Introduction
Glioblastoma (GBM) is the most malignant primary
brain tumor in humans. It is highly aggressive and heterogeneous,
remaining a major therapeutic challenge, since patients have a mean
overall survival (OS) of only 14 months and a progression-free
survival (PFS) time of 7–10 months (1). First-line postsurgical therapy for GBM
consists of temozolomide (TMZ) combined with regional fractionated
radiotherapy followed by adjuvant TMZ (2,3). The
introduction of TMZ as first-line treatment enhanced the quality of
life and OS of patients (2). However,
TMZ resistance has emerged and there is no standard of care
treatment for recurrence (3). The use
of chemotherapy for relapse cases yields response rates below 15%
(1,4).
New therapeutic strategies to overcome treatment failure and
improve the OS of GBM patients are required. In this context, the
synthetic pterocarpanquinones are promising compounds (5). Among them, LQB-118 has been revealed to
have antitumor activity in myeloid leukemia cells, promoting cell
death regardless of their resistance mechanisms (5–8). Likewise,
LQB-118 cytotoxicity was demonstrated in prostate cancer cells
resistant to androgen-based therapy, in vitro and in
vivo (9,10). The basic chemical property of the
compound may involve reduction of the paranaphtoquinone moiety in
the mitochondria, producing ROS or a reaction product that acts
like an alkylating agent (5). A
comprehensive toxicology study demonstrated good tolerability of
LQB-118 as confirmed by the absence of clinical, biochemical, or
hematological parameter changes (11). Furthermore, the dose that induced
subacute toxicity was 5 times higher than the therapeutic dose used
to treat prostate xenografts in nude mice (11,12).
LQB-118 also induced reactive oxygen species (ROS) formation and
apoptotic cell death by the intrinsic and endoplasmic reticulum
stress pathways (10,12). The compound regulated NF-κB, FOXO3a
and FOXM1 transcription factors without toxicity to mouse bone
marrow-derived cells (8,13). MAPKs and Akt pathways are regulators
of the aforementioned transcriptional factors and have been linked
to GBM heterogeneity, invasiveness and treatment resistance
(14–16). Therefore, understanding LQB-118
effects and mechanism of action has the potential to improve
therapeutic strategies. The present study evaluated the antitumor
activity of LQB-118 as a monotherapy and combined with radiotherapy
or chemotherapy in GBM monolayer and spheroid models.
Materials and methods
Cell lines
Human GBM cell lines, U251-MG, T98G and A172 were
kindly provided by Dr Vivaldo Moura-Neto. Cells were maintained in
Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12
(DMEM-F12; Gibco®; Thermo Fisher Scientific, Inc.)
supplemented with penicillin (100 UI/ml), streptomycin (0.1 mg/ml)
and 10% fetal bovine serum (FBS) as a monolayer at 37°C with 5%
CO2. Cells were tested and authenticated by DNA (STR)
profiling and periodically tested for Mycoplasma. Cells seeded at
3×104 cells/cm2 were used for all 2D
experiments. Cells were allowed to adhere to culture flasks
overnight before drug treatments and experimental analysis.
Compound handling
The synthetic compound, LQB-118 was developed by the
Laboratory of Bioorganic Chemistry at the Natural Products Research
Institute (IPPN) of the Federal University of Rio de Janeiro (UFRJ)
(5). LQB-118 and TMZ (ITF chemical,
Brazil) were stocked in powder form while cisplatin was stocked as
a solution, all at room temperature (RT). TMZ was diluted in
dimethyl sulfoxide (DMSO; cat. no. D2650;
Sigma-Aldrich®; Merck KGaA) immediately before use.
LQB-118 was diluted in DMSO and stored at −20°C for no longer than
two weeks. DMSO was used as a vehicle control for all
experiments.
MTT assay
Cells were incubated with different concentrations
of TMZ (5.0, 25.0, 50.0, 100.0, 250.0 and 500.0 µM) or LQB-118
(3.0, 6.0, 9.0 and 12.0 µM) for 24, 48 and 72 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
cat. no. 20395; SERVA) was added 4 h before the end of incubation.
The formazan crystals formed were eluted in DMSO and the absorbance
was measured at 492 nm using a Beckman Coulter DTX800 multimode
spectrophotometer. Optical density of control cells was considered
as 100% of viability. Three independent experiments were performed
with four replicates for each experimental condition.
Trypan blue exclusion assay
Cells were treated with TMZ (50.0 and 100.0 µM) or
LQB-118 (6.0 and 12.0 µM) for 24 and 48 h. After treatment, the
supernatant was collected, cells were washed with
phosphate-buffered saline (PBS) and detached using trypsin 0,125%
(cat. no. 27250018; GIBCO®; Thermo Fisher Scientific,
Inc.). Trypan blue was added to all collected cells (floating and
detached by trypsin cells). Cells stained blue (trypan
blue-positive cells) and not stained blue (trypan blue-negative
cells) were counted under an optical microscope. The percentage of
trypan blue-negative cells (assumed as viable cells) was calculated
relative to the control, which was considered 100%. Additionally,
the amount of trypan blue-positive cells (assumed as dead cells)
was calculated relative to the total number of cells present in
each condition.
