Introduction
Glioblastoma (GBM) remains one of the most common
types of primary brain tumors in the adult central nervous system
(CNS), with ≤5% of patients living >5 years after the first time
of diagnosis (1,2). GBM exhibits an evidently increased
incidence rate according to a study on Chinese patients with glioma
from 2000 to 2011, and cancer of the brain and the CNS are among
the 10 most common cancer types in China (3). The percentage of gliomas diagnosed in
the USA among all brain and CNS tumors and malignant brain and CNS
tumors is 31 and 81%, respectively (4). Currently, we see an increase in the
incidence of GBM, which is the highest grade of glioma
classification, ranging from 0.59 to 3.69 per 100,000 individuals
and with the highest increase observed in ages ranging from ~75–84
years old. In contrast to GBM, the highest incidence of
oligodendroglia and oligoastrocytomas [World Health Organization
(WHO) grade III] is in patients 35–44 years of age (2,5,6). The
highly invasive feature of GBM cells contributes to the migration
of glioma cells into the surrounding brain parenchyma excluding
surgical resection treatment, which may explain its dismal
prognosis, with a median overall survival of only 15 months, and
tumor recurrence (7). Recently, WHO
modified the Classification of Tumors of the Central Nervous System
based on molecular parameters in addition to histology of tumors
(6,8). Grades I–IV are assigned to GBM,
generally with an increasing malignancy from grade I to grade IV,
and ~55% of all gliomas are classified as grade IV GBM, despite
being relatively rare (2–3 cases per 100,000 adults in the USA and
Europe annually) (9). As
aforementioned, 81% of the CNS malignant types in USA are gliomas,
and almost half of them are highly aggressive GBMs according to the
list of brain tumors produced by WHO (1,8,10).
Surgery, radiotherapy and treatment with temozolomide accompanied
by tumor histopathology is the current standard therapy for GBM
(11–13). This highly malignant tumor creates a
serious social and economic burden, and is associated with high
mortality and morbidity (14).
Thus, improved therapeutic strategies or drugs are required.
Previous studies have highlighted the great
importance of the PI3K/AKT/mTOR signaling pathways in GBM (15,16),
which are mainly the consequence of the loss of PTEN in >50% of
GBMs (17). Certain inhibitors have
exhibited great inhibition effects in glioma cells (17,18).
Class I PI3Ks, which consist of the catalytic subunits p110α,
p110β, p110δ and PI3Kγ, are lipid kinases that, alongside mTOR,
play critical roles in a variety of cellular processes, including
differentiation, metabolism, migration and survival in
physiological and pathophysiological conditions, and can be
activated by cell surface receptors such as receptor tyrosine
kinases (RTKs), immunoglobulin receptors and G-protein-coupled
receptors (19–22). The PI3K family is overactive in
multiple types of tumors, including glioma, and has three different
subtypes, which are classified by sequence homology and substrate
specificity. The PI3K family can activate AKT [also known as
protein kinase B (PKB)] and mTOR (23). PKB/AKT and
3′-phosphoinositide-dependent kinase-1 can be activated by PI3K
through their pleckstrin-homology domains.
Changes in cell surface receptors, such as HER2,
oncogenes, protein tyrosine phosphatase non-receptor type 12,
presence of activating hotspot mutations in PIK3CA, and
inactivation of the lipid phosphatase PTEN can also lead to the
activation of the PI3K, PKB/AKT and mTOR signaling pathways
(23,24). Previous studies have revealed that
certain PI3K/AKT/mTOR pathway inhibitor monotherapies or in
combination with other drugs suppressed GBM proliferation in
vitro and in xenografts (17,18,24).
Overactivation of the PI3K/mTOR signaling pathway not only promotes
tumor cell growth and angiogenesis, but also is associated with
resistance to chemotherapy related to RTK inhibitors (23,25).
PQR309 is a PI3K/mTORC1/2 targeted inhibitor. Its
inhibitory concentration towards PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ is
33, 661, 708 and 451 nM, respectively. It has been revealed that
PQR309 may be a major inhibitor of PI3Kα, which is the most
effective therapeutic target in human tumors (17,23).
PQR309 exhibited a great tumor inhibition effect and minor side
effects in phase II clinical trials for different solid tumors such
as Ewing sarcoma, colorectal, breast, ovarian, Bartholin's gland
and lung carcinomas, mesothelioma, endometrial carcinoma, squamous
cell cancer of the tongue, thyme carcinoma, sinonasal carcinoma and
cervical carcinoma. Patients who tried this drug had positive
results, and reported common adverse events such as fatigue,
hyperglycaemia, nausea, diarrhoea, constipation, rash, anorexia and
vomiting (22). Considering the
small number of patients in the study, the conclusion is not
convincing. Its antitumor activity in refractory lymphoma in
combination with other drugs has made PQR309 a novel and promising
compound that is worth developing in the clinical setting of
lymphoma (26). In addition, PQR309
has a great ability to cross the blood brain barrier (BBB), it is
orally bioavailable, and has exhibited great pharmacokinetic
parameters and an antitumor proliferative effect in mice, rats and
dogs, both in vitro and in vivo (23). The great efficiency of this molecule
to suppress the activation of malignant tumors accompanied by its
safety profile and pharmacokinetic parameters, reveals the great
potential of PQR309 to be applied to brain tumors (23). However, the effect of PQR309 in
human GBM cells has not been studied to date. Thus, the present
study treated U87 cells, which have no mutant PTEN, and U251 cells,
which is the most common type of GBM cell line exhibiting mutant
PTEN, with PQR309 to evaluate whether PQR309 has an effect on GBM
cells.
Materials and methods
Cell culture
Human GBM cell lines (U87 and U251) were purchased
from the Cell Bank Type Culture Collection of the Chinese Academy
of Sciences. All the cell lines in our laboratory were identified
by short tandem repeat profiling by Procell Life Science &
Technology Co., Ltd. In addition, the U87 cell line used in the
present study is of the ATCC type, and has been reported to be a
glioblastoma of unknown origin. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; GINOM Co., Ltd.)
containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 37°C in a humidified atmosphere
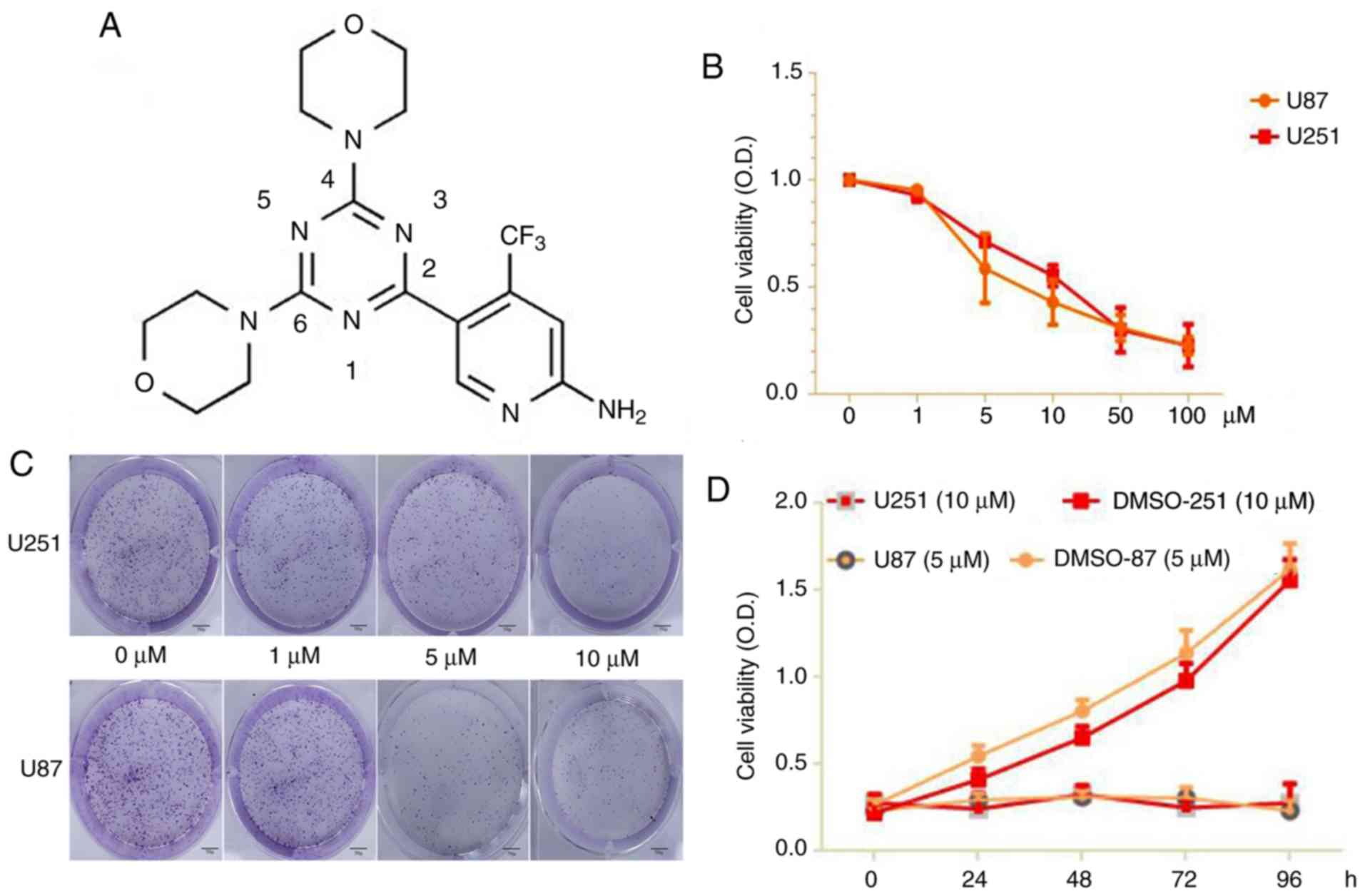
containing 5% carbon dioxide. PQR309 (Fig. 1) was purchased from Selleckchem, and
was dissolved in dimethyl sulfoxide (DMSO; Merck KGaA).
Antibodies
The antibodies used were as follows: Anti-Akt
(product no. 4691) and phospho-Akt (product no. 4060; both from
Cell Signaling Technology, Inc.), Bcl-2 (cat. no. GTX100064;
GeneTex, Inc.), Bcl-xL (product no. 2764), Bad (product no. 9239),
Bax (product no. 5023) and cyclin D1 (product no. 2978; all from
Cell Signaling Technology, Inc.), cleaved caspase-3 (product code
ab32042; Abcam), MMP-9 (product no. 13667), MMP-2 (product no.
40994) and GAPDH (product no. 5174; all from Cell Signaling
Technology, Inc.).
Cell viability
Cell viability was assessed using Cell Counting
Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.), to determine
the inhibitory effect of PQR309 on U87 and U251 cells according to
the manufacturer's instructions. Approximately 5×103
cells were seeded in a volume of 100 µl DMEM with 10% FBS, and
PQR309 was added into each well of a 96-well plate. Various
concentrations of PQR309 (0, 1, 5, 10, 20, 50 and 100 µM), as well
as a certain concentration applied for different time-points (24,
48, 72 and 96 h) were evaluated. Next, 10 µl CCK-8 was added, and
the cells were incubated for 1 h at 37°C. The absorbance value of
every well was measured with a spectrophotometric plate reader at
450 nm. Each group was assessed in triplicate.
Colony formation assay
U87 and U251 cells (~1,000) were plated on a 6-well
plate and cultured in 2 ml DMEM with 10% FBS. Then, the cells were
treated with different concentrations of PQR309 (0, 1, 5 and 10 µM)
for ~2 weeks at 37°C with 5% CO2. The cells were fixed
with 2 ml 5% paraformaldehyde at room temperature for 15 min and
then stained with 0.5% crystal violet at room temperature for 30
min. Then, each well was photographed with a camera.
5-Ethynyl-2′-deoxyuridine (Edu)
incorporation assay
A Cell-Light Edu DNA Cell Proliferation kit was
purchased from Guangzhou RiboBio Co., Ltd. Approximately
5×103 cells were seeded in a volume of 100 µl DMEM
containing 10% FBS into each well of a 96-well plate and treated
with various concentrations of PQR309 (0, 1, 5, 10 and 20 µM) for
72 h. Then, the cells were cultured with 50 µM Edu for 1 h at 37°C
in the presence of 5% CO2, and fixed with 4%
paraformaldehyde for 30 min at room temperature, according to the
manufacturer's protocol. The cells were then treated with 0.5%
Triton X-100 for 20 min and washed with PBS (3 times/5 min each).
Then, the cells were incubated with 100 µl 1X Apollo®
reaction cocktail for 30 min, and the cell nuclei were stained for
30 min with 5 µg/ml Hoechst 33342. Fluorescence images were
visualized under a fluorescence microscope at an ×200 magnification
(Olympus BX51; Olympus Corporation).
Cell cycle distribution analysis
Flow cytometry was used to determine the cell cycle
distribution using a cell cycle kit with PI staining (BD
Biosciences). U87 and U251 cells were plated in 6-well plates and
treated with various concentrations (0, 1, 5, 10 and 20 µM) of
PQR309 for 72 h. Then, the cells were collected by centrifugation
at 167.7 × g for 5 min at room temperature. Subsequently, the cells
were washed and fixed with PBS and cold 70% ethanol for 24 h at
4°C. Then, the cells were treated with 50 µl 100 µg/ml RNase at
37°C, washed twice with PBS, centrifuged at 167.7 × g for 5 min and
stained with 5 µl PI (50 mg/ml stock solution). The results were
analyzed by BD FACSAria (BD Biosciences). The data were quantified
using ModFit LT 4.0 (Verity Software House, Inc.).
Wound-healing assay
U87 and U251 cells were seeded into 6-well plates at
an appropriate density for ~70–80% confluence as a monolayer. Then,
the cells were scratched with a pipette tip to create straight
wound lines in each well. Subsequently, the floating cells were
removed by PBS. Various concentrations of PQR309 (0, 5 or 10 µM)
were added to 2 ml DMEM supplemented with 1% FBS for an additional
24 or 48 h. The images were visualized under a fluorescence
microscope (magnification, ×100; Olympus BX51; Olympus Corp.). The
wound distance was evaluated by ImageJ software (version 1.8.0;
National Institutes of Health).
Cell migration and invasion
assays
Migration and invasion assays were performed using a
Transwell chamber with an 8.0-µm pore polycarbonate membrane. U87
and U251 cells were seeded into the top chambers, where FBS had not
been added to DMEM, and were either coated with Matrigel or not
coated with Matrigel for invasion and migration, respectively.
Then, the chambers were placed into a 24-well plate, and medium
containing 10% FBS was added. After incubation for 24 h, the cells
were fixed and stained with 0.5% crystal violet at room temperature
for 30 min, which penetrated the underside surfaces of the
membranes, while the cells that had not crossed the membranes were
gently removed with cotton swabs. Subsequently, the cells were
quantified under a fluorescence microscope (magnification, ×100;
Olympus BX51; Olympus Corporation).
