Introduction
Gastric cancer (GC) is one of the leading causes of
death due to cancer. Yet, treatment outcomes have improved with the
development and improvement in multidisciplinary therapy (1,2). The
occurrence, development and prognosis of GC are closely related to
the crosstalk among different immune cells in the tumor
microenvironment (3–7). The relationship between GC progression
and immune cells has recently been receiving increased attention
(8).
Neutrophils are the most abundant type of white
blood cell, and are an essential component of the innate immune
system (9). They characteristically
arrive rapidly at sites of infection, injury, and tumors and
release a variety of cytokines and toxic molecules to eliminate
pathogens and elicit an acute inflammatory response (8–10). In
particular, intratumoral neutrophils (tumor-associated neutrophils:
TANs) are involved in angiogenesis and lymphangiogenesis, which
lead to tumor progression (4,6–8,11,12). We
previously reported that TANs in GC tissue are correlated with
lymph node metastasis and systemic inflammatory markers such as the
neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and
lymphocyte-monocyte ratio. Various immune cells including T cells,
natural killer cells, M2 macrophages, dendritic cells (DCs), and
neutrophils often infiltrate cancer tissues.
Although tumor-specific CD4+ T
lymphocytes play an indispensably important role in the antitumor
immune response at the tumor site, regulatory T cells and myeloid
suppressor cells are the major components of the immune suppressive
cellular network. Some types of TANs have similar immunosuppressive
functions as those of G-myeloid suppressor cells (6,13–19); however, the underlying mechanism is
still unclear. The present study aimed to investigate the
immunosuppressive ability of neutrophils in GC tissue and to
explore the influence of neutrophils on the proliferation of
CD4+ T cells.
Materials and methods
Neutrophil isolation and culture
Human neutrophils were isolated from peripheral
blood of healthy volunteers using Polymorphprep (Axis-Shield) and
centrifuged (400 × g and 20 min), resulting in a purity of ≥85%.
Cells were washed three times in complete RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA) with 100 U/ml penicillin, 100 µg/ml
streptomycin, L-glutamine (HyClone; GE Healthcare), and 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Neutrophils
were then incubated in complete RPMI-1640 medium (Sigma-Aldrich;
Merck KGaA) at 37°C in 5% CO2 for 16 h.
CD4+ T cell isolation and
culture
Peripheral blood mononuclear cells were also
isolated by density centrifugation (400 × g and 20 min) using
Polymorphprep, washed three times in complete RPMI-1640 medium, and
separated into CD4-positive and -negative cells using a CD4
Isolation Kit (Miltenyi Biotec) according to the manufacturer's
instructions, resulting in a purity of ≥90%. Following this,
CD4+ T cells were incubated in complete RPMI-1640 at
37°C in 5% CO2 for 24 h.
Preparation of tumor tissue culture
supernatants (TTCSs), non-tumor tissue culture supernatants (NTCSs)
and supernatant-conditioned neutrophils
A single-cell suspension was prepared from surgical
specimens from patients who underwent gastrectomy at the Osaka City
University (Osaka, Japan). Supernatants purified from tumor tissue
or the human scirrhous GC cell line, OCUM-12 (20), were defined as TTCSs, whereas the
supernatants purified from non-tumor tissues at least 5 cm distant
from the tumor site were defined as NTCSs. Both TTCSs and NTCSs
were purified after culturing 1×106 cells/ml in complete
RPMI-1640 at 37°C in 5% CO2 for 24 h. To generate
supernatant-conditioned neutrophils, neutrophils from healthy
volunteers were cultured with 50% TTCS or NTCS for 16 h, and then
washed with complete RPMI-1640 three times. Neutrophils cultured in
RPMI-1640 medium were used as controls.
Neutrophil stimulation with TTCS or
NTCS
Neutrophils from healthy volunteers were stimulated
with 50% TTCS or 50% NTCS for 16 h. After stimulation, the
percentage of 7-amino-actinomycin D-positive cells and the
expression of programmed cell death ligand-1 (PDL-1) and human
leukocyte antigen-DR (HLA-DR) were measured. In addition, the
expression of PDL-1 in neutrophils infiltrating the GC tissue was
compared with expression in neutrophils infiltrating normal mucosa
(at least 5 cm distant from the tumor site) with flow cytometry
(LSRII; BD Biosciences). H2O2 levels were
measured using a Hydrogen Peroxide Colorimetric Detection Kit (Enzo
Life Science).
In vitro neutrophil-CD4+ T
cell co-culture system
CD4+ T cells (1×105) were
seeded in 500 µl (20) complete
RPMI-1640 in 24-well plates. Neutrophils incubated with 50% TTCS or
50% NTCS, adjusted to 1×105 cells per 500 µl complete
RPMI-1640 medium, were co-cultured with CD4+ T cells
using Cell Culture Inserts (BD Falcon; BD Biosciences) at 37°C in
5% CO2 for 16 h. Expression of programmed cell death-1
(PD-1) and CD25 in CD4+ T cells was measured with flow
cytometry.
