Introduction
Pancreatic cancer (PC) is characterized by high
malignant potential, insidious onset and typically a poor patient
prognosis (1). Due to its high rate
of metastasis, ~80% of patients cannot receive radical treatment
(1,2). Despite intensive postoperative
interventions, including drug treatment and other methods, the
5-year survival rate is ≤7%, and PC has been identified as one of
the most serious types of malignant tumors that endanger human
health (3). Thus, understanding the
dynamics of the molecular mechanisms underlying metastasis is
crucial for designing better treatment options and improving the
outcomes of this fatal disease.
Heparanase (HPSE) is recently discovered important
functional enzyme and it is the only member of the endoglycosidase
family that can degrade the heparan sulfate (HS) chain in
glycosaminoglycans, and the only that specifically recognizes HS
side chains. As the most biologically active of the various
proteoglycans, HS participates in the multiple stages of the cell
adhesion reaction process and interacts with adhesion molecules,
cytokines and intercellular signaling molecules, thereby affecting
cell proliferation, differentiation, migration and morphology. HS
proteoglycan (HSPG) also plays an important role in various dynamic
interactions and pathways associated with inflammation and
ischemia-reperfusion injury, thrombosis and tumor metastasis
(4,5). HPSE can promote cell infiltration,
metastasis, tumor cell division, chemotaxis and microvascular
formation (6,7). HPSE is the only hydrolase found in
mammals that can cleave the HS side chain on HSPG and is thus an
ideal candidate for use as antitumor treatment. Therefore, the main
goal of the present study was to investigate the role of HPSE in
pancreatic cancer, the effects of HPSE silencing, and the
mechanisms underlying the observed dynamics of HPSE.
Materials and methods
Clinical tissue samples
Overall, PC samples and corresponding adjacent
non-cancer tissues were obtained from 6 patients (3 women and 3
men; mean age, 60.50±9.42 years; range, 48–72 years) who were
diagnosed at Anhui Provincial Hospital (Hefei, China) between June
2013 and June 2016, and the mRNA levels of HPSE were detected.
Detailed pathological and clinical data (including patient sex and
age, tumor size and location, vascular invasion, degree of
differentiation and TNM stage) from 128 patients between June 2013
and June 2016 were obtained from the medical records. Samples were
included in the present study based on the 8th edition of the Union
for International Cancer Control TNM staging system (8). Patients who had received radiotherapy
or chemotherapy prior to surgery were not included. The specimens
were fixed in 4% formalin at 37°C for 2 h and embedded in paraffin
for pathological analysis and confirmation of the diagnosis. The
clinical follow-up data of the patients were obtained from the PC
database of Anhui Provincial Hospital. The protocol of the present
study was approved by the Ethics Committee of the Anhui Provincial
Hospital (certification no. 2019-P-032) and all patients provided
written informed consent.
Cell lines and culture
The human HPDE6-C7, Capan-2, PANC-1, AsPC-1 and
BxPC-3 pancreatic cancer cell lines were obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were maintained in DMEM or RPMI-1640 (Biological Industries)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). All cells were cultured in an atmosphere of 5%
CO2 and at a temperature of 37°C. All cells were
authenticated by STR profiling before the experiment.
Stable transfection of pancreatic
cancer cell
HPSE-shRNA and control sh-NC were synthesized by
Sigma-Aldrich; Merck KGaA. For the construction of the β-catenin
vector, β-catenin cDNA was cloned into Flag-tagged-pcDNA3.1
(GenePharma). As regards the transfection of HPSE-shRNA, the
transfection of control sh-NC and β-catenin-vector in PANC-1 and
BxPC-3 cells was performed by Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in accordance with the instructions
of the manufacturer. The established stable cell lines were
verified by RT-qPCR and western blot analyses and used for the
subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from cells using
TRIzol® reagent following the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). A cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to synthesize the first-strand cDNA with the
following profile: 25°C for 10 min, 37°C for 120 min, 85°C for 5
min and held at 4°C. qPCR was performed with SYBR Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) to detect
the levels of HPSE transcription as follows: 48°C for 30 min, 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min and held at 4°C. The following primer sets were used for
analysis of the levels of HPSE expression: Forward
5′-ATGCTGCTGCGCTCGAA-3′ and reverse 5′-AGATGCAAGCAGCAACTTTGGC-3′.
For GAPDH, the primer set was as follows: Forward
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-GGGGTCGTTGATGGCAACA-3′.
The relative levels of expression were quantified using the
2−ΔΔCq method (9).
Western blot analysis
For the detection of levels of protein expression,
western blotting was performed as previously described (10). Briefly, the cells were lysed in RIPA
cell lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology, Inc.) with 1 mM phenylmethylsulfonyl fluoride at 4°C
for 30 min, with vortexing every 10 min, followed by centrifugation
at 13,800 × g for 10 min at 4°C. A BCA kit (cat. no. P0009;
Beyotime Institute of Biotechnology, Inc.) was used to quantify the
protein concentration. A total of 30 µg of denatured protein for
each sample was separated on a 10% SDS PAGE gel and transferred
onto a PVDF membrane. The membranes were blocked with 5% non-fat
milk in 0.05% Tween-20 in TBS (TBST) for 2 h at room temperature
and blotted with the following primary antibodies: HPSE (cat. no.
ab85543, 1:500, Abcam), E-cadherin (cat. no. SAB4503751, 1:500,
Sigma-Aldrich; Merck KGaA); vimentin (cat. no. SAB1305433, 1:1,000,
Sigma-Aldrich; Merck KGaA); Snail (cat. no. SAB4502825, 1:800,
Sigma-Aldrich; Merck KGaA); β-catenin (cat. no. SAB4500541, 1:800,
Sigma-Aldrich; Merck KGaA); glycogen synthase kinase (GSK)-3β (cat.
no. sc-81462, 1:1,000, Santa Cruz Biotechnology, Inc.);
phosphorylated (p-)GSK-3β (cat. no. 9336, 1:800, Cell Signaling
Technology, Inc.); and/or GAPDH (cat. no. 10494-1-AP, 1:5,000,
ProteinTech Group, Inc.). After washing with TBST, the membranes
were incubated with anti-rabbit or anti-mouse secondary antibodies,
conjugated with horseradish peroxidase (cat. nos. A0208 and A0216;
1:1,000, Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room
temperature. Following 3 washes, the membrane was visualised with
an enhanced chemiluminescence system (cat. no. P0018AM; Beyotime
Institute of Biotechnology, Inc.).
