Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
seventh most common cancer in the world, with ~600,000 cases each
year. The 5-year survival rate is generally <50% (1,2). HNSCC
is divided into two types, human papilloma virus (HPV)-positive and
HPV-negative, which is dependent on whether HNSCC is caused by HPV
(3). Due to the special anatomical
location of HNSCC, radiotherapy is one of the most important
treatment for patients with advanced HNSCC (4,5).
Unfortunately, ~50% of patients with HNSCC are insensitive to
radiation therapy and therefore do not survive, suggesting that
HNSCC is resistant to radiation. HPV-negative HNSCC is more
radioresistant than HPV-positive (6,7).
However, the cause of radioresistance in HPV-negative HNSCC remains
unclear. Therefore, it is critical to investigate the mechanisms of
radioresistance in HPV-negative HNSCC in order to improve the
survival rates of patients.
The present findings indicated that a potential
cause of radioresistance in patients with HPV-negative HNSCC is M2
macrophages. With the renaissance of tumor immunotherapy in recent
years, the immune system is considered to be a major regulator of
the tumor microenvironment (TME) in HNSCC (8) Numerous researchers have proposed the
important role of TME macrophages in radiotherapy (9,10).
Tumor-associated macrophages (TAMs) are a key component of TME and
play an important role in accelerating cancer progression (11). TAMs are divided into M1 type with
antitumor activity and M2 type with tumor invasion and metastasis
activity (12). Moreover, a number
of studies have suggested that the high density of M2 macrophages
in tumors is significantly associated with poor prognosis in
patients with HNSCC (13–16). It was reported that the low ratio of
M1 to M2 macrophages was associated with radiosensitivity of
HPV-positive HNSCC (17); however,
its mechanism of action remains unclear. Some researchers have
demonstrated that macrophages can enhance the DNA damage response
of nearby cells by releasing human heparin-binding epidermal growth
factor (HB-EGF) (18). However, it
is unclear which macrophages are the main source of HB-EGF. In the
present study, potential targets related to radioresistance were
screened in HNSCC clinical samples from The Cancer Genome Atlas
(TCGA) database and experimental procedures. It was hypothesized
that M2 macrophages can enhance DNA damage repair via the
HB-EGF-mediated epidermal growth factor receptor (EGFR) pathway.
These findings may reveal a novel immune cell-mediated system that
could contribute to increase the resistance of HNSCC cells to DNA
damage at the tissue level.
Materials and methods
Cell culture and co-culture
An HPV-positive cell line (SCC090) was purchased
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. An HPV-negative cell line (CAL27) was provided
by Professor Fu (Harbin Medical University). The human monocytic
cell line (THP1) was obtained from the authors' stock at Harbin
Medical University. SCC090 cells were maintained in 89% McCoy's 5A
(Gibco; Thermo Fisher Scientific, Inc.) and THP1 cells were
maintained in 89% RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.), which was supplemented with 9% fetal bovine serum (CellMax)
and 1% penicillin in a 37°C atmosphere containing 5%
CO2.
THP1 cells were cultured in 0.4-µm-pore-size
Transwells (Corning Inc.), in which 1.5×105 cells were
seeded and cultured in each chamber containing 100 ng/ml PMA (cat.
no. 16561-29-8; Sigma-Aldrich; Merck KGaA) for 24 h at 37°C. Then,
100 ng/ml lipopolysaccharides (cat. no. L8880; Solarbio Life
Sciences) and 20 ng/ml interferon-γ (cat. no. 300-02-20UG;
PeproTech, Inc.) or 20 ng/ml interleukin (IL)-4 (cat. no.
96-200-04-5; PeproTech, Inc.) and 20 ng/ml IL-13 (cat. no.
96-200-13-2; PeproTech, Inc.) were added to induce the polarization
of THP1 cells to M1 or M2, respectively. After a 48 h-polarizing
process of THP1, 2×105 CAL27 cells were seeded and
co-cultured in each basal chamber for 24 h, followed by 20 ng/ml
HB-EGF (cat. no. 259-HE-050; R&D Systems, Inc.) added in the
basal chamber under M1 cells and 3 µg/ml anti-HB-EGF (cat. no.
AF-259-NA; R&D Systems, Inc.) under M2 cells.
Sample collection
The clinical tissue samples used in this study were
obtained from the Tumor Hospital of Harbin Medical University
(Harbin, China). A total of 52 cases (39 males and 13 females; mean
age, 59 years) of histopathological diagnosis were collected from
June 2016 to November 2018. Matched tumor and normal tissue
specimens were obtained from the 52 patients undergoing surgery for
HNSCC. Specimens at the time of surgery were obtained in accordance
with the World Medical Association Declaration of Helsinki ethical
guidelines and patients provided written informed consent. Ethical
approval was granted by the Ethics Review Committee of Harbin
Medical University. All collected specimens were partially embedded
in paraffin, partially frozen by tissue embedding agent (Tissue
Freezing Medium; Sakura Finetek Japan Co., Ltd.), and the remaining
was kept in liquid nitrogen for subsequent detection.
In situ hybridization (ISH)
HPV DNA-based ISH was performed using the high risk
-HPV 50 probe (Tianjin Institute of Industrial Biotechnology),
recognizing high risk-HPV types 16 and 18, in accordance with the
manufacturer's instructions. The slides were examined by a
microscope with a digital analysis system, including a BX53 Upright
microscope (Olympus Corporation) connected to an Olympus DP74
camera (Olympus Corporation) that was linked to a computer with the
image capture software.
RNA scope
The cases of p16 IHC and high risk-HPV DNA
ISH-positive were further tested by ISH for HPV E6/E7 mRNA, which
was performed by hand using the RNAscope 2.5 HD RNAscope (Advanced
Cell Diagnostics) in accordance with the manufacturer's
instructions.
Irradiation
The fresh clinical tissue was quickly cleaned of
blood stains with PBS under sterile conditions. Then, the tissue
was cut into two parts, the control group (0 Gy) and radiation
group (6 Gy), which were marked. The two groups were immediately
transferred and cultured in 10% RPMI-1640 medium, following which
the radiation group (6 Gy) received irradiation (2 Gy for 3 min) in
the lab using the Rad Source RS 2000 small animal irradiator (Rad
Source Technologies). The optimal dose of X-ray irradiation was 6
Gy, based on preliminary experiments that we performed as well as
literature research. Then, the specimens were cultured at 37°C, in
a humidified incubator for 24 h. After 24 h, the tissues were
rinsed with fresh PBS, dried with filter paper and rapidly frozen
in Optimal Cutting Temperature compound (Sakura Finetek Japan Co.,
Ltd.) with liquid nitrogen. The tissues were cut into slices with a
thickness of 3–5 µm and stored at −80°C in sealed slide boxes.