Allogeneic mixed lymphocyte
reaction
Immature DCs were prepared by culturing adherent
peripheral blood monocytes for 7 days in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), recombinant human
granulocyte macrophage colony-stimulating factor (rH GM-CSF) (50
ng/ml), and interleukin-4 (IL-4) (50 ng/ml). To induce maturation
of DCs, lipopolysaccharide (100 ng/ml) was added to the cell
culture for 24 h before harvesting. Allogenic DCs and neutrophils
incubated with TTCS or NTCS were added to CD4+ T cells
labelled with carboxyfluorescein diacetate succinimidyl ester
(CFDA-SE) (Tonbo Biosciences) at a ratio of 1:10:10, and
co-cultured for 4 days in complete RPMI-1640 medium at 37°C in 5%
CO2, followed by the analysis of CFDA-SE signal with
flow cytometry on gated CD4 lymphocytes to explore the
proliferative capacity of the CD4+ T cells.
Patients and surgical specimens
We retrospectively examined surgical specimens from
patients who underwent gastrectomy for GC with pathological stage
II or III at the Department of Surgical Oncology (Osaka City
University, Japan) from January 2007 to March 2013. The average age
is 64.11 years and the ratio of male to female is 84 to 31. The
specimens were formalin-fixed and paraffin-embedded tissues
obtained from 115 primary tumors, which were analyzed using
immunohistochemistry (IHC). Pathological staging was performed
according to the 7th edition of the International Union Against
Cancer Tumour-Node-Metastasis (TNM) classification (https://www.wiley.com/en-us/TNM+Classification+of+Malignant+Tumours%2C+7th+Edition-p-9781444358964).
Postoperative follow-ups were performed every 3 months for the
first 2 years, and then every 6 months during years 3–5. The
retrospective protocol of the present study was approved by the
Osaka City University Ethics Committee, and all patients provided
informed consent for collection and analysis of the specimens.
IHC
To assess the locational relationship between TANs
and PD-1+ T cells, IHC was performed. Sections with a
thickness of 4 µm were obtained from the paraffin-embedded blocks.
After incubation at 60°C for 10 min, the sections were
deparaffinized using xylene and rehydrated using a graded series of
ethanol. The slides were subsequently washed twice for 5 min in
phosphate-buffered saline (PBS). Endogenous peroxidase activity was
blocked for 15 min in absolute methanol containing 3% hydrogen
peroxide. After washing the sections in PBS, the samples were
microwaved for 10 min for antigen retrieval. Non-specific binding
was blocked using a non-specific staining blocking reagent (Dako;
Agilent Technologies, Inc.). The sections were then incubated
overnight at 4°C with mouse monoclonal antibodies [CD15 (cat. no.
ab17080) or PD-1 (cat. no. ab137132), dilution 1:100; Abcam], and
subsequently washed with PBS for 10 min before incubating with
appropriate secondary antibodies [goat anti-human IgG H&L
(HRP); cat. no. ab6858; dilution 1:100] or 10 min at room
temperature. After washing the sections with PBS, the samples were
visualized using 3–3′-diaminobenzidine for 5 min and then
counter-stained using hematoxylin before mounting. We obtained an
average value from five hot spot high-power fields, and median
values of both CD15 and PD-1 were calculated for the 115 patients.
Patients were divided into high and low CD15+ TAN or
PD-1+ T cell groups, based on each median number. The
correlation between CD15+ TANs and PD-1+ T
cells was examined using the Chi-square test and a scatter
plot.
Ethical approval and informed
consent
This study's retrospective protocol was approved by
the Osaka City University Ethics Committee (Osaka, Japan), and
informed consent was obtained in writing from all patients. All
volunteers provided oral and written informed consent and agreed to
the use of their samples in scientific research and all
participants for collection and analysis of the specimens in this
study.
Statistical analysis
Data are expressed as mean value ± standard
deviation (SD). Continuous variables were compared using the
Student's t-test, and categorical variables were compared using the
Chi-square test. Correlations between parameters were assessed
using the Pearson correlation analysis and linear regression
analysis as appropriate. Differences were considered statistically
significant at P-values of <0.05. All statistical analyses were
performed using JMP software (version 11; SAS Institute).
Results
Characteristic changes in neutrophils
in GC tissue
To evaluate the impact of GC tissue on neutrophils,
we analyzed the percentage of apoptotic cells and the expression of
PDL-1 in neutrophils conditioned with TTCS or NTCS.
TTCS-conditioned neutrophils showed a significantly lower
percentage of apoptotic cells (Fig.
1A) and higher expression of PDL-1 (Fig. 1B) compared to NTCS-conditioned
neutrophils. Neutrophils directly isolated from GC tissue showed
significantly higher expression of PDL-1 than those from normal
mucosa (Fig. 1C). Mature human
neutrophils have antigen-presenting ability similar to DCs
(21–23). Thus, we examined the
antigen-presenting ability of neutrophils conditioned with TTCS or
NTCS. Neutrophils treated with TTCS showed significantly reduced
expression of HLA-DR compared to those incubated with NTCS
(Fig. 1D).
 | Figure 1.Characteristic changes in neutrophils
in GC tissue. (A) Comparison of the apoptotic ratio of neutrophils
incubated with TTCS or NTCS. Neutrophils incubated with TTCS showed
a significantly decreased ratio of apoptotic cells compared to
neutrophils incubated with NTCS. In (A) Q1,
CD16b-positive/7-AAD-A-negative; Q2,
CD16b-positive/7-AAD-A-positive; Q3,
CD16b-negative/7-AAD-A-negative; Q4,
CD16b-negative/7-AAD-A-positive. (B) Induction of PDL-1 expression
in neutrophils by GC tissue. Neutrophils incubated with TTCS showed
clearly upregulated expression of PDL-1 compared to neutrophils
incubated with NTCS. GC, gastric cancer; TTCS, tumor tissue culture
supernatant; NTCS, non-tumor tissue culture supernatant; PDL-1,
programmed cell death ligand-1; HLA-DR, human leukocyte antigen-DR;
7-AAD-A, 7-amino-actinomycin D. (C) Comparison of the ratio of
PDL-1+ neutrophils in the tumor area and normal area.