Immunohistochemistry (IHC)
IHC was performed using a standard
streptavidin-biotin-peroxidase complex-based methodology according
to the manufacturer's guidelines (SA2010; Boster Biological
Technology). The tissue sections were incubated at 4°C in a moist
chamber overnight with the addition of anti-HPSE antibody (cat. no.
ab85543, 1:200, Abcam). The expression levels of HPSE were also
quantified by determining the percentage of positive tumor cells
and the intensity of positive staining. Staining intensity was
scored as follows: Negative, 0; bordering, 1, weak, 2; moderate, 3;
and strong, 4. Additionally, staining was scored in accordance with
the percentage of stained tumor cells in the field as follows:
Negative, 0; 0–25%, 1; 26–50%, 2; 51–75%, 3; and 76–100%, 4. The
product of the intensity score and percentage of stained cells was
considered as the overall IHC score (range, 0–16). Two independent
pathologists observed and evaluated the staining process and
results.
Cell migration assay
A total of 2.0×105 cells were seeded in
24-well plates and grown to >80% confluence. Following overnight
culture of cells in 5% CO2 at 37°C, a 200-µl pipette tip
was used to create a longitudinal scratch in the middle of the
bottom of the sample well. Detached cells were washed away using
PBS and serum-free medium was added. Images were captured at 0 and
24 h after wounding to monitor healing using a light microscope
(Ti-S, Nikon Corporation) at a magnification of ×4.
Cell invasion assay
The effect of HPSE on metastatic ability of cancer
cells was evaluated by using a 24-well Transwell assay (8 µm pore
size; EMD Millipore) as previously described (10). In brief, 2.0×105 cells in
serum-free medium were added to the upper culture chambers that had
been precoated with Matrigel at 37°C. The bottoms of culture
chambers were filled with DMEM supplemented with 10% FBS by volume.
After incubation in 5% CO2 at 37°C for 48 h, staining of
the invading cells was performed using 0.5% crystal violet solution
for 10 min at room temperature and examined with a light microscope
(Ti-S, Nikon Corporation) at a magnification of ×4.
Tumor metastasis assay
A total of 100 male BALB/c nude mice (aged 6 weeks
and weighing ~18.30 g) were purchased from the Institute for
Experimental Animals of the Chinese Academy of Medical Sciences
(Beijing, China). The nude mice were maintained under pathogen-free
conditions, at 20–26°C, 40–70% humidity and a 12/12 light/dark
cycle, with access to food and water ad libitum. PANC-1
cells (2.0×106) transfected with sh-HPSE-luciferase or
sh-NC-luciferase were injected into the tail vein of mice (n=6)
after 1 month of acclimation. At 50 days after the injection, the
mice were sacrificed and their lungs were removed. Observation of
metastatic nodules in lung tissues was performed using the IVIS
Lumina II high-sensitivity imaging system (PerkinElmer, Inc.). The
duration of the experiment was 57 days and the health and behavior
of the mice were monitored daily. All mice were sacrificed by
cervical dislocation after inhaling 40% carbon dioxide. Death was
verified by cardiac arrest (monitoring was continued for 5–6 min
after breathing stopped). All animal experiments were approved by
the Ethics Committee of Anhui Provincial Hospital (certification
no. 2019-P-032).
Hematoxylin and eosin (HE)
staining
The tissue samples were cut into 4-µm sections and
mounted on silanized glass slides. Following deparaffinization and
hydration, the sections were stained with hematoxylin solution for
3 min in 35°C followed by 5 dips in 0.5% acid ethanol (1% HCl in
70% ethanol) and then rinsed in distilled water. Subsequently, the
sections were stained with eosin solution for 1 min in 35°C and
followed by dehydration with graded alcohol and clearing in xylene.
The sections were then examined under a light microscope (Ti-S,
Nikon Corporation) at a magnification of ×20.
Statistical analysis
SPSS version 19.0 (SPSS Inc.) software was used to
perform analysis of statistical data. Determination of levels of
variation and significance of differences for values among cohorts
was determined using a paired two-tailed Student's t-test and
Dunnett's t-test. Values for P<0.05 were considered to be of
statistical significance.