Immunofluorescence staining
Cells and frozen slices were first exposed to
radiation, then they were soaked in 4% paraformaldehyde for 15 min
at room temperature, in 0.5% Triton X-100 for 5 min, and blocked in
5% BSA (Beijing Solarbio Science & Technology Co., Ltd.) for 30
min. Samples were incubated with the γ H2A histone family member X
(H2AX) mouse monoclonal antibody (product code ab26350; Abcam) at
1:500 dilution, primary rabbit polyclonal antibodies against RAD51
recombinase (RAD51; product code ab133534; Abcam) at 1:200, and
phosphorylated (p)-DNA-dependent protein kinase (DNA-PK; phosphor
S2056; product code ab124918; Abcam) at 1:200 and EGFR inhibitor
gefitinib (ZD1839; cat. no. HY-50895; MedChemExpress) overnight at
4°C. After samples were washed three times with PBS (Shanghai
Bio-Tech Co., Ltd.), they were incubated for 1 h at room
temperature with the following secondary antibodies (dilution
1:200): Goat anti-mouse IgG (cat. no. BA1126; Wuhan Boster
Biological Technology, Ltd.) and Alexa Fuor 488 goat anti-rabbit
IgG (H+L) (cat. no. A0423; Beyotime Biotechnology). Samples were
then counterstained with DAPI (Beijing Solarbio Science &
Technology Co., Ltd.) to observe the nuclei. The area where the
green fluorescence emitted by the γ-H2AX foci overlapped with the
blue-emitting DAPI-stained nucleus was considered to be a
radiosensitivity predictor of HNSCC. The proportion of the γ-H2AX
epidemic area to the blue-fluorescent DAPI-stained nuclei was used
as the y-axis, and the t-test method was used to calculate the
statistical difference. The control group was untreated HNSCC
tissue. The green fluorescent γ-H2AX overlapping with the
blue-emitting DAPI-stained nuclei was used as the baseline number
of foci per nucleus. The results were visualized by an Olympus BX53
Upright light microscope using a magnification of ×400. γ-H2AX,
RAD51 and p-DNA-PK foci were counted using ImageJ v1.8.0 software
(National Institutes of Health). A total of three fields were
analyzed for all analyses of immunostaining.
Hematoxylin and eosin (H&E)
staining and immunohistochemistry (IHC)
Tumor and normal tissues were fixed in formalin for
2 h at room temperature. Then, they were processed and embedded in
paraffin, and 3 µm-thick sections were cut and baked at 60°C for 30
min. Sections were deparaffinized in three changes of xylene
(Tianjin FUYU Fine chemicals Co., Ltd.) for 5 min each and were
then rehydrated using graded concentrations of alcohol. Sections
were stained with H&E (Beijing Solarbio Science &
Technology Co., Ltd.) for 2 min at room temperature and then sealed
with neutral gelatin (item no. G8590; Solarbio Life Sciences).
The expression of p16 (cat. no. TA500036; OriGene
Technologies, Inc.), inducible nitric oxide synthase (iNOS; 1:100;
product code ab53769; Abcam), CD163 (1:100; cat. no. ab87099;
Abcam) and EGFR (1:100; product code ab52894; Abcam) in tumor and
normal tissues was determined by IHC analysis. Antigen retrieval
was performed using a pressure cooker with 10 mM citrate buffer (pH
6.0; Beijing Solarbio Science & Technology Co., Ltd.), and
endogenous peroxidase activity was blocked for 30 min at room
temperature by incubation in 3% hydrogen peroxide solution
(Guangzhou Jiangshun Chemical Technology Co., Ltd.). The slides
were incubated with antibodies against p16, iNOS, CD163 and EGFR
overnight at 4°C. The slides were washed with buffer and were
incubated with goat anti-mouse/rabbit IgG (cat. no. TA130015;
OriGene Technologies, Inc.) for 60 min at room temperature. ImageJ
v1.8.0 software was used to analyze digital images. A total of
three fields were analyzed for all analyses of IHC.
Immunofluorescence staining
detection
Extracted proteins from HPV-positive and
HPV-negative tumor tissues were used for analysis. Conditioned
medium was aliquoted and stored frozen (−80°C) for subsequent
analysis. A total of four cytokines [for IL-10, tumor necrosis
factor (TNF)-α, HB-EGF and EGF] were detected by bead-based
immunoassays with multi-cytokine detection kit (Human Magnetic
Luminex Performance Assay Base Kit; cat. no. LHSCM000; R&D
Systems, Inc.) following the manufacturer's guidelines.
Western blotting
Cell lysates were obtained 24 h after irradiation
with RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) and protease and phosphatase inhibitors (PMSF; cat.
no. 90100; Solarbio Life Sciences). Protein concentrations were
determined by a BCA kit (Beyotime Institute of Biotechnology).
Equal proteins (10 mg) were subjected to electrophoresis at 180 V
via 10% SDS-PAGE and were electrotransferred onto polyvinylidene
fluoride membranes (Bio-Rad Laboratories, Inc.) at 4°C and 20 V for
3 h. Membranes were blocked with 5% BSA overnight at 4°C.
Subsequently, the membranes were incubated with the following
primary antibodies: EGFR (1:2,000; product code ab245362; Abcam);
p-EGFR (Tyr1173; 1:2,000; cat. no. 44-794G; Thermo Fisher
Scientific, Inc.); and β-actin (1:2,000; cat. no. TA-09; ZSGB-BIO;
OriGene Technologies, Inc.) at 4°C overnight. Membranes were rinsed
in Tris-buffered saline (Beijing Solarbio Science & Technology
Co., Ltd.) with 0.1% Tween-20 (Beijing Solarbio Science &
Technology Co., Ltd.), followed by incubation with anti-rabbit or
anti-mouse IgG antibody (1:5,000; cat. no. BF03008; BOAOLONG) at
room temperature for 1 h. Protein bands were visualized using the
BeyoECL Plus kit (cat. no. P0018S; Beyotime Institute of
Biotechnology. Finally, the membranes were exposed to a gel imaging
system ChemiDox XRS+System (ECL developer; cat. no. 1708265;
Bio-Rad Laboratories, Inc.). Gray values of protein blots were
calculated using ImageJ v1.8.0 software. β-actin served as the
internal control.
TCGA data analysis
RNA-HTSeq-Counts data on 518 HNSCC tissue samples
and the corresponding clinical information were downloaded from
TCGA (http://www.tcga.org/). The data with
total RNA of which expression were detectable in >90% samples,
was as described by GENCODE (Release 28). A total of 98 patients
that were HPV-positive and 420 patients that were HPV-negative were
divided by the HPV status according to the clinical information of
the HNSCC project from TCGA. The expression of HB-EGF and EGF in 98
HPV-positive and 420 HPV-negative patients was compared using
Student's t-test.
Statistical analysis
Data are expressed as the means of at least three
independent experiments. P-values were analyzed using Student's
t-test with a two-tailed method. For comparisons between multiple
groups, the P-value analysis used ANOVA with Tukey's multiple
comparisons test. Pearson's rank test was used for correlation
assessments. P<0.05 was considered to indicate a statistically
significant difference. GraphPad Prism 7 software (GraphPad
Software, Inc.) was used to analyze data.