Neutrophils infiltrating the tumor area showed significantly
increased expression of PDL-1. In (B and C) Q1,
CD16b-positive/PDL-1-negative; Q2, CD16b-positive/PDL-1-positive;
Q3, CD16b-negative/PDL-1-negative; Q4,
CD16b-negative/PDL-1-positive. (D) Reduction of HLA-DR in
neutrophils by TTCS. Neutrophils incubated with TTCS tended to show
decreased expression of HLA-DR compared to neutrophils incubated
with NTCS. In (D) Q1, HLA-DR-positive/CD16b-negative; Q2,
HLA-DR-positive/CD16b-positive; Q3, HLA-DR-negative/CD16b-negative;
Q4, HLA-DR-negative/CD16b-positive. GC, gastric cancer; TTCS, tumor
tissue culture supernatant; NTCS, non-tumor tissue culture
supernatant; PDL-1, programmed cell death ligand-1; HLA-DR, human
leukocyte antigen-DR; 7-AAD-A, 7-amino-actinomycin D. |
Impact of neutrophils treated with
TTCS on CD4+ T cells
To explore the impact of TANs on CD4+ T
cells, we co-cultured CD4+ T cells and neutrophils. T
cells co-cultured with TTCS-conditioned neutrophils showed
significantly higher expression of PD-1 than T cells co-cultured
with NTCS-conditioned neutrophils (Fig.
2A and B). Next, we focused on CD25, which is an activating
factor of T cells. CD4+ T cells co-cultured with
neutrophils conditioned with TTCS showed lower expression of CD25
(Fig. 2C). Next, we examined whether
neutrophils possess immunosuppressive ability. To examine the
impact of neutrophils on CD4+ T cell proliferation, a
co-culture assay was performed using CD4+ T cells, DCs
and neutrophils. CD4+ T cells isolated from a healthy
volunteers proliferated when co-cultured with allogeneic DCs. After
further co-culturing of these T cells with autologous neutrophils
conditioned with NTCS or TTCS for 4 days, the change in their
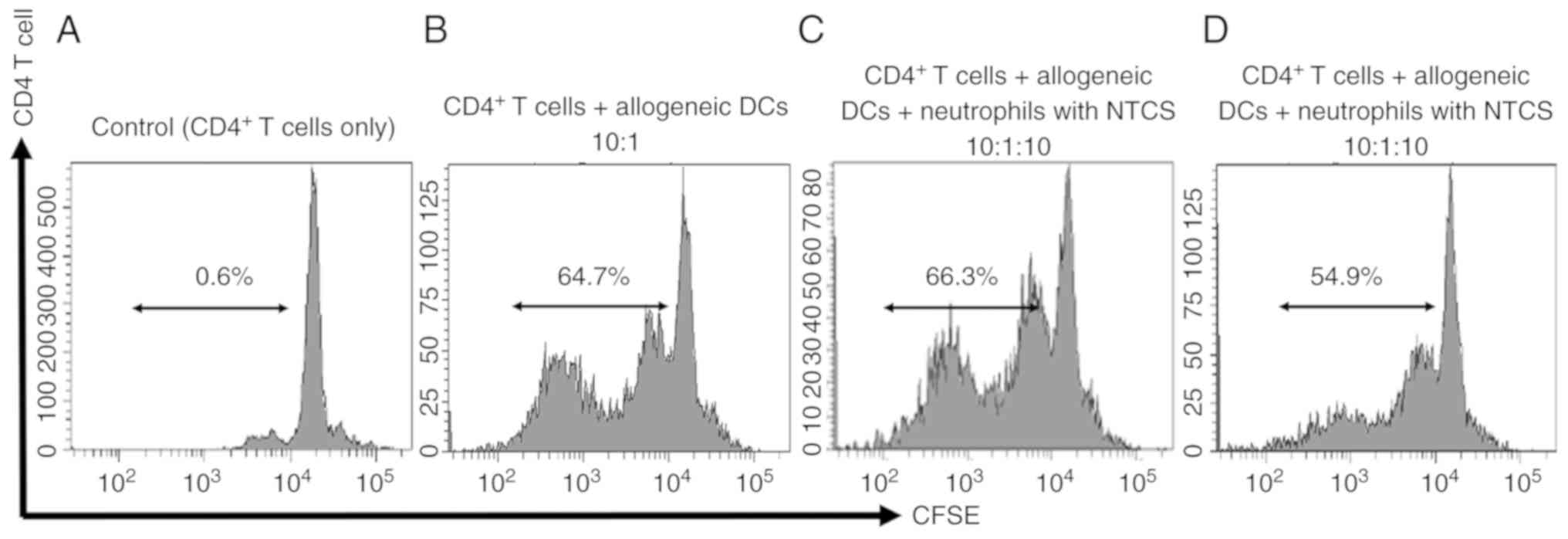
proliferative ability was examined. Proliferation of
CD4+ T cells co-cultured with neutrophils conditioned
with TTCS was suppressed. On the other hand, the proliferative
ability of CD4+ T cells co-cultured with neutrophils
conditioned with NTCS was not affected (Fig. 3).