Flow cytometric analysis of apoptosis
with PE/7-amino-actinomycin (7-ADD) staining and TUNEL assay
An Apoptosis Annexin V-PE/7-AAD kit (BD Biosciences)
was used to analyze the apoptosis of cells treated with various
concentrations (0, 1, 5, 10 and 20 µM) of PQR309 for 72 h. The
cells, including those floating in DMEM, were washed twice in PBS
after being collected by centrifugation at 167.7 × g for 5 min at
room temperature, according to the manufacturer's protocol. Then,
the cells were suspended in 100 µl 1X binding buffer (0.1 mM
HEPES/NaOH, 1.4 M NaCl and 25 mM CaCl2, pH 7.4) and
stained with 5 µl PE-Annexin V and 5 µl 7-ADD for 15 min in the
dark at room temperature. Then, 400 µl 1X binding buffer was added
to each tube. Analysis of the results was carried out with BD
FACSAria (BD Biosciences). Data were quantified with FlowJo
software (version 10.4.0; FlowJo LLC), while the sum of the upper
right and lower right quadrants was used for calculating total
apoptosis rates and subjected to statistical analysis. A TUNEL
assay was performed to detect DNA fragmentation in apoptotic cells
according to the manufacturer's protocol (Roche Molecular
Diagnostics). Images were obtained with an Olympus BX51
fluorescence microscope (Olympus Corp.).
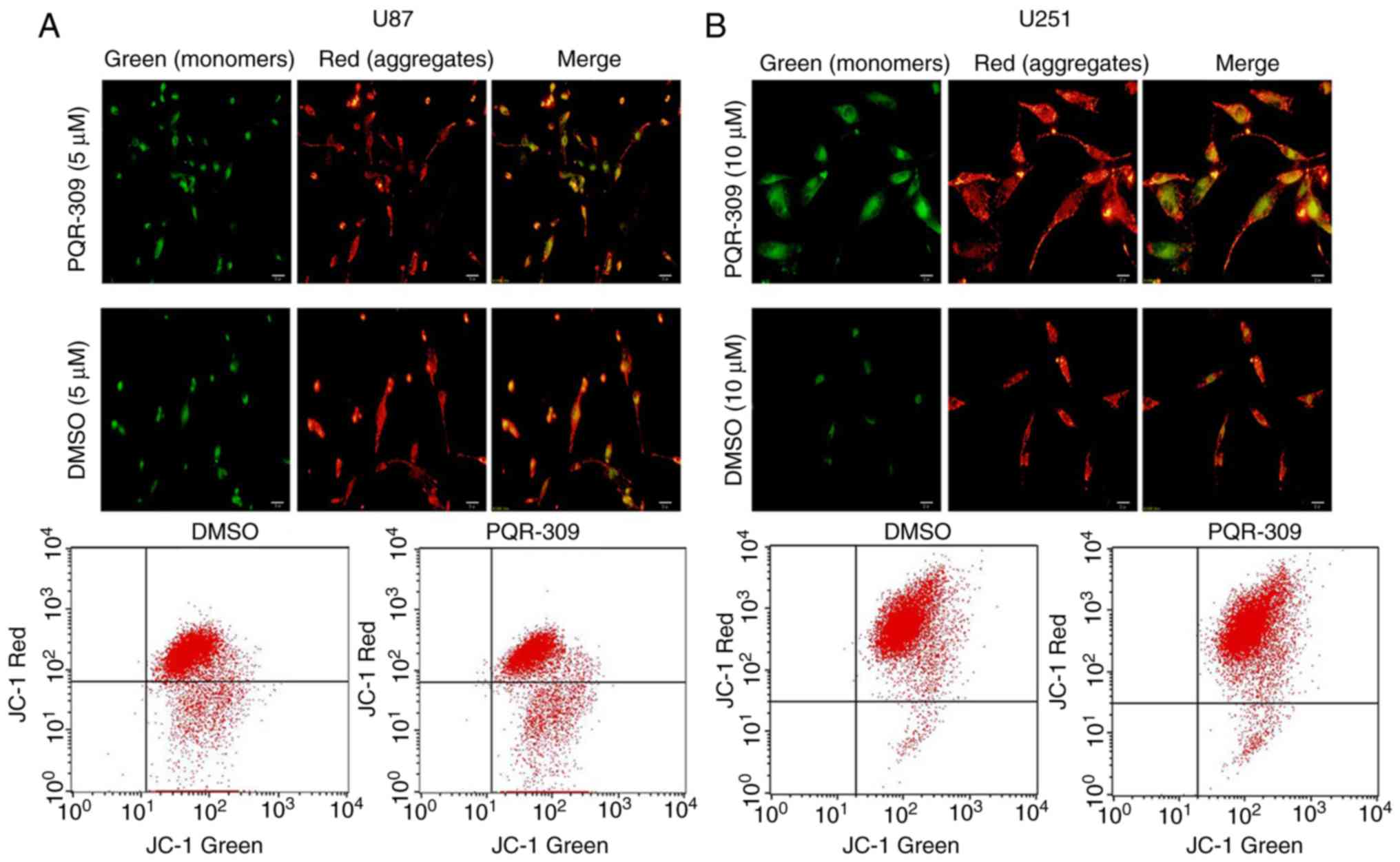
Mitochondrial membrane potential (ΔΨm)
assay
Considering that the loss of the ΔΨm of the
mitochondrial membrane is a hallmark event of early-stage
apoptosis, JC-1 staining [Yeasen Biotechnology (Shanghai) Co.,
Ltd.] was used to assess the ΔΨm. Cells were plated in 6-well
plates which consisted of a glass slide placed at the bottom of
each well, and treated with or without PQR309 for 72 h. Next, an
Olympus BX51 fluorescence microscope (Olympus Corp.) was used to
photograph the cells, according to the manufacturer's instructions.
A decrease in the ratio of red/green fluorescence intensity
detected by flow cytometry and microscopy indicated loss of
ΔΨm.
Western blot analysis
Cells were treated with 0, 5, 10 or 20 µM PQR309 for
72 h and then lysed in RIPA buffer (Shanghai Yeasen Biotechnology,
Co., Ltd.) for ~20 min on ice. BCA was used to test the
concentrations of each sample then, the protein samples were loaded
onto 10 or 12% SDS-PAGE for 40 µg per lane and electro-transferred
to PVDF membranes (Merck KGaA) for 60 or 90 min. After the
transfer, the membrane was blocked with 5% skim milk and then
incubated with the primary antibody (all used at 1:1,000) overnight
at 4°C. Subsequently, the membranes were incubated with Alexa Fluor
680/790-labelled goat anti-rabbit or goat anti-mouse IgG secondary
antibodies (cat. nos. 926-68021 and 926-68020; Li-COR Biosciences)
(all used at 1:1,000; LI-COR Biosciences) for 1 h. The bands were
visualized using the LI-COR Odyssey Infrared Imaging System (Li-COR
Biosciences) and the results were normalized to GAPDH.
Statistical analysis
Statistical analyses were conducted with SPSS 19.0
software (IBM Corp.) and GraphPad Prism 6.0 software (GraphPad
Software, Inc.). Data from the experiments are presented as the
mean ± SD. The comparisons among the different groups (>2
groups) were analyzed by one-way ANOVA and Tukey's post hoc test,
while Student's t-test was used for comparisons between 2 groups.
The results presented are representative of 3 independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
PQR309 suppresses the proliferation of
U87 and U251 cells
The results of a CCK-8 assay revealed a significant
suppressive effect of PQR309 on U87 and U251 cells. The results
indicated that the viability of the cells was significantly
(P<0.05) suppressed in a dose- and time-dependent manner after
the cells were treated with PQR309 (0, 1, 5, 10, 50 and 100 µM)
after 72 h (Fig. 1B). The colony
formation rates of treated U87 and U251 cells decreased in various
concentration groups compared to the control (Fig. 1C-D). According to these results, the
IC50 values of PQR309 were 7.104 (95% CI, 5.6–8.5) and
11.986 (95% CI, 10.6–13.4) in U87 and U251 cells, respectively.