Results
Clinical information
Surgical treatment information was collected from 52
patients with HNSCC. Statistical analysis revealed that patients
with HNSCC that were HPV-positive were mostly male and HPV
infection was related to smoking and alcohol consumption (Table I).
 | Table I.Demographic and clinical
characteristics in head neck cancer. |
Table I.
Demographic and clinical
characteristics in head neck cancer.
|
Characteristics | Total no. | HPV+
n=19 | HPV−
n=33 | P-value |
|---|
| Sex |
|
|
| 0.937 |
|
Male | 39 | 15 | 24 |
|
|
Female | 13 | 4 | 9 |
|
| Age |
|
|
| 0.770 |
|
<50 | 5 | 1 | 4 |
|
|
>50 | 47 | 28 | 19 |
|
| Smoking |
|
|
| 0.007 |
|
Never | 15 | 3 | 12 |
|
| <20
years | 5 | 5 | 0 |
|
| >20
years | 32 | 7 | 25 |
|
| Alcohol |
|
|
| 0.006 |
|
Never | 36 | 11 | 27 |
|
| <20
years | 2 | 1 | 1 |
|
| >20
years | 14 | 8 | 6 |
|
| Node status |
|
|
| 0.770 |
|
N0-N1 | 50 | 13 | 37 |
|
|
N2-N3 | 2 | 1 | 1 |
|
| Differentation |
|
|
| 0.390 |
|
Well | 21 | 10 | 11 |
|
|
Moderate | 18 | 7 | 11 |
|
|
Poor | 13 | 1 | 12 |
|
The histomorphology of tumor and normal tissues was
observed by H&E staining (Fig.
1A). HPV infection was detected by p16 IHC, HPV DNA ISH and
RNAscope (Fig. 1B). All 52
specimens, including 20 HPV-positive and 32 HPV-negative, p16 IHC
and HPV DNA ISH results were consistent. Then, one additional
HPV-positive case was found (no=20) through RNAscope. Finally, 19
HPV-positive cases and 33 HPV-negative cases were defined. The
information of patients was collected in Table I.
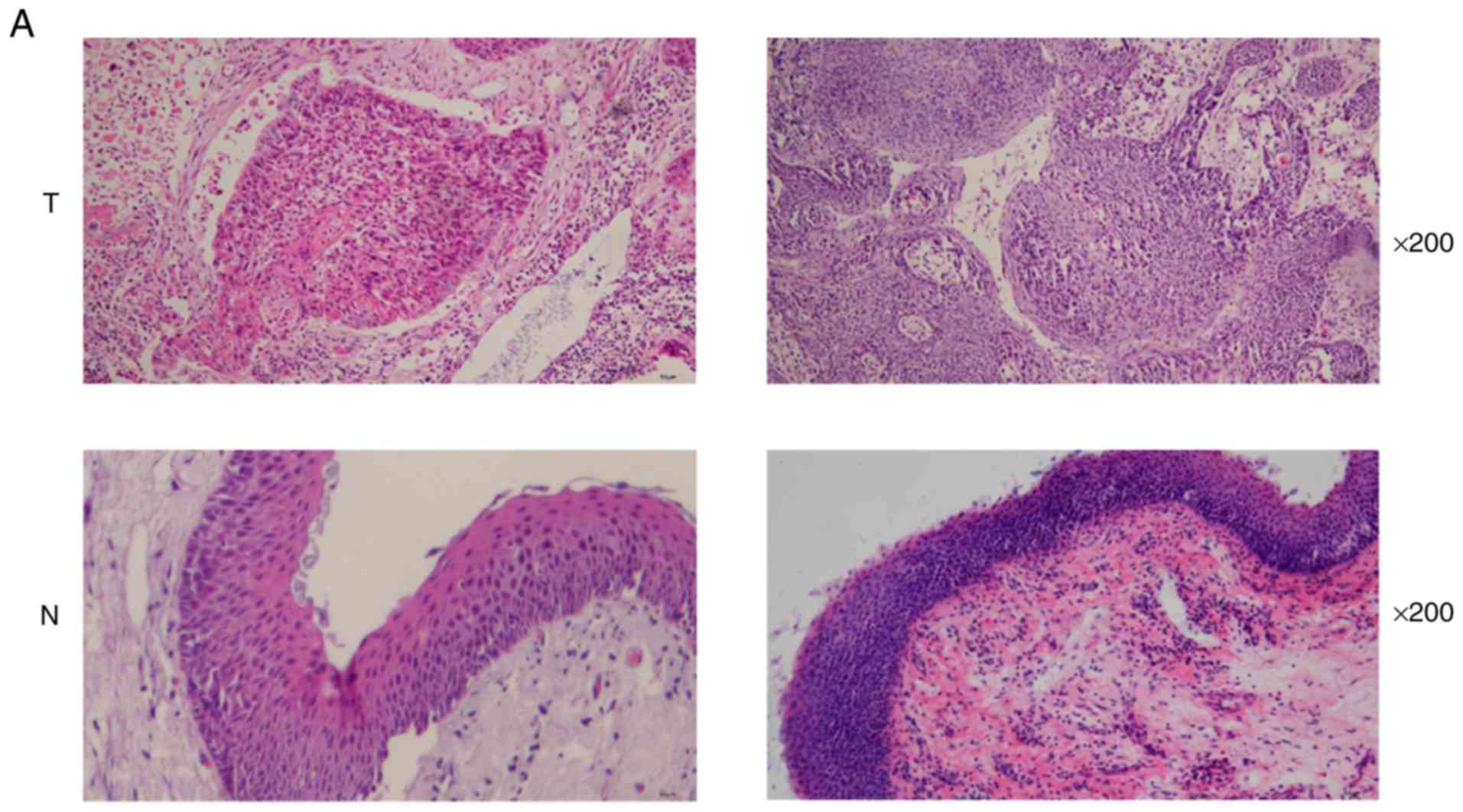 | Figure 1.Detection of HPV infection and
radiosensitivity in HNSCC tissues. (A) Representative images of
H&E staining using magnification, ×200 (T, HNSCC tissues; N,
head and neck normal tissues). (B) Representative images of
negative and positive HPV-16/18 detected by DNA ISH. Negative and
positive HPV16 RNA ISH detected by RNAscope. Negative and positive
p16 detected by p16 IHC using magnification, ×200 (HPV+,
HPV-positive tumor tissues; HPV− T, HPV-negative tumor
tissues; HPV+/HPV− N, head and neck normal
tissues; P, positive control-cervical cancer tissues; N, negative
control-glioma tissues). (C) Representative images of the γ-H2AX
staining of frozen HNSCC sections used to evaluate the extent of
radiosensitivity; magnification, ×200 (green, γ-H2AX staining;
blue, DAPI). (D) Number of the γ-H2AX foci per cell; HPV-positive
cases (n=19), HPV-negative cases (n=33). Results are presented as
the mean ± SD. **P<0.01. HPV, human papilloma virus; HNSCC, head
and neck squamous cell carcinoma; H&E, hematoxylin and eosin;
ISH, in situ hybridization; IHC, immunohistochemistry;
γ-H2AX, γ H2A histone family member X. |
The γ-H2AX foci were regarded as a predictor of
radiosensitivity in HNSCC (Fig.
