Introduction
Filamin B (FLNB) is a member of the filamin family,
which is a group of highly conserved actin-binding proteins that is
responsible for anchoring the actin network to cellular membranes
and transmembrane receptors. The FLNB gene was first isolated using
a yeast two-hybrid system by Takafuta et al (1). The FLNB gene is located at chromosome
3p14.3 and encodes a protein of 2,602 amino acids (2). FLNB, as a cytoskeletal protein, is
broadly distributed across the entire cell (more in the cytoplasm
than in the nucleus) and expressed in different tissues, such as
blood vessels, colon, breast, prostate and skeletal muscle
(3,4). FLNB consists of an N-terminal
actin-binding domain, followed by immunoglobulin-like repeat
domains that form a receptor-binding region at the C-terminus
(5). The FLNB structure facilitates
execution of dual functions in two ways: Assisting to form a
three-dimensional network of actin through the actin-binding
domain; and acting as scaffolding proteins for receptor activation
and signal transduction, then directing various cell functions,
including membrane stability, ion channel transport, adhesion,
proliferation, protrusion and motility (6). FLNB has been identified to play vital
roles in skeletal disorders. It was previously identified that FLNB
mutations or deficiencies cause multiple skeletal malformations,
including scoliosis, spondylocarpotarsal synostosis, Larsen
syndrome, atelosteogenesis, boomerang dysplasia, clubfoot, joint
dislocation and other unique skeletal abnormalities (7,8).
It has been recently demonstrated that FLNB
plays an important role in cancer. Several previous studies
demonstrated that FLNB expression was highly correlated with
tumor proliferation, metastasis and invasiveness. For example,
Bandaru et al (9) identified
that a FLNB gene deficiency in mouse embryonic fibroblasts
increased the expression and proteolytic activity of matrix
metalloproteinase-9 (MMP-9), as well as cell invasion mediated by
the RAS/ERK pathway. Another previous in vitro study
demonstrated that FLNB is highly expressed in several cancer
cells, such as A549 (adenocarcinomic human alveolar basal
epithelial cells) and HT1080 (fibrosarcoma cell line) cells, which
exhibit high invasiveness (10). In
particular, an alternative splicing (AS) switch in FLNB
promotes the epithelial-to-mesenchymal transition (EMT) in human
breast cancer (11).
Notably, Baltz et al (12) identified that FLNB functions
as an RNA-binding protein (RBP) using a photoreactive
nucleotide-enhanced UV cross-linking and oligo(dT) purification
method. RBPs are involved in virtually all steps of this
post-transcriptional regulation, including RNA splicing,
polyadenylation, stability, localization, translation and
degradation, and defects in RBPs have been linked to many human
disorders, including cancer, immunologic disorders and
neurodegenerative diseases (13–15).
Therefore, it was hypothesized that FLNB has important
regulatory functions in regulation of AS and transcription in
cancer, which has not been previously reported, to the best of the
authors’ knowledge.
In the present study, FLNB was knocked down
using short hairpin RNA (shRNA) in HeLa cells derived from human
cervical cancer cells. It was demonstrated that cell apoptosis was
significantly inhibited. Then, high-throughput RNA-sequencing
(RNA-seq) was performed to comprehensively analyze the
transcriptome changes of the knocked-down FLNB compared with
controls. The present results identified that FLNB regulated
the transcription and AS of subsets of genes, especially those
involved in apoptosis, proliferation and chondrocyte development
signaling pathways. The present results may provide insight for the
current understanding of FLNB in regulating gene
transcription and AS in cancer.
Materials and methods
Sample preparation and FLNB
knockdown
Human HeLa cells (CCTCC@GDC0009) were obtained from
The China Center for Type Culture Collection. HeLa cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 100
µg/ml streptomycin, 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), and 100 U/ml penicillin at 37°C in 5% CO2.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to perform plasmid transfection of HeLa,
according to the manufacturer's protocol. In total, 2.5 µg plasmid
was used to transfect ~5×105 Hela cells in each well of
a 6-well plate. The shRNA sequence for silencing FLNB was
5′-CCTTCAGGAATCGGGATTAA-3′. The primer was synthesized by Sangon
Biotech Co., Ltd. HeLa cells which were transfected with empty
vector were used as controls.
FLNB knockdown efficiency was assessed by
reverse transcription-quantitative (RT-qPCR) by comparing shFLNB
groups and control groups with Student's t-test, and GAPDH was used
as a control after 48 h of transfection. RNA was extracted using
TRIzol® kit (Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using M-MLV Reverse Transcriptase (Vazyme
Biotech Co., Ltd.). The RT procedure included incubation at 37°C
for 15 min and then 85°C for 5 sec. Real-time PCR was performed
with the StepOne RealTime PCR system (Thermo Fisher Scientific,
Inc.) using PrimeScript™ RT reagent kit (Takara Bio,
Inc). The PCR conditions consisted of denaturing at 95°C for 10
min, 40 cycles of denaturing at 95°C for 15 sec, annealing and
extension at 60°C for 30 sec. PCR amplifications were performed in
triplicate for each sample that normalized with the reference gene
GAPDH, and the data was assayed using the comparative Cq
(2−∆∆Cq) method (16).
Primers of FLNB and GAPDH for qPCR analysis are presented in
Table SI.