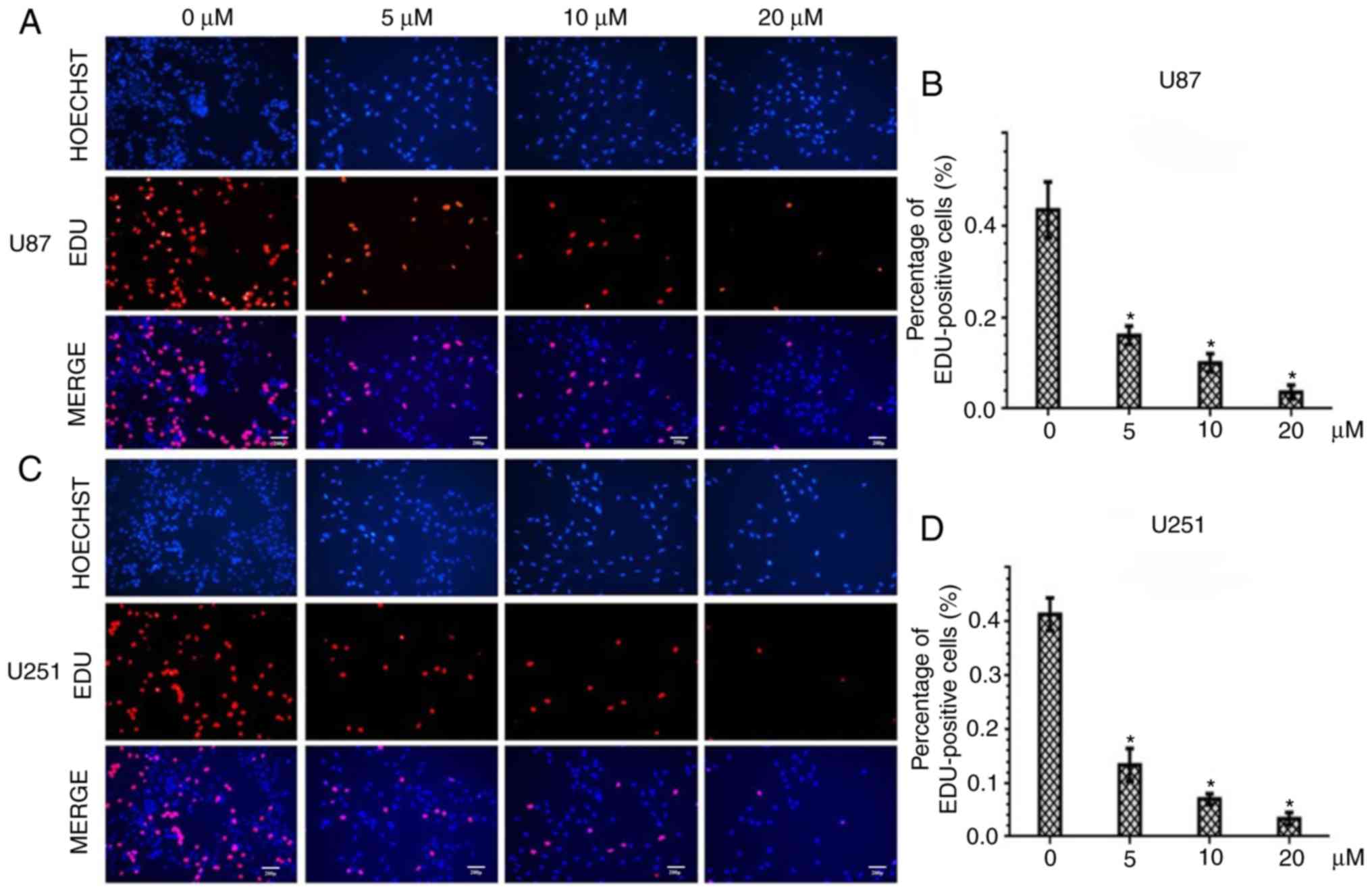
PQR309 induces EdU incorporation
decrease in glioma cells
To further assess the inhibitory effect of PQR309 in
U87 and U251 GBM cells, the DNA replication activity was assessed
by EdU incorporation assay. The results revealed a significant
suppression of cell proliferation in PQR309-treated U87 and U251
cells in a dose-dependent manner. The higher the concentration of
PQR309, the fewer cell nuclei with thymidine analog incorporation
were observed (Fig. 2A and C). The
total percentage of stained nuclei in cells treated with PQR309 was
lower than that in cells treated with DMSO (Fig. 2B and D). This indicated that DNA
replication was inhibited by PQR309. These results, along with the
viability data, confirmed the anti-proliferative effect of PQR309
on glioma cells.
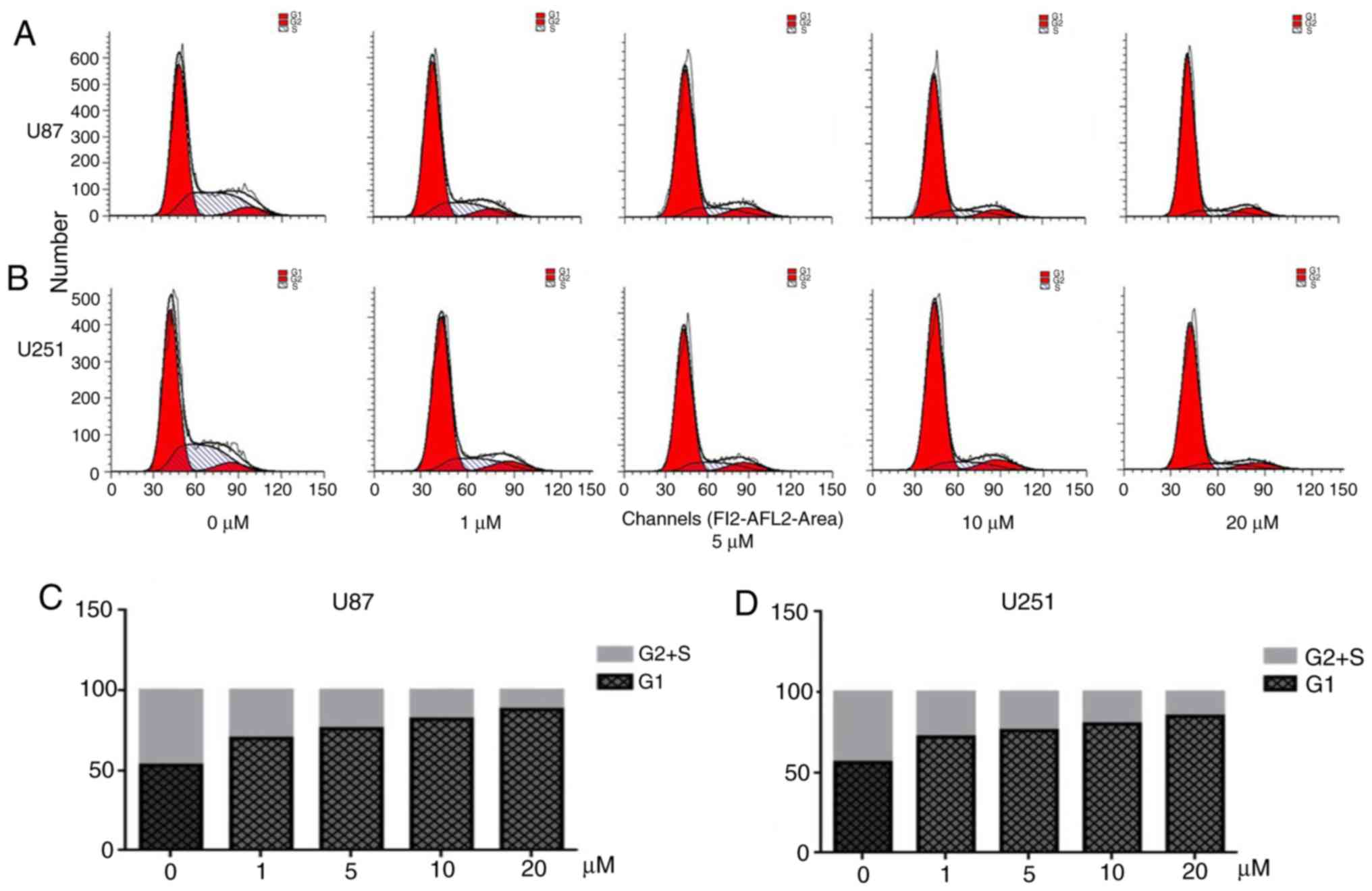
PQR309 causes cell cycle arrest in
glioma cells
Flow cytometry was performed to analyze the cell
cycle distribution (Fig. 3). PQR
309 induced a significant arrest at the G1 phase of the cell cycle
in U87 and U251 cells (Fig. 3A and
B). The cell cycle is associated with the viability of cells.