 | Figure 2.Impact of neutrophils on
CD4+ T cells. (A) CD4+ T cells co-cultured
with allogenic neutrophils and (B) co-cultured with autologous
neutrophils. CD4+ T cells co-cultured with allogenic or
autologous neutrophils incubated with TTCS showed significantly
upregulated expression of PD-1. In (A and B) Q1,
PD-1-positive/CD4-negative; Q2, PD-1-positive/CD4-positive; Q3,
PD-1-negative/CD4-negative; Q4, PD-1-negative/CD4-positive. (C)
Expression of CD25 in CD4+ T cells co-cultured with
autologous neutrophils. CD4+ T cells co-cultured with
autologous neutrophils incubated with TTCS tended to show
downregulation of CD25 expression. In (C) Q1,
CD2-positive/CD4-negative; Q2, CD25-positive/CD4-positive; Q3,
CD25-negative/CD4-negative; Q4, CD25-negative/CD4-positive. TTCS,
tumor tissue culture supernatant; PD-1, programmed cell
death-1. |
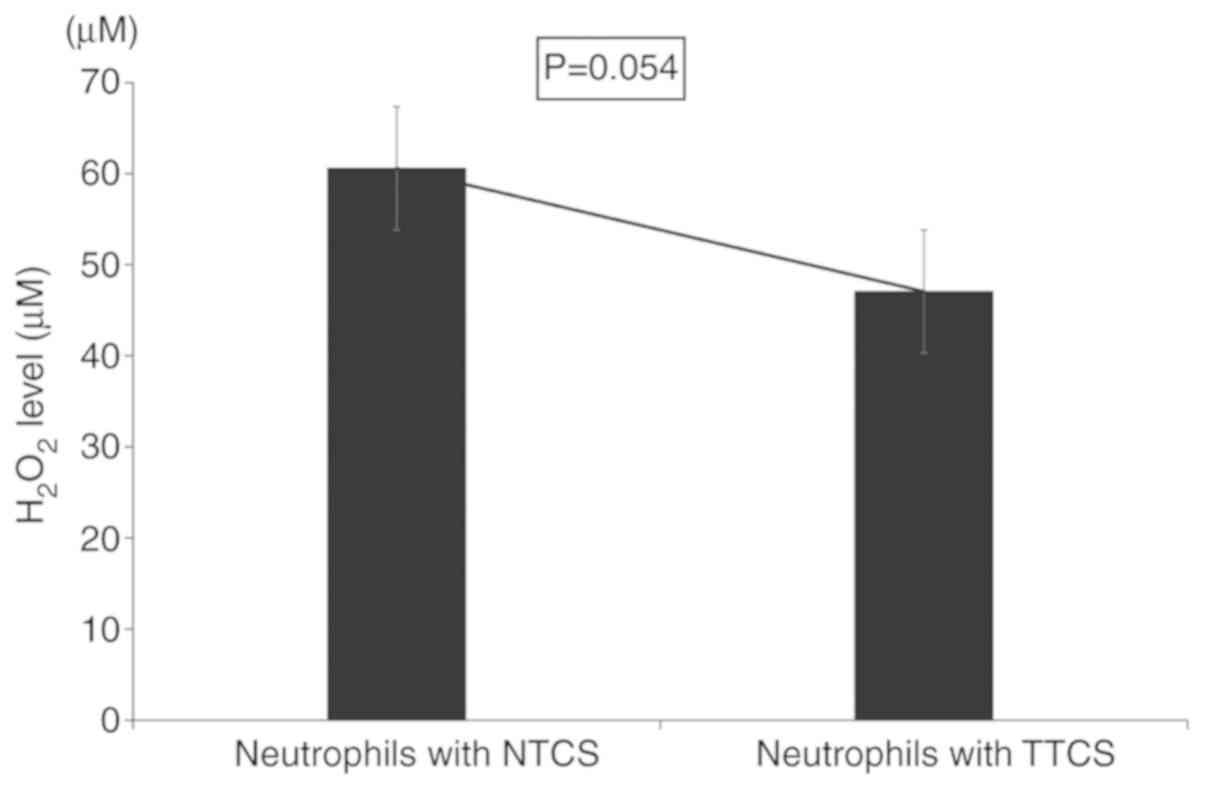
Furthermore, to investigate the ability of
neutrophils to kill cancer cells in the tumor microenvironment, we
measured the levels of reactive oxygen species such as
H2O2. TTCS-conditioned neutrophils had
significantly reduced levels of H2O2 compared
to NTCS-conditioned neutrophils (Fig.
4).