Annexin V/Propidium iodide (PI)
assay
Cells were treated with TMZ (50.0 and 100.0 µM) or
LQB-118 (6.0 and 12.0 µM) for 24 and 48 h. After treatment, the
cells were washed with PBS and detached from culture flasks using
trypsin 0,125% (Gibco®; Thermo Fisher Scientific, Inc.).
Then, all collected cells (floating and detached by trypsin cells)
were centrifuged (700 × g) and incubated with PBS supplemented with
2% bovine serum albumin (BSA) for 30 min at RT. After washing, the
cells were incubated with Annexin V-Alexa Fluor® 488
conjugated (cat. no. A13201; Invitrogen™; Thermo Fisher Scientific,
Inc.) for 15 min in the dark at RT. PI was added before event
acquisition and used to differentiate non-apoptotic cell death
(Annexin V−/PI+). The drug-induced apoptotic
rate (Annexin V+/PI− and Annexin
V+/PI+) was compared to the control
(spontaneous apoptosis). Events (10,000) were acquired using Cyan
ADP (Beckman Coulter) and data were analyzed using Summit 4.3
software (Beckman Coulter Inc.). Three independent experiments were
performed.
Western blot analysis
Cells were treated with TMZ (50.0 and 100.0 µM) or
LQB-118 (6.0 and 12.0 µM) for 24 h. After detachment by trypsin and
washing with PBS, the cells were lysed with Cell Extraction Buffer
(cat. no. FNN0011; Invitrogen™; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Protein concentration
was determined by Lowry method using a commercial Kit (DC™ Protein
Assay; cat. no. 500-0116; Bio-Rad Laboratories, Inc.). Western blot
analysis using 30 µg of protein was performed with 12% SDS-PAGE and
transferred to Hybond-P membranes (cat. no. 29047575; GE
Healthcare®) and immunoblotted. The membranes were
blocked with nonfat milk 5% for 1 h, washed with Tris-buffered
saline (TBS)-Tween 0.2%, and incubated with primary antibodies
(Abs) diluted in nonfat milk overnight at 4°C. The following day,
the membranes were incubated with secondary antibodies for 1 h at
RT. Primary antibodies used were anti-Akt (1:1,000 dilution; rabbit
polyclonal antibody (pAb); cat. no. 9272), anti-p-Akt (1:1,000
dilution; phosphorylation at Ser473; rabbit pAb; cat. no. 9271),
anti-p38 MAPK (1:1,000 dilution; rabbit pAb; cat. no. 9212),
anti-p-p38 MAPK (1:1,000 dilution; phosphorylation site: T180/Y182;
rabbit pAb; cat. no. 9211), anti-ERK1/2 (p44/42MAPK) (clone 137F5;
1:1,000 dilution; rabbit monoclonal antibody (mAb); cat. no. 4695;
all from Cell Signaling Technology, Inc.), anti-p-ERK1/ERK2 (clone
15H10L7; 1:1,000 dilution; phosphorylation at Thr185, Tyr187;
rabbit mAb; cat. no. 700012; ABfinity™; Invitrogen™; Thermo Fisher
Scientific, Inc.), anti-NRF2 (clone C-20; 1:1,000 dilution; rabbit
pAb; sc-722; Santa Cruz Biotechnology, Inc.), anti-pro-caspase-7
(clone MCH3101; 1:1,000 dilution; cat. no. MAB823; R&D
Systems®), anti-PARP (1:1,000 dilution; rabbit pAb; cat.
no. 9542 Cell Signaling Technology®, Inc.) and
anti-HSC70 (1:1,000 dilution; mouse mAb (B-6); cat. no. sc-7298;
Santa Cruz Biotechnology, Inc.). Secondary antibodies were
anti-mouse IgG and anti-rabbit IgG-HRP conjugated (1:20,000
dilution; Amersham ECL™ Western blotting Detection Reagents; GE
Healthcare®; cat. nos. respectively, A9169 and A9044).
ECL Prime Detection System (cat. no. RPN2236; Amersham
Biosciences™; GE Healthcare) was applied for protein detection by
C-Digit™ Blot Scanner, generating images in Image Studio Lite
software v3.1 (LI-COR Biosciences®). Three independent
experiments were performed and analyzed qualitatively. Protein
expression was normalized by HSC70 expression.
Spheroid model three-dimensional (3D)
culture
Multicellular tumor spheroids were formed from the
cell lines U251-MG and A172 using the liquid-overlay technique
(17). Cells (200 µl) (104
cells/ml) in DMEM-F12 medium supplemented with 10% of FBS were
seeded in a 96-well flat bottom plate previously coated with 1.5%
agarose type II (cat. no. A6877; Sigma-Aldrich®; Merck
KGaA). The outer wells were filled with PBS to avoid evaporation.
Cells were cultured for 4 days until tightly aggregated spheroids
of ~300 µm of diameter were fully formed.
Acid phosphatase (APH) assay (3D
culture)
Cell viability in spheroids was assessed using the
APH (cat. no. 71768; Sigma-Aldrich®; Merck KGaA) assay
(18). Fully formed spheroids were
treated with different concentrations of LQB-118 (3.0, 6.0, 9.0 and
12.0 µM) or TMZ (25.0, 50.0, 100.0 and 200.0 µM) for 72 h. Then,
spheroids were transferred to a 96-well plate without an agarose
coat and washed twice to remove the medium. Substrate solution
containing nitrophenylphosphate (2 mg/ml) and Triton X-100 in
citrate buffer (0.1M), diluted in PBS, was added and incubated for
90 min in an incubator at 37°C . Then, NaOH (1M) was added and the
absorbance measured at 405 nm using Beckman Coulter DTX800
multimode spectrophotometer. Optical densities were normalized
using the absorbance of spheroids treated with DMSO as a control.
