Of the 1.5 million new-onset breast cancer cases
diagnosed annually worldwide, a large subset are hormone receptor
(HR)-positive breast cancers [estrogen receptor (ER)-positive,
progesterone receptor-positive, or both, with normal human
epidermal growth factor receptor 2 (HER2) expression], accounting
for ~60-65% (1). Patients with this
disease (>70%) are significantly more commonly diagnosed at an
advanced stage compared with those with other subtypes of breast
cancer (2). Over several decades,
the mainstay in the treatment of women with advanced HR-positive
(HR+) breast cancer has been hormone therapy, which may
benefit patients initially, but eventually leads to drug resistance
and disease progression. Endocrine therapy-refractory patients are
not considered as suitable candidates for a combination of
endocrine therapy and chemotherapy, as this combination may
accentuate the expression of naïve regulatory T cells (Tregs) that
are associated with disease progression or death (3). Clinical studies have demonstrated that
everolimus [a mammalian target of rapamycin (mTOR) inhibitor] or
tucidinostat (a histone deacetylase inhibitor) in combination with
exemestane (an aromatase inhibitor) achieved significant
improvement of the progression-free survival (PFS) of patients with
HR+/HER2− advanced breast cancer (aBC), who
had become resistant to endocrine therapy (4,5). These
findings heightened the interest in the application of targeted
therapy for such patients.
Immunotherapy has also shown promising clinical
efficacy in breast cancer, specifically in triple-negative breast
cancer (TNBC), due to its immune-rich characteristics (6), and is currently the fifth treatment
strategy for breast carcinoma after surgery, chemotherapy,
radiotherapy and molecularly targeted therapy. By contrast,
HR+ breast tumor is an immunologically cold cancer.
Metastatic disease due to the escape from immune surveillance at
the primary tumor site contributes to the lower immunogenicity of
the metastases compared with the primary tumor. Moreover, previous
chemotherapy depletes immune-active tumors, giving rise to the
development of immunologically cold tumors in aBC. All these
underpinnings are associated with a weakened response of
HR+/HER2− aBC to immunotherapy. However, in
the context of high numbers of tumor-infiltrating lymphocytes
(TILs) and immune-related gene expression signatures, these
patients may benefit from systematic adjuvant therapy (7). Several clinical trials confirmed the
antitumor activities of inhibiting immune-checkpoints, such as
programmed death 1/programmed death ligand 1 (PD1/PD-L1) and
cytotoxic T lymphocyte antigen-4 (CTLA-4) in breast cancer
(8–11). The aim of the present study was to
evaluate the mechanisms of action and the therapeutic efficacy and
tolerability of targeted therapy and immunotherapy for
HR+/HER2− aBC.
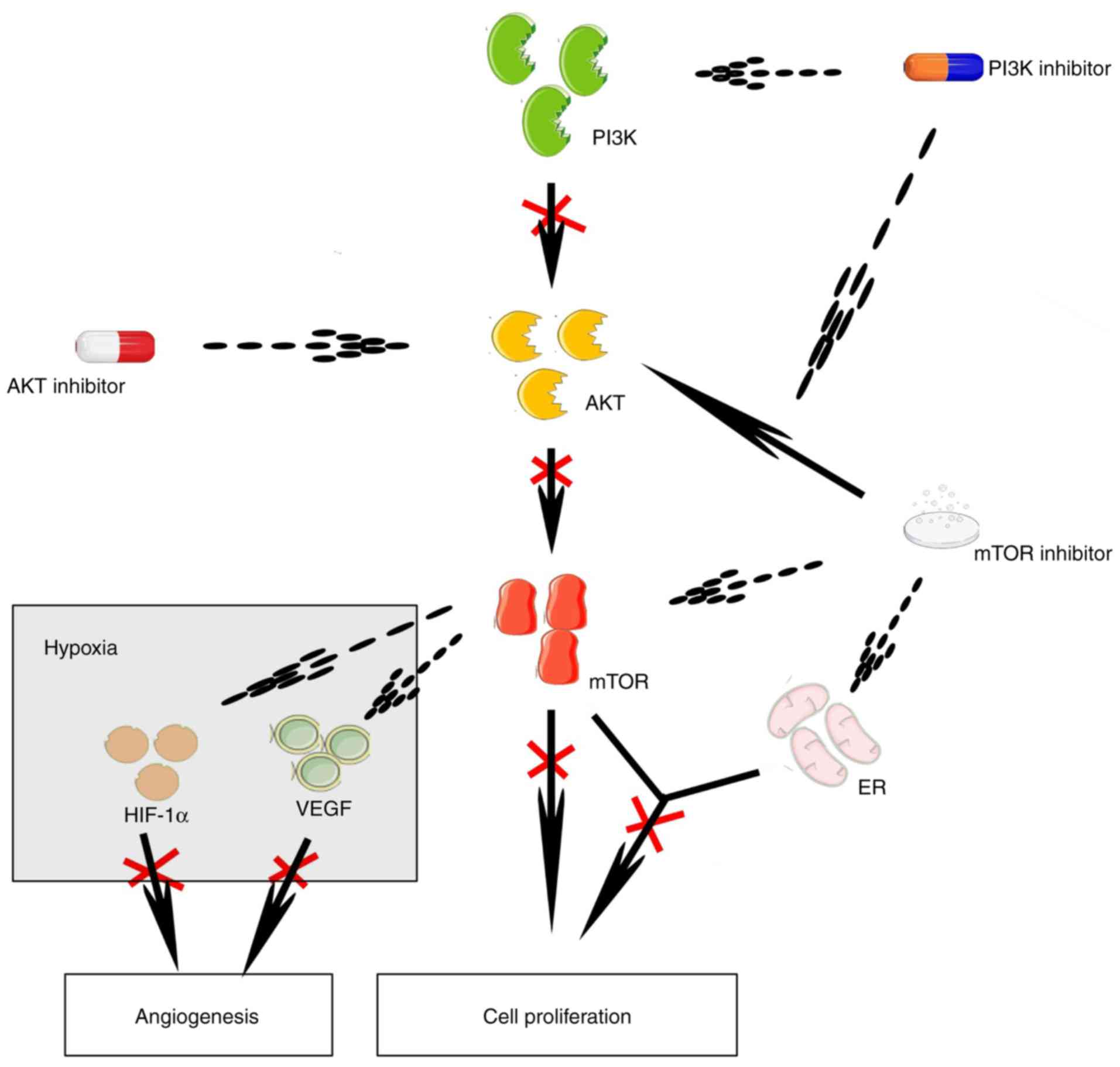
The phosphoinositide 3-kinase/protein kinase B
(PI3K/AKT) signaling pathway is crucial for cell survival and
proliferation via multiple downstream effectors, including
