Introduction
Oral tongue squamous cell carcinoma (OTSCC) is the
most common malignancy in the oral cavity and shows an aggressive
clinical behavior with high invasiveness and metastasis rates
(1–3). The GLOBOCAN database 2018 estimated
354,864 new cases of oral cancer (including lip cancer) globally
(4). Despite constant improvements
in strategies for detecting and treating cancers, the survival
rates of OTSCC patients remain dismal (5) and globally lip and oral cancers caused
approximately 177,384 deaths in 2018 (4). To improve patient diagnostics and
treatment, prediction of cancer metastasis and understanding of the
factors promoting or inhibiting cancer cell invasion are
paramount.
Cathelicidins are a family of vertebrate host
defence peptides, but in humans only human cationic antimicrobial
protein 18 (hCAP18) has been identified (6–10),
which is proteolytically processed to active leucine-leucine-37
(LL-37) (11,12). hCAP18/LL-37 is widely expressed in
epithelial and immune cells, shows potent antimicrobial activities
against various pathogens and has essential functions in
inflammation and modulation of the immune system (6–10). In
addition, human host defense cationic antimicrobial
peptide-18/antimicrobial peptide LL-37 (hCAP18/LL-37) plays a
complex role in carcinogenesis and its overexpression has been
implicated in the progression of ovarian, breast, pancreas,
prostate and lung cancers, as well as in malignant melanoma
(13–18). On the other hand, it has been shown
to suppress tumorigenesis in gastric, oral and haematological
cancer (19–21). Based on these findings, the effect
of LL-37 on cancer seems to depend on the origin and type of the
cancer (7).
LL-37-induced effects on host cells are mediated via
specific activation of various cell surface receptors, membrane
channels or intracellular targets as well as through an interaction
between the cell membrane (9,10).
LL-37 has been linked to the activation of N-formyl peptide
receptor 2 (FPR2) (10,18,22–24),
ERBB2 (25), purinergic receptor
P2X7R (18) and transactivation of
epidermal growth factor receptor (EGFR) (15,26,27).
Although LL-37 has been linked to multiple receptors, this
mechanism may not explain all of its complex functions in
activating or inhibiting cellular responses.
The hCAP18/LL-37 is present in normal human oral
mucosa and tongue at transcriptional and protein levels (28,29).
Recently, hCAP18/LL-37 was found to be weakly expressed in poorly
differentiated oral squamous cell carcinoma (OSCC) relative to
normal oral mucosa. The low expression of hCAP18/LL-37 was also
related to lymph node metastasis and tumor progression (21). Nonetheless, the role of hCAP18/LL-37
in OTSCC is still unclear. The present study aimed to determine the
expression of hCAP18/LL-37 in OTSCC cell lines and tissues and in
dysplastic samples of oral tongue. We also characterized the
effects of exogenous LL-37 on proliferation, migration and invasion
of three OTSCC cell lines. Furthermore, we determined the
activation of epidermal growth factor receptor (EGFR), the
mitogen-activated protein (MAP) kinase cascade and the PI3K/Akt
pathway following LL-7 treatment.
Materials and methods
Cell culture
The three human OTSCC cell lines HSC-3 [Japanese
Collection of Research Bioresources (JCRB) Cell Bank, Osaka, Japan,
JCRB0623], SCC-25 (ATCC, Wesel, Germany, CRL-1628) and SAS (JCRB
Cell Bank, JCRB0260) were all cultured in 1:1 Dulbecco's modified
Eagle's Medium (DMEM)/Ham's Nutrient Mixture F-12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100
µg/ml streptomycin, 50 µg/ml ascorbic acid, 250 ng/ml amphotericin
B and 0.4 ng/ml hydrocortisone (all from Sigma Aldrich; Merck
KGaA). Human papillomavirus HPV16 immortalized human oral
epithelial cells (IHGK) (30) were
cultured in Keratinocyte-SFM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 5 ng/ml human recombinant epidermal growth
factor (EGF 1–53) (Gibco; Thermo Fisher Scientific, Inc.), 50 µg/ml
bovine pituitary extract (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin, 100 µg/ml streptomycin, 250 ng/ml amphotericin
B and 100 µM CaCl2 (all from Sigma Aldrich; Merck KGaA).
All cells were maintained at 37°C with 5% CO2. Cells
were regularly mycoplasma tested with EZ-PCR Mycoplasma test kit
(Biological Industries, Beit-Haemek, Israel).
BrdU proliferation assay
The cells were seeded in normal culture media on
96-well culture plates at a density of 5,000 cells/well in
quintuplicate. After 24 h the compound 100 ng/ml EGF (positive
control) (ProSpec) or 0.5, 1.0, 5.0, 10.0 or 50.0 µg/ml LL-37 (Isca
Biochemicals) was added to the cells. The cell proliferation was
determined using the Cell Proliferation ELISA BrdU (cat. no.
11647229001, Roche) according to the manufacturer's protocol after
24, 48 and 72 h. Absorbance was measured at 450 nm using the
Victor2 Microplate Reader (Perkin Elmer Wallac). Media-only
containing wells were measured as a blank control. The results
represent the average of four separate experiments.
TUNEL cell death detection
The HSC-3 cells were seeded in normal media on an
8-well Lab-Tek® Chamber Slide™ System (Nalge Nunc
International) at a density of 12,500 cells/well in duplicate.
After 24 h the compound 100 ng/ml EGF (positive control) (ProSpec)
or 50 µg/ml LL-37 (Isca Biochemicals) was added to the cells. After
48 h, DNA fragmentation was determined using the In situ
Cell Death Detection Kit, Fluorescein (Roche) according to the
manufacturer's protocol. Cells were mounted with VECTASHIELD
Antifade Mounting Medium with DAPI (Vector Laboratories, Inc.), and
images were captured using an EVOS Digital Inverted Microscope (AMG
Life Technologies).
Transwell migration/invasion
assays
Transwell migration and invasion assays were
performed in 6.5-mm inserts with an 8-µm pore size (Corning, Inc.).
For the invasion assays, the membranes were coated with 50 µl of
MyoGel (2.4 mg/ml) (31,32) solidified with type I collagen from
rat tail (0.8 mg/ml, Corning, Inc.). Cells (70,000 cells/well) were
plated into the upper chamber in 200 µl of serum-free media
(OTSCCs) or supplement-free media (IHGK) with 0.5% lactalbumin
(Sigma Aldrich; Merck KGaA) and the indicated amounts of EGF
(positive control) or LL-37. As a chemoattractant, 500 µl of media
supplemented with 10% FBS (OTSCCs) or supplements (IHGK) was used
in the lower chamber. Experiment times varied between 24 h for
migration assays and 2–6 days for invasion assays. The cells were
fixed in 4% neutral-buffered formalin and stained with 1% toluidine
blue in 1% borax solution. The dye excess was washed out and the
non-invading cells were gently removed from the upper part of the
membrane with a cotton swab. The stained cells were then eluted in
1% SDS solution and absorbance was measured at 650 nm using the
Victor2 Microplate Reader (Perkin Elmer Wallac). Results represent
the average of three independent experiments, performed in
triplicate.
Immunoblotting
For analysis of the hCAP18/LL-37 amount, cells were
cultured for 72 h in normal culture medium. For signal transduction
analysis, cells were starved for 24 h and then stimulated with the
indicated amounts of compounds for 1 h. For EMT analysis, cells
were cultured with compounds for 48 h in normal culture medium.
Cells were lysed with elution buffer [50 mM Tris-HCl pH 7.5, 10 mM
CaCl2, 150 mM NaCl, 0.05% (v/v) Brij-35 (Sigma Aldrich;
Merck KGaA)] including Complete EDTA-free protease inhibitor
cocktail (Roche). For signal transduction analysis, also a
PhosSTOP™ phosphatase inhibitor (Roche) was added. The cell debris
was removed by centrifugation and protein concentrations were
measured with a DC Protein assay (Bio-Rad). For analysis of
hCAP18/LL-37, 50 µg of soluble protein, and for signal transduction
and EMT analysis 30 µg of soluble protein were separated under
reducing conditions on a 15 and 10% SDS-PAGE gel, respectively. The
proteins were transferred to an Immobilon-P membrane (Millipore),
which was blocked with 5% milk powder (Bio-Rad) or for the
phosphorylated antibodies with 5% BSA (Roche) in Tris-buffered
saline and 0.1% Tween 20. Membranes were incubated overnight with
hCAP18/LL-37 (dilution 1:500, HM2070, Hycult Biotech), EGF receptor
(dilution 1:1,000, 4267), phospho-EGF receptor (dilution 1:1,000,
Tyr1068, 3777), p44/42 MAPK (dilution 1:1,000, Erk1/2, 9102),
phospho-p44/42 MAPK (dilution 1:2,000, Erk1/2, Thr202/Tyr204,
9106), Akt (dilution 1:1,000, 9272), phospho-Akt (dilution 1:1,000,
Ser473, 9271) antibodies (all from Cell Signaling Technology,
Inc.), E-cadherin antibody (dilution 1:1,000, 4065, Cell Signaling
Technology), mouse anti-vimentin (dilution 1:750, M0725, Dako) or
anti-β-actin (dilution 1:2,000, ab8226, Abcam), followed by a
biotinylated anti-rabbit IgG (dilution 1:5,000, cat. no. E035301-2;
Dako) or anti-mouse IgG (dilution 1:5,000; cat. no. E035401-2;
Dako) and Vectastain ABC kit (Vector Laboratories). Immunocomplexes
were visualized using a Pierce ECL Western blotting substrate
(Thermo Fisher Scientific, Inc.) and the Luminescent image analyser
LAS-3000 (Fujifilm). Quantification of protein levels was performed
with Fiji software 1.51w (33) and
β-actin was used to normalize the results. The results represent
the average of two to four independent experiments in triplicate,
separated two to three times on SDS-PAGE gels.