The experiment was repeated at least 3 times with 8 replicates
each.
Migration assay (3D culture)
Spheroids were transferred to a 24-well plate coated
with 0.1% gelatin and culture medium containing LQB-118 (1.0 and
3.0 µM) or TMZ (25.0, 50.0, 100.0 and 200.0 µM). Migration was
assessed after 24, 48 and 72 h. To avoid cell proliferation, a
medium containing only 2% FBS was used in the experiments, carried
out in triplicate. Quantification of the migration index was
performed manually using the software ImageJ v.1.5 (19) and the migration index was calculated
using the following formula: (Area of the bigger halo)/(area of the
spheroid at 0 h).
Drug interaction analysis
U251-MG and A172 cells were treated with LQB-118
(3.0 and 6.0 µM) combined with ionizing radiation (4 Gy), TMZ (50
and 100 µM) or cisplatin (CDDP, 3.0 and 30.0 nM) for 48 h. Cell
viability and cell death were evaluated by MTT, trypan blue
exclusion and Annexin V/PI assays as aforementioned. The
significance of concurrent combination treatment by MTT was
evaluated by combination index (CI) value calculated by CompuSyn
software (ComboSyn, Inc; www.combosyn.com) derived from the Chou-Talalay method
(20,21). Statistical analysis of trypan blue
exclusion and Annexin V/PI were realized by GraphPad Prism software
version 5.0 (GraphPad Software® Incorporated) as the
limited number of conditions did not allow synergism analysis.
Statistical analysis
Statistical and graphical information was determined
using the GraphPad Prism software version 5.0. One-way ANOVA test
followed by Bonferroni post hoc test was used to compare treatment
groups. Significant variance of ANOVA F-values ranged from 4.63 to
49.61. DMSO was used as a reference group to determine statistical
significance and P-values were reported at 95% confidence
intervals. A P-value of <0.05 was considered to indicate a
statistically significant difference and denoted as P<0.05.
Statistical significance in the figures was represented by
*P<0.05, **P<0.01 and ***P<0.001. The results are
presented as the mean of independent experiments ± standard
error.
Results
LQB-118 reduces cell viability and
induces apoptosis in three GBM cell lines
U251-MG, A172 and T98G cells were treated with
different concentrations of LQB-118 to assess cell line
sensibility. Initially, LQB-118 reduced cell viability by MTT of
all cell lines (Fig. 1). LQB-118 6.0
µM significantly reduced U251-MG and A172 cell viability after 48
and 72 h, but not of T98G cells (Fig.
1A-C). Higher concentrations of LQB-118 (9.0 and 12.0 µM)
reduced cell viability by almost 90% after 48 and 72 h of treatment
in U251-MG and A172 (Fig. 1A and B).
In T98G cells, LQB-118 9.0 and 12.0 µM reduced cell viability by 60
and 80%, respectively (Fig. 1C).
Therefore, a concentration that had a moderate effect (6.0 µM) and
one (12.0 µM) that demonstrated a high reduction of cell viability
were selected to perform further experiments for 24 and 48 h of
treatment. Corroborating the viability results, LQB-118 6.0 and
12.0 µM reduced cell viability by approximately 50 and 80%,
respectively, as quantified by trypan blue exclusion assay
(Fig. 1D). However, only 12.0 µM
induced cell death (Fig. 1E).
Furthermore, cell detachment from culture flasks was observed in
all cell lines after treatment with 12.0 µM, but not in the
controls and LQB-118 6.0 µM groups (Fig.
S1). Apoptotic cell death induction by LQB-118 was evaluated by
Annexin V/PI labeling (Fig. 2). In
U251-MG and T98G cells, only 12.0 µM induced significant 60%
Annexin V labeling after 48 h (Fig. 2A
and C). A172 cells were sensitive to 9.0 and 12.0 µM,
presenting 40 and 80% of Annexin V labeling after 48 h,
respectively (Fig. 2B). The
collective results demonstrated the antitumoral activity of LQB-118
against three GBM-derived cell lines.
Temozolomide has a minor cytotoxic
effect in GBM cell lines
U251-MG, A172 and T98G sensibility to first line
chemotherapy were evaluated by MTT. The highest TMZ concentration,
500 µM, reduced cell viability in U251-MG cells while no effect was
observed in A172 and T98G cells (Fig.
3A-C). In the studied conditions, the cell lines demonstrated a
resistance profile to TMZ while they were sensitive to LQB-118. The
conventional dose schedule of TMZ reaches ~50 µM in plasma
(22). Therefore, 50.0 and 100.0 µM
were selected for further experiments in U251-MG cells since this
cell line has been revealed to be tumorigenic in nude mice and
resistant to TMZ by p38/NRF2 axis activation (15,23). TMZ
did not induce cell detachment from culture flasks, nor cell death
by trypan blue exclusion assay or Annexin V labeling, but induced a
slight reduction in pro-caspase-7 (Fig.