mammalian target of rapamycin (mTOR) (12). In HR+ breast cancer,
mutations of the activated PI3K, catalytic, a polypeptide
(PIK3CA) occur frequently, increase the AKT level and may
give rise to the insensitivity to antitumor therapy (12,13).
Several cytotoxic agents and mTOR inhibitors are available that can
reduce the size and even eliminate tumors, although activation of
ATK signaling reduces their therapeutic effectiveness (14–17).
Estrogen-induced cell proliferation in breast tumors depends on
mTOR signaling (18), and mTOR
inhibition can downregulate the expression of ER. Therefore,
inhibition of mTOR signaling may effectively reduce cell
proliferation in HR+ breast tumors, even when they have
been proven to be resistant to hormone therapy (19). In breast cancer xenograft models, a
PI3K inhibitor can abolish the activation of ATK signaling induced
by mTOR inhibition. Under hypoxic conditions, inhibition of the
PI3K/ATK/mTOR signaling pathway can reduce the expression of
hypoxia-inducible factor 1a and the synthesis of vascular
endothelial growth factor, both of which promote angiogenesis in
tumors (Fig. 1) (16). These mechanisms constitute the
conceptual framework for investigating the antitumor activity of
PI3K inhibitors, ATK inhibitors and mTOR inhibitors for the
treatment of patients with HR+ breast cancer.
Buparlisib (BKM120), an oral reversible PI3K
inhibitor, manifests antitumor efficacy as monotherapy and in
combination with endocrine therapy for the treatment of patients
with HR+ breast cancer independently of the presence of
PIK3CA mutations (20,21).
Two phase III randomized controlled trials (RCTs), BELLE-2
(22) and BELLE-3 (23), demonstrated that
buparlisib-fulvestrant significantly prolonged the median PFS of
HR+/HER2− endocrine treatment-refractory aBC
postmenopausal patients compared with fulvestrant (Table I). However, no additional studies
were launched to explore the clinical benefit and safety in this
setting, due to the serious toxicity of the combined treatment; the
most common grade 3–4 adverse events (AEs) were increased alanine
aminotransferase and aspartate aminotransferase levels, and
hyperglycemia (Table I). As a
result, the clinical application of buparlisib has been limited and
several other trials have been initiated to test a-specific PI3K
inhibitors in patients.
Alpelisib (BYL719) is an orally selective,
bioavailable, α-specific PI3K small-molecule inhibitor, which acts
synergistically with hormone therapy against HR+
PIK3CA-mutant breast cancer (24). The maximum tolerated dose (MTD) of
alpelisib combined with letrozole or fulvestrant is different, 300
and 400 mg daily, respectively (24,25).
Both drug combinations have manageable safety profiles, with
reversible toxicity. Additionally, these combinations are
associated with greater clinical benefits in PIK3CA-mutated
HR+/HER2− aBC compared with
PIK3CA-wild-type tumors. However, FGFR1 amplification
is an adverse factor in the antitumor activity of alpelisib in
HR+/PIK3CA-mutant breast cancer (24), suggesting that alpelisib should not
be administered to patients with coexisting genomic alterations.