Cell proliferation and apoptosis
assay
Cell proliferation was assessed using an MTT assay.
In total, 5×103 cell/well HeLa cells were cultured in
96-well plates. Then, cells were transfected with the vector using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
incubation at 37°C for 48 h, MTT solution (5 mg/ml; 0.025 ml) was
added to each well. The cells were incubated for another 4 h and
the supernatant was removed from each well. The colored formazan
crystals produced by MTT were dissolved in DMSO (0.15 ml) and the
optical density was measured at 490 nm.
For the flow cytometric analysis of cell apoptosis,
the transfected cells were incubated at 37°C for 48 h, and the live
cells were harvested and washed twice with ice-cold 1X PBS. Viable
cells were double-stained with 7-amino actinomycin D and
FITC-conjugated Annexin V (Beijing 4A Biotech Co., Ltd.). The
percentage of cell apoptosis was calculated as the sum of the right
lower and upper quadrants. The number of stained cells was
quantified using a flow cytometer (CytoFLEX; Beckman Coulter,
Inc.). Cell cycle distribution was quantified using multi-cycle
software (FlowJo 10.5.3; FlowJo, LLC).
Statistical analysis
Student's t-test was used to assess differences
between the FLNB knockdown and control groups. The results of each
experiment are presented as the mean ± SD of three experiments. The
data were analyzed using SPSS 19.0 software (IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
RNA-seq library preparation and
sequencing
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and then was purified
with two phenol-chloroform treatments. The RNA-seq library was
prepared with 1 µg total RNA for each sample using the VAHTS
Stranded mRNA-seq Library Prep kit (Vazyme Biotech Co., Ltd.). The
libraries were then applied to the Illumina HiSeq X Ten system
(Illumina, Inc.) for 150 nt paired-end sequencing.
RNA-Seq and differentially expressed
gene (DEG) analysis
Adaptors were removed from raw reads using cutadapt
and low-quality bases were trimmed using FASTX-Toolkit (version
0.0.13; http://hannonlab.cshl.edu/fastx_toolkit/index.html).
Reads >16 nt were aligned to the human GRch38 genome using
Tophat2 (17). Uniquely mapped
reads were used for counting gene reads. Fragments per kilobase of
transcript per million fragments mapped (FPKM) (18) was calculated for the gene expression
level. edgeR (19) and DESeq2
(20) was utilized to screen out
the DEGs. A false discovery rate (FDR)<0.05 and log2 fold change
≥1 or ≤-1 were set as the cut-off criteria for identifying DEGs.
Pearson's correlation analysis was performed to assess sample
distance.
AS analysis
The AS analysis was performed using the ABLas
pipeline, as previously described (21,22).
ABLas detects 10 types of alternative splicing events (ASEs) based
on the splice junction reads and uniquely mapped reads. These ASEs
include exon skipping (ES), alternative 3′splice site (A3SS),
intron retention, alternative 5′splice site (A5SS), mutually
exclusive exons (MXEs), mutually exclusive 3′untranslated regions
(UTRs), mutually exclusive 5′UTRs, cassette exon, A3SS&ES and
A5SS&ES.
To assess RBP-regulated ASEs, ABLas used Student's
t-test to evaluate the significance of the ratio alteration of
ASEs. Those events that were significant at the cut-off of FDR≤5%
were considered RBP-regulated ASEs.
Validation of DEGs and ASEs using
RT-qPCR and western blotting
RT-qPCR reactions were performed for validation of
transcription levels and AS changes. The primer information is
presented in Table SI. Total RNA
remaining from the RNA-seq library preparation was used for RT-qPCR
as previously described. The level of RNA expression for all genes
was normalized against GAPDH. For ASEs validation, the primers for
detecting ASEs are presented in Table
SI. To detect alternative isoforms, a boundary-spanning primer
of constitutive and alternative exons was used, as well as an
opposing primer in one constitutive exon. The boundary-spanning
primer of the alternative exon was designed according to a ‘model
exon’ to detect model splicing, or an ‘altered exon’ to detect
altered splicing.
Western blot analysis was performed for validation
of protein expression. Radioimmunoprecipitation assay lysis buffer
with protease inhibitors (Sigma-Aldrich; Merck KGaA) was used to
lyse treated cells on ice for 30 min, according to the
manufacturer's protocol. The protein concentration was determined
by the bicinchoninic acid protein assay kit (Sangon Biotech Co.,
Ltd.). Equal amounts (30 µg/load) of protein samples were resolved
by SDS-PAGE on on 10% gels (Bio-Rad Laboratories, Inc.) and
transferred to polyvinylidene fluoride difluoride membranes (Merck
KGaA). Subsequently, 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) was selected to block the membranes for 1 h at
room temperature, which were then incubated with primary antibodies
against FLNB (ABclonal Biotechnology Co., Ltd.; 1:500, cat. no.