3D-G). Furthermore, treatment with LQB-118 induced caspase-7
activation as suggested by the reduced expression of its
pro-caspase form (Fig. 3G). LQB-118
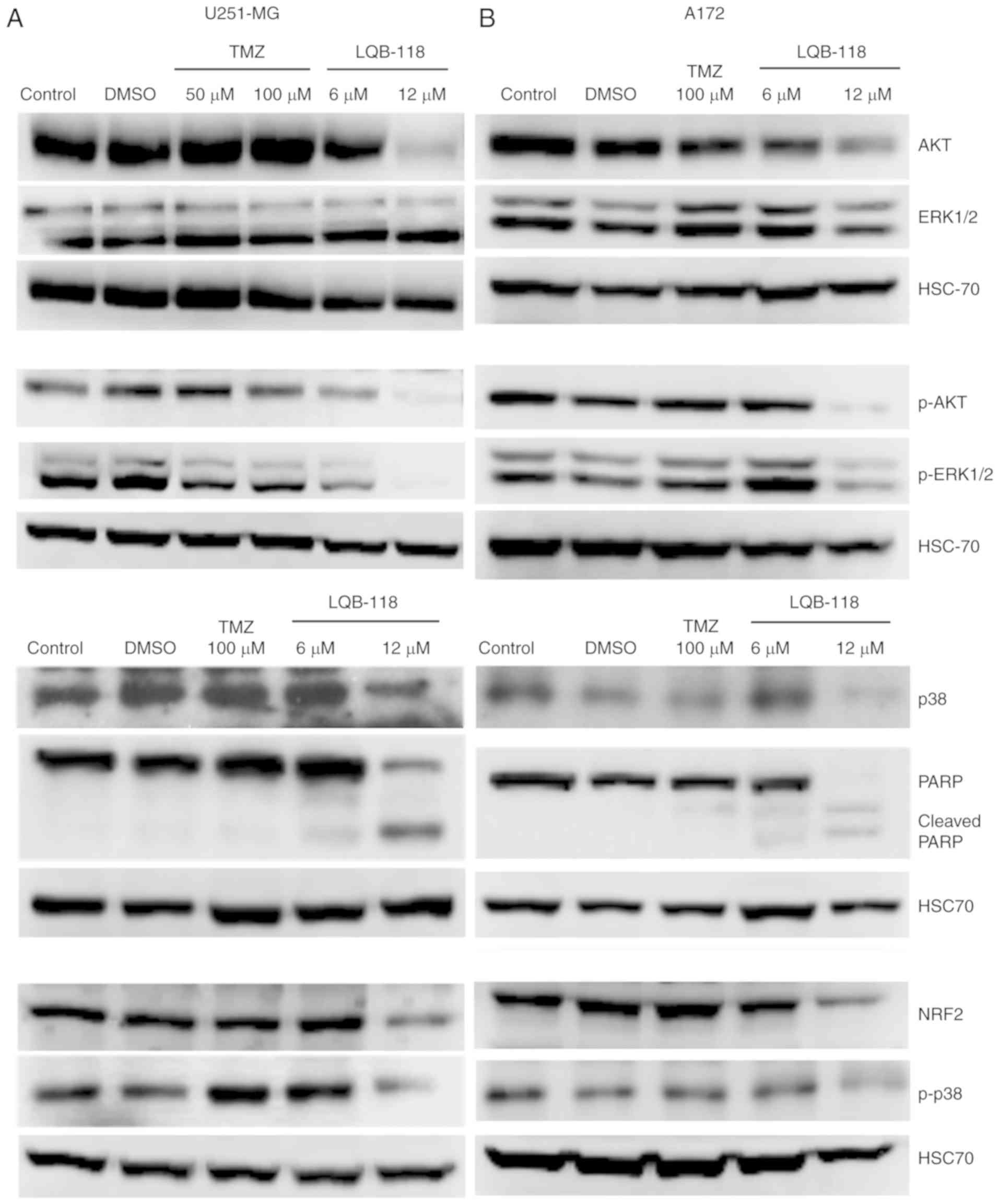
also promoted PARP cleavage in U251-MG and A172 cells (Fig. 5). These results corroborated apoptosis
induction by the compound LQB-118.
LQB-118 is cytotoxic and reduces cell
migration in spheroids of GBM cell lines
A 3D cell culture system is a great tool for drug
screening (24). LQB-118
antineoplastic activity was also evaluated in 3D cultures. LQB-118
concentrations of 9.0 and 12.0 µM significantly reduced U251-MG
spheroid viability, while TMZ did not (Fig. 4A). Cell lines maintained the
resistance to TMZ observed in the monolayer and LQB-118 maintained
its cytotoxicity, overcoming TMZ resistance. GBM is highly
infiltrative, therefore, the ability of LQB-118 to impair cell
migration was evaluated in a 3D culture. Lower LQB-118
concentrations were used (1.0 and 3.0 µM) to guarantee that the
effect observed was derived exclusively from migration inhibition
and not an artefact of proliferation inhibition or death induction.
The results revealed that LQB-118 significantly reduced the
migratory potential of GBM cells while TMZ had no effect,
corroborating the LQB-118 effectiveness in GBM cells (Fig. 4B and C). A similar profile was
observed for A172 spheroids (Fig.
4D-F).
Cytotoxic effect of LQB-118 is
associated with downregulation of survival pathways
The PI3K/Akt and MAPK pathways are markedly relevant
in GBM invasion, progression and treatment resistance (14,25,26).