Immunofluorescence staining
The HSC-3 cells were seeded in normal media on an
8-well Lab-Tek® Chamber Slide™ System (Nunc) at a
density of 12,500 cells/well in duplicate. After a 24 h culture,
cells were starved for 24 h and then stimulated with 100 ng/ml EGF
(positive control) (ProSpec) and 50 µg/ml LL-37 (Isca Biochemicals)
in serum-free media for 1 h. Cells were washed two times with PBS
and fixed with 4% neutral-buffered formalin for 30 min and washed
three times with PBS. After permeabilization in 0.1% TritonX-100 in
PBS, the samples were blocked in 1% BSA, PBS-0.1% Tween 20 (PBS-T)
for 1 h at room temperature (RT). The cells were incubated with EGF
receptor antibody (dilution 1:50; cat. no. 4267; Cell Signaling
Technology, Inc.) overnight at 4°C, followed by incubation with
Alexa Fluor 594 anti-rabbit secondary antibody (Invitrogen; Thermo
Fisher Scientific, Inc.) in 1% BSA/PBS-T in a humidified chamber
for 1 h at RT. Cell nuclei were stained with DAPI (Thermo Fisher
Scientific, Inc.), followed by rinsing three times with PBS. Cells
were embedded with Immu-Mount (Thermo Fisher Scientific, Inc.) and
examined using a Zeiss LSM 510 Meta confocal microscope with LSM
software (Zeiss).
Zymography
Approximately 400,000 cells/well on a 6-well plate
were treated with the indicated amounts of compounds in OptiMEM
(Gibco; Thermo Fisher Scientific, Inc.) for 24 h. Seventy-five
microliters of conditioned medium was concentrated with a speed-vac
device and samples were analyzed with gelatin zymography in 10%
SDS-PAGE, casted in the presence of 1 mg/ml fluorescently labelled
gelatin [2-methoxy-2,4- diphenyl-3-(2H) furanone (Fluka)]. After
electrophoresis, SDS was removed by 2.5% Triton X-100 to renature
the gelatinases, and gels were incubated in 50 mM Tris-HCl buffer,
pH 7.8, 150 mM NaCl, 5 mM CaCl2, 1 µM ZnCl2
overnight at 37°C. The degradation of fluorescent gelatin was
visualized using Molecular imager ChemiDoc XRS+ (Bio-Rad) (34). The intensities of bands were
quantified with Fiji software (33). The results represent the average of
two separate sample sets in triplicate, each analyzed three times.
The band intensities were normalized to the cellular soluble
protein concentration.
Patients and sample collection
This retrospective study was approved by the Ethics
Committee of the Northern Ostrobothnia Hospital District, Finland
(49/2010, 56/2010, 46/2013) and the Finnish National Supervisory
Authority for Welfare and Health (6865/05.01.00.06/2010,
7449/06.01.03.01/2013). OTSCC tissue specimens were obtained from
75 patients diagnosed and treated at the Oulu University Hospital,
Finland, between 1990 and 2016. Clinical and pathological
information were collected from a retrospective review of patient
medical records, and tumor histological grade was defined in
accordance with the World Health Organization classification
(Table I). Samples of normal tongue
tissue and tongue dysplasia were obtained from 26 patients
diagnosed and treated at the Oulu University Hospital, Finland, and
were histopathologically graded as normal/mild (n=9) or
moderate/severe (n=16) (Table
II).
 | Table I.Clinicopathological features of the
patients with OTSCC (N=75). |
Table I.
Clinicopathological features of the
patients with OTSCC (N=75).
|
Characteristics | Data n (%) |
|---|
| Sex |
|
|
Male | 44 (59) |
|
Female | 31 (41) |
| Age at diagnosis
(years) |
|
|
≤65 | 34 (45) |
|
>65 | 41 (55) |
|
Range | 26-88 |
|
Mean | 65 |
| Follow-up |
|
|
Time | Months |
|
Range | 2-251 |
|
Mean | 40 |
| Cause of death |
|
|
Alive | 41 (55) |
|
Cancer | 19 (25) |
|
Other | 12 (15) |
| No
information | 4 (5) |
| Clinical stage |
|
|
I–II | 39 (52) |
|
III–IV | 36 (48) |
| Histopathological
grade |
|
| 1 | 14 (19) |
| 2 | 41 (55) |
| 3 | 16 (21) |
| No
information | 4 (5) |
| Lymph node
status |
|
|
pN0 | 37 (49) |
|
pN+ | 25 (33) |
| No
information | 13 (55) |
| Neck
dissection |
|
|
Yes | 55 (73) |
| No | 19 (25) |
| No
information | 1 (1) |
| Adjuvant
treatment |
|
| No | 37 (49) |
|
Radiotherapy | 25 (33) |
| Radio +
chemotherapy | 12 (16) |
| Recurrence |
|
|
Yes | 21 (28) |
| No | 50 (67) |
| No
information | 4 (5) |
| hCAP18/LL-37
immunostaining |
|
|
Negative | 40 (53) |
|
Positive | 35 (47) |
 | Table II.Clinicopathological features of the
patients with dysplasia (N=26). |
Table II.
Clinicopathological features of the
patients with dysplasia (N=26).
|
Characteristics | Data n (%) |
|---|
| Sex |
|
|
Male | 14 (54) |
|
Female | 12 (46) |
| Age (years) |
|
|
≤60 | 11 (42) |
|
>60 | 15 (52) |
|
Range | 27-92 |
|
Mean | 60 |
| hCAP18/LL-37
immunostaining |
|
|
Normal/mild dysplasia |
|
|
Negative | 1 (11) |
|
Positive | 8 (89) |
|
Moderate/severe dysplasia |
|
|
Negative | 9 (53) |
|
Positive | 8 (47) |
Immunohistochemistry
Patient samples were fixed with 10% neutral buffered
formalin, embedded in paraffin, and serially sectioned at 4 µm.
Immunohistochemistry for hCAP18/LL-37 was performed using REAL
EnVision detection system, peroxidase/DAB+, Rabbit/Mouse kit from
Dako as described elsewhere (35).
Briefly, the sections were deparaffinized in xylene and rehydrated
with graded ethanol solutions. Following peroxidase blocking with
peroxidase blocking solution (Dako) for 10 min, the sections were
incubated with primary, monoclonal antibody to human LL-37/hCAP18
clone 3D11 (dilution 1:300; cat. no. HM2070; Hycult Biotech) for 30
min at RT, followed by incubation with streptavidin-biotinylated
horseradish peroxidase (StreptABComplex/HRP; cat. no. K5007; Dako)
for 30 min. The reactions were developed with diaminobenzydine
(DAB), and the sections were then counterstained with Mayer's
haematoxylin (Reagena). An isotype-specific immunoglobulin mouse
IgG1 (dilution 1:300; cat. no. X0931; Dako) was used as a negative
control. LL-37/hCAP18 antibody-stained immune cells in tumor stroma
were assessed similarly as shown previously (14). This was considered as a positive
control of LL-37/hCAP18 staining.
Semi-quantitative assessment
All samples were examined by three independent
observers (MR, MV and PCR) blinded to the patient clinical
information. Discrepancies in the scoring were settled by an
experienced oral pathologist (TS). In OTSCC, immunopositivity for
hCAP18/LL-37 was evaluated according to overall staining intensity
and area (percentage of positively stained tissues) from the whole
sample with ×10 magnification and the most representative fields
were selected and cells counted using ×20 magnification. The
existence of yellow/brown-stained cells was defined as expression
of hCAP18/LL-37. If the staining intensity was heterogeneous, then
scoring was based on the greatest degree of intensity. An
immunoreactive score (IRS) was used for the hCAP18/LL-37 scoring.