1C). There were no significant differences in the number of
γ-H2AX foci in HPV-positive and HPV-negative tumor tissues that
were treated by 0 Gy. However, when the tumor tissues were treated
by 6 Gy X-ray, the number of γ-H2AX foci in HPV-positive tumor
tissues was significantly higher than the HPV-negative ones
(P=0.0048; Fig. 1D). In summary,
the results indicated that HPV-positive HNSCC tissues exhibited
higher radiosensitivity.
M2 macrophages are highly infiltrated
in HPV-negative HNSCC and released HB-EGF
To investigate whether the infiltration of M1 and M2
macrophages varies in different types of HNSCC, IHC was performed
with antibodies against iNOS (a marker of M1 macrophages) and
against CD163 (a marker of M2 macrophages; Fig. 2A). The results demonstrated that M1
infiltration in HPV-positive tumor tissues was higher compared with
HPV-negative tissues (P=0.0024; Fig.
2B); M2 infiltration was higher in HPV-positive tumor tissues
compared with HPV-negative tumor tissues (P=0.0045; Fig. 2C).
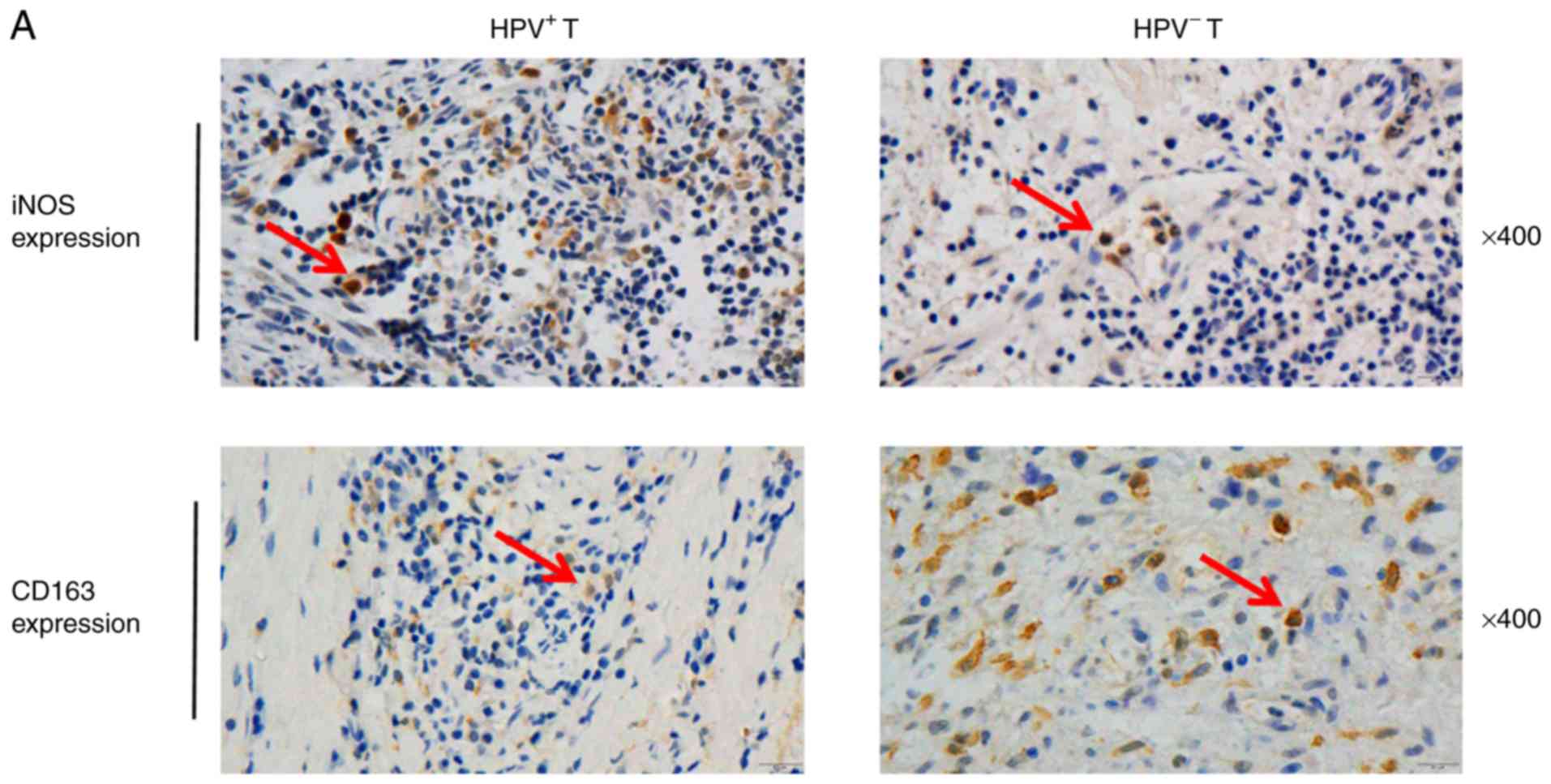 | Figure 2.Infiltration of M2 macrophages is
high in HPV-negative HNSCC and M2 macrophages release HB-EGF. (A)
IHC staining of HNSCC tumor and normal tissues using anti-iNOS and
anti-CD163 antibodies; magnification, ×400 (HPV+ T,
HPV-positive tumor tissues; HPV− T, HPV-negative tumor
tissues). Arrows indicate dot-like hybridization signals in tumor
cell nuclei. (B and C) iNOS and CD163 IHC score. HPV-positive cases
(n=19), HPV-negative cases (n=33). **P<0.01, ***P<0.001. (D)
Expression profiles of HB-EGF and EGF from 9 HNSCC clinical samples
were assessed by multiple-cytokine detection. *P<0.05. (E) Box
plot showing differences in the expression of HB-EGF and EGF in
HPV-positive (n=98) and HPV-negative (n=420) HNSCC in the TCGA
database. ***P<0.001. Infiltration of M2 macrophages is high in
HPV-negative HNSCC and M2 macrophages release HB-EGF. (F)
Expression profiles of TNF-α and IL-10 from 12 HNSCC clinical
samples were detected by the multiple-cytokine detection method.
(G) Expression profiles of HB-EGF and EGF from the supernatants of
M1 and M2 were detected by the multiple-cytokine detection method.
Results are presented as the mean ± SD. *P<0.05, **P<0.01.