Considering the effect of LQB-118 on cell migration and viability,
and to further understand its mechanism, Akt, ERK and p38 pathways
were investigated by western blotting. TMZ 50.0 and 100.0 µM did
not regulate p38 and total Akt and phosphorylated protein levels,
while it slightly reduced ERK phosphorylation in U251-MG cells
(Fig. 5A). Conversely, 12.0 µM of
LQB-118 reduced total protein expression and phosphorylated levels
of p38 and Akt and ERK phosphorylation, while the levels of total
ERK were not reduced (Fig. 5A).
Furthermore, LQB-118 reduced NRF2 expression and concurrently
inhibited MAPK and Akt pathways, reinforcing the potential of
LQB-118 against this heterogeneous disease. As aforementioned for
the 3D assays, a similar profile was observed for the A172 cell
line (Fig. 5B).
Synergic effect of LQB-118 and
cisplatin reduces viability and increases apoptosis
GBM patients present resistance, disease progression
and recurrence. Salvage therapies are not effective and LQB-118
downregulates radio- and chemoresistance-associated pathways.
Therefore, its effect in association with DNA damage inducers,
ionizing radiation, CDDP and TMZ was evaluated, in order to obtain
a possible less toxic and more effective treatment approach. First,
LQB-118 6.0 or TMZ 50.0 µM where concurrently used with ionizing
radiation. In U251-MG cells, 6.0 µM of LQB-118 combined with 4 Gy
significantly reduced cell viability by ~40% as determined by
trypan blue exclusion assay in comparison to isolated treatments,
while TMZ 50.0 µM with 4 Gy reduced cell viability by ~27%
(Fig. 6A). However, this effect was
not observed by Annexin V labeling for both treatment strategies
(Fig. 6B), demonstrating that LQB-118
and TMZ have similar results when combined with radiotherapy. In
A172 cells, 4 Gy of ionizing radiation alone reduced cell viability
and no additional effect was observed after combination with
LQB-118 or TMZ in these cells (Fig. 6C
and D).
Subsequently, LQB-118 was concurrently treated with
CDDP or TMZ (Fig. 7). Previous data
from our group demonstrated that cisplatin 3.0 µM had no effect
while 30.0 µM reduced cell viability by 50% as determined by MTT
(unpublished data). Therefore, these concentrations were selected
for combination experiments. In U251-MG cells, LQB-118 (3.0 and 6.0
µM) demonstrated a synergic effect with CDPP (3.0 and 30.0 µM) and
TMZ (50.0 and 100.0 µM) as revealed by MTT after 48 h (Fig. 7A and B; Table SI). The combination of LQB-118 3.0 µM
with CDDP 3.0 µM significantly enhanced cell death as demonstrated
by trypan blue exclusion and Annexin V/PI assays (Fig. 7C and D). Only 20% of U251-MG cells
were viable after combined treatment (data not shown). In A172
cells, LQB-118 3.0 µM demonstrated an additive effect when
associated with CDDP 3.0 and 30.0 µM (CI=1.01 and CI=0.95,
respectively) (Fig. 7E) and an
antagonist effect when treated with TMZ (Fig. 7F; Table
SI). No significant enhancement in cell death was observed
after treatment with CDPP (Fig. 7G)
neither with TMZ (Fig. 7H). However,
the combination of LQB-118 (3.0 µM) with CDDP (3.0 µM) reduced cell
viability by ~24% as determined by trypan blue exclusion assay
(data not shown). These data indicated that LQB-118 combined with
CDDP has a notable effect on cell proliferation, revealing LQB-118
as a potential alternative for a subset of patients with disease
recurrence.
Discussion
GBM is one of the most aggressive tumors and has a
five-year survival rate of only 5.1% (27). TMZ can improve OS, however the
majority of patients cannot complete treatment due to toxicity and
resistance has become a clinical problem (2,28,29). Despite the therapeutic advances made
in recent decades, patients relapse and progress to death. In the
context of new drug development to overcome resistance and improve
treatment, the present study evaluated the antitumoral effect of
LQB-118, a synthetic compound. LQB-118 significantly reduced cell
viability and induced high levels of apoptosis, while plasmatic
concentrations of TMZ did not promote cell death, suggesting an
intrinsic resistance of these cells to TMZ. GBM cell line response
to TMZ is not uniform in literature mainly due to different
treatment procedures and exposure times. In the present study, the
same time-points were used for the compound to guarantee an
unbiased comparison parameter. Under the studied conditions,
LQB-118 induced cell death in different GBM cell lines while TMZ
was not efficient in promoting cell death.
Accordingly, LQB-118 induced apoptosis in leukemia
cell lines with multidrug resistance (MDR) phenotype (6,7,12) and in androgen-resistant prostate
cancer cells (9), demonstrating its
great potential to overcome MDR mechanisms in different tumor
types. In addition, our group demonstrated the antineoplastic role
of LQB-118 in peripheral blood samples obtained from leukemia
patients (7), and LQB-118 reduced
growth of prostate, melanoma and Erlich tumors, in vitro and
in vivo (9,10,30).
Moreover, LQB-118 presented no toxicity for bone marrow and spleen
cells from healthy mice, primary and secondary organs of the immune
system and activated human PBMC (peripheral blood mononuclear
cells). These data demonstrate its selectivity for tumor cells and
great potential for treatment of patients non-responsive to
conventional therapy (8,30,31).