The NEO-ORB phase II clinical study (26) indicated that alpelisib-letrozole did
not improve the response of postmenopausal women with early
HR+/HER2− breast cancer compared with
placebo-letrozole in the neoadjuvant setting, regardless of the
patients' PIK3CA mutation status (Table I). By contrast,
alpelisib-fulvestrant compared with placebo-fulvestrant
significantly prolonged the median PFS, increased the overall
response rate (ORR; complete or partial response) to ≥24 weeks and
increased the clinical benefit rate (ORR + stable disease) to ≥24
weeks (CBR) in postmenopausal patients with endocrine-refractory
HR+/HER2− PIK3CA-mutant aBC; however,
no difference was observed in patients with PIK3CA-wild-type
tumors (SOLAR-1 trial) (27). The
most frequent grade 3–4 treatment-associated AEs in the alpelisib
group were hyperglycemia, rash and maculopapular rash (Table I). These findings collectively
suggest that alpelisib in combination with endocrine therapy should
only be used for the treatment of patients with
HR+/HER2−, PIK3CA-mutant aBC.
Capivasertib (AZD5363) is another orally selective
and potent AKT inhibitor, the sensitivity of cancer to which is
increased by the presence of the PIK3CA mutation (32). Estrogen blockade may be crucial for
its antitumor activity, as capivasertib does not improve the median
PFS of patients with HR+/HER2− aBC receiving
paclitaxel without endocrine agents, even in the
PIK3CA-mutant subpopulation (BEECH trial; Table I) (33). Capivasertib exerts a synergistic
effect with fulvestrant in delaying tumor progression in xenograft
models of ER+ breast cancer (34); further investigation of the clinical
activity and safety of capivasertib-fulvestrant in patients with
HR+/HER2− aBC is ongoing (NCT01992952).
Everolimus (Afinitor), an orally selective and
potent allosteric mTOR inhibitor (35), acts synergistically with letrozole
to suppress cell proliferation and trigger apoptosis in
ER+ breast cancer cells (18). In the neoadjuvant setting,
everolimus can significantly increase the ORR of patients with
early HR+/HER2− breast cancer who receive
letrozole monotherapy (36).
Numerous phase II and III clinical trials, including the 4EVER
trial (37), GINECO study (38), BOLERO-2 trial (4), BOLERO-4 trial (39) and BOLERO-6 trial (40), have explored the antitumor efficacy
and safety profile of everolimus combined with aromatase inhibitors
in HR+/HER2− aBC (Table I). Taken together, the combination
significantly prolongs the median PFS of postmenopausal patients
with HR+/HER2− aBC compared with aromatase
inhibitors or everolimus alone, with acceptable toxicity. This
combined strategy appears to be a good first- and second-line
treatment option for patients with HR+/HER2−
aBC, as it is more effective and less toxic compared with most
chemotherapy protocols (41),
particularly as first-line therapy, as its median PFS is longer
compared with that of second-line therapy (39).
The interaction of CDK4/6 with D-type cyclins
phosphorylates the retinoblastoma (Rb) tumor suppressor protein to
promote the cell cycle progression from the G1 to the S phase
(42). However, the cell cycle can
be inhibited by CDK4/6 inhibitors, thereby suppressing tumor
proliferation (43). The regulation
of CDK4/6 activity is associated with the ubiquitination and
phosphorylation of endogenous cell inhibitors of the INK4 family
(44). However, the regulation is
commonly disrupted in tumors by a number of mechanisms, leading to
increased CDK4/6 activity that delays senescence (45–47).
CDK4/6 inhibitors can promote senescence of cancer cells by
decreasing the activity of these kinases. These inhibitors exert a
stronger inhibitory effect on the proliferation of
immunosuppressive Tregs compared with any other type of T cells,
thereby shifting the local immune balance over time to increase
tumor immunogenicity (48). They
also promote the expression of endogenous retrovirus elements,
which is associated with the high level of intracellular
double-stranded RNA that stimulates the production of type III
interferon, ultimately bolstering the presentation of tumor
antigens (48). The expression of
MHC I-presented neoantigens may be activated by the CDK4/6
inhibitors, resulting in the release of proinflammatory cytokines
by fibroblasts (Fig. 2) (48). Currently, three CDK4/6 inhibitors
have been approved by the Food and Drug Administration in the
United States for the treatment of HR+/HER2−
aBC.
The second small-molecule CDK4/6 inhibitor is
ribociclib (LEE011), which is characterized by its oral
bioavailability and high selectivity (54). A preclinical study demonstrated the
antitumor efficacy of ribociclib combined with letrozole and PI3K
inhibitors in HR+ breast cancer in vivo (55). Based on these findings, a phase Ib
clinical trial of ribociclib plus an endocrine agent also
demonstrated clinical efficacy with acceptable toxicity in
postmenopausal patients with advanced HR+ breast tumor
(56). This combination also
significantly improved the PFS of premenopausal women with
HR+/HER2− aBC compared with those on placebo
plus endocrine therapy (57). Two
well-designed phase III RCTs, MONALEESA-2 (58) and MONALEESA-3 (59), demonstrated that ribociclib combined
with hormone therapy (letrozole or fulvestrant) outperformed
hormone therapy alone in postmenopausal patients with
HR+/HER2− aBC with regard to the median PFS,
ORR and CBR. The drug toxicity in the combination cohort was
tolerable, with similar grade 3–4 AEs as those of
palbociclib-letrozole (Table II).