When cells were treated with various concentrations of PQR309 for
72 h, the number of EdU-positive cells decreased, while the
percentage of cells in G1 phase was increased compared with that in
the control groups treated with DMSO, while the percentage of cells
in the S and G2 phases was decreased (Fig. 3C and D). Notably, the percentage of
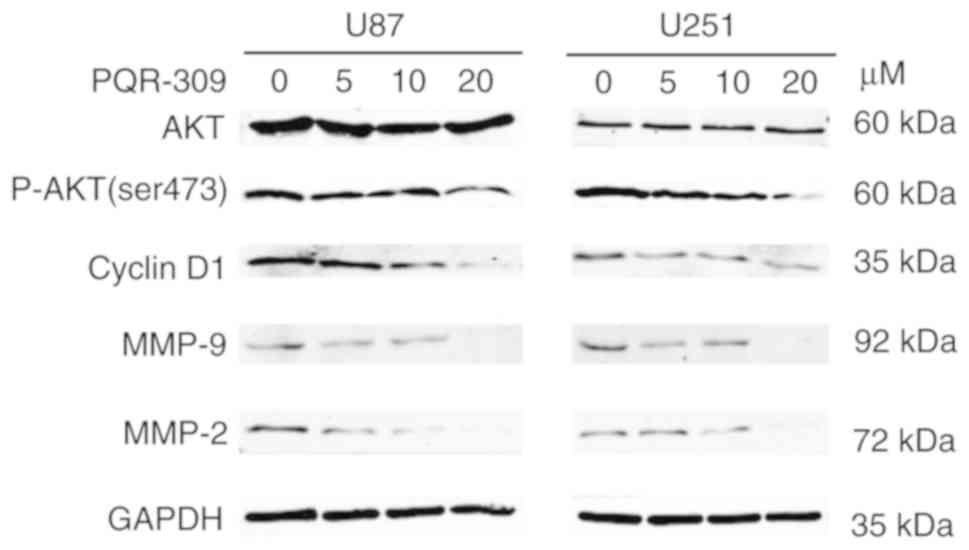
cells in the S and G2 phases, and the level of expression of cyclin
D1 and p-AKT decreased in a dose-dependent manner in GBM cells
(Fig. 4). Another PI3K-AKT
inhibitor used in our previous study revealed a similar phenomenon;
therefore, PQR309 may induce G1 arrest in U87 and U251 cells via
the PI3K/AKT signaling pathway (27).
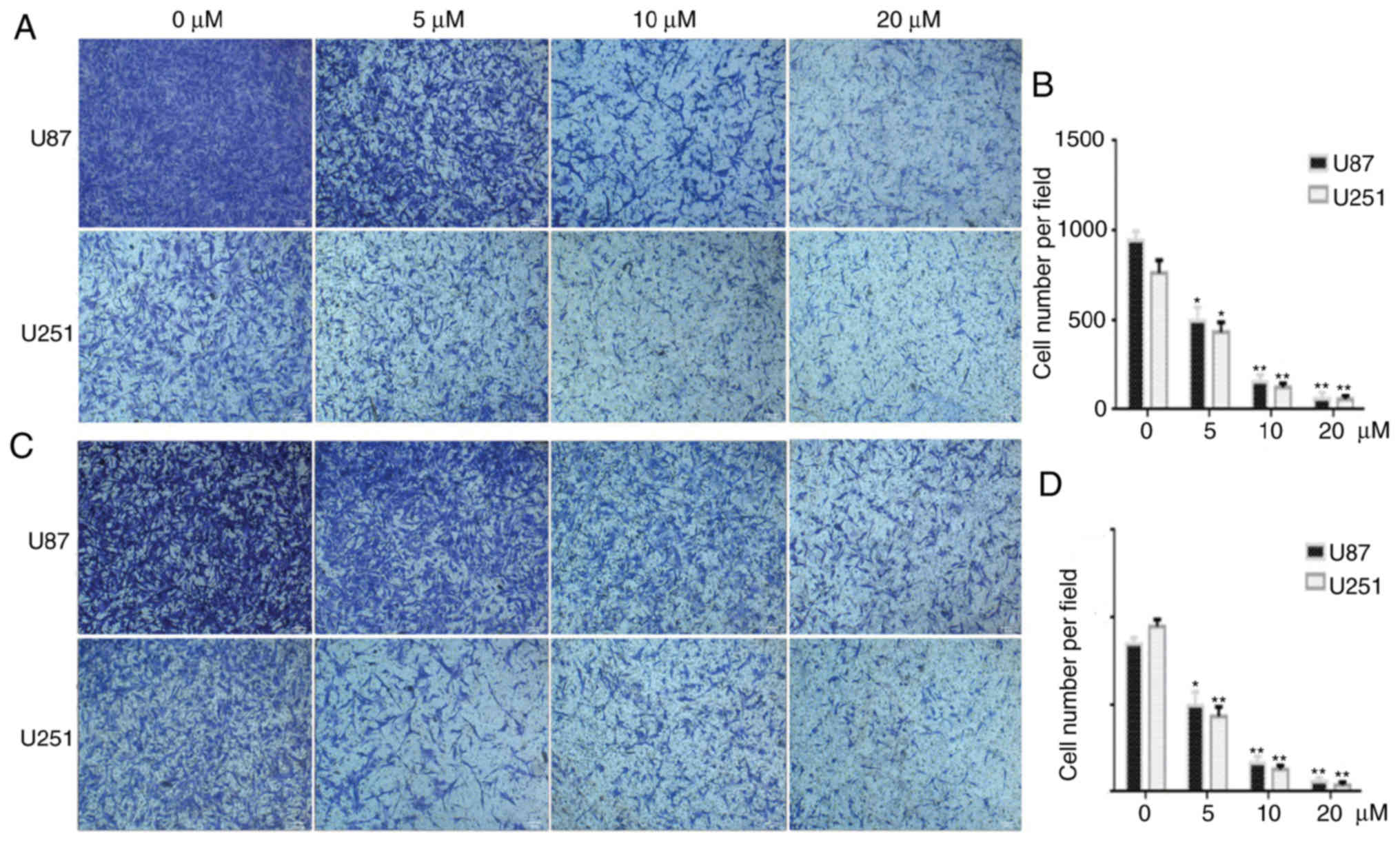
PQR309 inhibits the migration and
invasion of U87 and U251 cells
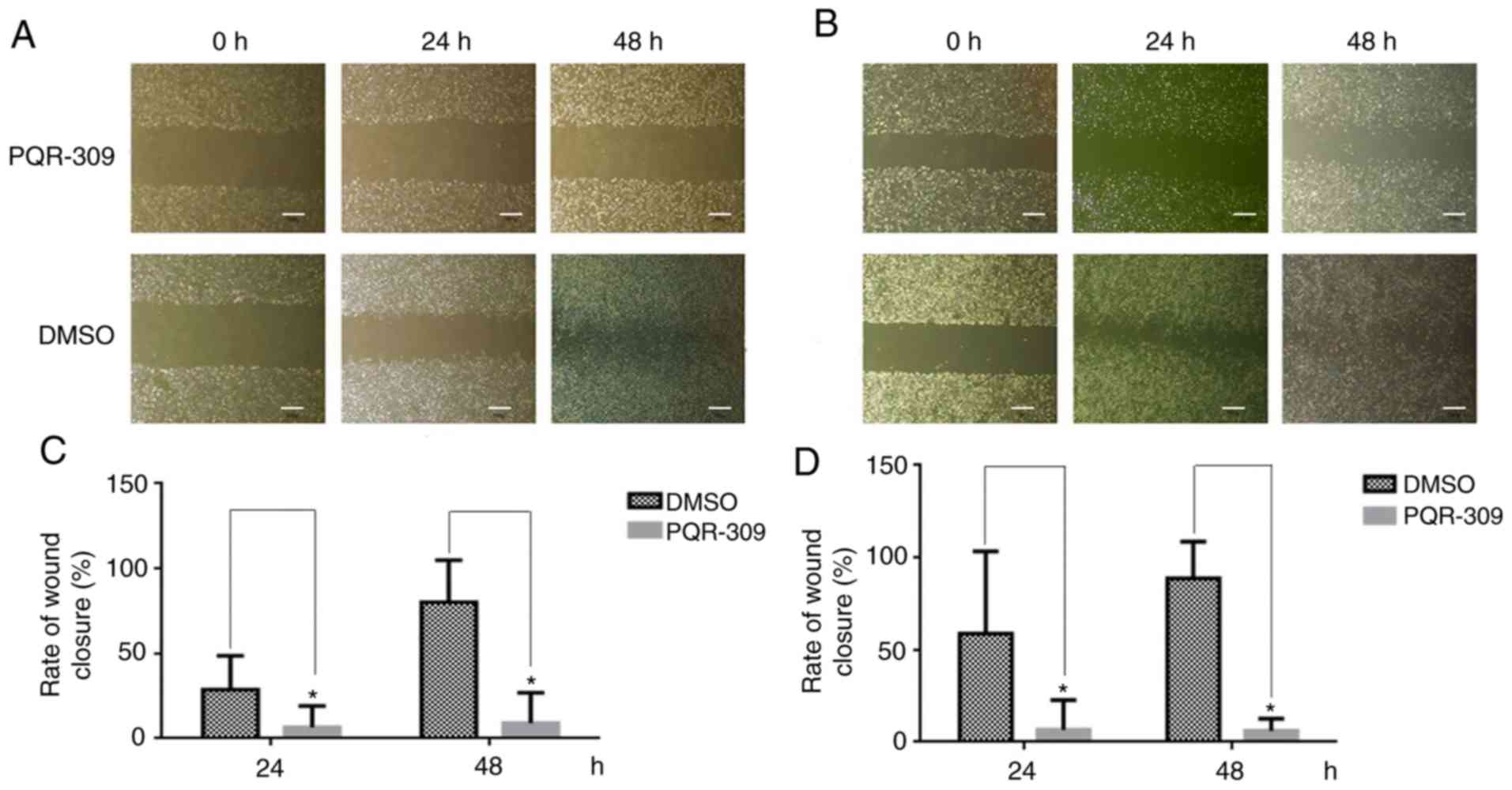
Wound-healing, migration and invasion assays were
performed to investigate the effect of PQR309 on glioma metastasis.
U87 and U251 cells were treated with various concentrations (0, 5,