Results
Association of HPSE expression with PC
clinicopathological parameters and prognosis
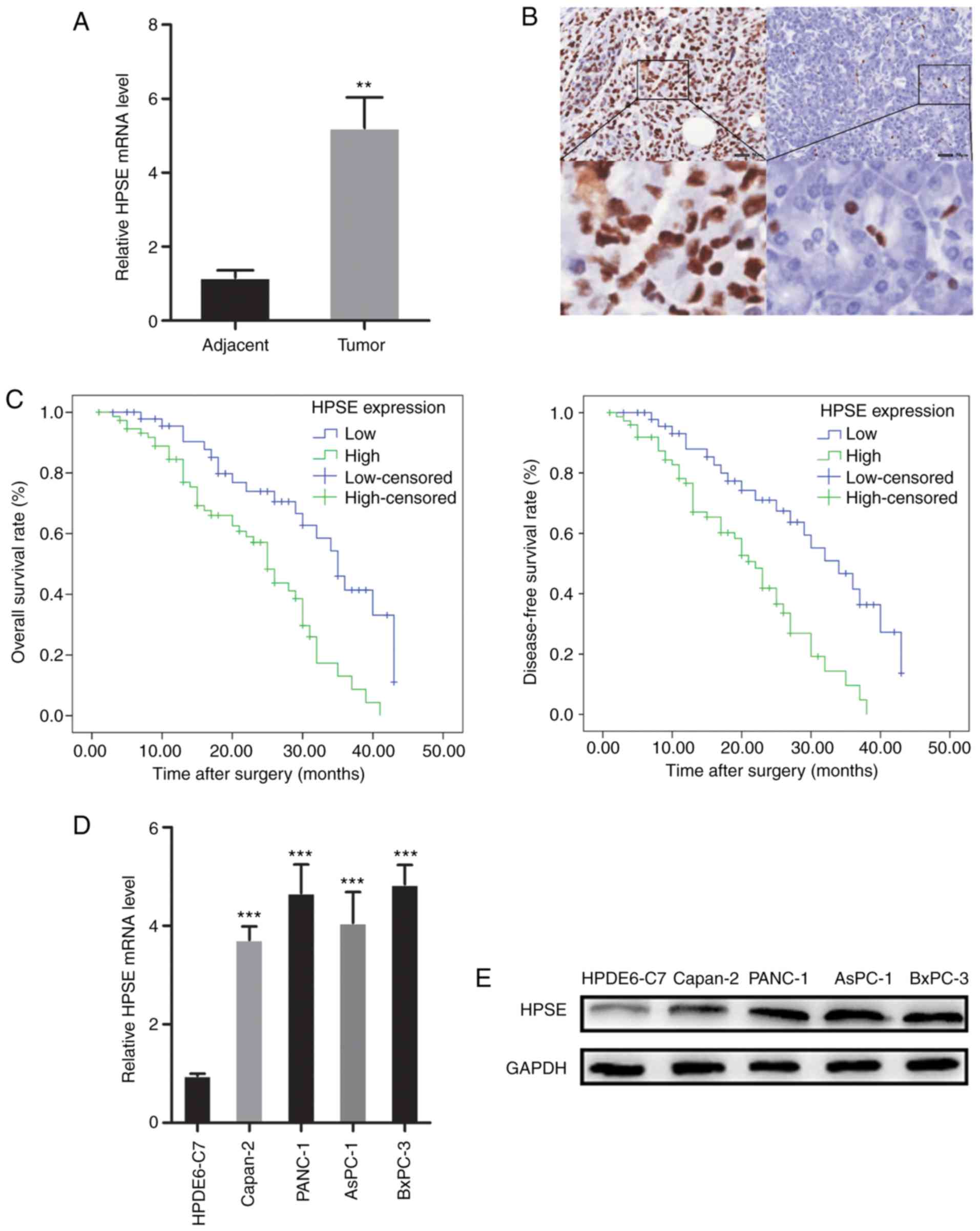
RT-qPCR analysis and IHC were employed to evaluate
the expression of HPSE in PC tissue samples. The mRNA and protein
expression of HPSE were also examined in pancreatic cancer cell
lines. High expression of HPSE was observed in PC tissues and
cancer cell lines (Fig. 1). In
order to evaluate the biological significance of HPSE in PC, the
associations between PC tissue HPSE levels and clinicopathological
parameters were analyzed. As shown in Table I, increased HPSE expression was
significantly associated with the presence of vascular invasion
(P=0.025), poor differentiation (P=0.012) and higher TNM stage
(P=0.024). Kaplan-Meier survival curves were plotted to compare the
overall survival (OS) and disease-free survival (DFS) of PC
patients according to HPSE expression (Fig. 1). Patients with high HPSE expression
had poorer prognosis compared with those with low HPSE expression
(OS, P=0.001; DFS, P=0.000). Multivariate survival analysis further
revealed that intratumoral HPSE expression (OS, P=0.009; DFS,
P=0.004) was an independent poor prognostic marker for OS and DFS
(Tables II and III).
 | Table I.Association between HPSE protein
expression (immunohistochemical staining) in pancreatic cancer and
clinicopathological variables. |
Table I.
Association between HPSE protein
expression (immunohistochemical staining) in pancreatic cancer and
clinicopathological variables.
|
|
| HPSE expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Total | Low (n=53) | High (n=75) | χ2 | P-value |
|---|
| Sex |
|
|
|
| 0.760 |
| Male | 85 | 36 | 49 | 0.093 |
|
|
Female | 43 | 17 | 26 |
|
|
| Age (years) |
|
|
|
| 0.391 |
| ≤60 | 54 | 20 | 34 | 0.735 |
|
|
>60 | 74 | 33 | 41 |
|
|
| Size (cm) |
|
|
|
| 0.373 |
| ≤4 | 104 | 45 | 59 | 0.793 |
|
|
>4 | 24 | 8 | 16 |
|
|
| Tumor location |
|
|
|
| 0.493 |
| Head and
neck | 85 | 37 | 48 | 0.470 |
|
| Body and
tail | 43 | 16 | 27 |
|
|
| Vascular
invasion |
|
|
|
| 0.025 |
|
Negative | 61 | 19 | 42 | 5.055 |
|
|
Positive | 67 | 34 | 33 |
|
|
| Differentiation |
|
|
|
| 0.012 |
|
High/moderate | 58 | 31 | 27 | 6.339 |
|
| Poor and
undifferentiated | 70 | 22 | 48 |
|
|
| TNM stage |
|
|
|
| 0.023 |
|
I–II | 62 | 32 | 30 | 5.163 |
|
|
III–IV | 66 | 21 | 45 |
|
|
 | Table II.Univariate and multivariate analysis
of the correlation between clinicopathological parameters and
overall survival of patients with pancreatic cancer. |
Table II.