Of note, the therapeutic efficacy of ribociclib was independent of
PIK3CA or TP53 mutations, total Rb, Ki67 or p16 protein expression,
and the CDKN2A, CCND1, or ESR1 mRNA levels (60).
Abemaciclib (LY2835219) is the third oral,
small-molecule CDK4/6 inhibitor, which is structurally distinct
from palbociclib and ribociclib and appears to be more selective
for CDK4 compared with CDK6 (61).
As a single agent, abemaciclib produced an outstanding tumor
response with an acceptable safety profile in advanced
HR+ breast cancer (62),
which warrants its further development, as monotherapy or in
combination with other therapies. The MONARCH 1 phase II single-arm
study (63) and the MONARCH 2 phase
III RCT (64) investigated the
monotherapy with abemaciclib and its combination with fulvestrant,
respectively, for patients with HR+/HER2−,
endocrine-refractory aBC. Analysis of the results demonstrated the
clinical efficacy and good tolerability of abemaciclib, both as a
single agent and combined with fulvestrant. Of note, significant
improvement of the median PFS, ORR and CBR was observed in the
abemaciclib-fulvestrant cohort compared with fulvestrant alone.
Leukopenia and neutropenia were the most common grade 3–4 AEs of
abemaciclib (Table II).
PD1 is most frequently expressed in TNBC compared
with other subsets of breast tumors (70). Previous clinical studies have mainly
focused on applying anti-PD1/PD-L1 treatment to advanced TNBC,
which demonstrated clinical efficacy (71–73).
Recently, two phase I trials, KEYNOTE-028 (8) and JAVELIN Solid Tumor (9), documented that anti-PD1/PD-L1
monotherapy with pembrolizumab (MK-3475) or avelumab (MSB0010718C)
also achieved a modest but lasting antitumor response in
HR+/HER2− aBC (Table III). These findings may provide a
new perspective for the treatment of patients with this disease; a
rational recommendation is that future studies aim to evaluate the
clinical efficacy and safety profile of anti-PD1/PD-L1 therapy in
combination with other therapies, particularly hormone therapy, for
patients with HR+/HER2− aBC.
The CD28-B7 immunoglobulin superfamily is a critical
signature in T-cell activation and tolerance, in which CD28 and
CTLA-4 are two immunoregulatory molecules that competitively share
their two ligands (B7.1 and B7.2) (74). CD28 is expressed in 90% of human
CD4+ T cells and 50% of human CD8+ T cells,
sending a costimulating signal upon TCR binding that plays a key
role in the transmission of a productive immune response in many
cases (75). The alteration of the
number of bound TCRs is consistent with that of the CD28
presentation. However, CTLA-4 is an important negative regulator of
the CD28-dependent T-cell response, with a 500- to 2,500-fold
higher affinity for both B7 ligands compared with CD28 (75). As such, an increase in the CTLA-4
expression ultimately suppresses the immune response (Fig. 3). CTLA-4 blockade, in turn,
increases the level of CD28 expression, which can enhance the
immune response of chronic tumor-reactive T cells in tumors to
achieve an antitumor effect (76).
Tremelimumab (CP-675,206) is a fully humanized IgG2
anti-CTLA-4 monoclonal antibody that accentuates the immune
activity of human T-cells by blocking the binding of CTLA-4 to B7.1
and B7.2 (80). The antitumor
activity of tremelimumab as a single agent in advanced melanoma has
been confirmed in numerous clinical trials (80–84),
but it is inferior to that of chemotherapy (85). It appears that tremelimumab in
combination with other treatments is likely to achieve maximum
clinical efficacy. A single-arm pilot study of tremelimumab plus
durvalumab for the treatment of 18 patients with aBC (11 with
HR+ breast cancer and 7 with TNBC) demonstrated that ORR
was only observed in TNBC patients (43%), but not in HR+
breast cancer patients (10). These
findings mirror the results of a previous phase I clinical trial of
tremelimumab combined with exemestane in 26 patients with
HR+ aBC to investigate its MTD, clinical efficacy and
safety (11). The best therapeutic
benefit was observed in 11 patients with stable disease for ≥12
weeks; however, no partial or complete response was documented. The
MTD of exemestane was 6 mg/kg every 90 days. Diarrhea was the most
common grade 3–4 treatment-related AE, but it was not observed in
patients treated at the MTD. The treatment benefit was positively
associated with the increased expression of inducible costimulator
by peripheral CD4+ and CD8+ T cells that may
be secondary to the immune activation following CTLA-4 blockade.
These disappointing outcomes have limited the utility of
tremelimumab for HR+/HER2− aBC patients.
Articles published in English were searched in the
PubMed and Embase databases using the search terms ‘immunotherapy’
or ‘targeted therapy’ and ‘breast cancer’ and ‘clinical trial’ and
‘estrogen receptor-positive’. The publications were retrieved on
July 3, 2019. Clinical studies that evaluated the clinical efficacy
and safety profile of targeted therapy or immunotherapy for
HR+/HER2− aBC met the inclusion criteria. We
also retrieved relevant clinical studies currently underway in the
ClinicalTrial.gov database on July 26, 2019.
Not applicable.
No funding was received.
Not applicable.