10 and 20 µM) of PQR309, and the group treated only with DMSO was
used as a control for comparisons. As revealed in Fig. 5, the PQR309-treated group of U87 and
U251 cells exhibited less wound closure compared with that of the
untreated group after 48 h. Furthermore, the migration and invasion
abilities of GBM cells were inhibited after treatment with PQR309
(Fig. 6). These results were
further confirmed using western blot analyses. The expression
levels of MMP-9 and MMP-2 in glioma cells were gradually decreased
with increasing concentrations of PQR309 (Fig. 4).
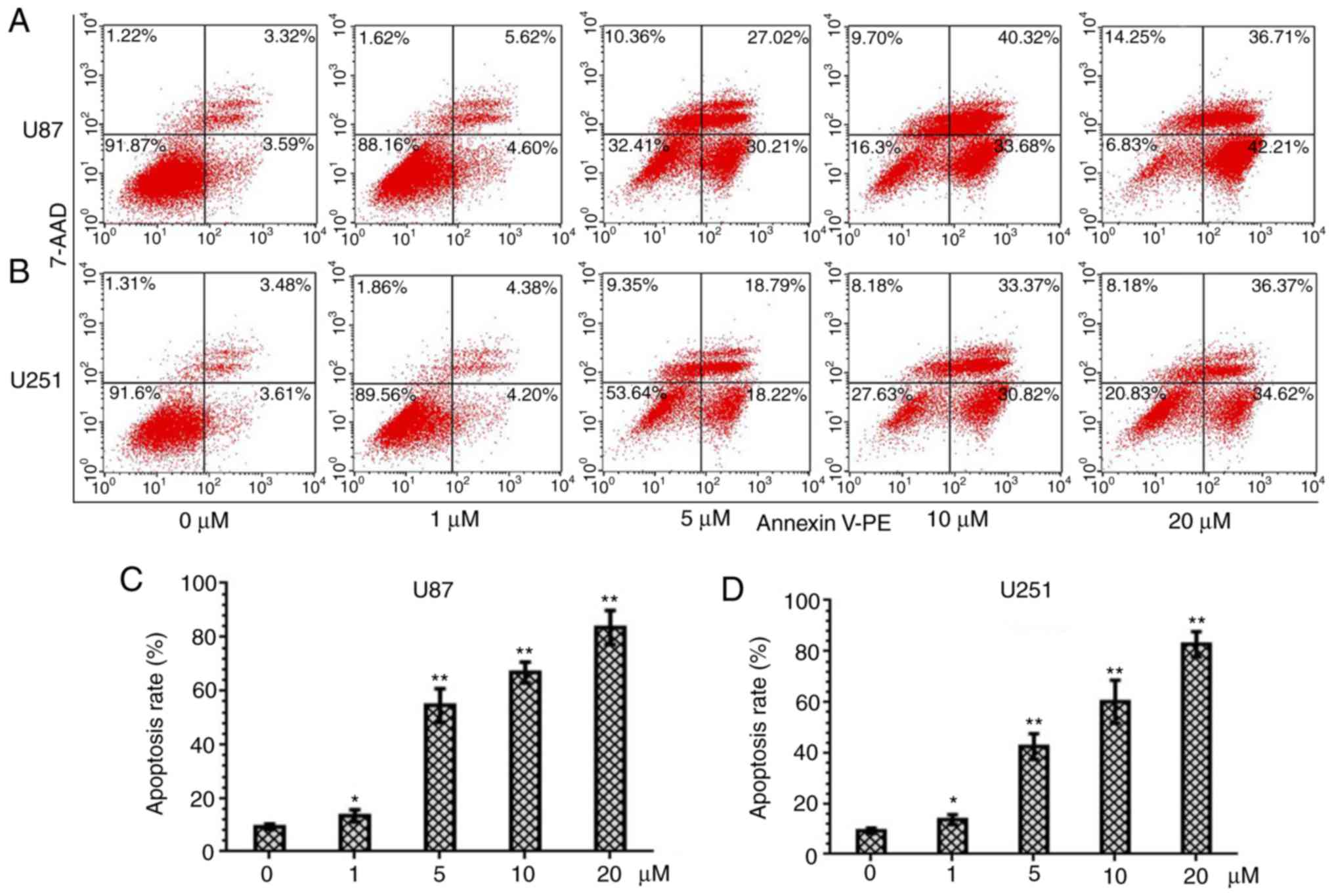
PQR309 induces apoptosis in U87 and
U251 cells
PQR309 was revealed to play a marked role in the
inhibition of glioma cells. The present study demonstrated its
ability to kill cells. Glioma cells were treated with various
concentrations (0, 1, 5, 10 and 20 µM) of PQR309 to observe its
influence on cell apoptosis. The results revealed that the
apoptotic cell population was increased with increasing
concentrations of PQR309 (Fig. 7).