A2481), NLR family apoptosis inhibitory protein (NAIP; BIOSS;
1:1,000; cat. no. bs-5804R), mitogen-activated protein kinase
kinase 7 (MAP2K7; ABclonal Biotechnology Co., Ltd.; 1:1,000; cat.
no. A2186) and GAPDH (ABclonal Biotechnology Co., Ltd.; 1:50,000;
cat. no. AC036) at 4°C overnight with gentle rocking. The membranes
were then washed three times, and then incubated with a secondary
antibody [peroxidase-conjugated AffiniPure goat Anti-Rabbit IgG
(H+L); 1:5,000; cat. no. ZB-2301; Origene Technologies, Inc.] at
room temperature for 2 h, and immunoreactivity was detected with
ECL substrate (Thermo Fisher Scientific, Inc.). GAPDH was used as
an internal control.
Functional enrichment analysis
To determine functional categories of DEGs, Gene
Ontology (GO; http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG Database 2017/6/5; http://www.genome.jp/kegg/) pathways enrichment
analysis was performed using the KOBAS 2.0 server (23). The most enriched 10 terms were
selected and plotted.
Results
FLNB knockdown significantly inhibits
apoptosis of HeLa cells
Previous studies demonstrated that FLNB
regulates proliferation of chondrocytes (24) and FLNB plays a causal role in
the regulation of EMT in human breast cancer (11). Therefore, it was hypothesized that
FLNB might regulate proliferation or apoptosis in cancer
cells. A cell model was constructed by knocking down FLNB using
shRNA in HeLa cells. The expression of FLNB was examined in
HeLa cells, which were transfected with empty vector or shRNA with
three experimental repeats by RT-qPCR. The mRNA level of
FLNB was decreased in shRNA-transfected cells (Fig. 1A). Cell proliferation was
significantly increased by knockdown of FLNB (shFLNB;
P=0.025; Fig. 1B); however, shFLNB
significantly reduced cell apoptosis (P<0.05; Fig. 1C and D). These results suggested
that shFLNB significantly inhibited apoptosis in HeLa cells.
RNA-seq and DEG analysis
To investigate FLNB-mediated transcriptional
regulation, cDNA libraries on shFLNB and control cells were
constructed for RNA-seq. Utilizing the Illumina HiSeq X Ten
platform, two biological replicates were used and yielded a total
of 81.7±8.6 M 150 nucleotide paired-end raw reads per sample. After
removing adaptors and low-quality reads, around 78.3±8.4 M
high-quality reads were generated, 84.15–89.69% of the reads from
each sample could be uniquely aligned to the human GRCH38 genome
using TopHat2 (Table SII).
Uniquely mapped reads were used to estimate the normalized
transcription level as FPKM. In total, 23,763 expressed genes were
detected from RNA-seq (Table
SIII). The effective knockdown of FLNB was further
confirmed with RNA-seq data (Fig.
2A), which was consistent with the RT-qPCR results (Fig. 1A). FPKM values for all 23,763 genes
were used to generate a Pearson's distance correlation matrix to
compare the transcriptomes from each sample. The heatmap
demonstrated that shFLNB and the control were distinguished, and
two biological replicates were highly correlated (Fig. 2B).
The DEGs between the shFLNB and control cells were
investigated using the edgeR package (19). In total, 826 upregulated and 363
downregulated DEGs (Table SIV)
were identified (log2 fold change≥1 and FDR <5%). The DEGs
associated with shFLNB are presented in a volcano plot (Fig. 2C). The expression heatmap of DEGs
indicated that more genes were upregulated upon shFLNB transfection
(Fig. 2D). It was additionally
identified that the mRNA expression of MMP-9 was
downregulated after knockdown of FLNB in HeLa cells (Table SIII), which is not consistent with
that reported in mouse embryonic fibroblasts (9).
DESeq2 (20) was
used to detect DEGs, to compare the results with edgeR. A total of
120 upregulated and 12 downregulated DEGs were identified (FDR
<5%; Fig. 2E). This suggested
that DESeq2 was more stringent than edgeR in recovering DEGs. The
Venn diagram demonstrated that 63% of DEGs based on DESeq2 were
also identified by edgeR (Fig. 2F).
DESeq2 is more inclined to identify DEGs with higher expression
compared with edgeR (Fig. 2G).
Knockdown of FLNB in HeLa cells
preferentially regulates transcription level variation of
tumorigenesis and cartiliage development-related genes
To further examine the potential biological roles of
these DEGs regulated by FLNB, GO and KEGG enrichment
analysis was performed. The top 10 biological process terms of the
GO analysis demonstrated that shFLNB-upregulated genes were
strongly enriched in ‘membrane depolarization involved in
regulation of action potential’, ‘transmembrane transport’,
‘negative regulation of neuron apoptotic process’, ‘cartilage
development’ and ‘extracellular matrix organization’ (Fig. 3A). The downregulated genes were
enriched in ‘signal transduction’, ‘cell adhesion’, ‘angiogenesis’,
‘inflammatory response’, ‘innate immune response’ and ‘cell
differentiation’ (Fig. 3B). Based
on the KEGG analysis (Fig. 3C), the
pathways of upregulated gene sets were mainly associated with the
‘GABAergic synapse’, ‘ABC transporters’ and ‘ECM-receptor
interaction’ signaling pathway. ‘Leukocyte transendothelial
migration’, ‘cell adhesion molecules (CAMs)’ and ‘transcriptional
misregulation in cancer’ pathways were significantly enriched in
downregulated gene sets (Fig. 3D;
Table SV).