HPV, human papilloma virus; HNSCC, head and neck squamous cell
carcinoma; HB-EGF, heparin-binding epidermal growth factor; iNOS,
inducible nitric oxide synthase; EGF, epidermal growth factor;
TCGA, The Cancer Genome Atlas; TNF, tumor necrosis factor; IL,
interleukin; IHC, immunohistochemistry. |
The expression of HB-EGF and EGF was measured in
HPV-positive and HPV-negative tumor tissues. The results revealed
that the HB-EGF expression was higher than EGF in both HPV-positive
and HPV-negative tumor tissues. In addition, the expression of
HB-EGF in HPV-negative HNSCC tissues was higher than HPV-positive
HNSCC tissues (P=0.0332; Fig. 2D).
To further evaluate the expression of HB-EGF and EGF in
HPV-positive and HPV-negative HNSCC tissues at the transcriptome
level, the data of HPV-positive patients (n=98) and HPV-negative
HNSCC patients (n=420) was analyzed from the TCGA database. As
anticipated, HB-EGF and EGF were upregulated in HPV-negative HNSCC
and downregulated in HPV-positive HNSCC (P<0.001). Moreover, the
expression of HB-EGF was higher than EGF in both HPV-positive and
HPV-negative HNSCC (Fig. 2E).
Next, the expression of TNF-α and IL-10 was
detected, causing the macrophages to polarize into the M1 or M2
type, respectively, in HPV-positive and HPV-negative HNSCC tissues.
The expression of TNF-α was significantly higher in HPV-positive
tumor tissues than that in HPV-negative tumor tissues (P=0.0185;
Fig. 2F), but the expression of
IL-10 was lower in HPV-positive tumor tissues (P=0.0402; Fig. 2F). Then, the supernatant was
collected from cultured M1 and M2 macrophages and the expression of
HB-EGF and EGF was assessed. In the supernatant from both types of
macrophages, there was a higher expression level of HB-EGF than
EGF. Notably, M2 macrophages secreted significantly higher levels
of HB-EGF than M1 macrophages (P=0.007; Fig. 2G).
Based on these results, the infiltration of M2
macrophages was higher in HPV-negative HNSCC tissues. Additionally,
M2 macrophages were the main source of HB-EGF.
M2 macrophages reduce the
radiosensitivity of HPV-negative HNSCC by releasing HB-EGF
The HB-EGF expression against the γ-H2AX foci within
24 h after 6 Gy irradiation is presented in Fig. 3A, indicating a significant negative
correlation between the expression of HB-EGF and γ-H2AX foci in
HNSCC tissues (r=0.-8131).
 | Figure 3.M2 macrophages reduce the
radiosensitivity of HPV-negative HNSCC by releasing HB-EGF. (A)
Correlation analyses between the fraction of the γ-H2AX foci and
the expression of HB-EGF. (B) Representative images of fluorescent
staining. Immunofluorescence analyses revealing the expression of
γ-H2AX foci in CAL27 and SCC90 cells with or without HB-EGF (20
ng/ml) 5 h after 4 Gy radiation; magnification, ×200 (green, γ-H2AX
staining; blue, DAPI). (C) Fraction of CAL27 and SCC90 cells with
the residual γ-H2AX foci (%) with or without HB-EGF (50 ng/ml).
**P<0.01. (D) Immunofluorescence analyses of the γ-H2AX foci of
M2 macrophages co-cultured with CAL27 or SCC90 and anti-HB-EGF
antibody (3 µg/ml), and M1 macrophages co-cultured with CAL27 or
SCC90 and HB-EGF (20 ng/ml); magnification ×200 (green, γ-H2AX
staining; blue, DAPI). M2 macrophages reduce the radiosensitivity
of HPV-negative HNSCC by releasing HB-EGF. (E) Number of the γ-H2AX
foci of M1 and M2 macrophages co-cultured with CAL27. (F) Number of
the γ-H2AX foci of M2 macrophages co-cultured with CAL27 cells with
anti-HBEGF-neutralizing antibody. (G) Number of the γ-H2AX foci of
M1 macrophages co-cultured with CAL27 cells with HB-EGF. Results
are presented as mean ± SD. *P<0.05. HPV, human papilloma virus;
HNSCC, head and neck squamous cell carcinoma; HB-EGF,
heparin-binding epidermal growth factor; γ-H2AX, γ H2A histone
family member X. |
To determine the role of HB-EGF in radiation
responses, the radiosensitivity of the SCC90 cell line
(HPV-positive) and the CAL27 cell line (HPV-negative) was detected
with or without HB-EGF (Fig. 3B).
In the SCC90 cell line, the radiation sensitivity was reduced when
HB-EGF was added (P=0.0011), and the results of CAL27 were
consistent (P=0.0037; Fig. 3C).
To establish the M2
macrophage-HB-EGF-radiosensitivity model, CAL27 cells co-cultured
with polarized macrophages for 24 h were subjected to a
radiosensitivity assay (Fig. 3D).
The CAL27 cells co-cultured with M1 macrophages exhibited higher
radiosensitivity compared with CAL27 cells co-cultured with M2
macrophages (P=0.0012; Fig. 3E).
Notably, M2 macrophages co-cultured with CAL27 cells exhibited
higher sensitivity to radiation therapy when the
anti-HBEGF-neutralizing antibody was added (P=0.0127; Fig. 3F). The CAL27 cells co-cultured with
M1 macrophages exhibited a significant decrease in radiosensitivity
after treatment with HB-EGF (P=0.0155; Fig. 3G). These results supported the model
in which M2 macrophages decreased radiosensitivity in HPV-negative
HNSCC by secreting HB-EGF.
Inhibition of EGFR promotes the
non-homologous end- joining (NHEJ) repair pathway
To further determine the downstream pathway by which
HB-EGF decreased radiosensitivity, the phosphorylation of EGFR was
detected in CAL27 cells and CAL27 cells treated with HB-EGF.
Western blotting experiments using phosphospecific antibodies to
recognize the activated EGFR revealed that when only HB-EGF was
added, the p-EGFR level was significantly increased in CAL27 cells.
However, under 4 Gy irradiation, p-EGFR expression was increased in
the HB-EGF group compared with the non-HB-EGF group (EGFR,
P=0.0049; p-EGFR, P=0.0044; Fig. 4A and
B, respectively).
 | Figure 4.In CAL27 cells, inhibition of EGFR by
small molecule inhibitor results in reduced NHEJ repair capacity.
(A) CAL27 and CAL27 with HB-EGF (50 ng/ml) were assessed by the
western blot analysis with anti-EGFR and anti-p-EGFR antibodies.