Univariate and multivariate analysis
of the correlation between clinicopathological parameters and
overall survival of patients with pancreatic cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.868 | 0.525–1.432 | 0.579 |
|
|
|
|
Male |
|
|
|
|
|
|
|
Female |
|
|
|
|
|
|
| Age (years) | 0.768 | 0.468–1.260 | 0.296 |
|
|
|
|
≤60 |
|
|
|
|
|
|
|
>60 |
|
|
|
|
|
|
| Size (cm) | 1.149 | 0.633–2.088 | 0.648 |
|
|
|
| ≤4 |
|
|
|
|
|
|
|
>4 |
|
|
|
|
|
|
| Tumor location | 1.221 | 0.724–2.059 | 0.455 |
|
|
|
| Head
and neck |
|
|
|
|
|
|
| Body
and tail |
|
|
|
|
|
|
| Vascular
invasion | 1.812 | 1.109–2.962 | 0.018a |
|
|
|
|
Negative |
|
|
|
|
|
|
|
Positive |
|
|
|
|
|
|
|
Differentiation | 2.397 | 1.455–3.947 | 0.001a | 1.919 | 1.220–3.916 | 0.014a |
|
High/moderate |
|
|
|
|
|
|
| Poor
and undifferentiated |
|
|
|
|
|
|
| TNM stage | 1.671 | 1.027–2.721 | 0.039a |
|
|
|
|
I–II |
|
|
|
|
|
|
|
III–IV |
|
|
|
|
|
|
| HPSE
expression | 2.682 | 1.536–4.684 | 0.001a | 2.186 | 1.140–3.229 | 0.009a |
|
Low |
|
|
|
|
|
|
|
High |
|
|
|
|
|
|
 | Table III.Univariate and multivariate analysis
of the correlation between clinicopathological parameters and
disease-free survival of patients with pancreatic cancer. |
Table III.
Univariate and multivariate analysis
of the correlation between clinicopathological parameters and
disease-free survival of patients with pancreatic cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.961 | 0.584–1.579 | 0.874 |
|
|
|
|
Male |
|
|
|
|
|
|
|
Female |
|
|
|
|
|
|
| Age (years) | 0.762 | 0.463–1.254 | 0.286 |
|
|
|
|
≤60 |
|
|
|
|
|
|
|
>60 |
|
|
|
|
|
|
| Size (cm) | 1.177 | 0.650–2.134 | 0.590 |
|
|
|
| ≤4 |
|
|
|
|
|
|
|
>4 |
|
|
|
|
|
|
| Tumor location | 1.293 | 0.759–2.202 | 0.344 |
|
|
|
| Head
and neck |
|
|
|
|
|
|
| Body
and tail |