FLNB regulates AS in HeLa cells
Since RNA-seq technology provides an opportunity to
study AS, transcriptome data were used to examine ASEs that might
be regulated by FLNB in HeLa cells. Spliced reads account for
48.46–53.99% of all uniquely mapped reads obtained from shFLNB and
control cells (Table SII). Of the
367,321 annotated exons from the reference genome, 224,199 were
detected. Using Tophat2, 151,708 annotated splice junctions and
108,767 novel splice junctions were detected, accounting for 40% of
the total. A total of 16,783 known ASEs (defined in the reference
genome) and 46,959 new splicing events (not including intron
retention) were identified using the previously published ABLas
software tool (Table SVI). These
results suggested that splicing in human is more complex than
previously hypothesized.
For each ASE, the differential splicing patterns
usage between shFLNB and control cells was calculated. A total of
402 significant differential ASEs in 363 genes was identified (AS
ratio difference ≥0.2 and P-value ≤0.05; Table SVII). In detail, these ASEs include
48 ES, 49 cassette exon, 75 A5SS, 65 A3SS and 119 intron retention
(Fig. 4A). Compared with the
control cells, an enrichment of ES and a depletion of cassette exon
in shFLNB were observed, suggesting that exon inclusion is
suppressed by FLNB silencing (Fig.
4A). To examine the changes in ASEs attributed to transcription
regulation, genes with differential expression level and ASEs were
overlapped. The present results identified that there were only
four DEGs with significantly altered splicing. Notably, metastasis
associated lung adenocarcinoma transcript 1 (MALAT1), as a
well-known long non-coding RNA associated with cancer metastasis,
demonstrated changes in expression and AS after FLNB knockdown
(Fig. 4B).
 | Figure 4.Alternative splicing analysis by
shFLNB. (A) Classification of significant changes in alternative
splicing after FLNB knockdown. (B) Overlap of DEGs and genes with
differential alternative splicing (RASG). Top 10 (C) GO biological
process terms and (D) KEGG pathways of genes with differential
alternative splicing events between shFLNB and the control.
Rectangles around GO terms /KEGG pathways indicate notable
apototic-related and cartilage development terms. sh, short
hairpin; FLNB, filamin B; DEG, differentially expressed genes; GO,
Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes;
3pMXE, mutually exclusive 3′untranslated regions; 5pMXE, mutually
exclusive 5′untranslated regions; A3SS, alternative 3′splice site;
A5SS, alternative 5′splice site; ES, exon skipping; IntronR, intron
retention; RASE, regulated alternative splicing events; RASG, genes
with differential alternative splicing; KIF9, kinesin family member
9; SLC25A36, solute carrier family 25 member 36; MALAT1, metastasis
associated lung adenocarcinoma transcript 1. |
GO functional clustering analysis demonstrated that
the alternative spliced genes were enriched in ‘organ
morphogenesis’, ‘apoptotic process’, ‘cell death’ and ‘signal
transduction’ (Fig. 4C). KEGG
pathways were most enriched in those involved in ‘Adherens
junctions’, ‘GnRH signaling pathway’, ‘Axon guidance’, ‘Bacterial
invasion of epithelial cells’ and ‘Mineral absorption’ pathways
(Fig. 4D; Table SVIII). These results suggested that
FLNB knockdown markedly regulated AS of apoptosis-related
genes and genes involved in skeletal development.
RT-qPCR validation of DEGs and ASEs by
FLNB knockdown in HeLa cells
A number of important tumorigenesis and skeletal
development-related DEGs and ASEs in HeLa cells were further
validated by RT-qPCR. A total of nine DEGs, including ATPase copper
transporting α (ATP7A), bone morphogenetic protein 7 (BMP7),
collagen type II α 1 chain (COL2A1), matrix metallopeptidase 13
(MMP13), interleukin 23 subunit α (IL23A), MALAT1, NAIP, solute
carrier family 25 member 36 and ghrelin and obestatin prepropeptide
(GHRL) were evaluated, although expression of GHRL did not yield
sufficient PCR signals. The results of all other eight genes upon
FLNB silencing matched well with the RNA-seq data,
suggesting that FLNB-regulated DEGs identified from RNA-seq
data are acceptable (Fig. 5A and
B). A total of two apoptotic-related proteins were detected by
western blotting in the shFLNB and control cells; NAIP (negative
regulator of apoptosis) was upregulated and MAP2K7 (positive
regulator of apoptosis) was downregulated in the shFLNB cells
compared with the control cells (Fig.