The IRS was obtained by multiplying the intensity score (0, no
staining; 1, weak staining; 2, moderate staining; 3, strong
staining) by the area score (1, ≤25%; 2, >25 to ≤50% and 3,
>50% of cancer cells), yielding an IRS range from 0 to 9. An IRS
score ≤1 was considered as negative hCAP18/LL-37 expression, while
≥2 was classified as positive hCAP18/LL-37 immunoexpression. For
statistical analysis, the OTSCC patients were classified into two
groups, ‘low expression’ included those with negative or weak
expression (IRS score ≤1) and ‘high expression’ included those with
moderate or strong expression (IRS score ≥2). The outcomes were
categorized as overall survival: Time from treatment initiation
until death or last known date alive; disease-specific survival:
Time from treatment initiation until death due to cancer or last
known date alive and disease-free survival: Time from treatment
initiation until diagnosis of the first recurrence (local, regional
or distant) or last follow-up information for those without
recurrence. Normal and dysplastic samples were evaluated by
intensity of staining on epithelial layers (0, no staining; 1, weak
staining; 2, moderate staining; 3, strong staining). A score ≤1 was
considered as negative hCAP18/LL-37 expression, while ≥2 was
classified as positive for hCAP18/LL-37. For statistical analysis,
samples were grouped as ‘normal tongue tissue or mild dysplasia’
and ‘moderate or severe dysplasia’. By creating these two broad
groups, we aimed to reduce the subjectivity inherent to grading
dysplasia.
Statistical analysis
Calculations were performed with IBM SPSS Statistics
26 (IBM Corp.). In all in vitro cell experiments, P-values
were calculated with the independent-samples Kruskal-Wallis test
and significance was adjusted by the Bonferroni correction for
multiple tests. In in vitro experiments the results are
presented as average ± standard deviation (SD). For finding
correlations between the hCAP18/LL-37 staining in
immunohistochemistry samples and patient clinicopathological
parameters, the Spearman's rank correlation coefficient was used.
Furthermore, a Kaplan-Meier test was used to construct the survival
curves. To determine the significance of expression of hCAP18/LL-37
in OTSCC and normal/tongue dysplasia tissues, the Kruskal-Wallis
test with Dunn's post hoc test was used. P-value <0.05 was
considered indicative of a statistically significant result.
Results
Human cationic antimicrobial
protein-18 (hCAP18) is expressed in OTSCC cells and exogenous LL-37
has a fluctuating effect on the cells
The human cationic antimicrobial
protein-18/antimicrobial peptide LL-37 (hCAP18/LL-37) has been
found in varying degrees at the mRNA and protein levels in several
cancer cell lines (14–16). We first evaluated the hCAP18/LL-37
expression in human OTSCC cell lines HSC-3, SCC-25 and SAS, and in
dysplastic papillomavirus HPV16 immortalized human oral epithelial
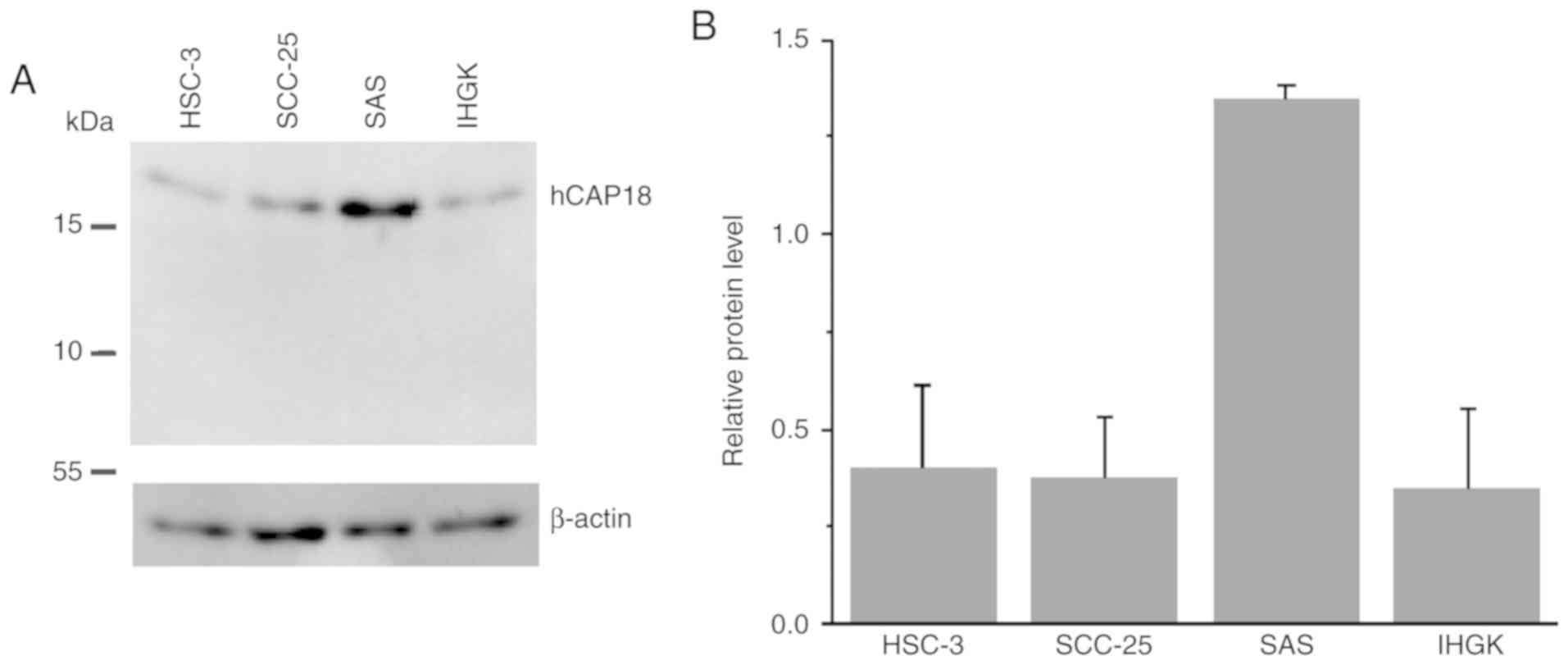
(IHGK) cells by immunoblotting (Fig.
1). Our analysis revealed that only hCAP18 (19 kDa) was present
in all cells, while the LL-37 peptide (5 kDa) was absent (Fig. 1A). The immunoblot results showed
that the OTSCC cell line SAS contained the highest amount of hCAP18
among the cell lines tested (Fig. 1A
and B). Our analysis disclosed that OTSCC cells express hCAP18,
which may be further cleaved to LL-37 in the extracellular
space.
LL-37 has been shown to affect cell proliferation of
several cancer cell lines (13-16,23,36-43). Thus, we assessed the
effect of the recombinant LL-37 on proliferation of HSC-3, SCC-25,
SAS and IHGK cell lines (Fig. 2).
Cells were treated with increasing doses of synthetic LL-37 peptide
in the presence of serum since the effects of the peptide appear to
be dependent on serum (14,43). Epidermal growth factor (EGF) was
used as a positive control (44).
After 24 and 48 h, the highest dose (50 µg/ml) of LL-37 reduced the
proliferation of OTSCC and dysplastic IHGK cell lines compared with
the corresponding untreated controls (Fig. 2A and B). Doses of 0.5–10 µg/ml had
reducing effects on SCC-25 cells at 24 h and on SAS and IHKG cells
at 48 h, otherwise these doses had no marked impact on
proliferation. The addition of 100 ng/ml EGF, however, reduced the
proliferation of all cell lines at 24 and 48 h. Interestingly,
after 72 h the effects of LL-37 and EGF were somewhat reversed.
Especially in SAS cells the longer incubation time with all doses
of LL-37 induced cell proliferation and in HSC-3 cells with a dose
of 50 µg/ml. Also at this time point, no marked effect was observed
on the other cell lines.
To further assess possible apoptotic cell death
effects of LL-37 and to validate the BrdU proliferation assay
results, we performed a TUNEL assay for HSC-3 control, 100 ng/ml
EGF and 50 µg/ml LL-37-treated cells (Fig. S1). After 48 h a notable amount of
stained cells with DNA fragmentation was noted only in the
LL-37-treated cells, indicating that reduction in proliferation was
probably caused by apoptosis. Our data suggest that LL-37,
especially at high doses, mostly reduces proliferation of OTSCC
cells, but its effects may fluctuate depending on incubation time
and cell line.