|
|
|
|
|
|
| Vascular
invasion | 1.685 | 1.034–2.746 | 0.036a |
|
|
|
|
Negative |
|
|
|
|
|
|
|
Positive |
|
|
|
|
|
|
|
Differentiation | 2.493 | 1.506–4.126 | 0.000a | 2.052 | 1.220–3.452 | 0.007a |
|
High/moderate |
|
|
|
|
|
|
| Poor
and undifferentiated |
|
|
|
|
|
|
| TNM stage | 1.598 | 0.983–2.597 | 0.059 |
|
|
|
|
I–II |
|
|
|
|
|
|
|
III–IV |
|
|
|
|
|
|
| HPSE
expression | 2.762 | 1.581–4.825 | 0.000a | 2.309 | 1.296–4.112 | 0.004a |
|
Low |
|
|
|
|
|
|
|
High |
|
|
|
|
|
|
Establishment of HPSE silencing in PC
cells
In order to investigate the function of HPSE in PC,
the levels of HPSE expression were knocked down in PANC-1 and
BxPC-3 with the use of shRNA interference. The successful
construction of PANC-1 and BxPC-3 cells with HPSE silencing was
verified by RT-qPCR and western blot analyses (Fig. S1).
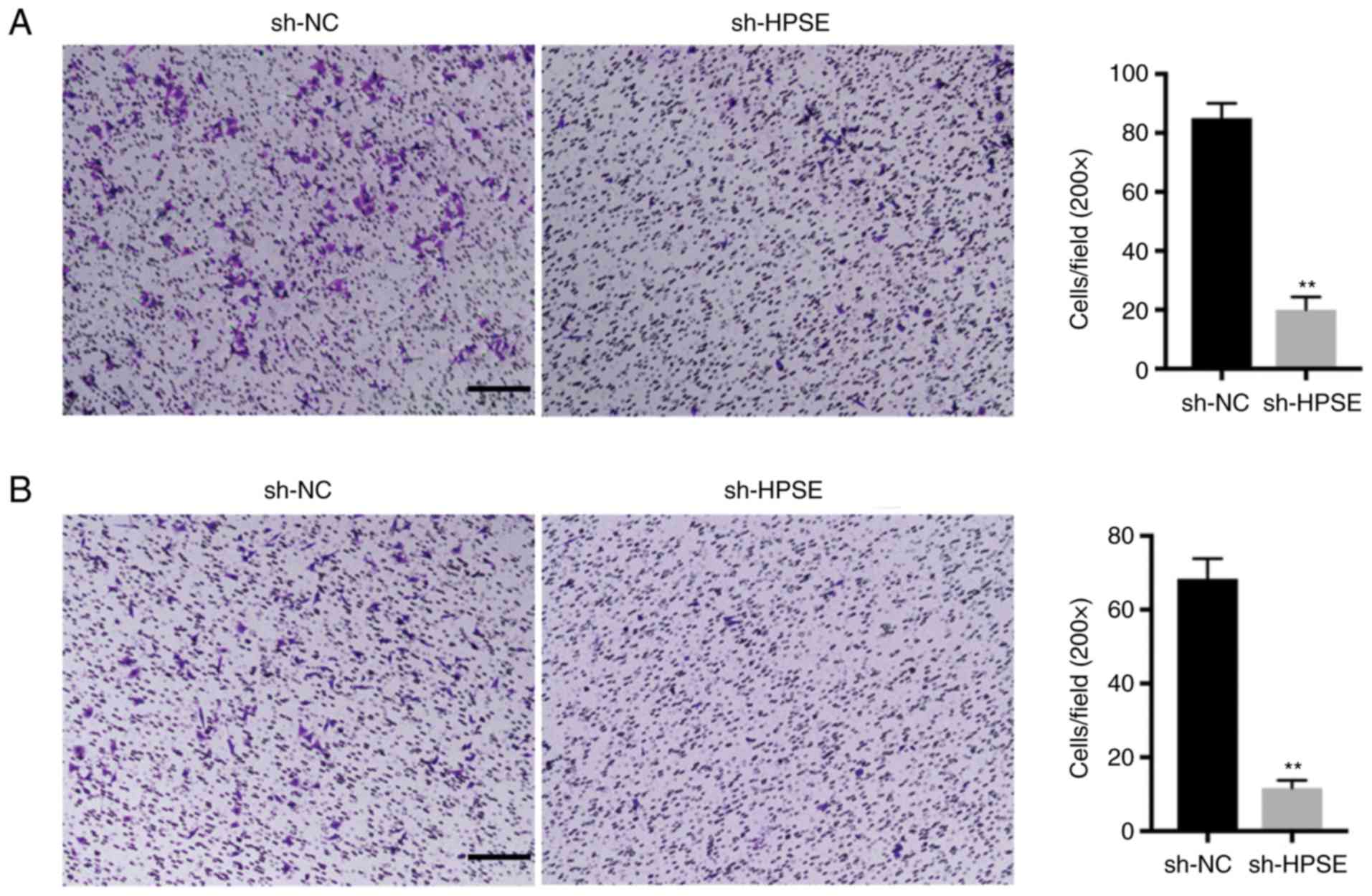
HPSE modulates the migration and
invasion of PC cells
The inhibition of HPSE expression in PANC-1 and
BxPC-3 cells markedly delayed wound closure compared with controls
(Fig. 2). Additionally, the impact
of changes in the levels of expression of HPSE on the invasion
potential of the PANC-1 and BxPC-3 PC cell lines was analyzed. In
the Transwell invasion assay, PANC-1 and BxPC-3 cells with
suppressed HPSE expression exhibited reduced invasion and migration
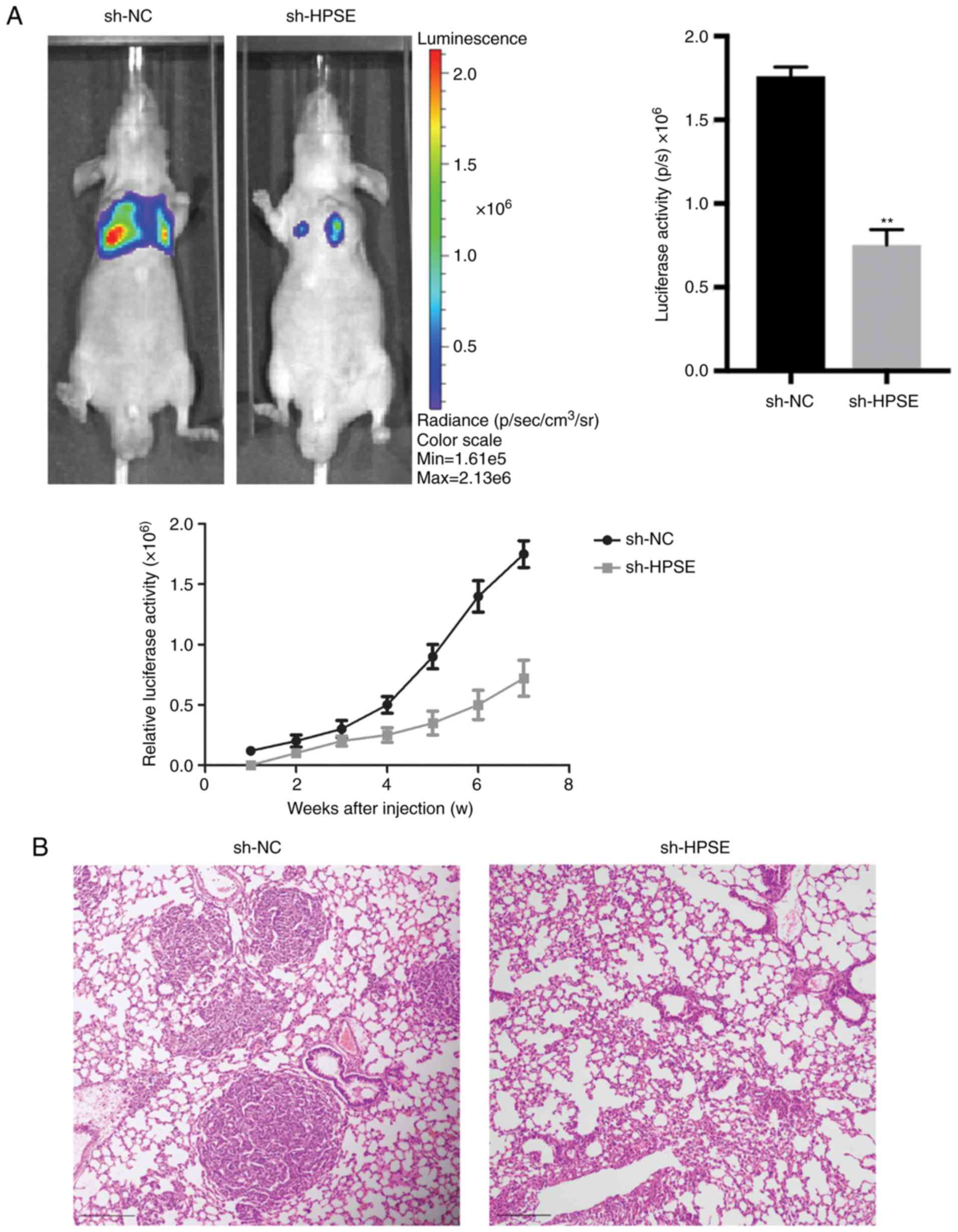
ability in comparison with the control (Fig. 3). In order to validate the role of
HPSE in the dynamics of tumorigenesis, a lung metastasis model in
nude mice was developed using PANC-1 cells and the effect of
endogenous HPSE on metastasis was evaluated in vivo. The
results indicated that cells with downregulated HPSE had a
significantly reduced ability of inducing tumor formation in the
lungs compared with the controls (Fig.