YS: Conception and design, writing and final
approval of the manuscript. LH: Writing the manuscript and figure
charting. YW: Writing the manuscript and drawing the tables. QW:
Writing the manuscript. WH: Writing the manuscript and drawing the
tables. All authors have reviewed and approved the final version of
the manuscript prior to submission.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64, 1. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Invasive breast cancer version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:324–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwa M, Li X, Novik Y, Oratz R, Jhaveri K,
Wu J, Gu P, Meyers M, Muggia F, Speyer J, et al: Serial
immunological parameters in a phase II trial of exemestane and
low-dose oral cyclophosphamide in advanced hormone
receptor-positive breast canc. Breast Cancer Res Treat. 168:57–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esteva FJ, Hubbard-Lucey VM, Tang J and
Pusztai L: Immunotherapy and targeted therapy combinations in
metastatic breast cancer. Lancet Oncol. 20:e175–e186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bianchini G, Qi Y, Alvarez RH, Iwamoto T,
Coutant C, Ibrahim NK, Valero V, Cristofanilli M, Green MC,
Radvanyi L, et al: Molecular anatomy of breast cancer stroma and
its prognostic value in estrogen receptor-positive and -negative
cancers. J Clin Oncol. 28:4316–4323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rugo HS, Delord JP, Im SA, Ott PA,
Piha-Paul SA, Bedard PL, Sachdev J, Le Tourneau C, van Brummelen
EMJ, Varga A, et al: Safety and antitumor activity of pembrolizumab
in patients with estrogen receptor-positive/human epidermal growth
factor receptor 2-negative advanced breast cancer. Clin Cancer Res.
24:2804–2811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dirix LY, Takacs I, Jerusalem G,
Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME,
Somer R, Smakal M, et al: Avelumab, an anti-PD-L1 antibody, in
patients with locally advanced or metastatic breast cancer: A phase
1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 167:671–686.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santa-Maria CA, Kato T, Park JH, Kiyotani
K, Rademaker A, Shah AN, Gross L, Blanco LZ, Jain S, Flaum L, et
al: A pilot study of durvalumab and tremelimumab and immunogenomic
dynamics in metastatic breast cancer. Oncotarget. 9:18985–18996.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vonderheide RH, LoRusso PM, Khalil M,
Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J,
Mariani GL, et al: Tremelimumab in combination with exemestane in
patients with advanced breast cancer and treatment-associated
modulation of inducible costimulator expression on patient T cells.
Clin Cancer Res. 16:3485–3494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindsley CW: The Akt/PKB family of protein
kinases: A review of small molecule inhibitors and progress towards
target validation: A 2009 update. Curr Top Med Chem. 10:458–477.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stemke-Hale K, Gonzalez-Angulo AM, Lluch
A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, et
al: An integrative genomic and proteomic analysis of PIK3CA, PTEN,
and AKT mutations in breast cancer. Cancer Res. 68:6084–6091. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang GS, Brouwer-Visser J, Ramirez MJ,
Kim CH, Hebert TM, Lin J, Arias-Pulido H, Qualls CR, Prossnitz ER,
Goldberg GL, et al: Insulin-like growth factor 2 expression
modulates Taxol resistance and is a candidate biomarker for reduced
disease-free survival in ovarian cancer. Clin Cancer Res.
16:2999–3010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pérez-Tenorio G and Stål O; Southeast
Sweden Breast Cancer Group, : Activation of AKT/PKB in breast
cancer predicts a worse outcome among endocrine treated patients.
Br J Cancer. 86:540–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Zhao M, Hao M, Sun X, Wang J, Mao
Y, Zu L, Liu J, Shen Y, Wang J and Shen K: Dual inhibition of PI3K
and mTOR mitigates compensatory AKT activation and improves
tamoxifen response in breast cancer. Mol Cancer Res. 11:1269–1278.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mabuchi S, Ohmichi M, Kimura A, Hisamoto
K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E,
Nakatsuji Y, et al: Inhibition of phosphorylation of BAD and Raf-1
by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol
Chem. 277:33490–33500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boulay A, Rudloff J, Ye J, Zumstein-Mecker
S, O'Reilly T, Evans DB, Chen S and Lane HA: Dual inhibition of
mTOR and estrogen receptor signaling in vitro induces cell death in
models of breast cancer. Clin Cancer Res. 11:5319–5328. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lui A, New J, Ogony J, Thomas S and
Lewis-Wambi J: Everolimus downregulates estrogen receptor and
induces autophagy in aromatase inhibitor-resistant breast cancer
cells. BMC Cancer. 16:4872016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayer IA, Abramson VG, Isakoff SJ, Forero
A, Balko JM, Kuba MG, Sanders ME, Yap JT, Van den Abbeele AD, Li Y,
et al: Stand up to cancer phase Ib study of
pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole
in estrogen receptor-positive/human epidermal growth factor
receptor 2-negative metastatic breast cancer. J Clin Oncol.