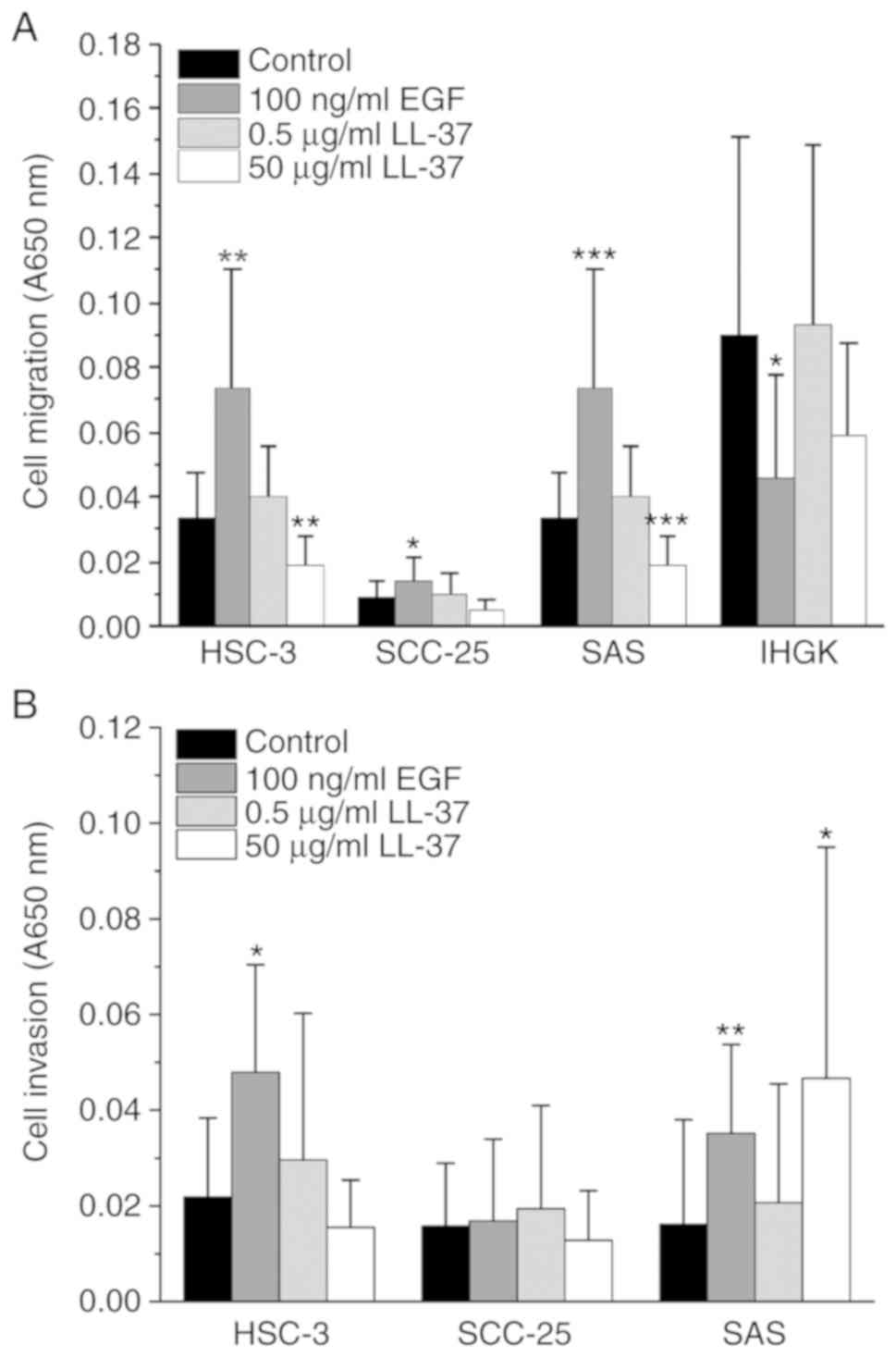
LL-37 stimulates the migration and
invasion of OTSCC cell lines
It has been reported that LL-37 promotes migration
and invasion of several cancer cell lines (14,16,36–38,43).
We assayed the effect of LL-37 on OTSCC cell line migration using
Transwell inserts (Fig. 3A). To
elucidate the effects on invasion, we used Transwells coated with
Myogel (31) and type I collagen
mixture (Fig. 3B). The OTSCC cell
lines tested display different aggressiveness and invasive ability
(45–49). Because dysplastic IHGK cells did not
invade, their migration was only assayed (Fig. 3A). All of the cancer cell lines
showed a slightly increased migration and invasion when treated
with 0.5 µg/ml LL-37 (Fig. 3A and
B). For IHGK cells, the addition of 0.5 µg/ml LL-37 only
marginally increased their migration. In SAS cells, 50 µg/ml LL-37
clearly increased the invasion but not migration, whereas in the
other cell lines this dose led to decreased invasion or migration
compared with the controls. The treatment with our positive control
100 ng/ml EGF increased the migration and invasion of all three
OTSCC cell lines compared with the control. On the other hand, the
migration of IHGK cells decreased with the addition of 100 ng/ml
EGF. Our data suggest that LL-37 induces migration and invasion of
OTSCC cells, but the effect depends on the cell line.
LL-37 affects the amount of EGFR on
the plasma membrane
In normal epithelial and lung cancer cells, LL-37
appears to mediate its actions on cell proliferation and migration
mostly via EGFR by the activation of downstream pathways (15,26,27,43,50).
Since EGFR appears to have a prognostic value in oral cancer
(51) and results demonstrate that
LL-37 treatment affects cell proliferation, migration and invasion,
we tested phosphorylation of EGFR, extracellular signal-regulated
protein kinases 1 and 2 (Erk1/2) and the serine/threonine kinase
Akt by immunoblotting (Fig. 4).
Phosphorylated EGFR was only detected in the HSC-3 and SCC-25
cells, not in the SAS or IHGK cells. Phosphorylation of EGFR in
HSC-3 cells increased only when our positive control 100 ng/ml EGF
was added, but in SCC-25 cells it also slightly increased with the
addition of 50 µg/ml LL-37. The most evident finding was that the
amount of total EGFR markedly increased in all cell lines when
cells were stimulated with 50 µg/ml LL-37. To further study the
localization of EGFR in HSC-3 cells, we used immunocytochemistry
(Fig. S2). Our staining showed
that in starved control cells EGFR localized both to the plasma
membrane and to the cytosol close to the nucleus. In EGF-stimulated
cells, the brightest staining was observed as droplets close to the
nucleus. In LL-37-treated cells, the strong EGFR staining lined the
plasma membrane of HSC-3 cells. These data suggest that a high
concentration of LL-37 affects the stability of the EGFR protein
rather than its expression and phosphorylation.
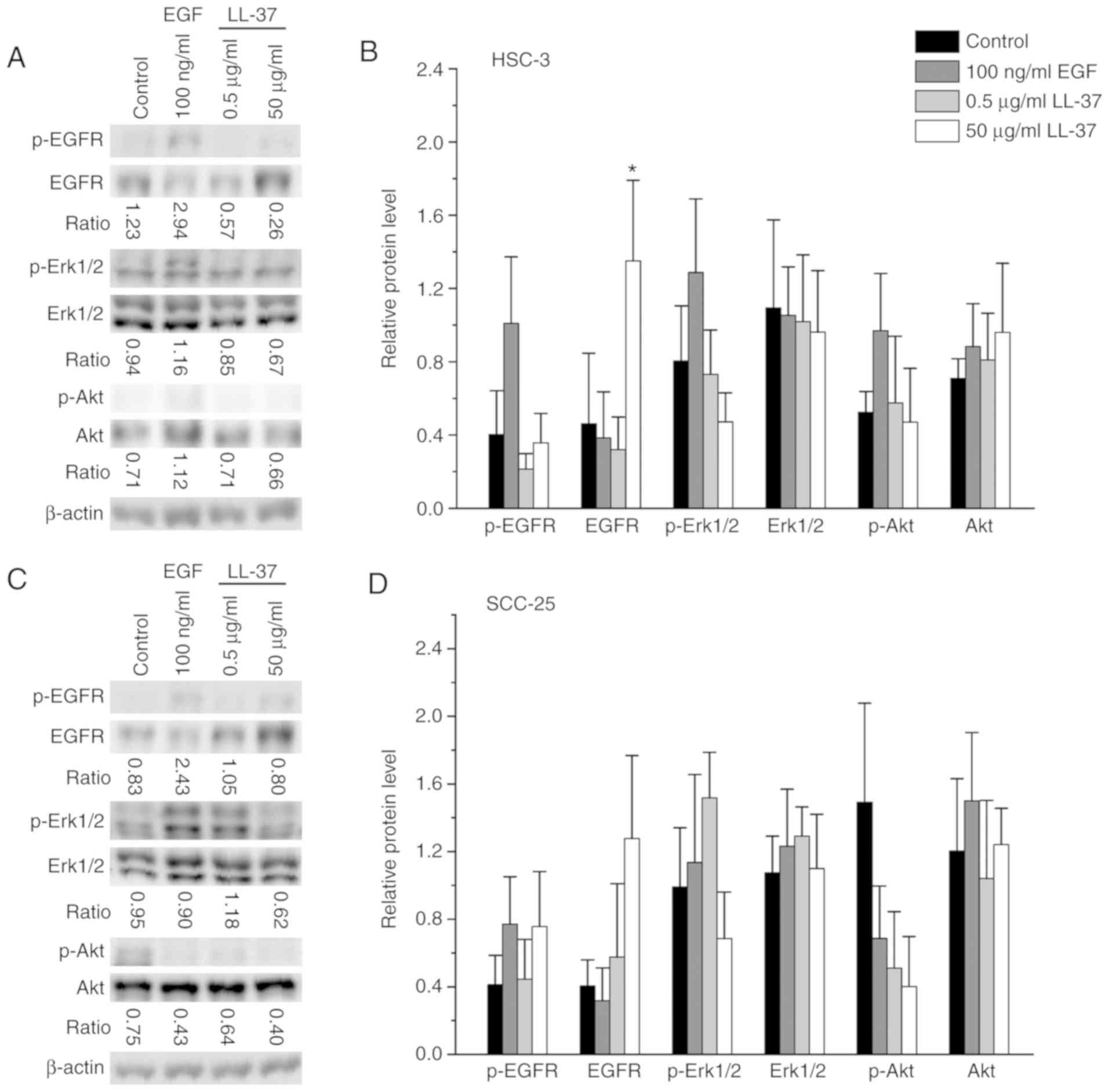 | Figure 4.Immunoblot analysis of EGFR, Erk1/2
and Akt activation in EGF- or LL-37-treated OTSCC cell lines and
IHGK cells. Cells were starved for 24 h and stimulated with the
indicated doses of EGF or LL-37 for 1 h. Starved non-stimulated
cells were used as a control. Cell homogenates (30 µg of soluble
protein) were separated on SDS-PAGE gels. The amounts of EGFR,
Erk1/2 and Akt and their phosphorylated forms were analyzed with
immunoblots in (A) HSC-3 and (C) SCC-25 cells. In A and C,
representative immunoblots are shown. The average ratio between the
optical density of phosphorylated protein/total protein was
calculated. β-actin was used as a control to normalize the
quantities of proteins in (B) HSC-3 and (D) SCC-25 cells using Fiji
software. The results represent average ± SD of two independent
experiments, separated three times on SDS-PAGE gels. P-values were
calculated with the independent-samples Kruskal-Wallis test and
significance was adjusted by the Bonferroni correction for multiple
tests. *P<0.05, compared with the control. Immunoblot analysis
of EGFR, Erk1/2 and Akt activation in EGF- or LL-37-treated OTSCC
cell lines and IHGK cells. Cells were starved for 24 h and
stimulated with the indicated doses of EGF or LL-37 for 1 h.