The decrease in ΔΨm of the mitochondrial membrane accompanies the
early stage of apoptosis. Changes in ΔΨm were assessed by JC-1
staining according to the manufacturer's instructions after
treating the cells for 72 h with various concentrations of PQR309.
The flow cytometric results and images are presented in Fig. 8. The results revealed that the
change in the ratio of red/green fluorescence intensity indicated
the loss of ΔΨm (Fig. 8). Moreover,
the western blot results revealed that the expression levels of
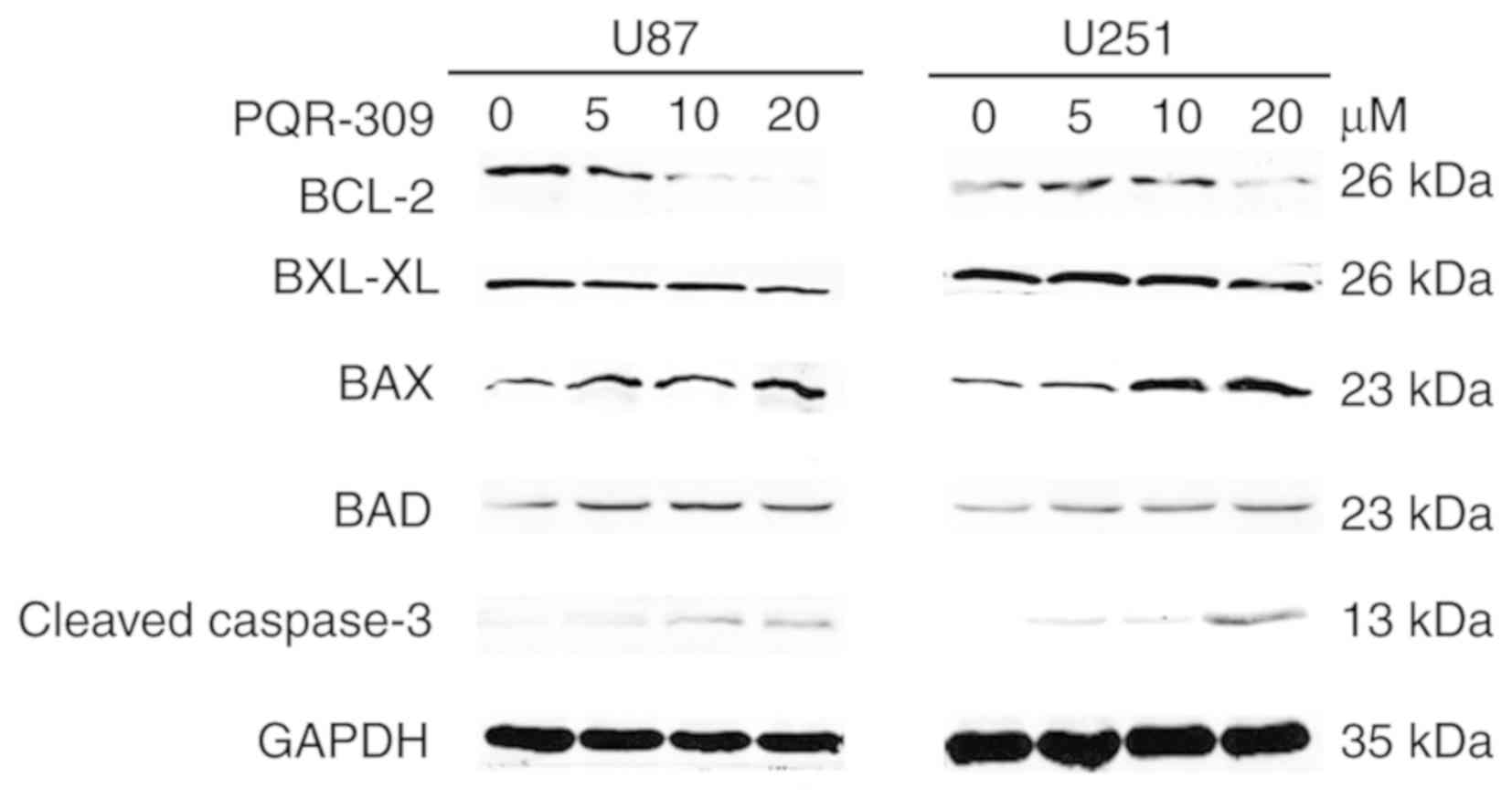
Bcl-2 (Fig. 9) and p-AKT (Fig. 4) were downregulated, in contrast to
those of Bax, Bad, cleaved caspase-3 which were increased (Fig. 9) with increasing drug concentration.
These results revealed that PQR309 had the ability to cause
apoptosis of glioma cells through the PI3K/AKT signaling pathway in
U87 and U251 cells, as revealed in Fig.
9. In addition, the results of TUNEL assay support the
apoptosis effect of PQR309 on U87 and U251 cells (Fig. 10).
Discussion
GBM has a poor prognosis, with a median survival
time of 15 months, and only 27% of patients survive >2 years
(28). Although tremendous efforts
have been made, the exact pathogenesis of glioma remains unknown
(14). Loss of the tumor-suppressor
PTEN and activation of the RTKs, such as EGF receptor, c-Met, PDGF
receptor and VEGF receptor, contribute to molecular dysfunctions
associated with glioma malignancy (29). The PI3K signaling pathway is one of
the most critical signaling pathways involved in the development of
human GBM pathogenesis (16,30).
PI3K activation initiates a signaling transduction pathway that
stimulates differentiation, metabolism, migration, cell
proliferation and survival (22).
One of the principal downstream effectors of PI3K is mTOR, which is
an important regulator of cell growth and proliferation (31,32).
In summary, different cell signaling pathways, including activation
of RTKs, constitutive recruitment and activation by Ras, activation
of the p110a subunit (PI3KCA), loss or inactivating mutations of
PTEN, G-protein-coupled receptors and chemotherapy resistance, are
the result of dysregulation of the PI3K/mTOR signaling pathway,
which may be a potential therapy for glioma via targeted PI3K/mTOR
inhibitors, such as PQR309.
A previous study reported that PQR309 is orally
available, crosses the BBB and is a PI3K/mTORC1/2 inhibitor
(23). Although a phase 1 trial
evaluated its clinical activity and revealed that patients benefit
from PQR309 trial medication in other solid tumors, such as breast
cancer and lung carcinoma (22).