(B) EGFR and EGFR phosphorylation expression in CAL27 cells with or
without HB-EGF after 4 Gy radiation. NC, CAL27 cells. (C)
Representative images of the number of the p-DNA-PK foci per cell
in 5 h after irradiation (4 Gy) in CAL27 cells with or without the
EGFR inhibitor; magnification, ×200 (green, p-DNA-PK staining;
blue, DAPI). (D) Representative images of the number of the RAD51
foci per cell in 5 h after irradiation (4 Gy) in CAL27 cells with
or without the EGFR inhibitor; magnification ×200 (green, p-DNA-PK
staining; blue, DAPI). *P<0.05. In CAL27 cells, inhibition of
EGFR by small molecule inhibitor results in reduced NHEJ repair
capacity. (E-a) Number of p-DNA-PK foci of NHEJ specific markers in
CAL27 cells with or without EGFR inhibitors; (E-b) Number of RAD51
foci of HR specific markers in CAL27 cells with or without EGFR
inhibitors. (F) IHC analyses of EGFR in cancer and normal tissues;
magnification, ×400 (HPV+ T, HPV-positive tumor tissues;
HPV-T, HPV-negative tumor tissues; HPV+/HPV−
N, head and neck normal tissues). (G) EGFR IHC score. HPV-positive
cancer tissues (n=19), HPV-negative cases (n=33). (H) Expression of
EGFR between HPV-positive (n=98) and HPV-negative (n=420) HNSCC in
the TCGA database. Results are presented as the mean ± SD.
*P<0.05. HPV, human papilloma virus; HNSCC, head and neck
squamous cell carcinoma; EGFR, epidermal growth factor receptor;
NHEJ, non-homologous end-joining; HB-EGF, heparin-binding epidermal
growth factor; p-, phosphorylated; DNA-PK, DNA-dependent protein
kinase; RAD51, RAD51 recombinase; IHC, immunohistochemistry; TCGA,
The Cancer Genome Atlas. |
To investigate which pathway of the two principal
DNA double-strand break (DSB) repair pathways [NHEJ and homologous
recombination (HR)] is the main pathway activated by the
combination of HB-EGF and EGFR, RAD51 (a specific marker for HR)
and p-DNA-PK (a specific marker for NHEJ) expression levels were
detected 5 h after CAL27 cells treated with HB-EGF and the EGFR
inhibitor were exposed to radiation (Fig. 4C and D). Immunofluorescence analysis
showed that the number of p-DNA-PK foci of CAL27 cells treated with
HB-EGF alone was significantly higher than that of the untreated
group (P=0.04531), and the number of p-DNA-PK foci was
significantly reduced after the addition of EGFR inhibitor
(P=0.04319) indicating that HB-EGF activated EGFR and then promoted
the induction of NHEJ (Fig. 4E-a).
In contrast, there was no significant difference in the number of
RAD51 lesions after the addition of HB-EGF (P=0.07562), and there
was no significant difference after the addition of EGFR inhibitor
(P=0.08866; Fig. 4E-b). These
findings indicated that NHEJ was the main pathway activated by the
interaction of HB-EGF and EGFR.
To eliminate the effect of HPV infection on EGFR
activation, the expression of EGFR was detected in HPV-positive and
HPV-negative tumors, which was then compared to normal tumors. In
both HPV-positive and HPV-negative HNSCC, the expression of EGFR
was higher in tumor tissues. Notably, the expression of EGFR was
significantly higher in HPV-negative HNSCC than HPV-positive
(P=0.0492; Fig. 4F and G). To
confirm the differences in EGFR expression, data from the TCGA
database were analyzed, which revealed the upregulation of EGFR in
HPV-negative HNSCC and downregulation in HPV-positive HNSCC
(P=0.0312; Fig. 4H).
Discussion
The present study revealed that HPV-positive HNSCC
tissues were more sensitive to radiotherapy (19). In HPV-negative HNSCC tissues, there
was increased M2 macrophage infiltration compared with HPV-positive
HNSCC tissues, and these macrophages released HB-EGF to induce
radiotherapy resistance. This was related to the interaction
between HB-EGF and EGFR, and the induction of the NHEJ pathway.
HNSCC can be divided into HPV-positive and
HPV-negative (20). In recent
years, a number of studies have revealed that patients with
HPV-positive HNSCC have increased sensitivity to radiotherapy and
chemotherapy, and they exhibited improved prognosis rates (21,22).
However, patients with HPV-negative HNSCC exhibited higher levels
of resistance to radiotherapy and chemotherapy, which causes tumor
recurrence and poor prognosis rates (23). Prevc et al (24) revealed that immunodeficient mice
with HPV-positive tumors had a 30% higher radiosensitivity level.
Arenz et al (25)
demonstrated that the increased radiosensitivity of HPV-positive
HNSCC cell lines was a result of cell cycle dysregulation and the
induction of apoptosis, however the underlying mechanism was
unclear. Since in HPV-positive cell lines such as SCC090, HPV
appears in multiple copies in a free or integrated state, and each
cell has multiple oncogenes, such as E6 and E7, it is therefore
difficult to achieve full gene knockout of the HPV virus (26). In addition, some research results
revealed that the knockout of HPV16-E7 by the CRISPR/CAS system
induced apoptosis and growth inhibition in HPV16-positive cells
(27,28). In order to avoid the effect of gene
knockout on cell changes in subsequent experiments, our experiments
did not use the HPV-positive HNSCC cell line combined with knockout
technology, but instead it used the HPV negative HNSCC cell line
binding inhibitor for mechanism research.
Recently, studies have revealed that the tumor
immune microenvironment plays an important role in the treatment of
HNSCC (29,30). Compared with HPV-negative tumors, an
increased number of immune cells infiltrate HPV-positive tumors,
among which TAMs play an important role (13). TAMs are mainly divided into M1
macrophages that secrete pro-inflammatory cytokines, such as TNF-α
or IL-1β, and M2 macrophages that secrete inhibitory cytokines,
such as IL-10 or TGF-β (31). Most
researchers have hypothesized that the infiltration of M2
macrophages leads to poor prognosis in patients with HPV-negative
HNSCC, and data from TCGA revealed that high infiltration of M2
macrophages decreased the survival rates of patients with HNSCC
(32,33). Consistent with this statement, Rades
et al (34) revealed that
radioresistance led to poor prognosis in HNSCC. In the present
study, IHC with the M1 and M2 surface markers iNOS and CD163 was
used to detect M1 and M2 in patient tissues. Higher levels of M2
macrophage infiltration was revealed in HPV-negative tissues, and
the radiosensitivity of HPV-negative HNSCC tissues was lower
compared with positive tissues. Therefore, it is proposed that the
infiltration of M2 macrophages may be related to radioresistance of
HPV-negative HNSCC.
In the present study, it was demonstrated that M2
macrophages released higher levels of HB-EGF compared with M1
macrophages. The expression of HB-EGF was significantly higher in
HPV-negative tissues. HB-EGF was first discovered in 1990, and
macrophages are the main source of HB-EGF (17). It has been reported that HB-EGF
alleviates DNA damage responses in tumor cells and enhances
radioresistance in the intestinal canal (18,35).