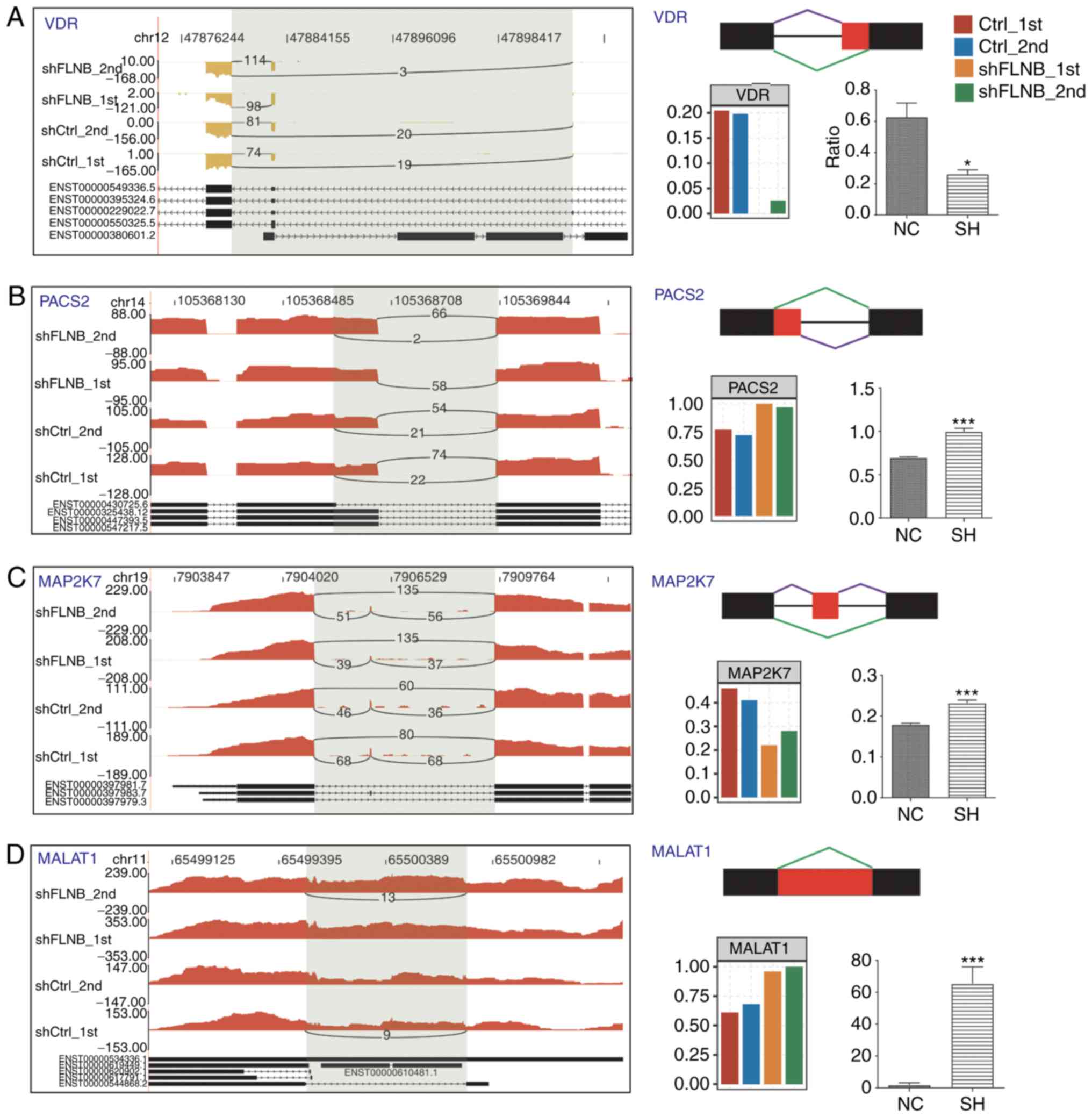
5C). A total of four FLNB-regulated splicing events located in
vitamin D receptor (VDR), phosphofurin acidic cluster sorting
protein 2 (PACS2), MAP2K7 and MALAT1 were validated by designing
PCR primer pairs (Table SI), which
amplify both long and short splicing isoforms in the same reaction
(Fig. 6). These four ASEs validated
by qPCR were in agreement with the RNA-seq results. The ratio of
two splicing patterns in each ASE were significantly altered after
FLNB-knockdown. These experimental results further suggested that
FLNB plays an important regulatory role in gene expression
and AS in HeLa cell.
 | Figure 5.Validation of FLNB-regulated
genes (DEGs). (A) Relative expression level (FPKM, up) and RT-qPCR
measurement (down) of cartilage development-related DEGs. (B)
Related expression level (FPKM, up) and RT-qPCR measurement (down)
of apoptotic-related DEGs. (C) Western blot analysis of two
apoptotic-related proteins in shFLNB and Ctrl HeLa cells.
FLNB, filamin B; DEGs, differentially expressed genes; FPKM,
fragments per kilobase of transcript per million fragments mapped;
RT-qPCR, reverse transcription-quantitative PCR; sh, short hairpin;
Ctrl, control; ATP7A, ATPase copper transporting α; BMP7, bone
morphogenetic protein 7; COL2A1, collagen type II α 1 chain; MMP13,
matrix metallopeptidase 13; IL23A, interleukin 23 subunit α;
MALAT1, metastasis associated lung adenocarcinoma transcript 1;
NAIP, NLR family apoptosis inhibitory protein; SLC25A36, solute
carrier family 25 member 36; MAP2K7, mitogen-activated protein
kinase kinase 7. |
Discussion
The present study is the first study, to the best of
the authors’ knowledge, to perform entire transcriptome analysis
with the knockdown of FLNB in a cancer cell line (HeLa
cells). The widespread effects of inactivation of FLNB on
multiple biological functions were identified, especially
tumorigenesis and skeletal development. After FLNB knockdown
in HeLa cells, compared with the control, it was identified that
the level of apoptosis significantly decreased, consistent with the
suppressive role in tumor progression of FLNB as reported in
mouse embryonic fibroblasts (9).