A preclinical study evaluated LQB-118 subacute
toxicity and oral administration did not demonstrate clinical signs
of toxicity (11). A dose, five times
higher than the therapeutic dose induced liver focal necrosis,
which was not accompanied by alterations in hepatic enzymes
(11). A theoretical analysis of the
pharmacokinetic properties of LQB-118 demonstrated it does not
violate the Lipinski's rule of five (Ro5) and has a favorable
profile, being more likely to progress to market (11). Furthermore, LQB-118 has 96%
probability of crossing the blood brain barrier (BBB) and 100%
probability of human intestine absorption (11). Corroborating the potential in crossing
the BBB, LQB-118 is effective in cells overexpressing
P-glycoprotein (Pgp) and multidrug resistance protein (MRP) with
enhanced efflux pump activity (7,12). These
proteins are one of the most important components of BBB,
protecting the brain from xenobiotics in physiological conditions.
In the tumor context, the identification of modifications in BBB
developed the concept of blood-brain tumor barrier (BBTB). The BBTB
is characterized by angiogenesis, which generates abnormal leaky
vessels and BBTB disruption allowing tumor infiltration in
parenchyma among other alterations (32). LQB-118 is a potential drug to overcome
BBTB protection, promoting an effective treatment and sensitizing
tumor cells to conventional therapy. Accordingly, using an in
vivo model of glioblastoma would be relevant to further confirm
the potential of LQB-118 against this lethal malignancy.
Cell-based assays are important tools for novel
compound identification. However, most assays used to assess the
biological activity of novel compounds rely on traditional
two-dimensional (2D) cell culture, having experimental limitations.
The 3D architecture creates an environment with oxygen and nutrient
gradients that ultimately alters gene expression resembling the
tumor gene profile and microenvironment (33–35). The
3D cell culture system often exhibits different sensitivity to
treatment, being better predictors of drug response (36). In the present study, LQB-118
maintained the cytotoxic effect observed in 2D cultures when
assessed in spheroids. Furthermore, low doses of LQB-118 that did
not reduce cell viability neither induce cell death, where able to
reduce the migration of spheroids as observed in a 3D conformation.
These data support the effect of LQB-118 exclusively on cell
migration in the concentrations of choice. Migration, observed in a
3D conformation, partially mimics the ability of cells to invade
the parenchyma since the assay requires cells to move in a
semisolid medium without any chemoattractive factor to induce it.
Furthermore, GBM invades the surrounding parenchyma, but does not
evade the central nervous system, reinforcing the importance to
evaluate migration capacity more than invasion by conventional
assays since this tumor does not systemically metastasize.
Therefore, migration inhibition is an important mechanism to impair
parenchyma infiltration by tumor cells, the main reason for disease
recurrence after tumor resection.
Previous literature has indicated an important role
for mitochondrial metabolism in LQB-118 activity. The
paranaphtoquinone moiety is reduced in the mitochondria and the
resulting product can act like an alkylating agent or transfer
electrons to molecular oxygen, producing ROS and inducing lipid
peroxidation, in vitro (5,10,12). Considering that an MTT assay evaluates
mitochondrial enzyme activity, this could explain the intensified
effect of LQB-118 observed in GBM. Corroborating our findings of
Akt and MAPK pathway downregulation, our group demonstrated that
FOXM1, FOXO3a and NF-κB transcriptional factors are regulated by
LQB-118 in leukemic cell models (6,8,13). Therefore, LQB-118 may be regulating
the transcription factors by PI3K/AKT and MAPK pathway inhibition
or may be regulating these pathways indirectly by endoplasmic
reticulum (ER) stress due to protein misfolding culminating with
apoptosis.
GBM is a highly heterogeneous disease and p53,
receptor tyrosine kinase (RTK), PI3K/PTEN and MAPK are core
pathways in gliomagenesis (25,37). The
selected cell lines present survival pathways constitutively
activated at different levels, in which T98G and U251-MG cells are
TP53 mutated whereas A172 is TP53 wild-type (23). Strategies that combine the inhibition
of ERK and PI3K pathways, concurrently, have been demonstrated to
be more efficient for glioma treatment (38,39).
Recent literature studies have demonstrated that the p38/NRF2 axis
is associated with TMZ resistance (15,40).
PI3K/AKT and MAPK inhibition and NFR2 knockdown was revealed to
sensitize GBM cells to radiation and TMZ treatment (15,38,40,41).
The present study demonstrated that LQB-118 concurrently inhibited
ERK, Akt and p38 activation, followed by reduction of NRF2 levels.
These data indicate a potential effect of LQB-118 to sensitize
cells to TMZ and radiation, mainly because there is no standard of
care for GBM relapse. Therefore, it was determined whether LQB-118
combined with ionizing radiation or chemotherapy would be more
effective, in vitro. The results demonstrated that LQB-118
treatment used with radiation reduced cell viability, while
combination with low doses of cisplatin significantly enhanced cell
death. This effect was cell line-dependent, demonstrating the
importance of understanding the molecular basis of the disease for
treatment decision. These data demonstrated that LQB-118 has a
potent cytotoxic effect as monotherapy as well as combined with
other antitumor agents, mainly cisplatin.