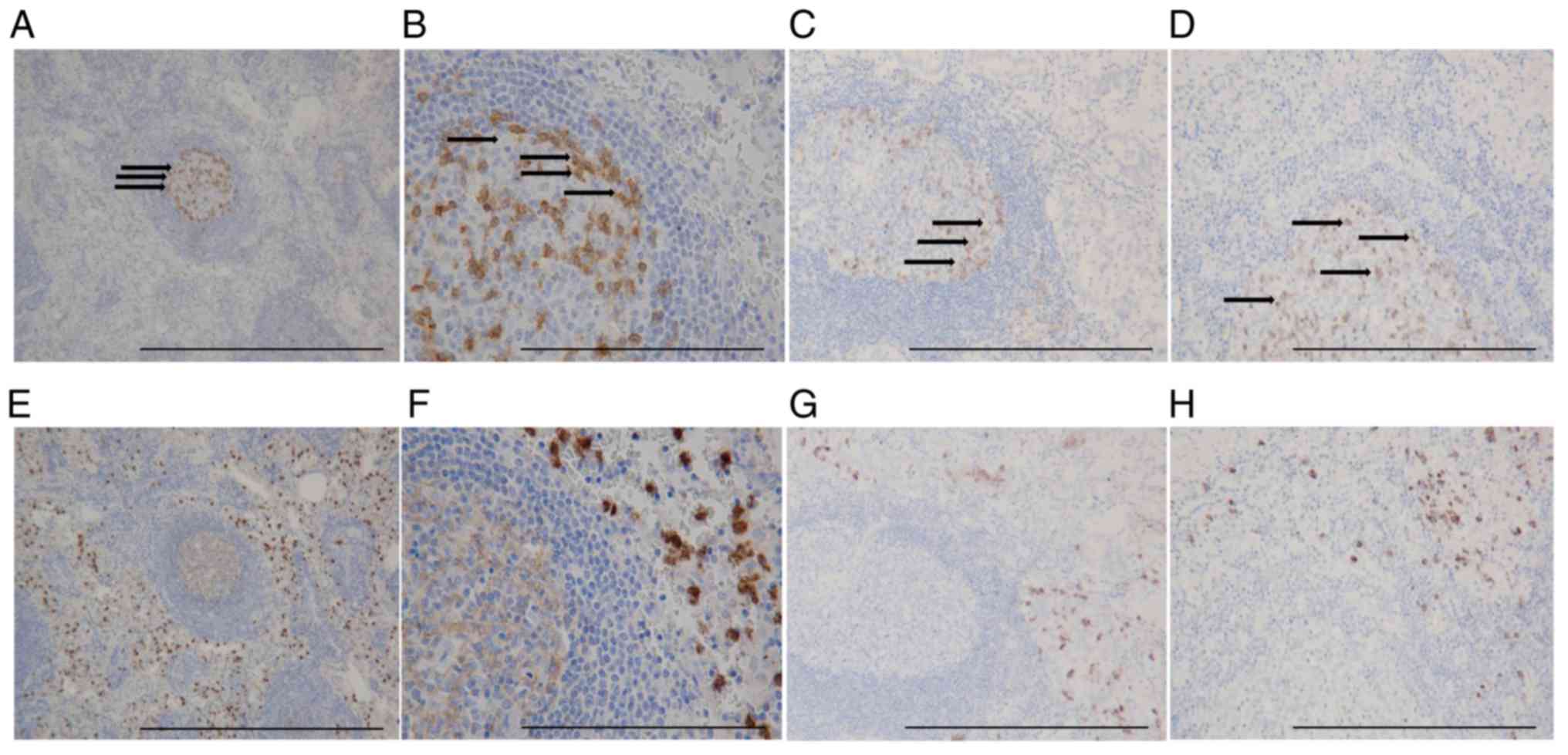
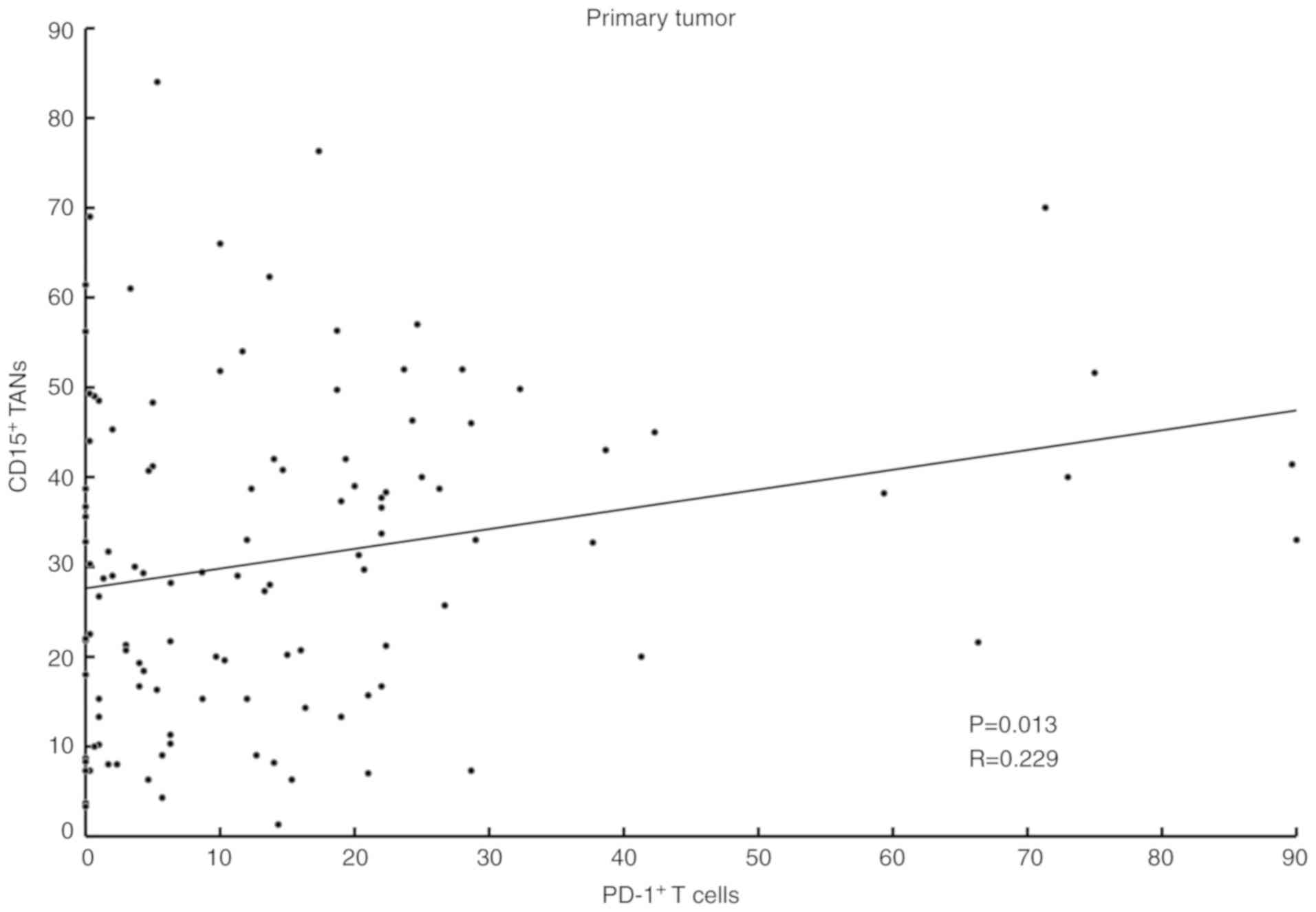
Distribution of PD-1+ cells
and TANs in GC tissue
IHC showed that PD-1+ T cells formed
clusters both in primary tumors and lymph nodes (Fig. 5A-D), and CD15+ TANs
infiltrated around the clusters (Fig.
5E-H). Our analysis indicated a positive correlation between
the expression of PD-1+ T cells and the number of
infiltrating CD15+ TANs in GC tissue (Table I). A scatter plot additionally showed
a weak positive correlation between CD15+ TANs and
PD-1+ T cells (Fig.
6).
 | Table I.Correlation between the expression of
PD-1+ T cells and the number of infiltrating
CD15+ TANs in gastric cancer tissue. |
Table I.
Correlation between the expression of
PD-1+ T cells and the number of infiltrating
CD15+ TANs in gastric cancer tissue.
|
|
| PD-1+ T
cells |
|
|---|
|
|
|
|
|
|---|
| CD15+
TANs | N | High | Low | P-value |
|---|
| High | 58 | 36 | 22 | 0.006a |
| Low | 57 | 21 | 36 |
|
Discussion
We previously reported that neutrophils in GC tissue
(TANs) are associated with poor prognosis. In that report, we
showed the relationship between infiltration of TANs and the
systemic neutrophil-lymphocyte ratio, which could be reflective of
the immune response (6,7). The present study revealed that
neutrophils conditioned with TTCS displayed altered characteristics
such as prolonged lifespan, upregulation of programmed cell death
ligand-1 (PDL-1) expression, downregulation of human leukocyte
antigen-DR (HLA-DR) expression and production of
H2O2. PDL-1 expression on neutrophils is
elevated in various cancer types such as GC, HIV-associated
cancers, breast cancer and lung cancer (8–12).
Extension of the neutrophil lifespan by cancer cells
has also been pointed out in previous studies (24–28), and
the same result was obtained in our experiments. These results
suggest that neutrophils in the local tumor microenvironment are
part of the immunosuppressive mechanism mediated by the PD-1/PDL-1
pathway (25,28,31) and
function over a long period of time to allow tumor growth. We
previously reported that patients in the high CD15+ TAN