Furthermore, the transcriptome analysis demonstrated that
FLNB knockdown led to upregulation of genes that can
negatively regulate the apoptotic process, including genes such as
NAIP, while significantly downregulating inflammatory and
immune-related genes. Additionally, FLNB knockdown resulted
in the deregulation of the AS of hundreds of genes; many were
enriched in the top three GO functional terms associated with
apoptosis. Notably, it was observed that FLNB-regulated the
expression of genes in skeletal development-related pathways, such
as ‘cartilage development’ and ‘mineral absorption’ at both the AS
and mRNA levels. These findings together suggested that FLNB
can extensively regulate pre-mRNA AS in cancer cells, which may be
exerted via its RNA binding capability. The FLNB-regulated
mRNA expression level could be resulted from either
FLNB-regulated mRNA stability or transcription directly or
indirectly. The present data supported a model that FLNB-knockdown
can inhibit cancer cell apoptosis via regulation of AS and mRNA
levels of the apoptotic genes. The present results demonstrated
that FLNB regulates the AS and mRNA levels of functional
related genes, which may provide insight for the current
understanding for FLNB biology and regulatory mechanisms, as
well as for FLNB-targeted anti-cancer therapies.
A number of previous studies demonstrated that
FLNB mutations or deficiencies result in multiple skeletal
hypogenesis; the clinical symptoms usually manifest as a reduction
of bone mineral density (BMD), ossification of intervertebral disc,
disturbance of proliferation, differentiation and apoptosis in
chondrocytes, and impairment of angiogenesis (24–26).
The present DEG analysis identified that FLNB knockdown
increased the expression of genes relevant to cartilage
development, including MMP13, COL2A1, BMP7 and ATP7A
in HeLa cells, which need further verification in cartilage cells
(27). Additionally, previous
studies (28,29) demonstrated that FLNB
mutations are associated with a reduction in BMD; however, the
effect of FLNB mutations on bone remodeling is largely
unclarified based on functional studies. In the present study, it
was observed that knockdown of FLNB altered the splicing
pattern of VDR. VDR is a member of the nuclear hormone
receptor superfamily, and is a ligand-inducible transcription
factor (30). A recent study
suggested that alternatively spliced transcript variants encoding
different isoforms in VDR genes are important in regulating
the metabolic processes that affect BMD (31). Indeed, the VDR gene FokI and
BsmI polymorphisms are significantly associated with low BMD
(32). These results provide
insight into the molecular mechanism of BMD caused by FLNB
dysfunction, and cartilage cells should be used in future studies
to verify the regulatory role of FLNB.
Recently, FLNB has been considered one of the
biomarkers for cancer. Splicing variants of FLNB expression
have been reported to play key roles in the proliferation and
differentiation of tumor cells (33). A recent study demonstrated that in
HMLE cells (immortalized human mammary epithelial cells),
FLNB interacts with forkhead box C1 (FOXC1) to form a
complex (11). FLNB exon
skipping leads to a decrease in FLNB protein located in the
nucleus, thus the reduced interaction between FLNB, FOXC1 and PBX
homeobox 1 leads to the release of FOXC1 to promote EMT (11). Th{Li, 2018 #58}is finding suggested
that FLNB plays an important regulatory role in
tumorigenesis. In the present study, it was demonstrated that
FLNB also had transcriptional and post-transcriptional
regulatory functions in HeLa cells.
DEGs between shFLNB and control HeLa cells were
obtained. GO and KEGG enrichment analyses demonstrated that
upregulated genes were enriched in the' negative regulation of the
neuron apoptotic process' pathway, and downregulated genes were
enriched in ‘inflammatory response’ and ‘innate immune response’,
which may contribute to the survival of cancer cells. Notably, it
was verified that FLNB knockdown increased the expression of
NAIP and MALAT1.
IL23A associates with interleukin 12B to form
interleukin (IL)-23, a heterodimeric cytokine which induces
autoimmune inflammation, and is related to tumor incidence and
growth (34). Low concentrations of
IL-23 promote the proliferation of lung cancer cells, whereas high
concentrations of IL-23 inhibit proliferation (35). This is consistent with the fact that
FLNB knockdown promotes cell proliferation. NAIP is one of
eight human inhibitors of apoptosis proteins that have been
identified (36). NAIP has
been suggested to have an important role in protecting cells
against apoptosis (37). Although
NAIP is generally thought to be expressed in nerve cells,
NAIP is weakly linked to unfavorable prognosis in multiple
cancer types, which indicates the precise role of NAIP in
the dysregulation of apoptosis in cancer (38). The present results suggested that
FLNB may control the expression of NAIP, and thus
affect apoptosis of cancer cells. MALAT1, a long non-coding
RNA, is a transcriptional regulator for numerous genes, including
some genes related with cancer metastasis and cell migration, and
MALAT1 is involved in cell cycle regulation (39). Upregulation of MALAT1 in
multiple cancerous tissues was associated with the proliferation
and metastasis of tumor cells (39). In conclusion, FLNB knockdown
results in abnormal expression of many important genes associated
with tumorigenesis, demonstrating the important regulatory role of
FLNB in the process of cancer.