Starved non-stimulated cells were used as a control. Cell
homogenates (30 µg of soluble protein) were separated on SDS-PAGE
gels. The amounts of EGFR, Erk1/2 and Akt and their phosphorylated
forms were analyzed with immunoblots in (E) SAS and (G) IHGK cells.
In E and G, representative immunoblots are shown. The average ratio
between the optical density of phosphorylated protein/total protein
was calculated. β-actin was used as a control to normalize the
quantities of proteins in (F) SAS and (H) IHGK cells using Fiji
software. The results represent average ± SD of two independent
experiments, separated three times on SDS-PAGE gels. P-values were
calculated with the independent-samples Kruskal-Wallis test and
significance was adjusted by the Bonferroni correction for multiple
tests. *P<0.05, compared with the control. EGF, epidermal growth
factor; EGFR, epidermal growth factor receptor; OTSCC, oral tongue
squamous cell carcinoma; LL-37, antimicrobial peptide
leucine-leucine-37. |
An increased phosphorylation of Erk1/2 compared with
the starved control was observed in all cancer cell lines when
positive control 100 ng/ml EGF was added, but not in dysplastic
IHGK cells (Fig. 4). The addition
of 0.5 µg/ml LL-37 led to a marked increase in the phosphorylation
of Erk1/2 in SCC-25 cells, but in the other cell lines it had a
slightly decreasing effect or no effect at all. Addition of 50
µg/ml LL-37 decreased the phosphorylation of Erk1/2 in all cell
lines, and it was statistically significant in SAS cells. No
significant changes were seen in the total amount of Erk1/2 with
any of the treatments. The phosphorylation of Akt increased with
positive control 100 ng/ml EGF only in HSC-3 and SAS cells. In
HSC-3 and SCC-25 cells, there was a dose-dependent decrease in Akt
phosphorylation with LL-37 stimulation. Interestingly, in SAS and
IHKG cells the addition of 50 µg/ml LL-37 increased the Akt
phosphorylation, even though 0.5 µg/ml LL-37 slightly reduced the
Akt phosphorylation. For the total protein amount of Akt, the
treatments seemed not to have a significant effect. Our data
suggest that OTSCC and IHKG cell lines respond differently to the
EGF and LL-37 treatments with regard to the downstream
pathways.
LL-37 induces secretion of matrix
metalloproteinase (MMP)2 and MMP9
LL-37 has been shown to affect
epithelial-to-mesenchymal transition (EMT) (18,52)
and to induce the amount of matrix metalloproteinases (MMPs)
(14,17,53).
Our invasion assay results suggest that LL-37 also induces EMT or
activates expression of MMPs in our cell lines. We therefore
analyzed common epithelial marker E-cadherin and mesenchymal marker
vimentin by immunoblotting and used zymography to evaluate the
amounts of pro- and active forms of MMP2 and MMP9. Additionally,
EGF is known to induce EMT and also MMP expression in cancer cell
lines (54). LL-37 treatments only
slightly reduced or induced the amount of E-cadherin in the
different cell lines (Fig. S3A-H).
EGF reduced E-cadherin levels in all cell lines, significantly in
SCC-25 cells (Fig. S3C and D).
Treatment with 0.5 µg/ml LL-37 reduced or had no effect on the
level of vimentin, while 50 µg/ml LL-37 induced vimentin in HSC-3
and SCC-25 cells. EGF markedly induced vimentin level only in
SCC-25 cells (Fig. S3C and D),
while in other cell lines it somewhat reduced the vimentin
level.
On zymogram gels, we found bands corresponding to
pro-MMP9 and pro- and active MMP2 in the HSC-3 and SCC-25 cells
(Fig. S4A and C), but in SAS and
IHGK cells (Fig. S4E and G) only
pro-forms of MMP2 and MMP9 were detected. Quantification of the
bands showed that 50 µg/ml LL-37 increased the amount of these MMPs
in the HSC-3, SAS and IHGK cells (Fig.
S4B, F and H), but in SCC-25 cells it slightly decreased the
MMPs (Fig. S4D). In addition, 0.5
µg/ml LL-37 mostly induced the amount of MMPs. Furthermore, 100
ng/ml EGF increased the amount of pro-MMP9 in the HSC-3, SAS and
IHGK cells. For pro-MMP2, EGF had a slightly reducing effect or no
effect in all cell lines. However, the level of active MMP2
slightly increased in the HSC-3 and SCC-25 cells. Our results
suggest that LL-37 may induce the amount of MMP2 and MMP 9,
however, LL-37 did not induce marked EMT in the tested cell
lines.
hCAP18/LL-37 is expressed in OTSCC
tissues and tongue dysplasia but is not associated with
clinicopathological characteristics and/or outcome of the
patients
The tissue expression of hCAP18/LL-37 has been
analyzed in several cancer types (13-19,21,40,41). Our
immunohistochemistry results showed positive immunostaining for
hCAP18/LL-37 in 35/75 OTSCC samples (47%) (Table I). In positive samples, both
peripheral and central cells of neoplastic islands were stained
(Fig. 5A). In oral tongue samples,
8/9 normal/mild (89%) and 8/17 moderate/severe oral dysplasia (47%)
showed positivity for hCAP18/LL-37 (Table II), and positive staining was
detected within the epithelial layers of tissue (Fig. 5B). The inter-observer k-value,
representing the degree of agreement among readers, was 0.88 for
OTSCC samples and 0.95 for normal and tongue dysplasia samples. The
hCAP18/LL-37 levels were higher in normal/mild dysplasia samples
than in OTSCC samples (P<0.05). In addition, the hCAP18/LL-37
expression was higher in normal/mild dysplasia than in
moderate/severe dysplasia, albeit not significantly (P=0.059,
Fig. 5C). However, no differences
between OTSCC and moderate/severe dysplasia were observed
(P>0.05). To determine the correlation between levels of
hCAP18/LL-37 and various clinicopathological features, patients of
the OTSCC cohort were divided into low and high expression
subgroups. As shown in Table III,
the expression of hCAP18/LL-37 in OTSCC tissues was not correlated
with the clinicopathological characteristics of the cancer
patients. We next investigated the association between expression
of hCAP18/LL-37 and clinical prognosis of OTSCC patients.
hCAP18/LL-37 survival analyses based on univariate log-rank test
revealed no significant association with overall survival (Fig. S5A), disease-specific survival
(Fig. S5B) or disease-free
survival (Fig. S5C). Taken
together, our results suggest that hCAP18/LL-37 shows a lower
expression in OTSCC tissues than in normal/mild dysplasia samples,
but its amount is not correlated with clinicopathological features
and/or outcome of patients with OTSCC.
 | Table III.Spearman correlation between
immunohistochemical expression of hCAP18/LL-37 and
clinicopathological variables. |
Table III.