Collectively, the present data provided consistent
evidence of the effectiveness of LQB-118 against GBM cell lines in
a monolayer and 3D conformation. The cytotoxic effect was
associated with the inhibition of PI3K and MAPK pathways, well
described resistance pathways mutated in 60% of GBM cases (25). This is the first study to demonstrate
the effect of LQB-118 in GBM as monotherapy and in combination,
revealing a potential therapeutic alternative for patients
resistant to standard protocol.
Supplementary Material
Supporting Data
Acknowledgements
Authors would like to acknowledge Dr Vivaldo
Moura-Neto from the Instituto Estadual do Cérebro (IEC), Rio de
Janeiro, Brazil for kindly providing the studied cell lines. Also,
the Laboratório de Diagnósticos por DNA - DECOL - IBRAG from UERJ
for performance of STR profiling, the fellowships from the
Ministério da Saúde/INCA that supported the work of authors PSB and
FCCF and the Fundação de Amparo à Pesquisa do Rio de Janeiro
(FAPERJ-nota 10) that supported the Master's degree of GHCG.
Funding
The present study was supported by funds from the
Conselho Nacional de Desenvolvimento Científico e Tecnológico
(CNPq-304565/2016-4) and the Fundação de Amparo à Pesquisa do Rio
de Janeiro (FAPERJ; E-26/202-798/2017).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PSB was responsible for the study design, cell line
monolayer experiments and manuscript writing. GHCG and FCCF
performed western blotting and GMCL performed spheroid experiments.
GPFL provided insights in the study design and revised it
critically for important intellectual content. PRRC and CDN
synthetized and provided LQB-118, revised the final version and
agreed to be accountable for all aspects of the work. RCM was
responsible for study conception and orientation. All authors
critically revised and approved the final manuscript.
Ethics approval and consent to
participate
The present study does not contain any studies with
human participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M, van den Bent M, Tonn JC, Stupp
R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E,
Balana C, Chinot O, et al: European Association for Neuro-Oncology
(EANO) guideline on the diagnosis and treatment of adult astrocytic
and oligodendroglial gliomas. Lancet Oncol. 18:e315–e329. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dresemann G: Temozolomide in malignant
glioma. Onco Targets Ther. 3:139–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Netto CD, da Silva AJ, Salustiano EJ,
Bacelar TS, Riça IG, Cavalcante MC, Rumjanek VM and Costa PR: New
pterocarpanquinones: Synthesis, antineoplasic activity on cultured
human malignant cell lines and TNF-alpha modulation in human PBMC
cells. Bioorg Med Chem. 18:1610–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Souza Reis FR, de Faria FC, Castro CP,
de Souza PS, da Cunha Vasconcelos F, Bello RD, da Silva AJ, Costa
PR and Maia RC: The therapeutical potential of a novel
pterocarpanquinone LQB-118 to target inhibitor of apoptosis
proteins in acute myeloid leukemia cells. Anticancer Agents Med
Chem. 13:341–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maia RC, Vasconcelos FC, de Sá, Bacelar T,
Salustiano EJ, da Silva LF, Pereira DL, Moellman-Coelho A, Netto
CD, da Silva AJ, Rumjanek VM and Costa PR: LQB-118, a
pterocarpanquinone structurally related to lapachol
[2-hydroxy-3-(3-methyl-2-butenyl)-1,4-naphthoquinone]: A novel
class of agent with high apoptotic effect in chronic myeloid
leukemia cells. Invest New Drugs. 29:1143–1155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nestal de Moraes G, Castro CP, Salustiano
EJ, Dumas ML, Costas F, Lam EW, Costa PR and Maia RC: The
pterocarpanquinone LQB-118 induces apoptosis in acute myeloid
leukemia cells of distinct molecular subtypes and targets FoxO3a
and FoxM1 transcription factors. Int J Oncol. 45:1949–1958. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martino T, Magalhães FC, Justo GA, Coelho
MG, Netto CD, Costa PR and Sabino KC: The pterocarpanquinone
LQB-118 inhibits tumor cell proliferation by downregulation of
c-Myc and cyclins D1 and B1 mRNA and upregulation of p21 cell cycle
inhibitor expression. Bioorg Med Chem. 22:3115–3122. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martino T, Kudrolli TA, Kumar B, Salviano
I, Mencalha A, Coelho MGP, Justo G, Costa PRR, Sabino KCC and
Lupold SE: The orally active pterocarpanquinone LQB-118 exhibits
cytotoxicity in prostate cancer cell and tumor models through
cellular redox stress. Prostate. 78:140–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunha-Júnior EF, Martins TM,
Canto-Cavalheiro MM, Marques PR, Portari EA, Coelho MG, Netto CD,
Costa PR, Sabino KC and Torres-Santos EC: Preclinical studies
evaluating subacute toxicity and therapeutic efficacy of LQB-118 in
experimental visceral leishmaniasis. Antimicrob Agents Chemother.