Previous studies have demonstrated that M2 macrophages induced the
invasion and migration of myxoid liposarcoma cells and induced the
proliferation of ovarian cancer cells by releasing HB-EGF (36–39).
The present results also revealed the negative association between
the expression of HB-EGF and radiosensitivity. HPV-positive and
-negative cell lines SCC090 and CAL27 were selected, and
cytological methods were used to co-culture M1 and M2 macrophages
with CAL27 cells, and combined HB-EGF inhibitors were used to study
whether the secretion of HB-EGF by M2 macrophages decreases the
radiation sensitivity of HNSCC cells. Consistent with previous
research, it was demonstrated that M2 macrophages played an
important role in the development of radioresistance in
HPV-negative HNSCC by releasing HB-EGF.
HB-EGF has been revealed to be the ligand with the
highest affinity for EGFR in cervical cancer, and HB-EGF could
activate EGFR phosphorylation (40). Several previous studies have
revealed that EGFR triggers a downstream signal cascade via
autophosphorylation (41–43). In the present study, western
blotting, using phosphorylation-specific antibodies to identify
activated EGFR, revealed that p-EGFR was significantly increased in
CAL27 cells when HB-EGF was added, indicating that EGFR was
activated by HB-EGF. However, under 4 Gy irradiation, p-EGFR
expression was increased in the HB-EGF group compared with the
non-HB-EGF group.
Both classical pathways for DSB repair (NHEJ and HR)
are affected by EGFR signaling. A study by Kriegs et al
(44) demonstrated that radiation
and the activation of EGFR stimulated the MAPK pathway in order to
regulate the NHEJ repair pathway, while Toulany et al
(45) demonstrated that
PI3K/Akt/mTOR signaling was activated to regulate the HR repair
pathway after EGFR was activated. The present results revealed that
the NHEJ pathway, but not the HR pathway, was stimulated after EGFR
was activated. Regarding DNA damage repair and related pathways,
only the EGFR and NHEJ pathways have been studied, and deeper
mechanisms have yet to be explored.
In summary, the present study indicated that M2
macrophages can induce radioresistance of HPV-negative HNSCC by
releasing HB-EGF to activate EGFR that can enhance the NHEJ repair
pathway. This finding further explores the causes of radiotherapy
resistance in patients with HPV-negative HNSCC, indicating that M2
macrophages and HB-EGF are possible regulatory targets for future
treatment of HNSCC, and provides new insights into HNSCC
radiotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672670), the
Heilongjiang Province Outstanding Youth Foundation (grant no.
JC2018023), the Graduate Innovation Research Project of Harbin
Medical University (grant no. YJSCX2017-8HYB) and the Medical and
Health Science and Technology Plan Project of Longgang District,
Shenzhen (LGKCYLWS2019000100).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
The study was conceived and designed by EF and LW.
JG, PY, JS provided the clinical specimens. EF, TL, XM, XC, LS, HL,
FM, SZ, JG, PY, JS and SH conducted the experiments and analyzed
data. The manuscript was written by EF, SY and TL. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval was granted by the Ethics Review
Committee of Harbin Medical University. Specimens at the time of
surgery were obtained in accordance with the World Medical
Association Declaration of Helsinki ethical guidelines and patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park S, Jang WJ and Jeong CH:
Nano-biomechanical validation of epithelial-mesenchymal transition
in oral squamous cell carcinomas. Biol Pharm Bull. 39:1488–1495.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Solomon B, Young RJ and Rischin D: Head
and neck squamous cell carcinoma: Genomics and emerging biomarkers
for immunomodulatory cancer treatments. Semin Cancer Biol.
52:228–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosca L, Pagano M, Ilisso CP, Cave DD,
Desiderio V, Mele L, Caraglia M, Cacciapuoti G and Porcelli M:
AdoMet triggers apoptosis in head and neck squamous cancer by
inducing ER stress and potentiates cell sensitivity to cisplatin. J
Cell Physiol. 234:13277–13291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ou D, Adam J, Garberis I, Blanchard P,
Nguyen F, Levy A, Casiraghi O, Gorphe P, Breuskin I, Janot F, et
al: Influence of tumor-associated macrophages and HLA class I
expression according to HPV status in head and neck cancer patients
receiving chemo/bioradiotherapy. Radiother Oncol. 130:89–96. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mirghani H, Amen F, Tao Y, Deutsch E and
Levy A: Increased radiosensitivity of HPV-positive head and neck
cancers: Molecular basis and therapeutic perspectives. Cancer Treat
Rev. 41:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian L, Yi X, Dong Z, Xu J, Liang C, Chao
Y, Wang Y, Yang K and Liu Z: Calcium bisphosphonate nanoparticles
with chelator-free radiolabeling to deplete tumor-associated
macrophages for enhanced cancer radioisotope therapy. ACS Nano.
12:11541–11551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
She L, Qin Y, Wang J, Liu C, Zhu G, Li G,
Wei M, Chen C, Liu G, Zhang D, et al: Tumor-associated macrophages
derived CCL18 promotes metastasis in squamous cell carcinoma of the
head and neck. Cancer Cell Int. 18:1202018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barikbin R, Berkhout L, Bolik J,
Schmidt-Arras D, Ernst T, Ittrich H, Adam G, Parplys A, Casar C,
Krech T, Karimi K, et al: Early heme oxygenase 1 induction delays
tumour initiation and enhances DNA damage repair in liver
macrophages of Mdr2−/− mice. Sci Rep. 8:162382018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vitale I, Manic G, Coussens LM, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petty AJ and Yang Y: Tumor-associated
macrophages: Implications in cancer immunotherapy. Immunotherapy.
9:289–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Yan B, Lou H, Shen Z, Tong F, Zhai
A, Wei L and Zhang F: Immunological network analysis in HPV
associated head and neck squamous cancer and implications for
disease prognosis. Mol Immunol. 96:28–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lau SK, Chu PG and Weiss LM: CD163: A
specific marker of macrophages in paraffin-embedded tissue samples.
Am J Clin Pathol. 122:794–801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Shintani S, Terakado N, Nakashiro K
and Hamakawa H: Infiltration of tumor-associated macrophages in
human oral squamous cell carcinoma. Oncol Rep. 9:1219–1223.
2002.PubMed/NCBI
|
|
16
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Fu E, Lou H, Mao X, Yan B, Tong F,
Sun J and Wei L: IL-6 induced M1 type macrophage polarization
increases radiosensitivity in HPV positive head and neck cancer.