group demonstrated a worse prognosis than those in the low
CD15+ TAN group, and that high CD15+ TAN
infiltration in tumor-draining lymph nodes is an independent
prognostic factor for patients with GC (6,7). Such an
immunosuppressive function of TANs may contribute to the poor
prognosis of patients with GC.
Furthermore, neutrophils have antigen-presenting
ability similar to DCs (23,24), and reduced expression of HLA-DR
suggests a reduction in this ability. To the best of our knowledge,
this is the first demonstration of a reduction in HLA-DR on
neutrophils. Additionally, we showed that neutrophils conditioned
with TTCS induced PD-1 expression in CD4+ T cells and
decreased the proliferation of CD4+ T cells, indicating
that neutrophils that have gained an immunosuppressive function are
present in GC tissue (24,25). Several reports have shown that immune
cells sensitized to tumors suppress T cell proliferation. Our
research on neutrophils showed similar results (25,26,31,32).
Moreover, the positional relationship between CD15+ TANs
and PD1+ T cells as observed with IHC revealed that
clusters of PD1+ T cells are at the center of the immune
response mediated by TANs. Tumor-infiltrating lymphocytes including
T cells and B cells are mostly organized as clusters, and various
immune reactions are thought to occur in GC tissue (26). Our IHC showed that the
PD-1+ lymphocyte population exists in clusters, and
their activity may be affected by crosstalk with TANs. TANs may
induce the PD-1/PDL-1 pathway and suppress the proliferation of
CD4+ T cells. PDL-1 expression is associated with
aggressive subtypes of several types of cancer and poor prognosis
(3,27–29).
Blockade of the PD-1/PDL-1 pathway has also attracted attention in
GC as a new promising therapeutic approach in oncology (5,8,32,33).
Many immune cells such as tumor-associated
macrophages, cancer-associated fibroblasts and regulatory T cells
migrate into the tumor to form an immunosuppressive environment
(5,30–32). Of
the immune cells reported, the immunosuppressive capacity of
neutrophils through PDL-1 expression has not been well studied.