32:1202–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma CX, Luo J, Naughton M, Ademuyiwa F,
Suresh R, Griffith M, Griffith OL, Skidmore ZL, Spies NC, Ramu A,
et al: A Phase I trial of BKM120 (Buparlisib) in combination with
fulvestrant in postmenopausal women with estrogen receptor-positive
metastatic breast cancer. Clin Cancer Res. 22:1583–1591. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baselga J, Im SA, Iwata H, Cortés J, De
Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, et
al: Buparlisib plus fulvestrant versus placebo plus fulvestrant in
postmenopausal, hormone receptor-positive, HER2-negative, advanced
breast cancer (BELLE-2): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:904–916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Leo A, Johnston S, Lee KS, Ciruelos E,
Lønning PE, Janni W, O'Regan R, Mouret-Reynier MA, Kalev D, Egle D,
et al: Buparlisib plus fulvestrant in postmenopausal women with
hormone-receptor-positive, HER2-negative, advanced breast cancer
progressing on or after mTOR inhibition (BELLE-3): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
19:87–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayer IA, Abramson VG, Formisano L, Balko
JM, Estrada MV, Sanders ME, Juric D, Solit D, Berger MF, Won HH, et
al: A phase Ib study of alpelisib (BYL719), a PI3Kα-specific
inhibitor, with letrozole in ER+/HER2-metastatic breast cancer.
Clin Cancer Res. 23:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juric D, Janku F, Rodón J, Burris HA,
Mayer IA, Schuler M, Seggewiss-Bernhardt R, Gil-Martin M, Middleton
MR, Baselga J, et al: Alpelisib plus fulvestrant in PIK3CA-Altered
and PIK3CA-wild-type estrogen receptor-positive advanced breast
cancer: A phase 1b clinical trial. JAMA Oncol. 5:e1844752019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayer IA, Prat A, Egle D, Blau S, Fidalgo
JAP, Gnant M, Fasching PA, Colleoni M, Wolff AC, Winer EP, et al: A
phase II randomized study of neoadjuvant letrozole plus alpelisib
for hormone receptor-positive, human epidermal growth factor
receptor 2-negative breast cancer (NEO-ORB). Clin Cancer Res.
25:2975–2987. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
André F, Ciruelos E, Rubovszky G, Campone
M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al:
Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced
breast cancer. N Engl J Med. 380:1929–1940. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y: MK-2206: A potent oral allosteric
AKT inhibitor. Cancer Res. 69:DDT01–1. 2009.
|
|
29
|
Ma CX, Sanchez C, Gao F, Crowder R,
Naughton M, Pluard T, Creekmore A, Guo Z, Hoog J, Lockhart AC, et
al: A phase I study of the AKT inhibitor MK-2206 in combination
with hormonal therapy in postmenopausal women with estrogen
receptor-positive metastatic breast cancer. Clin Cancer Res.
22:2650–2658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing Y, Lin NU, Maurer MA, Chen H, Mahvash
A, Sahin A, Akcakanat A, Li Y, Abramson V, Litton J, et al: Phase
II trial of AKT inhibitor MK-2206 in patients with advanced breast
cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN
loss/PTEN mutation. Breast Cancer Res. 21:782019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma CX, Suman V, Goetz MP, Northfelt D,
Burkard ME, Ademuyiwa F, Naughton M, Margenthaler J, Aft R, Gray R,
et al: A phase II trial of neoadjuvant MK-2206, an AKT inhibitor,
with anastrozole in clinical stage II or III PIK3CA-mutant
ER-positive and HER2-negative breast cancer. Clin Cancer Res.
23:6823–6832. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Banerji U, Dean EJ, Pérez-Fidalgo JA,
Batist G, Bedard PL, You B, Westin SN, Kabos P, Garrett MD, Tall M,
et al: A Phase I open-label study to identify a dosing regimen of
the Pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in
PIK3CA-mutated breast and gynecologic cancers. Clin Cancer Res.
24:2050–2059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turner NC, Alarcón E, Armstrong AC, Philco
M, López Chuken YA, Sablin MP, Tamura K, Gómez Villanueva A,
Pérez-Fidalgo JA, Cheung SYA, et al: BEECH: A dose-finding run-in
followed by a randomised phase II study assessing the efficacy of
AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in
patients with estrogen receptor-positive advanced or metastatic
breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol.