Spearman correlation between
immunohistochemical expression of hCAP18/LL-37 and
clinicopathological variables.
| Variable | hCAP18/LL-37
(correlation coefficient/P-value) |
|---|
| Age | −0.061/0.604 |
| Sex | 0.025/0.829 |
| Smoking habit | 0.129/0.397 |
| T stage | 0.011/0.927 |
| N stage | 0.099/0.446 |
| Histopathological
grade | −0.018/0.882 |
| Recurrence | 0.040/0.741 |
| Treatment | 0.053/0.654 |
| Neck
dissection | −0.123/0.296 |
Discussion
Human host defense cationic antimicrobial
peptide-18/antimicrobial peptide LL-37 (hCAP18/LL-37) has been
detected in healthy human tongue (28) and oral squamous cell carcinoma
(OSCC) (21), but its role remains
unclear. Recently a study reported that low expression of
hCAP18/LL-37 in poorly differentiated OSCC was related to lymph
node metastasis and tumor progression promotion (21). Therefore, we analyzed the effect of
recombinant LL-37 on one oral dysplastic and three oral tongue
squamous cell carcinoma (OTSCC) cell lines and determined the
expression of hCAP18/LL-37 in normal/dysplastic and OTSCC patient
samples. Our data suggest that LL-37 has a fluctuating effect on
proliferation and invasion of OTSCC cell lines, and the
hCAP18/LL-37 levels are higher in normal/mild dysplastic samples of
oral tongue when compared with levels in OTSCC samples.
All three OTSCC cell lines as well as the dysplastic
IHGK cell line expressed hCAP18, the precursor form of LL-37.
Similarly, only the precursor has been detected in ovarian cancer
cell lines (14); however, in HaCaT
cells LL-37 was found also in the cell lysate (55). We also analyzed hCAP18/LL-37 from
conditioned medium of one OTSCC cell line (data not shown), but we
did not detect LL-37 nor hCAP18, which was also the case with HaCaT
cells (55). In general, hCAP18 is
considered to be cleaved extracellularly to LL-37 by proteinases
(11,12). In our case, however, it appeared
that hCAP18/LL-37 were secreted in low levels or degraded rapidly
after secretion, since they were not present in the conditioned
medium of the OTSCC cell lines. The OTSCC cell lines seemed to
produce different amounts of hCAP18, while the SAS cell line had
the highest endogenous protein amount of the three cancer cell
lines tested. Lung cancer and malignant melanoma cell lines were
also found to express varying amounts of hCAP18 (15,16).
Our analysis revealed that a high dose (50 µg/ml) of
recombinant LL-37 had a suppressive effect on the proliferation of
OTSCC and dysplastic cell lines at early time points, which also
leads to induced DNA fragmentation. Lower doses (0.5–10 µg/ml)
reduced the proliferation or had no considerable effects. In many
cases, similar concentrations of LL-37 were previously found to
induce the proliferation of cancer cells (14-16,36-38,41,43).
Nevertheless, in previous experiments higher concentrations of
recombinant LL-37 (>5-50 µg/ml) seemed to decrease cell
proliferation (15,36–41,43)
and even induce necrotic cell death (43) or caspase-independent apoptosis
(40,56). Furthermore, a truncated (27-mer)
peptide of hCAP18 was found to induce caspase-independent apoptosis
of the highly invasive clone of SAS (42). In addition, a shortened fragment of
LL-37, KI-21-3 caused considerable anti-proliferative and
caspase-3-dependent apoptotic properties on SCC-4 cells (57). Interestingly, OTSCC cell lines
responded somewhat differently to the LL-37 treatments after longer
incubation. Especially with the SAS cell line, which had the
highest cellular hCAP18 amount, the LL-37 peptide first markedly
reduced proliferation, but then the effect was reversed and
proliferation significantly increased. Regarding colon cancer cell
lines, LL-37 was suggested to have different cytotoxicity towards
p53 wild-type cells than towards p53-mutant cells (40). This finding could also partly
explain our result since HSC-3 and SCC-25 have been reported to
carry p53 mutations (58,59), while the SAS cell line has wild-type
p53 (60,61). However, our results suggest that
LL-37 has a fluctuating reducing effect on proliferation of OTSCC
cell lines.
Although LL-37 decreased cell proliferation of OTSCC
cells, our experiments indicate that the peptide could induce cell
migration and invasion. The cell lines tested are considered to
exhibit varying ability to migrate and invade, HSC-3 and SAS being
more aggressive than SCC-25 (45–49).
As expected, the migration and invasion ability of aggressive OTSCC
cell lines was significantly induced by our positive control EGF
(62). In addition, all cell lines
showed slightly increased migration and invasion with a low dose of
LL-37, and the effect on invasion was pronounced with a high dose
in SAS cells, which also have a high endogenous amount of hCAP18.
Our results are concordant with earlier results showing that LL-37
promotes cancer cell motility (14,16,18,25,36–38,43).
Nevertheless, the OTSCC cell lines had a fluctuating response to
the LL-37 peptide and the effect seemed not to fully correlate with
the aggressiveness of the cell line.
LL-37 has been shown to transactivate EGFR in
various epithelial cells and also in lung cancer cells (15,26,27,43,50) in
a process that involves stimulation of a membrane-anchored
metalloproteinase, thereby releasing EGFR ligands (26,27,50).
In our experiments, we did not find EGFR phosphorylation with all
of our epithelial origin cell lines even with positive control EGF.
Surprisingly, in all cell lines the high dose of LL-37 increased
the amount of total EGFR. Further analysis revealed that this
increase was probably due to stabilization of the receptor to the
plasma membrane. Others have reported that the total EGFR amount is
unaffected by LL-37 treatment (27), and therefore, this is a new effect
seen with LL-37.
Phosphorylation of Erk1/2, which is the key
regulator of cell growth and cell cycle progression (63), decreased with the high dose of LL-37
in our studies, consistent with the proliferation results. In
addition, activation of Akt, a master regulator of many metastatic
processes, including cell growth, proliferation, motility and
epithelial mesenchymal transition (EMT) (64), in most cases decreased with LL-37
treatment. However, in SAS cells the high dose of LL-37 induced Akt
activation, which would explain the induced invasion of SAS cells.
In previous studies, LL-37 has been shown to activate Erk1/2 or
other MAP kinase pathways (15,26,50,53)
and Akt (41,50,53,65).
Furthermore, in these studies activation was linked to increased
cell growth and/or motility (15,41,50,53).
LL-37 also affected the activation of these pathways in our cell
lines, thereby changing their behavior. In addition, the amount of
MMP2 and MMP9 was induced in OTSCC and dysplastic cell lines, but
this increase was not directly associated with cell invasion,
differing from earlier results (14,53).
Our results also suggest that LL-37 does not induce pronounced EMT
in OTSCC cell lines. Previous findings concerning impact of LL-37
on EMT markers have been contradictory (18,52),
suggesting that LL-37 actions fluctuate depending on the cell
line.
The in vitro results encouraged us to
evaluate the immunoexpression and prognostic value of hCAP18/LL-37
in OTSCC patient samples and in oral tongue dysplasia. In the
present study, we found that hCAP18/LL-37 expression was
significantly lower in OTSCC tissues when compared with that in
normal/mild dysplasia samples. Additionally, moderate/severe
dysplasia showed lower expression of hCAP18/LL-37 than mild
dysplasia, although the difference was not significant. This is in
accordance with an earlier study showing that hCAP18/LL-37 is
downregulated in oral cancer (21).
Furthermore, in gastric and colon cancers the expression of
hCAP18/LL-37 was previously found to be lower in cancer cells than
in normal epithelium (6,19,40,41).
For these cancer cell line types, the hCAP18/LL-37 peptide had also
a decreasing effect on cell proliferation (39,41),
as was mostly observed with our OTSCC cell lines. In a previous
study, low hCAP18/LL-37 expression was associated with lymph node
metastasis and tumor progression in OTSCC (21). However, with the limitations imposed
by sample size, our observations suggest no correlation between
cancer progression and/or patient outcome and expression of
hCAP18/LL-37, even though hCAP18/LL-37 may have a suppressive
effect on dysplasia and SCC in oral tongue epithelium.
This study has potential limitations. The effects of
recombinant LL-37 were analyzed using only three OTSCC cell lines.
However due to the unavailability of resources we were not able to
include more cell lines to our experiments. Use of additional cell
lines would have strengthened our conclusions concerning the main
effects of LL-37 on OTSCC cell proliferation, migration/invasion
and activation of the EGFR and its downstream pathways. In the
analysis of the OTSCC patient samples, the sample number and the
lack of complete data of all prominent confounding factors,
resulted in limitations to the statistical analysis. In addition,
the Kaplan-Meier analysis found that there were no differences
between the positive and negative hCAP18/LL-37 groups. Thereby we
were not able to perform multivariate analysis creating hazard
ratios. Furthermore, the Spearman's rank correlation was used to
calculate correlation between immunohistochemical expression of
hCAP18/LL-37 and clinicopathological variables, since in our data
the variables were not normally distributed.