Thus, investigation of PDL-1 expression induced by TANs in cancer
cells has important implications. Which tumor-infiltrating immune
cells initiate the immunosuppressive microenvironment remains
unclear. Neutrophils may be the first to infiltrate a tumor since
neutrophils are the first immune cells that respond to infection
and are involved in removal of foreign bodies (9). Therefore, our findings suggest that
PDL-1+ TANs may be a new target for the treatment of GC
in the future.
The present study has several limitations. First, in
this experimental model, the detailed mechanism of how TAN
infiltration of GC tissue results in immunosuppression and
suppression of CD4+ T cell function is lacking. Because
our study did not provide direct evidence for neutrophils as the
first immune cells to form an immunosuppressive environment in GC
tissue, further in vitro experiments are required to
evaluate immune regulation by TANs. Second, TANs have two
phenotypes: Anti-tumorigenic (N1) or pro-tumorigenic (N2) (34–37), and
our study did not address which type of TAN contributed to the
results obtained in this study. Hence, further investigation
regarding the phenotypes of TANs in relation to our study results
is warranted.
In conclusion, our study demonstrated that GC cells
possess the potential to alter the characteristics of neutrophils
and induce upregulation of immune check point molecules to inhibit
T cell proliferation. Therefore, our findings suggest that
regulation of tumor-infiltrating neutrophils is critical to
overcome the immunosuppressive microenvironment of GC.
Acknowledgements
Not applicable.
Funding
HT was supported by Grants-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology (grant no. 26461990) for this study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SH, JN, YY and CS performed experiments described in
the study. TT and KM contributed to the collection of samples,
analysis, and data management. MY provided the materials and
carried out additional experiments of revision with SH. HT
contributed to analysis and interpretation of the data and review
of the article. KH and MO supervised and aided in the conduction of
the experiments. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental procedures were approved (no. 3138)
by the Osaka City University Ethics Committee, and all patients
provided informed consent for collection and analysis of the
specimens.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests regarding this study.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
TANs
|
tumor-associated neutrophils
|
|
TTCS
|
tumor tissue culture supernatant
|
|
NTCS
|
non-tumor tissue culture
supernatant
|
|
DCs
|
dendritic cells
|
|
PBS
|
phosphate-buffered saline
|
|
PDL-1
|
programmed cell death ligand-1
|
|
PD-1
|
programmed cell death-1
|
|
HLA-DR
|
human leukocyte antigen-DR
|
|
7-AAD-A
|
7-amino-actinomycin D
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Ajani JA, Lee J, Sano T, Janjigian YY, Fan
D and Song S: Gastric adenocarcinoma. Nat Rev Dis Primers.
3:170362017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizrak Kaya D, Harada K, Shimodaira Y,
Amlashi FG, Lin Q and Ajani JA: Advanced gastric adenocarcinoma:
Optimizing therapy options. Expert Rev Clin Pharmacol. 10:263–271.
2017.PubMed/NCBI
|
|
3
|
Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan
J, Zhong X, Li X, Qian H and Wang X: PD-L1 and gastric cancer
prognosis: A systematic review and meta-analysis. PLoS One.
12:e01826922017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galdiero MR, Garlanda C, Jaillon S, Marone
G and Mantovani A: Tumor associated macrophages and neutrophils in
tumor progression. J Cell Physiol. 228:1404–1412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kataoka K, Deleersnijder A and Lordick F:
Will molecular target agents enable the multidisciplinary treatment
in stage IV gastric cancer? Eur J Surg Oncol. 43:1835–1845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiramatsu S, Tanaka H, Nishimura J,
Sakimura C, Tamura T, Toyokawa T, Muguruma K, Yashiro M, Hirakawa K
and Ohira M: Neutrophils in primary gastric tumors are correlated
with neutrophil infiltration in tumor-draining lymph nodes and the
systemic inflammatory response. BMC Immunol. 19:132018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tokumoto M, Tanaka H, Ohira M, Go Y, Okita
Y, Sakurai K, Toyokawa T, Kubo N, Muguruma K, Maeda K, et al: A
positive correlation between neutrophils in regional lymph nodes
and progression of gastric cancer. Anticancer Res. 34:7129–7136.
2014.PubMed/NCBI
|
|
8
|
Rakic A, Beaudry P and Mahoney DJ: The
complex interplay between neutrophils and cancer. Cell Tissue Res.
371:517–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aulakh GK: Neutrophils in the lung: ‘The
first responders’. Cell Tissue Res. 371:577–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosales C, Lowell CA, Schnoor M and
Uribe-Querol E: Neutrophils: Their role in innate and adaptive
immunity 2017. J Immunol Res. 2017:97483452017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurt B, Schulick R, Edil B, El Kasmi KC
and Barnett C Jr: Cancer-promoting mechanisms of tumor-associated
neutrophils. Am J Surg. 214:938–944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying HQ, Deng QW, He BS, Pan YQ, Wang F,
Sun HL, Chen J, Liu X and Wang SK: The prognostic value of
preoperative NLR, d-NLR, PLR and LMR for predicting clinical
outcome in surgical colorectal cancer patients. Med Oncol.