60:3794–3801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Sá, Bacelar T, da Silva AJ, Costa PR
and Rumjanek VM: The pterocarpanquinone LQB 118 induces apoptosis
in tumor cells through the intrinsic pathway and the endoplasmic
reticulum stress pathway. Anticancer Drugs. 24:73–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Faria FC, Leal ME, Bernardo PS, Costa
PR and Maia RC: NFkB pathway and microRNA-9 and −21 are involved in
sensitivity to the pterocarpanquinone LQB-118 in different CML cell
lines. Anticancer Agents Med Chem. 15:345–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molina JR, Hayashi Y, Stephens C and
Georgescu MM: Invasive glioblastoma cells acquire stemness and
increased Akt activation. Neoplasia. 12:453–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma L, Liu J, Zhang X, Qi J, Yu W and Gu Y:
p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma
cells against TMZ. Med Oncol. 32:692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Pore N, Cerniglia GJ, Mick R,
Georgescu MM, Bernhard EJ, Hahn SM, Gupta AK and Maity A:
Phosphatase and tensin homologue deficiency in glioblastoma confers
resistance to radiation and temozolomide that is reversed by the
protease inhibitor nelfinavir. Cancer Res. 67:4467–4473. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid-based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedrich J, Eder W, Castaneda J, Doss M,
Huber E, Ebner R and Kunz-Schughart LA: A reliable tool to
determine cell viability in complex 3-d culture: The acid
phosphatase assay. J Biomol Screen. 12:925–937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishii N, Maier D, Merlo A, Tada M,
Sawamura Y, Diserens AC and Van Meir EG: Frequent co-alterations of
TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human
glioma cell lines. Brain Pathol. 9:469–479. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shield K, Ackland ML, Ahmed N and Rice GE:
Multicellular spheroids in ovarian cancer metastases: Biology and
pathology. Gynecol Oncol. 113:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pelloski CE, Lin E, Zhang L, Yung WK,
Colman H, Liu JL, Woo SY, Heimberger AB, Suki D, Prados M, et al:
Prognostic associations of activated mitogen-activated protein
kinase and Akt pathways in glioblastoma. Clin Cancer Res.
12:3935–3941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (Suppl 4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stupp R, Brada M, van den Bent MJ, Tonn JC
and Pentheroudakis G; ESMO Guidelines Working Group, : High-grade
glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 25 Suppl 3:iii93–iii101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lwin Z, MacFadden D, Al-Zahrani A, Atenafu
E, Miller BA, Sahgal A, Menard C, Laperriere N and Mason WP:
Glioblastoma management in the temozolomide era: Have we improved
outcome? J Neurooncol. 115:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salustiano EJ, Dumas ML, Silva-Santos GG,
Netto CD, Costa PR and Rumjanek VM: In vitro and in vivo
antineoplastic and immunological effects of pterocarpanquinone
LQB-118. Invest New Drugs. 34:541–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
da Silva AJ, Buarque CD, Brito FV,
Aurelian L, Macedo LF, Malkas LH, Hickey RJ, Lopes DV, Noël F,
Murakami YL, et al: Synthesis and preliminary pharmacological
evaluation of new (+/-) 1,4-naphthoquinones structurally related to
lapachol. Bioorg Med Chem. 10:2731–2738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Tellingen O, Yetkin-Arik B, de Gooijer
MC, Wesseling P, Wurdinger T and de Vries HE: Overcoming the
blood-brain tumor barrier for effective glioblastoma treatment.
Drug Resist Updat. 19:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baharvand H, Hashemi SM, Kazemi Ashtiani S
and Farrokhi A: Differentiation of human embryonic stem cells into
hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol.
50:645–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barbone D, Van Dam L, Follo C, Jithesh PV,
Zhang SD, Richards WG, Bueno R, Fennell DA and Broaddus VC:
Analysis of gene expression in 3D spheroids highlights a survival
role for ASS1 in mesothelioma. PLoS One. 11:e01500442016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nelson CM and Bissell MJ: Modeling dynamic
reciprocity: Engineering three-dimensional culture models of breast
architecture, function, and neoplastic transformation. Semin Cancer
Biol. 15:342–352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hongisto V, Jernström S, Fey V, Mpindi JP,
Kleivi Sahlberg K, Kallioniemi O and Perälä M: High-throughput 3D
screening reveals differences in drug sensitivities between culture
models of JIMT1 breast cancer cells. PLoS One. 8:e772322013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan QW and Weiss WA: Targeting the
RTK-PI3K-mTOR axis in malignant glioma: Overcoming resistance. Curr
Top Microbiol Immunol. 347:279–296. 2010.PubMed/NCBI
|
|
39
|
McNeill RS, Canoutas DA, Stuhlmiller TJ,
Dhruv HD, Irvin DM, Bash RE, Angus SP, Herring LE, Simon JM,
Skinner KR, et al: Combination therapy with potent PI3K and MAPK
inhibitors overcomes adaptive kinome resistance to single agents in
preclinical models of glioblastoma. Neuro Oncol. 19:1469–1480.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li K, Ouyang L, He M, Luo M, Cai W, Tu Y,
Pi R and Liu A: IDH1 R132H mutation regulates glioma
chemosensitivity through Nrf2 pathway. Oncotarget. 8:28865–28879.
2017.PubMed/NCBI
|
|
41
|
Sato A, Sunayama J, Matsuda K, Seino S,
Suzuki K, Watanabe E, Tachibana K, Tomiyama A, Kayama T and
Kitanaka C: MEK-ERK signaling dictates DNA-repair gene MGMT
expression and temozolomide resistance of stem-like glioblastoma
cells via the MDM2-p53 axis. Stem Cells. 29:1942–1951. 2011.
View Article : Google Scholar : PubMed/NCBI
|