4A). A similar result was also observed based on the results of
HE staining (Fig. 4B). Furthermore,
the luciferase activity of lung metastatic nodules in the sh-NC
group was significantly higher compared with that in the sh-HPSE
group at 50 days after injection with PANC-1 cells (P=0.027;
Fig. 4A). Taken together, these
findings indicate that the downregulated expression of HPSE
markedly suppressed cell migration and invasion in vitro and
in vivo.
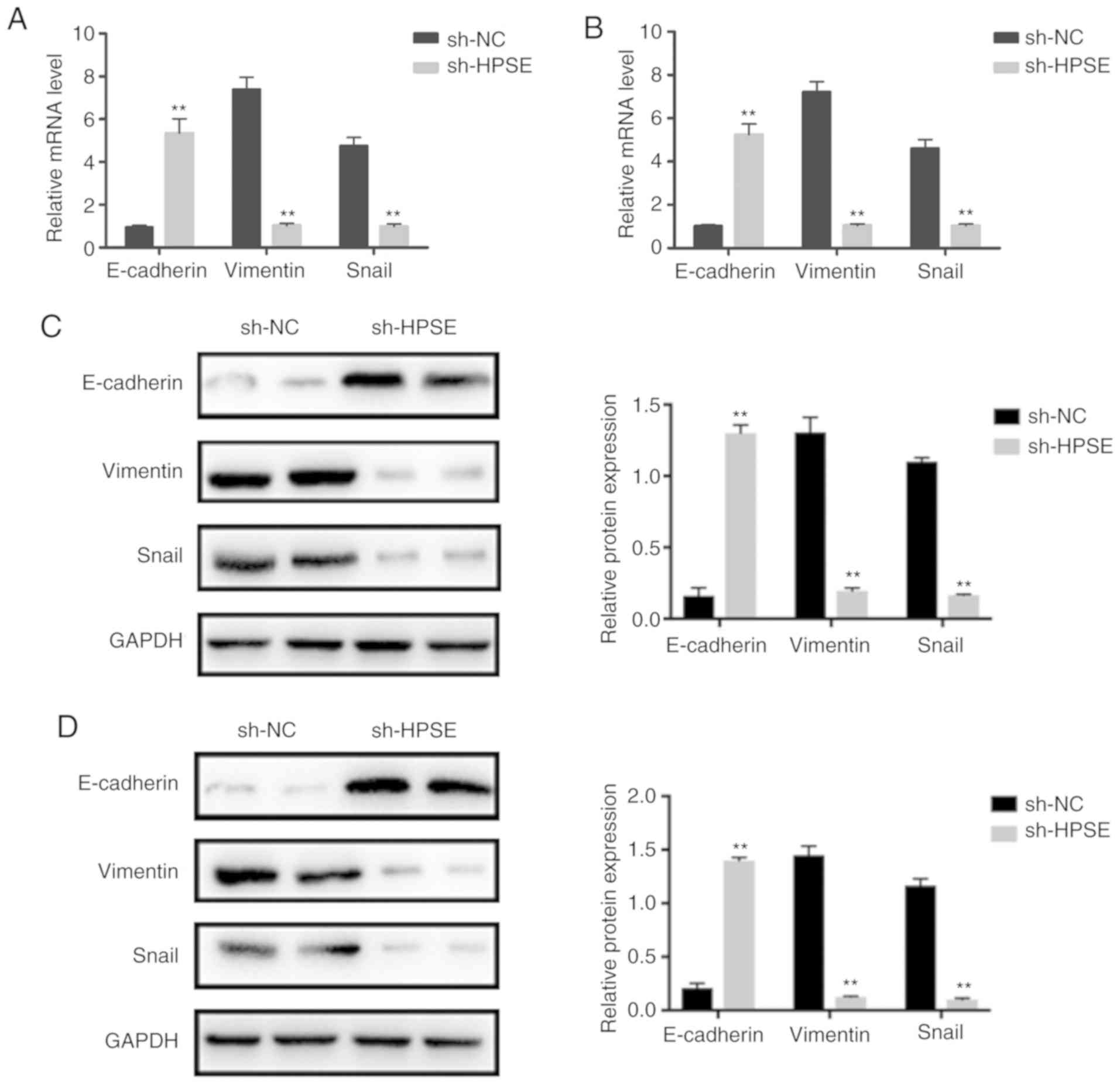
HPSE promotes
epithelial-to-mesenchymal transition (EMT) in PC cells
Accumulating evidence has indicated that the
migration and invasion of PC cells is regulated by the EMT process.
In order to investigate whether HPSE is associated with EMT, the
expression levels of epithelial (E-cadherin) and mesenchymal
(vimentin, Snail) markers were detected in PANC-1 and BxPC-3 cells
with HPSE knockdown. Western blot and RT-qPCR analyses indicated an
increase in the expression levels of E-cadherin compared with the
controls. By contrast, there was a marked decline in the expression
levels of vimentin and Snail in PANC-1 and BxPC-3 cells with HPSE
knockdown (Fig. 5). These results
indicate that HPSE likely promotes EMT in PC cells.