31:3052014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamoto R, Inagawa S, Sano N, Tadano S,
Adachi S and Yamamoto M: The neutrophil-to-lymphocyte ratio (NLR)
predicts short-term and long-term outcomes in gastric cancer
patients. Eur J Surg Oncol. 44:607–612. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng JF, Huang Y and Chen QX: Preoperative
platelet lymphocyte ratio (PLR) is superior to neutrophil
lymphocyte ratio (NLR) as a predictive factor in patients with
esophageal squamous cell carcinoma. World J Surg Oncol. 12:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cortez-Retamozo V, Etzrodt M, Newton A,
Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B,
Gorbatov R, et al: Origins of tumor-associated macrophages and
neutrophils. Proc Natl Acad Sci USA. 109:24919–2496. 2012.
View Article : Google Scholar
|
|
17
|
Zhou J, Nefedova Y, Lei A and Gabrilovich
D: Neutrophils and PMN-MDSC: Their biological role and interaction
with stromal cells. Semin Immunol. 35:19–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pillay J, Tak T, Kamp VM and Koenderman L:
Immune suppression by neutrophils and granulocytic myeloid-derived
suppressor cells: Similarities and differences. Cell Mol Life Sci.
70:3813–3827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nywening TM, Belt BA, Cullinan DR, Panni
RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE,
et al: Targeting both tumour-associated CXCR2(+) neutrophils and
CCR2(+) macrophages disrupts myeloid recruitment and improves
chemotherapeutic responses in pancreatic ductal adenocarcinoma.
Gut. 67:1112–1123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato Y, Yashiro M, Noda S, Tendo M,
Kashiwagi S, Doi Y, Nishii T, Matsuoka J, Fuyuhiro Y, Shinto O, et
al: Establishment and characterization of a new hypoxia-resistant
cancer cell line, OCUM-12/Hypo, derived from a scirrhous gastric
carcinoma. Br J Cancer. 102:898–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Immune suppression and infections. Keeping
a balance. Mayo Clin Health Lett. 31:4–5. 2013.
|
|
22
|
Ostanin DV, Kurmaeva E, Furr K, Bao R,
Hoffman J, Berney S and Grisham MB: Acquisition of
antigen-presenting functions by neutrophils isolated from mice with
chronic colitis. J Immunol. 188:1491–1502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cassatella MA: Human mature neutrophils as
atypical APC. Blood. 129:1895–1896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singhal S, Bhojnagarwala PS, O'Brien S,
Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L,
Deshpande C, et al: Origin and role of a subset of tumor-associated
neutrophils with antigen-presenting cell features in early-stage
human lung cancer. Cancer Cell. 30:120–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang TT, Zhao YL, Peng LS, Chen N, Chen W,
Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al: Tumour-activated
neutrophils in gastric cancer foster immune suppression and disease
progression through GM-CSF-PD-L1 pathway. Gut. 66:1900–1911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X and Xu W: Neutrophils diminish
T-cell immunity to foster gastric cancer progression: The role of
GM-CSF/PD-L1/PD-1 signalling pathway. Gut. 66:1878–1880. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakimura C, Tanaka H, Okuno T, Hiramatsu
S, Muguruma K, Hirakawa K, Wanibuchi H and Ohira M: B cells in
tertiary lymphoid structures are associated with favorable
prognosis in gastric cancer. J Surg Res. 215:74–82. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamura T, Ohira M, Tanaka H, Muguruma K,
Toyokawa T, Kubo N, Sakurai K, Amano R, Kimura K, Shibutani M, et
al: Programmed death-1 ligand-1 (PDL1) expression is associated
with the prognosis of patients with stage II/III gastric cancer.
Anticancer Res. 35:5369–5376. 2015.PubMed/NCBI
|
|
29
|
Abbas M, Steffens S, Bellut M, Becker JU,
Großhennig A, Eggers H, Wegener G, Kuczyk MA, Kreipe HH, Grünwald
V, et al: Do programmed death 1 (PD-1) and its ligand (PD-L1) play
a role in patients with non-clear cell renal cell carcinoma? Med
Oncol. 33:592016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bowers NL, Helton ES, Huijbregts RP,
Goepfert PA, Heath SL and Hel Z: Immune suppression by neutrophils
in HIV-1 infection: Role of PD-L1/PD-1 pathway. PLoS Pathog.
10:e10039932014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng Y, Li H, Deng Y, Tai Y, Zeng K,
Zhang Y, Liu W, Zhang Q and Yang Y: Cancer-associated fibroblasts
induce PDL1+ neutrophils through the IL6-STAT3 pathway
that foster immune suppression in hepatocellular carcinoma. Cell
Death Dis. 9:4222018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galdiero MR, Varricchi G, Loffredo S,
Mantovani A and Marone G: Roles of neutrophils in cancer growth and
progression. J Leukoc Biol. 103:457–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang X, Pan Y, Ma J, Kang Z, Xu X, Zhu Y,
Chen J, Zhang W, Chang W and Zhu J: Prognostic significance of the
infiltration of CD163+ macrophages combined with
CD66b+ neutrophils in gastric cancer. Cancer Med.
7:1731–1741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mishalian I, Bayuh R, Zolotarov L, Levy L,
Singhal S, Albelda SM and Fridlender ZG: Tumor-associated
neutrophils (TAN) develop protumorigenic properties during tumor
progression. Cancer Immunol Immunother. 62:1745–1756. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sagiv JY, Michaeli J, Assi S, Mishalian I,
Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et
al: Phenotypic diversity and plasticity in circulating neutrophil
subpopulations in cancer. Cell Rep. 10:562–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|