30:774–780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ribas R, Pancholi S, Guest SK, Marangoni
E, Gao Q, Thuleau A, Simigdala N, Polanska UM, Campbell H, Rani A,
et al: AKT antagonist AZD5363 influences estrogen receptor function
in endocrine-resistant breast cancer and synergizes with
fulvestrant (ICI182780) in vivo. Mol Cancer Ther. 14:2035–2048.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Efeyan A and Sabatini DM: mTOR and cancer:
Many loops in one pathway. Curr Opin Cell Biol. 22:169–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baselga J, Semiglazov V, van Dam P,
Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R,
Bianchi G, et al: Phase II randomized study of neoadjuvant
everolimus plus letrozole compared with placebo plus letrozole in
patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2630–2637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tesch H, Stoetzer O, Decker T, Kurbacher
CM, Marmé F, Schneeweiss A, Mundhenke C, Distelrath A, Fasching PA,
Lux MP, et al: Efficacy and safety of everolimus plus exemestane in
postmenopausal women with hormone receptor-positive, human
epidermal growth factor receptor 2-negative locally advanced or
metastatic breast cancer: Results of the single-arm, phase IIIB
4EVER trial. Int J Cancer. 144:877–885. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Royce M, Bachelot T, Villanueva C,
Özgüroglu M, Azevedo SJ, Cruz FM, Debled M, Hegg R, Toyama T,
Falkson C, et al: Everolimus plus endocrine therapy for
postmenopausal women with estrogen receptor-positive, human
epidermal growth factor receptor 2-negative advanced breast cancer:
A clinical trial. JAMA Oncol. 4:977–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jerusalem G, de Boer RH, Hurvitz S,
Yardley DA, Kovalenko E, Ejlertsen B, Blau S, Özgüroglu M, Landherr
L, Ewertz M, et al: Everolimus plus exemestane vs everolimus or
capecitabine monotherapy for estrogen receptor-positive,
HER2-negative advanced breast cancer: The BOLERO-6 randomized
clinical trial. JAMA Oncol. 4:1367–1374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Generali D, Venturini S, Rognoni C, Ciani
O, Pusztai L, Loi S, Jerusalem G, Bottini A and Tarricone R: A
network meta-analysis of everolimus plus exemestane versus
chemotherapy in the first- and second-line treatment of estrogen
receptor-positive metastatic breast cancer. Breast Cancer Res
Treat. 152:95–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Carnero A and Hannon GJ: The INK4 family
of CDK inhibitors. Curr Top Microbiol Immunol. 227:43–55.
1998.PubMed/NCBI
|
|
45
|
Hall M and Peters G: Genetic alterations
of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human
cancer. Adv Cancer Res. 68:67–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shapiro GI: Cyclin-dependent kinase
pathways as targets for cancer treatment. J Clin Oncol.
24:1770–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fry DW, Harvey PJ, Keller PR, Elliott WL,
Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK and
Toogood PL: Specific inhibition of cyclin-dependent kinase 4/6 by
PD 0332991 and associated antitumor activity in human tumor
xenografts. Mol Cancer Ther. 3:1427–1438. 2004.PubMed/NCBI
|
|
50
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cristofanilli M, Turner NC, Bondarenko I,
Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et
al: Fulvestrant plus palbociclib versus fulvestrant plus placebo
for treatment of hormone-receptor-positive, HER2-negative
metastatic breast cancer that progressed on previous endocrine
therapy (PALOMA-3): Final analysis of the multicentre,
double-blind, phase 3 randomised controlled trial. Lancet Oncol.
17:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim S, Loo A, Chopra R, Caponigro G, Huang
A, Vora S, Parasuraman S, Howard S, Keen N, Sellers W and Brain C.
LEE011: An orally bioavailable, selective small molecule inhibitor
of CDK4/6-Reactivating Rb in cancer. Mol Cancer Ther. 12 (11
Suppl):PR022013.
|
|
55
|
O'Brien NA, Tomaso ED, Ayala R, Tong L,
Issakhanian S, Linnartz R, Finn RS, Hirawat S and Slamon DJ: In
vivo efficacy of combined targeting of CDK4/6, ER and PI3K
signaling in ER+breast cancer. Cancer Res. 74 (19
Suppl):S47562014.
|
|
56
|
Juric D, Munster PN, Campone M,
Ismail-Khan R, García-Estevez L, Hamilton EP, Becerra C, De Boer
RH, Hui R and Goncalves A: Ribociclib (LEE011) and letrozole in
estrogen receptor-positive (ER+), HER2-negative (HER2-) advanced
breast cancer (aBC): Phase Ib safety, preliminary efficacy and
molecular analysis. J Clin Oncol. 34 (15 Suppl):S5682016.
View Article : Google Scholar
|
|
57
|
Tripathy D Sohn J, Im SA, Colleoni M,
Franke F, Bardia A, Harbeck N, Hurvitz S, Chow L, Lee KS, et al:
First-line ribociclib vs. placebo with goserelin and tamoxifen or a
non-steroidal aromatase inhibitor in premenopausal women with
hormone receptor-positive, HER2- negative advanced breast cancer:
Results from the randomized phase III MONALEESA-7 trial. Cancer
Res. 78 (4 Suppl):GS2–05. 2018.
|
|
58
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,
Winer EP, et al: Ribociclib as first-line therapy for HR-positive,
advanced breast cancer. N Engl J Med. 375:1738–1748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Slamon DJ, Neven P, Chia S, Fasching PA,
De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martín
M, et al: Phase III randomized study of ribociclib and fulvestrant
in hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin
Oncol. 36:2465–2472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2-negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gelbert LM, Cai S, Lin X, Sanchez-Martinez
C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack
RS, et al: Preclinical characterization of the CDK4/6 inhibitor
LY2835219: In-vivo cell cycle-dependent/independent anti-tumor
activities alone/in combination with gemcitabine. Invest New Drugs.