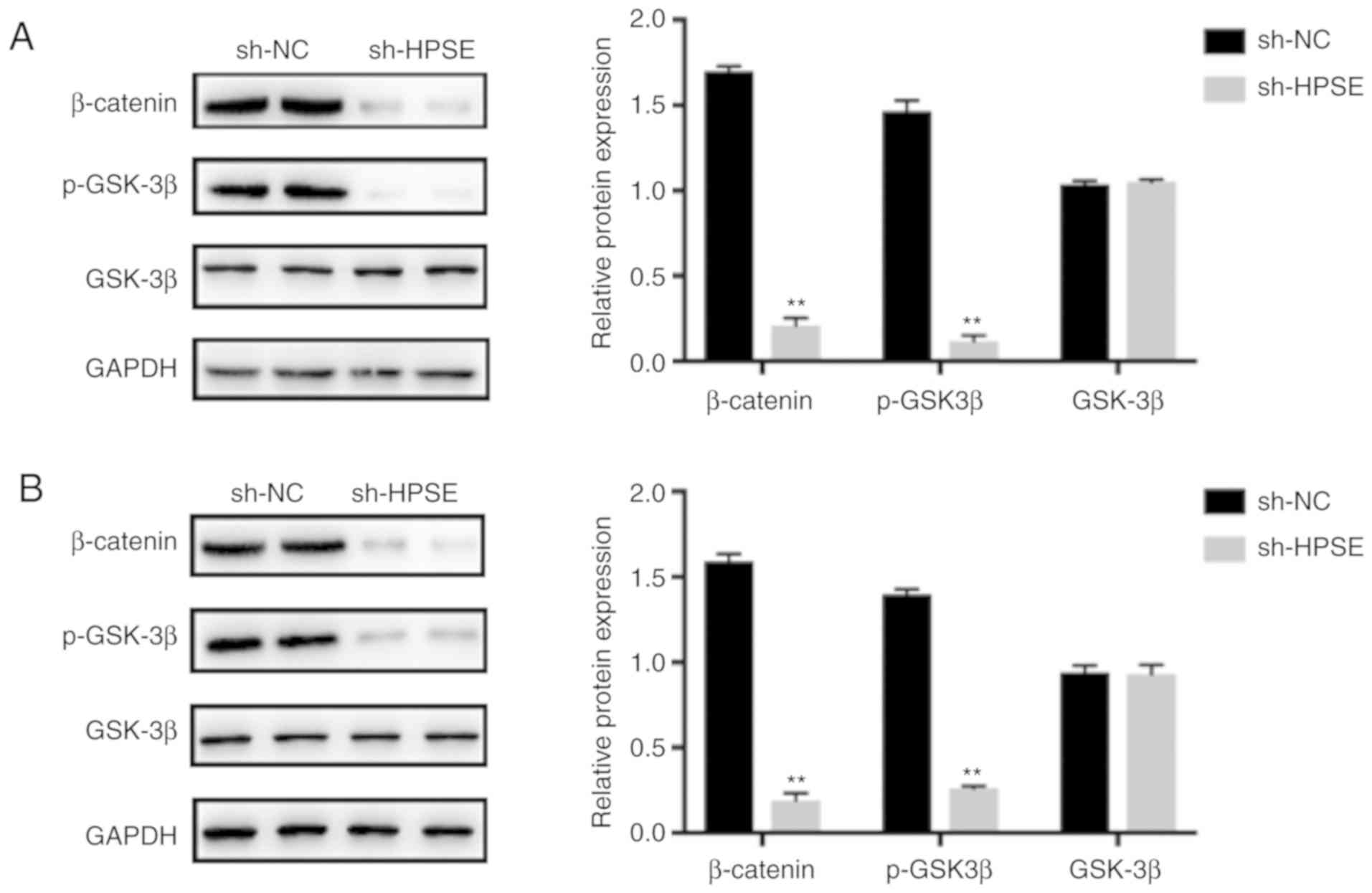
HPSE regulates EMT by activating the
Wnt/β-catenin signaling pathway
Wnt/β-catenin is among the main contributors to
EMT-related signaling pathways that are crucial for the growth and
progression of various cancers. To explore the association between
HPSE and the Wnt/β-catenin pathway, we first examined the
activation of GSK3β and β-catenin. As indicated by the results of
western blotting, HPSE interference affected the expression of
Wnt/β-catenin-associated proteins. The results demonstrated that
the expression levels of p-GSK3β and β-catenin were markedly
downregulated when HPSE was inhibited and also in comparison with
samples from the control group (Fig.
6). To determine whether the Wnt/β-catenin pathway plays an
important role in the pathway that links HPSE and EMT,
overexpression of β-catenin was induced in sh-HPSE PANC-1 cells
(Fig. S2). As verified by the
results of western blotting, β-catenin neutralized the effect of
HPSE on EMT, as demonstrated by changes in the expression levels of
EMT-related proteins (Fig. 7).
Collectively, the results of the present study suggest that HPSE
promotes EMT through activating the Wnt/β-catenin signaling
pathway, thereby promoting metastasis of PC.
Discussion
PC is a malignancy characterized by rapid
progression, poor prognosis and extremely low patient survival
rates. The biological characteristics of PC include a high
propensity for invading nerves, blood vessels and lymph nodes, a
high metastatic rate, a deep anatomical location that makes it
difficult to detect, lack of specific markers and lack of specific
symptoms at the early stages (11).
Over 75% of the patients clinically diagnosed with PC have
advanced-stage diseased. The surgical resection rate for patients
with PC is low, and PC is typically not sensitive to chemotherapy
and/or radiotherapy, with a mean 5-year OS rate of ~4% (11). Comprehensive treatment, including
surgery, chemotherapy and radiotherapy, the ability to predict
tumor recurrence and metastasis and evaluation of the prognosis of
PC are key to improving the survival rate of patients. To achieve
such a comprehensive treatment approach, it is necessary to
establish a specific index that predicts outcome for patients with
PC and determine the best course of treatment and the potential for
recurrence and metastasis. Thus far, domestic research on primary
PC has mainly focused on early diagnosis and achieving the best
treatment outcomes. There are currently no clearly identified and
accepted specific diagnostic markers for PC and there is no clear
clinical-based path for the evaluation of the prognosis of patients
with PC. Furthermore, research on metastasis, recurrence rates and
prognosis for patients with PC is in its early stages. However,
there is an emerging understanding of the value of research on
tumor markers related to metastasis and recurrence of PC and on
accurate determination of patient prognosis.