In summary, we demonstrated that the LL-37 peptide
reduced proliferation, and induced migration and invasion and MMP2
and MMP9 expression of OTSCC cell lines time- and cell
line-dependently. In all cell lines, a high dose of LL-37 increased
the amount of total EGFR. Furthermore, hCAP18/LL-37
immunoexpression was lower in OTSCC samples than that in the
normal/mild tongue dysplasia, but expression did not correlate with
patient outcome.
Supplementary Material
Supporting Data
Acknowledgements
We gratefully acknowledge Maija-Leena Lehtonen,
Tanja Kuusisto, Eeva-Maija Kiljander and Piia Mäkelä from
University of Oulu for expert technical assistance.
Funding
The present study was supported by research grants
from the Bayer Foundation, the Sigrid Juselius Foundation, the
Cancer Foundation of Finland, the Medical Research Center Oulu, and
research funds from the Medical Faculty of the University of Oulu
and Oulu University Hospital special state support for
research.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MV and MR conceived and designed the experiments,
performed the experiments, analyzed the data and wrote the paper.
PCR conceived and designed the experiments, analyzed the data and
wrote the paper. ES and MS contributed materials, analyzed the data
and wrote the paper. TS conceived and designed the experiments,
contributed reagents and wrote the paper. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of the Northern Ostrobothnia Hospital District, Finland
(49/2010, 56/2010, 46/2013) and the Finnish National Supervisory
Authority for Welfare and Health (6865/05.01.00.06/2010,
7449/06.01.03.01/2013).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that there are no competing
interests, and no financial or personal relationships with other
people or organizations that could inappropriately influence this
work.
References
|
1
|
Annertz K, Anderson H, Palmér K and
Wennerberg J: The increase in incidence of cancer of the tongue in
the Nordic countries continues into the twenty-first century. Acta
Otolaryngol. 132:552–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mroueh R, Haapaniemi A, Grénman R, Laranne
J, Pukkila M, Almangush A, Salo T and Mäkitie A: Improved outcomes
with oral tongue squamous cell carcinoma in Finland. Head Neck.
39:1306–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu WK, Wang G, Coffelt SB, Betancourt AM,
Lee CW, Fan D, Wu K, Yu J, Sung JJ and Cho CH: Emerging roles of
the host defense peptide LL-37 in human cancer and its potential
therapeutic applications. Int J Cancer. 127:1741–1747. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piktel E, Niemirowicz K, Wnorowska U,
Wątek M, Wollny T, Głuszek K, Góźdź S, Levental I and Bucki R: The
role of cathelicidin LL-37 in cancer development. Arch Immunol Ther
Exp (Warsz). 64:33–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xhindoli D, Pacor S, Benincasa M, Scocchi
M, Gennaro R and Tossi A: The human cathelicidin LL-37-A
pore-forming antibacterial peptide and host-cell modulator. Biochim
Biophys Acta. 1858:546–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuroda K, Okumura K, Isogai H and Isogai
E: The human cathelicidin antimicrobial peptide LL-37 and mimics
are potential anticancer drugs. Front Oncol. 5:1442015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verjans ET, Zels S, Luyten W, Landuyt B
and Schoofs L: Molecular mechanisms of LL-37-induced receptor
activation: An overview. Peptides. 85:16–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sørensen OE, Follin P, Johnsen AH, Calafat
J, Tjabringa GS, Hiemstra PS and Borregaard N: Human cathelicidin,
hCAP-18, is processed to the antimicrobial peptide LL-37 by
extracellular cleavage with proteinase 3. Blood. 97:3951–3959.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki K, Schauber J, Coda A, Lin H,
Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A
and Gallo RL: Kallikrein-mediated proteolysis regulates the
antimicrobial effects of cathelicidins in skin. FASEB J.
20:2068–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heilborn JD, Nilsson MF, Jimenez CIC,
Sandstedt B, Borregaard N, Tham E, Sørensen OE, Weber G and Ståhle
M: Antimicrobial protein hCAP18/LL-37 is highly expressed in breast
cancer and is a putative growth factor for epithelial cells. Int J
Cancer. 114:713–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coffelt SB, Waterman RS, Florez L, Höner
zu Bentrup K, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES,
Morris CA and Scandurro AB: Ovarian cancers overexpress the
antimicrobial protein hCAP-18 and its derivative LL-37 increases
ovarian cancer cell proliferation and invasion. Int J Cancer.
122:1030–1039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Haussen J, Koczulla R, Shaykhiev R,
Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner
A, Czubayko F and Bals R: The host defence peptide LL-37/hCAP-18 is
a growth factor for lung cancer cells. Lung Cancer. 59:12–23. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JE, Kim HJ, Choi JM, Lee KH, Kim TY,
Cho BK, Jung JY, Chung KY, Cho D and Park HJ: The antimicrobial
peptide human cationic antimicrobial protein-18/cathelicidin LL-37
as a putative growth factor for malignant melanoma. Br J Dermatol.
163:959–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hensel JA, Chanda D, Kumar S, Sawant A,
Grizzle WE, Siegal GP and Ponnazhagan S: LL-37 as a therapeutic
target for late stage prostate cancer. Prostate. 71:659–670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sainz B Jr, Alcala S, Garcia E,
Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo
I, Hidalgo M, Gomez-Lopez G, et al: Microenvironmental
hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by
activating its cancer stem cell compartment. Gut. 64:1921–1935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hase K, Murakami M, Iimura M, Cole SP,
Horibe Y, Ohtake T, Obonyo M, Gallo RL, Eckmann L and Kagnoff MF:
Expression of LL-37 by human gastric epithelial cells as a
potential host defense mechanism against Helicobacter
pylori. Gastroenterology. 125:1613–1625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An LL, Ma XT, Yang YH, Lin YM, Song YH and
Wu KF: Marked reduction of LL-37/hCAP-18, an antimicrobial peptide,
in patients with acute myeloid leukemia. Int J Hematol. 81:45–47.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Qi G, Qin M, Zou Y, Zhong K, Tang
Y, Guo Y, Jiang X, Liang L and Zou X: DNA methylation directly
downregulates human cathelicidin antimicrobial peptide gene (CAMP)
promoter activity. Oncotarget. 8:27943–27952. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coffelt SB, Tomchuck SL, Zwezdaryk KJ,
Danka ES and Scandurro AB: Leucine leucine-37 uses formyl peptide
receptor-like 1 to activate signal transduction pathways, stimulate
oncogenic gene expression, and enhance the invasiveness of ovarian
cancer cells. Mol Cancer Res. 7:907–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y,
Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of
macrophage polarization and tumorigenesis by formyl peptide
receptor-2. Oncogene. 30:3887–3899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Yang D, Chen Q, Schmidt AP, Anderson
GM, Wang JM, Wooters J, Oppenheim JJ and Chertov O: LL-37, the
neutrophil granule- and epithelial cell-derived cathelicidin,
utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to
chemoattract human peripheral blood neutrophils, monocytes, and T
cells. J Exp Med. 192:1069–1074. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber G, Chamorro CI, Granath F, Liljegren
A, Zreika S, Saidak Z, Sandstedt B, Rotstein S, Mentaverri R,
Sánchez F, et al: Human antimicrobial protein hCAP18/LL-37 promotes
a metastatic phenotype in breast cancer. Breast Cancer Res.
11:R62009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tjabringa GS, Aarbiou J, Ninaber DK,
Drijfhout JW, Sørensen OE, Borregaard N, Rabe KF and Hiemstra PS:
The antimicrobial peptide LL-37 activates innate immunity at the
airway epithelial surface by transactivation of the epidermal
growth factor receptor. J Immunol. 171:6690–6696. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tokumaru S, Sayama K, Shirakata Y,
Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M,
Nagai H, et al: Induction of keratinocyte migration via
transactivation of the epidermal growth factor receptor by the
antimicrobial peptide LL-37. J Immunol. 175:4662–4668. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frohm Nilsson M, Sandstedt B, Sørensen O,
Weber G, Borregaard N and Ståhle-Bäckdahl M: The human cationic
antimicrobial protein (hCAP18), a peptide antibiotic, is widely
expressed in human squamous epithelia and colocalizes with
interleukin-6. Infect Immun. 67:2561–2566. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khurshid Z, Naseem M, Yahya I Asiri F,
Mali M, Sannam Khan R, Sahibzada HA, Zafar MS, Faraz Moin S and
Khan E: Significance and diagnostic role of antimicrobial
cathelicidins (LL-37) peptides in oral health. Biomolecules.
