FC, Massuger LF, Sugahara
K and van Kuppevelt TH: Antibody GD3G7 selected against embryonic
glycosaminoglycans defines chondroitin sulfate-E domains highly
up-regulated in ovarian cancer and involved in vascular endothelial
growth factor binding. Am J Pathol. 171:1324–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Xie X, Zhou C, Peng S, Rao D and Fu
J: Which factors are associated with actual 5-year survival of
oesophageal squamous cell carcinoma? Eur J Cardiothorac Surg.
41:e7–e11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dedhar S: Cell-substrate interactions and
signaling through ILK. Curr Opin Cell Biol. 12:250–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: A cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shirley LA, McCarty S, Yang MC, Saji M,
Zhang X, Phay J, Ringel MD and Chen CS: Integrin-linked kinase
affects signaling pathways and migration in thyroid cancer cells
and is a potential therapeutic target. Surgery. 159:163–170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stegh AH: Targeting the p53 signaling
pathway in cancer therapy-the promises, challenges and perils.
Expert Opin Ther Targets. 16:67–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hein MY, Hubner NC, Poser I, Cox J,
Nagaraj N, Toyoda Y, Gak IA, Weisswange I, Mansfeld J, Buchholz F,
et al: A human interactome in three quantitative dimensions
organized by stoichiometries and abundances. Cell. 163:712–723.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Montalbano J, Lui K, Sheikh MS and Huang
Y: Identification and characterization of RBEL1 subfamily of
GTPases in the Ras superfamily involved in cell growth regulation.
J Biol Chem. 284:18129–18142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang H, Ji F, Sun J, Xie Y, Xu Y and Yue
H: RBEL1 is required for osteosarcoma cell proliferation via
inhibiting retinoblastoma 1. Mol Med Rep. 13:1275–1280. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hassan M, Alaoui A, Feyen O,
Mirmohammadsadegh A, Essmann F, Tannapfel A, Gulbins E,
Schulze-Osthoff K and Hengge UR: The BH3-only member Noxa causes
apoptosis in melanoma cells by multiple pathways. Oncogene.
27:4557–4568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar AS, Naruszewicz I, Wang P,
Leung-Hagesteijn C and Hannigan GE: ILKAP regulates ILK signaling
and inhibits anchorage-independent growth. Oncogene. 23:3454–3461.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leung-Hagesteijn C, Mahendra A,
Naruszewicz I and Hannigan GE: Modulation of integrin signal
transduction by ILKAP, a protein phosphatase 2C associating with
the integrin-linked kinase, ILK1. EMBO J. 20:2160–2170. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang K, Hitomi M and Stacey DW: Variations
in cyclin D1 levels through the cell cycle determine the
proliferative fate of a cell. Cell Div. 1:322006. View Article : Google Scholar : PubMed/NCBI
|