Cancer Lett. 456:69–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geiger-Maor A, Guedj A, Even-Ram S, Smith
Y, Galun E and Rachmilewitz J: Macrophages regulate the systemic
response to DNA damage by a cell nonautonomous mechanism. Cancer
Res. 75:2663–2673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fulcher CD, Haigentz M Jr and Ow TJ;
Education Committee of the American Head Neck Society (AHNS), :
AHNS Series: Do you know your guidelines? Principles of treatment
for locally advanced or unresectable head and necksquamous cell
carcinoma. Head Neck. 40:676–686. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brakenhoff RH, Wagner S and Klussmann JP:
Molecular patterns and biology of HPV-associated HNSCC. Recent
Results Cancer Res. 206:37–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foy JP, Bazire L, Ortiz-Cuaran S, Deneuve
S, Kielbassa J, Thomas E, Viari A, Puisieux A, Goudot P, Bertolus
C, et al: A 13-gene expression-based radioresistance score
highlights the heterogeneity in the response to radiation therapy
across HPV-negative HNSCC molecular subtypes. BMC Med. 15:1652017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pike LRG, Hwang WL, Royce TJ, Sanford NN
and Mahal BA: HPV status predicts for improved survival following
chemotherapy in metastatic squamous cell carcinoma of the
oropharynx. Oral Oncol. 86:69–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ziemann F, Seltzsam S, Dreffke K, Preising
S, Arenz A, Subtil FSB, Rieckmann T, Engenhart-Cabillic R, Dikomey
E and Wittig A: Roscovitine strongly enhances the effect of
olaparib on radiosensitivity for HPV neg. but not for HPV pos.
HNSCC cell lines. Oncotarget. 8:105170–105183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prevc A, Kranjc S, Cemazar M, Todorovic V,
Zegura B, Novak M, Filipic M, Flezar MS, Kirbis IS, Rotter A, et
al: Dose-modifying factor of radiation therapy with concurrent
cisplatin treatment in HPV-Positive squamous cell carcinoma: A
preclinical study. Radiat Res. 189:644–651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arenz A, Ziemann F, Mayer C, Wittig A,
Dreffke K, Preising S, Wagner S, Klussmann JP, Engenhart-Cabillic R
and Wittekindt C: Increased radiosensitivity of HPV-positive head
and neck cancer cell lines due to cell cycle dysregulation and
induction of apoptosis. Strahlenther Onkol. 190:839–846. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Speel EJ: HPV integration in head and neck
squamous cell carcinomas: Cause and consequence. Recent Results
Cancer Res. 206:57–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YC, Cai ZM and Zhang XJ: Reprogrammed
CRISPR-Cas9 targeting the conservedregions of HPV6/11 E7 genes
inhibits proliferation and induces apoptosis inE7-transformed
keratinocytes. Asian J Androl. 18:475–479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu Z, Yu L, Zhu D, Ding W, Wang X, Zhang
C, Wang L, Jiang X, Shen H, He D, et al: Disruption of HPV16-E7 by
CRISPR/Cas system induces apoptosis and growth inhibition in HPV16
positive human cervical cancer cells. Biomed Res Int.
2014:6128232014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moskovitz JM, Moy J, Seiwert TY and Ferris
RL: Immunotherapy for head and neck squamous cell carcinoma: A
review of current and emerging therapeutic options. Oncologist.
22:680–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HC, Chan LP and Cho SF: Targeting the
immune microenvironment in the treatment of head and neck squamous
cell carcinoma. Front Oncol. 9:10842019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yahaya MAF, Lila MAM, Ismail S, Zainol M
and Afizan NARNM: Tumour-associated macrophages (TAMs) in colon
cancer and how to reeducate them. J Immunol Res. 2019:23682492019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Gao K, Lei W, Dong L, Xuan Q, Feng
M, Wang J, Ye X, Jin T, Zhang Z and Zhang Q: Lymphocyte-to-monocyte
ratio is associated with prognosis of diffuse large B-cell
lymphoma: Correlation with CD163 positive M2 type tumor-associated
macrophages, not PD-1 positive tumor-infiltrating lymphocytes.
Oncotarget. 8:5414–5425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alves AM, Diel LF and Lamers ML:
Macrophages and prognosis of oral squamous cell carcinoma: A
systematic review. J Oral Pathol Med. 47:460–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rades D, Seidl D, Janssen S, Strojan P,
Karner K, Bajrovic A, Hakim SG, Wollenberg B and Schild SE:
Comparing two lower-dose cisplatin programs for radio-chemotherapy
of locally advanced head-and-neck cancers. Eur Arch
Otorhinolaryngol. 274:1021–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ozbilgin MK, Aktas C, Uluer ET, Buyukuysal
MC, Gareveran MS and Kurtman C: Influence of radiation exposure
during radiotherapy. Evidence for the increase of versican and
heparin-binding EGF-like growth factor concentrations. Anal Quant
Cytopathol Histpathol. 38:126–132. 2016.PubMed/NCBI
|
|
36
|
Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM,
Yu ZL and Zhao YF: Tumor associated macrophages induce epithelial
to mesenchymal transition via the EGFR/ERK1/2 pathway in head and
neck squamous cell carcinoma. Oncol Rep. 40:2558–2572.
2018.PubMed/NCBI
|
|
37
|
Zhao G, Liu L, Peek RM Jr, Hao X, Polk DB,
Li H and Yan F: Activation of epidermal growth factor receptor in
macrophages mediates feedback inhibition of M2 polarization and
gastrointestinal tumor cell growth. J Biol Chem. 291:20462–20472.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carroll MJ, Kapur A, Felder M, Patankar MS
and Kreeger PK: M2 macrophages induce ovarian cancer cell
proliferation via a heparin binding epidermal growth factor/matrix
metalloproteinase 9 intercellular feedback loop. Oncotarget.
7:86608–86620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nabeshima A, Matsumoto Y, Fukushi J, Iura
K, Matsunobu T, Endo M, Fujiwara T, Iida K, Fujiwara Y, Hatano M,
et al: Tumour-associated macrophages correlate with poor prognosis
in myxoid liposarcoma and promote cell motility and invasion via
the HB-EGF-EGFR-PI3K/Akt pathways. Br J Cancer. 112:547–555. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schrevel M, Osse EM, Prins FA, Trimbos
JBMZ, Fleuren GJ, Gorter A and Jordanova ES: Autocrine expression
of the epidermal growth factor receptor ligand heparin-binding
EGF-like growth factor in cervical cancer. Int J Oncol.
50:1947–1954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Psyrri A, Seiwert TY and Jimeno A:
Molecular pathways in head and neck cancer: EGFR, PI3K, and more.
Am Soc Clin Oncol Educ Book. 246–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z: ErbB receptors and cancer. Methods
Mol Biol. 1652:3–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kriegs M, Kasten-Pisula U, Rieckmann T,
Holst K, Saker J, Dahm-Daphi J and Dikomey E: The epidermal growth
factor receptor modulates DNA double-strand break repair by
regulating non-homologous end-joining. DNA Repair (Amst).
9:889–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toulany M, Baumann M and Rodemann HP:
Stimulated PI3K-AKT signaling mediated through ligand or
radiation-induced EGFR depends indirectly, but not directly, on
constitutive K-Ras activity. Mol Cancer Res. 5:863–872. 2007.
View Article : Google Scholar : PubMed/NCBI
|


