Furthermore, it was identified that AS genes
regulated by FLNB were significantly enriched in the
‘apoptotic process’ and ‘cell death’ pathways. The
FLNB-regulated AS genes in these pathways included PACS2,
MALAT1 and MAP2K7, which were validated by RT-qPCR. In
particular, PACS2 has been shown to be a multi-functional
sorting protein, which regulates endoplasmic reticulum-mitochondria
communication and Bid-mediated apoptosis (40). PACS2 also mediates tumor
necrosis factor-related apoptosis-inducing ligand lysosomes,
interacting with Bim and Bax to permeabilize lysosomes and induce
apoptosis (41). The present
findings regarding FLNB-mediated AS of apoptotic-regulating
genes suggested a more complex network among the apoptotic
regulation process in cancer, which supports a previous study
demonstrating that FLNB knockdown in mouse embryonic
fibroblasts leads to increased proteolytic activity of MMP-9
(9). In the present study, it was
identified that the mRNA expression of MMP-9 was
downregulated after knockdown of FLNB in HeLa cells. Further
research is required to determine whether or not FLNB
regulates transcription and AS by binding to RNA as an RBP.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Yi Zhang (ABLife
BioBigData Institute) for her helpful discussions.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81860746) and The
Joint Fund of the National Natural Science Foundation of China
(grant no. U1503221).
Availability of data and materials
RNA-sequencing data in the present study have been
deposited in NCBI's Gene Expression Omnibus and are accessible
through GEO (https://www.ncbi.nlm.nih.gov/geo) series (accession
no. GSE134769).
Authors' contributions
HRM and LC contributed to the study design. FW, CC,
RJ, HKZ, SW and ZQ conducted the experiments and/or performed data
analysis. HRM and CC prepared the manuscript. ZX made the part
analysis of bioinformation data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takafuta T, Wu G, Murphy GF and Shapiro
SS: Human beta-filamin is a new protein that interacts with the
cytoplasmic tail of glycoprotein Ibalpha. J Biol Chem.
273:17531–17538. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brocker F, Bardenheuer W, Vieten L,
Jülicher K, Werner N, Marquitan G, Michael D, Opalka B and Schütte
J: Assignment of human filamin gene FLNB to human chromosome band
3p14.3 and identification of YACs containing the complete FLNB
transcribed region. Cytogenet Cell Genet. 85:267–268. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stossel TP, Condeelis J, Cooley L, Hartwig
JH, Noegel A, Schleicher M and Shapiro SS: Filamins as integrators
of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2:138–145.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng Y and Walsh CA: The many faces of
filamin: A versatile molecular scaffold for cell motility and
signalling. Nat Cell Biol. 6:1034–1038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorlin JB, Yamin R, Egan S, Stewart M,
Stossel TP, Kwiatkowski DJ and Hartwig JH: Human endothelial
actin-binding protein (ABP-280, nonmuscle filamin): A molecular
leaf spring. J Cell Biol. 111:1089–1105. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baldassarre M, Razinia Z, Burande CF,
Lamsoul I, Lutz PG and Calderwood DA: Filamins regulate cell
spreading and initiation of cell migration. PLoS One. 4:e78302009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang CF, Wang CH, Siong H'ng W, Chang CP,
Lin WD, Chen YT, Wu JY and Tsai FJ: Filamin B loss-of-function
mutation in dimerization domain causes autosomal-recessive
spondylocarpotarsal synostosis syndrome with Rib anomalies. Hum
Mutat. 38:540–547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bicknell LS, Morgan T, Bonafe L, Wessels
MW, Bialer MG, Willems PJ, Cohn DH, Krakow D and Robertson SP:
Mutations in FLNB cause boomerang dysplasia. J Med Genet.
42:e432005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bandaru S, Zhou AX, Rouhi P, Zhang Y,
Bergo MO, Cao Y and Akyürek LM: Targeting filamin B induces tumor
growth and metastasis via enhanced activity of matrix
metalloproteinase-9 and secretion of VEGF-A. Oncogenesis.
3:e1192014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iguchi Y, Ishihara S, Uchida Y, Tajima K,
Mizutani T, Kawabata K and Haga H: Filamin B Enhances the
invasiveness of cancer cells into 3D collagen matrices. Cell Struct
Funct. 40:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Choi PS, Chaffer CL, Labella K,
Hwang JH, Giacomelli AO, Kim JW, Ilic N, Doench JG, Ly SH, et al:
An alternative splicing switch in FLNB promotes the mesenchymal
cell state in human breast cancer. Elife. 7:e371842018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baltz AG, Munschauer M, Schwanhausser B,
Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew
K, Milek M, et al: The mRNA-bound proteome and its global occupancy
profile on protein-coding transcripts. Mol Cell. 46:674–690. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kafasla P, Skliris A and Kontoyiannis DL:
Post-transcriptional coordination of immunological responses by
RNA-binding proteins. Nat Immunol. 15:492–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou H, Mangelsdorf M, Liu J, Zhu L and Wu
JY: RNA-binding proteins in neurological diseases. Sci China Life
Sci. 57:432–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wurth L and Gebauer F: RNA-binding
proteins, multifaceted translational regulators in cancer. Biochim
Biophys Acta. 1849:881–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Pertea G, Trapnell C, Pimentel H,
Kelley R and Salzberg SL: TopHat2: Accurate alignment of
transcriptomes in the presence of insertions, deletions and gene
fusions. Genome Biol. 14:R362013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robinson MD, McCarthy DJ and Smyth GK:
EdgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia H, Chen D, Wu Q, Wu G, Zhou Y, Zhang Y
and Zhang L: CELF1 preferentially binds to exon-intron boundary and
regulates alternative splicing in HeLa cells. Biochim Biophys Acta
Gene Regul Mech. 1860:911–921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin L, Li G, Yu D, Huang W, Cheng C, Liao
S, Wu Q and Zhang Y: Transcriptome analysis reveals the complexity
of alternative splicing regulation in the fungus Verticillium
dahliae. BMC Genomics. 18:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong
S, Kong L, Gao G, Li CY and Wei L: KOBAS 2.0: A web server for
annotation and identification of enriched pathways and diseases.