7(pii): E802017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oda D, Bigler L, Mao EJ and Disteche CM:
Chromosomal abnormalities in HPV-16-immortalized oral epithelial
cells. Carcinogenesis. 17:2003–2008. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salo T, Sutinen M, Hoque Apu E, Sundquist
E, Cervigne NK, de Oliveira CE, Akram SU, Ohlmeier S, Suomi F,
Eklund L, et al: A novel human leiomyoma tissue derived matrix for
cell culture studies. BMC Cancer. 15:9812015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salo T, Dourado MR, Sundquist E, Apu EH,
Alahuhta I, Tuomainen K, Vasara J and Al-Samadi A: Organotypic
three-dimensional assays based on human leiomyoma-derived matrices.
Philos Trans R Soc Lond B Biol Sci. 373(pii): 201604822018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nyberg P, Heikkilä P, Sorsa T, Luostarinen
J, Heljasvaara R, Stenman UH, Pihlajaniemi T and Salo T: Endostatin
inhibits human tongue carcinoma cell invasion and intravasation and
blocks the activation of matrix metalloprotease-2, −9, and −13. J
Biol Chem. 278:22404–22411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sundquist E, Kauppila JH, Veijola J,
Mroueh R, Lehenkari P, Laitinen S, Risteli J, Soini Y, Kosma VM,
Sawazaki-Calone I, et al: Tenascin-C and fibronectin expression
divide early stage tongue cancer into low- and high-risk groups. Br
J Cancer. 116:640–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang W, Zheng Y, Jia J, Li C, Duan Q, Li
R, Wang X, Shao Y, Chen C and Yan H: Antimicrobial peptide LL-37
promotes the viability and invasion of skin squamous cell carcinoma
by upregulating YB-1. Exp Ther Med. 14:499–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Jia J, Li C, Duan Q, Yang J, Wang
X, Li R, Chen C, Yan H and Zheng Y: Antimicrobial peptide LL-37
promotes the proliferation and invasion of skin squamous cell
carcinoma by upregulating DNA-binding protein A. Oncol Lett.
12:1745–1752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia J, Zheng Y, Wang W, Shao Y, Li Z, Wang
Q, Wang Y and Yan H: Antimicrobial peptide LL-37 promotes YB-1
expression, and the viability, migration and invasion of malignant
melanoma cells. Mol Med Rep. 15:240–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ,
Chu KM, Yu J and Cho CH: The host defense peptide LL-37 activates
the tumor-suppressing bone morphogenetic protein signaling via
inhibition of proteasome in gastric cancer cells. J Cell Physiol.
223:178–186. 2010.PubMed/NCBI
|
|
40
|
Ren SX, Cheng AS, To KF, Tong JH, Li MS,
Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, et al: Host immune
defense peptide LL-37 activates caspase-independent apoptosis and
suppresses colon cancer. Cancer Res. 72:6512–6523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li D, Liu W, Wang X, Wu J, Quan W, Yao Y,
Bals R, Ji S, Wu K, Guo J and Wan H: Cathelicidin, an antimicrobial
peptide produced by macrophages, promotes colon cancer by
activating the Wnt/β-catenin pathway. Oncotarget. 6:2939–2950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okumura K, Itoh A, Isogai E, Hirose K,
Hosokawa Y, Abiko Y, Shibata T, Hirata M and Isogai H: C-terminal
domain of human CAP18 antimicrobial peptide induces apoptosis in
oral squamous cell carcinoma SAS-H1 cells. Cancer Lett.
212:185–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shaykhiev R, Beisswenger C, Kändler K,
Senske J, Püchner A, Damm T, Behr J and Bals R: Human endogenous
antibiotic LL-37 stimulates airway epithelial cell proliferation
and wound closure. Am J Physiol Lung Cell Mol Physiol.
289:L842–L848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kamata N, Chida K, Rikimaru K, Horikoshi
M, Enomoto S and Kuroki T: Growth-inhibitory effects of epidermal
growth factor and overexpression of its receptors on human squamous
cell carcinomas in culture. Cancer Res. 46:1648–1653.
1986.PubMed/NCBI
|
|
45
|
Momose F, Araida T, Negishi A, Ichijo H,
Shioda S and Sasaki S: Variant sublines with different metastatic
potentials selected in nude mice from human oral squamous cell
carcinomas. J Oral Pathol Med. 18:391–395. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Matsumoto K, Matsumoto K, Nakamura T and
Kramer RH: Hepatocyte growth factor/scatter factor induces tyrosine
phosphorylation of focal adhesion kinase (p125FAK) and promotes
migration and invasion by oral squamous cell carcinoma cells. J
Biol Chem. 269:31807–31813. 1994.PubMed/NCBI
|
|
47
|
Ramos DM, Chen BL, Boylen K, Stern M,
Kramer RH, Sheppard D, Nishimura SL, Greenspan D, Zardi L and
Pytela R: Stromal fibroblasts influence oral squamous-cell
carcinoma cell interactions with tenascin-C. Int J Cancer.
72:369–376. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okumura K, Konishi A, Tanaka M, Kanazawa
M, Kogawa K and Niitsu Y: Establishment of high- and low-invasion
clones derived for a human tongue squamous-cell carcinoma cell line
SAS. J Cancer Res Clin Oncol. 122:243–248. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takahashi K, Kanazawa H, Akiyama Y, Tazaki
S, Takahara M, Muto T, Tanzawa H, Sato KI, Akiyama T, MUTO T, et
al: Establishment and characterization of a cell line (SAS) from
poorly differentiated human squamous cell carcinoma of the tongue.
J Jpn Stomatol Soc. 38:20–28. 1989.
|
|
50
|
Yin J and Yu FS: LL-37 via EGFR
transactivation to promote high glucose-attenuated epithelial wound
healing in organ-cultured corneas. Invest Ophthalmol Vis Sci.
51:1891–1897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ribeiro FA, Noguti J, Oshima CT and
Ribeiro DA: Effective targeting of the epidermal growth factor
receptor (EGFR) for treating oral cancer: A promising approach.
Anticancer Res. 34:1547–1552. 2014.PubMed/NCBI
|
|
52
|
Cheng M, Ho S, Yoo JH, Tran DH, Bakirtzi
K, Su B, Tran DH, Kubota Y, Ichikawa R and Koon HW: Cathelicidin
suppresses colon cancer development by inhibition of cancer
associated fibroblasts. Clin Exp Gastroenterol. 8:13–29.
2014.PubMed/NCBI
|
|
53
|
Carretero M, Escámez MJ, García M, Duarte
B, Holguín A, Retamosa L, Jorcano JL, Río MD and Larcher F: In
vitro and in vivo wound healing-promoting activities of human
cathelicidin LL-37. J Invest Dermatol. 128:223–236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Braff MH, Di Nardo A and Gallo RL:
Keratinocytes store the antimicrobial peptide cathelicidin in
lamellar bodies. J Invest Dermatol. 124:394–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mader JS, Mookherjee N, Hancock REW and
Bleackley RC: The human host defense peptide LL-37 induces
apoptosis in a calpain- and apoptosis-inducing factor-dependent
manner involving Bax activity. Mol Cancer Res. 7:689–702. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Açil Y, Torz K, Gülses A, Wieker H, Gerle
M, Purcz N, Will OM, Eduard Meyer J and Wiltfang J: An experimental
study on antitumoral effects of KI-21-3, a synthetic fragment of
antimicrobial peptide LL-37, on oral squamous cell carcinoma. J
Craniomaxillofac Surg. 46:1586–1592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sakai E and Tsuchida N: Most human
squamous cell carcinomas in the oral cavity contain mutated p53
tumor-suppressor genes. Oncogene. 7:927–933. 1992.PubMed/NCBI
|
|
59
|
Min BM, Baek JH, Shin KH, Gujuluva CN,
Cherrick HM and Park NH: Inactivation of the p53 gene by either
mutation or HPV infection is extremely frequent in human oral
squamous cell carcinoma cell lines. Eur J Cancer B Oral Oncol.
30B:338–345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kanata H, Yane K, Ota I, Miyahara H,
Matsunaga T, Takahashi A, Ohnishi K, Ohnishi T and Hosoi H: CDDP
induces p53-dependent apoptosis in tongue cancer cells. Int J
Oncol. 17:513–517. 2000.PubMed/NCBI
|
|
61
|
Ohnishi K, Ota I, Takahashi A and Ohnishi
T: Glycerol restores p53-dependent radiosensitivity of human head
and neck cancer cells bearing mutant p53. Br J Cancer.
83:1735–1739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ratushny V, Astsaturov I, Burtness BA,
Golemis EA and Silverman JS: Targeting EGFR resistance networks in
head and neck cancer. Cell Signal. 21:1255–1268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nijnik A, Pistolic J, Filewod NCJ and
Hancock REW: Signaling pathways mediating chemokine induction in
keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun.
4:377–386. 2012. View Article : Google Scholar : PubMed/NCBI
|