32:825–837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Patnaik A, Rosen LS, Tolaney SM, Tolcher
AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW,
Hilton JF, et al: Efficacy and safety of abemaciclib, an inhibitor
of CDK4 and CDK6, for patients with breast cancer, non-small cell
lung cancer, and other solid tumors. Cancer Discov. 6:740–753.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dickler MN, Tolaney SM, Rugo HS, Cortés J,
Diéras V, Patt D, Wildiers H, Hudis CA, O'Shaughnessy J, Zamora E,
et al: MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6
inhibitor, as a single agent, in patients with refractory
HR+/HER2−metastatic breast cancer. Clin
Cancer Res. 23:5218–5224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Iwai Y, Okazaki T, Nishimura H, Kawasaki
A, Yagita H and Honjo T: Microanatomical localization of PD-1 in
human tonsils. Immunol Lett. 83:215–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Okazaki T, Maeda A, Nishimura H, Kurosaki
T and Honjo T: PD-1 immunoreceptor inhibits B cell
receptor-mediated signaling by recruiting src homology
2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc
Natl Acad Sci USA. 98:13866–13871. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yokosuka T, Takamatsu M,
Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M and Saito T:
Programmed cell death 1 forms negative costimulatory microclusters
that directly inhibit T cell receptor signaling by recruiting
phosphatase SHP2. J Exp Med. 209:1201–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sheppard KA, Fitz LJ, Lee JM, Benander C,
George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR and
Chaudhary D: PD-1 inhibits T-cell receptor induced phosphorylation
of the ZAP70/CD3zeta signalosome and downstream signaling to
PKCtheta. Febs Lett. 574:37–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ali HR, Glont SE, Blows FM, Provenzano E,
Dawson SJ, Liu B, Hiller L, Dunn J, Poole CJ, Bowden S, et al:
PD-L1 protein expression in breast cancer is rare, enriched in
basal-like tumours and associated with infiltrating lymphocytes.
Ann Oncol y. 26:1488–1493. 2015. View Article : Google Scholar
|
|
71
|
Nanda R, Chow LQ, Dees EC, Berger R, Gupta
S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al:
Pembrolizumab in patients with advanced triple-negative breast
cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 34:2460–2467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Adams S, Schmid P, Rugo HS, Winer EP,
Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, et al:
Pembrolizumab monotherapy for previously treated metastatic
triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086
study. Ann Oncol. 30:397–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Adams S, Loi S, Toppmeyer D, Cescon DW, De
Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, et
al: Pembrolizumab monotherapy for previously untreated,
PD-L1-positive, metastatic triple-negative breast cancer: Cohort B
of the phase II KEYNOTE-086 study. Ann Oncol. 30:405–411. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Peggs KS, Quezada SA, Korman AJ and
Allison JP: Principles and use of anti-CTLA4 antibody in human
cancer immunotherapy. Curr Opin Immunol. 18:206–213. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Egen JG, Kuhns MS and Allison JP: CTLA-4:
New insights into its biological function and use in tumor
immunotherapy. Nat Immunol. 3:611–618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005.PubMed/NCBI
|
|
78
|
Mokyr MB, Kalinichenko T, Gorelik L and
Bluestone JA: Realization of the therapeutic potential of CTLA-4
blockade in low-dose chemotherapy-treated tumor-bearing mice.
Cancer Res. 58:5301–5304. 1998.PubMed/NCBI
|
|
79
|
Attia P, Phan GQ, Maker AV, Robinson MR,
Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE,
et al: Autoimmunity correlates with tumor regression in patients
with metastatic melanoma treated with anti-cytotoxic T-lymphocyte
antigen-4. J Clin Oncol. 23:6043–6053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tarhini AA and Kirkwood JM: Tremelimumab
(CP-675,206): a fully human anticytotoxic T lymphocyte-associated
antigen 4 monoclonal antibody for treatment of patients with
advanced cancers. Expert Opin Biol Ther. 8:1583–1593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ribas A: Overcoming immunologic tolerance
to melanoma: Targeting CTLA-4 with tremelimumab (CP-675,206).
Oncologist. 13 (Suppl 4):S10–S15. 2008. View Article : Google Scholar
|
|
82
|
Ribas A, Camacho LH, Lopez-Berestein G,
Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja
E, Parker CA, et al: Antitumor activity in melanoma and anti-self
responses in a phase I trial with the anti-cytotoxic T
lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J
Clin Oncol. 23:8968–8977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ribas A, Comin-Anduix B, Economou JS,
Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB,
Shintaku IP, Glaspy JA, et al: Intratumoral immune cell
infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients
with melanoma undergoing CTLA4 blockade. Clin Cancer Res.
15:390–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Camacho LH, Antonia S, Sosman J, Kirkwood
JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA,
Gomez-Navarro J and bRibas A: Phase I/II trial of tremelimumab in
patients with metastatic melanoma. J Clin Oncol. 27:1075–1081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ribas A, Hauschild A, Kefford R, Punt CJ,
Haanen JB, Marmol M, Garbe C, Gomez-Navarro J, Pavlov D and
Marshall M: Phase III, open-label, randomized, comparative study of
tremelimumab (CP-675,206) and chemotherapy [temozolomide (TMZ) or
dacarbazine (DTIC)] in patients with advanced melanoma. J Clin
Oncol. 26 (15 Suppl):LBA90112008. View Article : Google Scholar
|