Nucleic Acids Res. 39((Web Server Issue)): W316–W322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu J, Lu J, Lian G, Ferland RJ,
Dettenhofer M and Sheen VL: Formin 1 and Filamin B physically
interact to coordinate chondrocyte proliferation and
differentiation in the growth plate. Hum Mol Genet. 23:4663–4673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson SG, Jones MR, Mullin BH, Dick IM,
Richards JB, Pastinen TM, Grundberg E, Ljunggren O, Surdulescu GL,
Dudbridge F, et al: Common sequence variation in FLNB regulates
bone structure in women in the general population and FLNB mRNA
expression in osteoblasts in vitro. J Bone Miner Res. 24:1989–1997.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zieba J, Forlenza KN, Khatra JS,
Sarukhanov A, Duran I, Rigueur D, Lyons KM, Cohn DH, Merrill AE and
Krakow D: TGFβ and BMP dependent cell fate changes due to loss of
filamin B produces disc degeneration and progressive vertebral
fusions. PLoS Genet. 12:e10059362016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Lian G, Lenkinski R, De Grand A,
Vaid RR, Bryce T, Stasenko M, Boskey A, Walsh C and Sheen V:
Filamin B mutations cause chondrocyte defects in skeletal
development. Hum Mol Genet. 16:1661–1675. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mullin BH, Mamotte C, Prince RL, Spector
TD, Dudbridge F and Wilson SG: Conditional testing of multiple
variants associated with bone mineral density in the FLNB gene
region suggests that they represent a single association signal.
BMC Genet. 14:1072013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Q, Wu N, Cui L, Wu Z and Qiu G: Filamin
B: The next hotspot in skeletal research? J Genet Genomics.
44:335–342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Campbell MJ and Adorini L: The vitamin D
receptor as a therapeutic target. Expert Opin Ther Targets.
10:735–748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamichi Y, Udagawa N, Suda T and
Takahashi N: Mechanisms involved in bone resorption regulated by
vitamin D. J Steroid Biochem Mol Biol. 177:70–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao L, Chen M, Lei Y, Zhou Z, Shen H and
Le F: Association between vitamin D receptor BsmI polymorphism and
bone mineral density in pediatric patients: A meta-analysis and
systematic review of observational studies. Medicine (Baltimore).
96:e67182017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsui JC, Lau CP, Cheung AC, Wong KC, Huang
L, Tsui SK and Kumta SM: Differential expression of filamin B
splice variants in giant cell tumor cells. Oncol Rep. 36:3181–3187.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Langowski JL, Zhang X, Wu L, Mattson JD,
Chen T, Smith K, Basham B, McClanahan T, Kastelein RA and Oft M:
IL-23 promotes tumour incidence and growth. Nature. 442:461–465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Zhang L, Zhang J, Wei Y, Li K, Huang
L, Zhang S, Gao B, Wang X and Lin P: Interleukin 23 regulates
proliferation of lung cancer cells in a concentration-dependent way
in association with the interleukin-23 receptor. Carcinogenesis.
34:658–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davoodi J, Ghahremani MH, Es-Haghi A,
Mohammad-Gholi A and Mackenzie A: Neuronal apoptosis inhibitory
protein, NAIP, is an inhibitor of procaspase-9. Int J Biochem Cell
Biol. 42:958–964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chamaillard M, Girardin SE, Viala J and
Philpott DJ: Nods, Nalps and Naip: Intracellular regulators of
bacterial-induced inflammation. Cell Microbiol. 5:581–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Finlay D, Teriete P, Vamos M, Cosford NDP
and Vuori K: Inducing death in tumor cells: Roles of the inhibitor
of apoptosis proteins. F1000Res. 6:5872017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li ZX, Zhu QN, Zhang HB, Hu Y, Wang G and
Zhu YS: MALAT1: A potential biomarker in cancer. Cancer Manag Res.
10:6757–6768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Myhill N, Lynes EM, Nanji JA,
Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G
and Simmen T: The subcellular distribution of calnexin is mediated
by PACS-2. Mol Biol Cell. 19:2777–2788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Werneburg NW, Bronk SF, Guicciardi ME,
Thomas L, Dikeakos JD, Thomas G and Gores GJ: Tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) protein-induced
lysosomal translocation of proapoptotic effectors is mediated by
phosphofurin acidic cluster sorting protein-2 (PACS-2). J Biol
Chem. 287:24427–24437. 2012. View Article : Google Scholar : PubMed/NCBI
|