However, the sample size was too small to show its clinical benefit
for patients. The present study revealed for the first time that
this small molecule has a great clinical significance on GBM, and
is a potential new anti-glioma drug.
In the present study, U87 and U251 cells were
treated with PQR309 to examine the effect of PQR309 on GBM cells.
PQR309 inhibited the proliferation of U87 and U251 cells in a dose-
and time-dependent manner, with IC50 values of 7.104 and
11.986 µm, respectively. The concentration of PQR309 in blood 24 h
later was >2 µM after oral administration in female rats, while
no signs of liver toxicity were observed, accompanied with
antiproliferative action in vitro and antitumor activity
in vivo (23). PQR309 also
promoted marked G1 arrest and cell apoptosis in a dose-dependent
manner, and cell migration and invasion abilities were suppressed
too. The wound-healing, cell migration and cell invasion assays
confirmed that the migration and invasion of human glioma cell
lines were reduced by PQR309. These results were supported by the
expression of p-AKT, AKT, Bcl-2, Bcl-xL, Bax, Bad, cleaved
caspase-3, MMP-2, MMP-9 and cyclin D1, as determined by western
blotting. There are various signaling pathways involved in the
anti-proliferation effect of PI3K/mTOR inhibitors, including the
NF-κB, ERK/MAPK and PI3K/AKT signaling pathways, which can affect
cell survival, proliferation and apoptosis (33–35).
There are numerous molecules with antiproliferative activity
against GBM which exhibit similar effects to PQR309; however, the
majority of them may not have the ability to cross the BBB or may
have a significant toxicity in vitro and in vivo at
the working concentration. Thus, PQR309 should be investigated in
clinical trials.
Although it was evident that PQR309 suppressed the
proliferation and induced the apoptosis of GBM U87 and U251 cells
in the present study, several limitations exist, including the lack
of animal experiments to assess whether PQR309 is orally available
and crosses the BBB in a GBM model. Despite exhibiting favorable
pharmacokinetic parameters in mice, rats and dogs, a trial on
patients with glioma has not been performed yet; thus, a larger
number of clinical trials on CNS metastasis and and patients with
GBM are required. To the best of our knowledge, the present study
is the first to report that PQR309 suppresses cell proliferation
and invasion, and induces apoptosis and G1 cell cycle arrest in
glioma cells. The present results strongly support further clinical
investigation of PQR309 in GBM (NCT02850744).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572489).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KY and QXC conceived and designed the study. KY
conducted the experiments. KY, XJT, FFX, LG, QS, YQT, XD, BHL and
JHL performed the statistical analysis. KY wrote the manuscript.
XJT, YQT, XD, BHL and QXC reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zanders ED, Svensson F and Bailey DS:
Therapy for glioblastoma: Is it working? Drug Discov Today.
24:1193–1201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Taillibert S, Kanner A, Read W,
Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K,
et al: Effect of tumor-treating fields plus maintenance
temozolomide vs maintenance temozolomide alone on survival in
patients with glioblastoma: A randomized clinical trial. JAMA.
318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Gittleman H, Stetson L, Virk SM
and Barnholtz-Sloan JS: Epidemiology of gliomas. Cancer Treat Res.
163:1–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santosh V, Sravya P, Gupta T, Muzumdar D,
Chacko G, Suri V, Epari S, Balasubramaniam A, Radotra BD,
Chatterjee S, et al: ISNO consensus guidelines for practical
adaptation of the WHO 2016 classification of adult diffuse gliomas.
Neurol India. 67:173–182. 2019.PubMed/NCBI
|
|
7
|
Molinaro AM, Taylor JW, Wiencke JK and
Wrensch MR: Genetic and molecular epidemiology of adult diffuse
glioma. Nat Rev Neurol. 15:405–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verdecchia A, De Angelis G and Capocaccia
R: Estimation and projections of cancer prevalence from cancer
registry data. Stat Med. 21:3511–3526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linz U: Commentary on effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial (Lancet
Oncol. 2009;10:459-466). Cancer. 116:1844–1846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park AK, Kim P, Ballester LY, Esquenazi Y
and Zhao Z: Subtype-specific signaling pathways and genomic
aberrations associated with prognosis of glioblastoma. Neuro Oncol.
21:59–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wojtas B, Gielniewski B, Wojnicki K,
Maleszewska M, Mondal SS, Nauman P, Grajkowska W, Glass R, Schüller
U, Herold-Mende C and Kaminska B: Gliosarcoma Is driven by
alterations in PI3K/AKT, RAS/MAPK pathways and characterized by
collagen gene expression signature. Cancers (Basel). 11(pii):
E2842019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Yang JA, Liu BH, Liao JM, Yuan FE,
Tan YQ and Chen QX: TGX-221 inhibits proliferation and induces
apoptosis in human glioblastoma cells. Oncol Rep. 38:2836–2842.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu PF, Yang JA, Liu JH, Yang X, Liao JM,
Yuan FE, Liu BH and Chen QX: PI3Kβ inhibitor AZD6482 exerts
antiproliferative activity and induces apoptosis in human
glioblastoma cells. Oncol Rep. 41:125–132. 2019.PubMed/NCBI
|
|
19
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wicki A, Brown N, Xyrafas A, Bize V, Hawle
H, Berardi S, Cmiljanović N, Cmiljanović V, Stumm M, Dimitrijević
S, et al: First-in human, phase 1, dose-escalation pharmacokinetic
and pharmacodynamic study of the oral dual PI3K and mTORC1/2
inhibitor PQR309 in patients with advanced solid tumors (SAKK
67/13). Eur J Cancer. 96:6–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beaufils F, Cmiljanovic N, Cmiljanovic V,
Bohnacker T, Melone A, Marone R, Jackson E, Zhang X, Sele A,
Borsari C, et al:
5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine
(PQR309), a potent, brain-penetrant, orally bioavailable, pan-class
I PI3K/mTOR inhibitor as clinical candidate in oncology. J Med
Chem. 60:7524–7538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen H, Mei L, Zhou L, Shen X, Guo C,
Zheng Y, Zhu H, Zhu Y and Huang L: PTEN restoration and PIK3CB
knockdown synergistically suppress glioblastoma growth in vitro and
in xenografts. J Neurooncol. 104:155–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tarantelli C, Gaudio E, Arribas AJ, Kwee
I, Hillmann P, Rinaldi A, Cascione L, Spriano F, Bernasconi E,
Guidetti F, et al: PQR309 is a novel dual PI3K/mTOR inhibitor with
preclinical antitumor activity in lymphomas as a single agent and
in combination therapy. Clin Cancer Res. 24:120–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Li XM, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/ GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teodorczyk M and Martin-Villalba A:
Sensing invasion: Cell surface receptors driving spreading of
glioblastoma. J Cell Physiol. 222:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vastrad B, Vastrad C, Godavarthi A and
Chandrashekar R: Molecular mechanisms underlying gliomas and
glioblastoma pathogenesis revealed by bioinformatics analysis of
microarray data. Med Oncol. 34:1822017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of AKT/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang J and Manning BD: A complex
interplay between AKT, TSC2 and the two mTOR complexes. Biochem Soc
Trans. 37:217–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Xu Y, Zhou Q, Chen M, Zhang Y,
Liang H, Zhao J, Zhong W and Wang M: PI3K in cancer: Its structure,
activation modes and role in shaping tumor microenvironment. Future
Oncol. 14:665–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanker AB, Kaklamani V and Arteaga CL:
Challenges for the clinical development of PI3K inhibitors:
Strategies to improve their impact in solid tumors. Cancer Discov.
9:482–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yap TA, Bjerke L, Clarke PA and Workman P:
Drugging PI3K in cancer: Refining targets and therapeutic
strategies. Curr Opin Pharmacol. 23:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|