The HPSE gene (Hpa) encodes a mammalian β-D glucose
endoglycosidase, of which two isomeric isomers are known, namely
Hpa1 and Hpa2. The extracellular matrix (ECM) consists of core
proteins with HS side chains. The HPSE gene encodes a
endoglycosidase that degrades HS, resulting in ECM reconstitution
and plays a crucial role in important biological processes such as
angiogenesis and tumor metastasis (12). HPSE activates plasminogen and matrix
metalloproteinases by degrading HSPG and other proteolytic enzymes,
and promotes tumor angiogenesis and lymphangiogenesis through
auxiliary receptors such as basic fibroblast growth factor and
vascular endothelial growth factor, thereby promoting tumor
metastasis and recurrence (13).
This indicates that the level of expression of HPSE is closely
associated with the potential for tumor infiltration and metastatic
ability. Previous studies have also demonstrated that HPSE can
promote vascularization of tumor cells and can induce formation of
lymphoid tissue, ultimately promoting an increase in rates of tumor
invasion and metastasis (6,14,15).
In addition, HPSE appears to be of value in assessing tumor
invasiveness and metastatic ability (16,17).
In the present study, HPSE expression and the correlation with
clinicopathological data was examined in PC, in order to determine
the effects of HPSE on tumor size, location, vascular invasion,
differentiation and recurrence, and uncover the possible mechanism
of action. Our investigation may provide a new theoretical basis
for further exploration of indicators predicting PC recurrence and
prognosis.
The process of EMT involves the transdifferentiation
of epithelial cells to mesenchymal cells after the cells have been
subjected to particular physiological and pathological conditions,
which is accompanied by variations in cell morphology and the
levels of expression of associated genes, and affects the rates of
development and metastasis of PC (18). The changes in the levels of
expression of mesenchymal proteins increases the motility,
invasiveness and metastatic potential of PC cells. Cell surface HS
is mainly localized in lipid rafts. It reduces transforming growth
factor (TGF)-β responsiveness in these cells by facilitating
caveolae/lipid raft-mediated endocytosis and rapid degradation of
TGF-β bound to TGF-β receptors, thereby diminishing non-lipid
raft-mediated endocytosis and TGF-β-stimulated signaling (19). TGF-β is a potent stimulator of EMT
in cancer cells, such as PC cells. In addition, HPSE is an enzyme
that acts on the cell surface and within the ECM and are
responsible for degrading polymeric HS molecules into shorter-chain
oligosaccharides. This suggests that high levels of HPSE
expression, which reduce the expression of cell surface HS, may
activate TGF-β-stimulated non-Smad-dependent and Smad-dependent
signaling pathways that are involved in EMT (proliferation and
migration) of cancer cells. Furthermore, TGF-β activates the
Wnt/β-catenin pathway.
Furthermore, there are a number of critically
important pathways that contribute to the promotion of mesenchymal
protein expression, including RAS/RAF/MEK/ERK, PI3K/AKT/mTOR and
Wnt/β-catenin (20). The downstream
activity of these pathways incudes stimulating the expression of
EMT transcription determinants, such as Snail, Slug, Twist and Zeb,
ultimately promoting inhibition of the epithelial phenotype and
acquisition of mesenchymal characteristics (18). The growing evidence base has
uncovered that different types of small-molecule inhibitors and
phytochemicals can affect the progression of EMT and reversing the
underlying mechanisms, thereby inducing re-expression of epithelial
markers. The understanding of the association between EMT and PC
will likely contribute to the identification of novel therapeutic
targets for PC. Our future aim is to use RNAseq to assess the total
pathways in HPSE interference cell lines in order to determine
whether HPSE is an essential gene in PC cells in vivo, and
carry out follow-up research on the role of HPSE in PC.
In conclusion, the present study investigated
whether HPSE promotes EMT through the activation of the
Wnt/β-catenin signaling pathway in PC cells, and demonstrated that
downregulation of HPSE expression inhibited migration and invasion
of PC cells in vitro and in vivo. HPSE interference
increased the expression of E-cadherin and reduced the expression
of vimentin. Furthermore, the overexpression of β-catenin
neutralized the effect of HPSE on EMT, as indicated by the observed
changes in the protein levels of EMT-related molecular markers in
PC cells. Collectively, these findings indicate that HPSE likely
acts as an EMT inducer and may be of value as a target for
antimetastatic treatment in patients with PC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Anhui
Provincial Natural Science Foundation (grant no. 1708085MH228).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW conceived the study. CW, YW, YZ and GW performed
the experiments. JZ and KX supervised the experiments and edited
the images. CW, YW and GW reviewed the article before submission
for spelling and grammar, as well as intellectual content. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committee of the Anhui Provincial Hospital, Anhui Medical
University (Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halbrook CJ and Lyssiotis CA: Employing
metabolism to improve the diagnosis and treatment of pancreatic
cancer. Cancer Cell. 31:5–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hessmann E, Johnsen SA, Siveke JT and
Ellenrieder V: Epigenetic treatment of pancreatic cancer: Is there
a therapeutic perspective on the horizon? Gut. 66:168–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masola V, Bellin G, Gambaro G and Onisto
M: Heparanase: A multitasking protein involved in extracellular
matrix (ECM) remodeling and intracellular events. Cells. 7:2362018.
View Article : Google Scholar
|
|
5
|
Masola V, Zaza G, Gambaro G, Franchi M and
Onisto M: Role of heparanase in tumor progression: Molecular
aspects and therapeutic options. Semin Cancer Biol. 62:86–98. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caruana I, Savoldo B, Hoyos V, Weber G,
Liu H, Kim ES, Ittmann MM, Marchetti D and Dotti G: Heparanase
promotes tumor infiltration and antitumor activity of
CAR-redirected T lymphocytes. Nat Med. 21:524–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tran VM, Wade A, McKinney A, Chen K,
Lindberg OR, Engler JR, Persson AI and Phillips JJ: Heparan sulfate
glycosaminoglycans in glioblastoma promote tumor invasion. Mol
Cancer Res. 15:1623–1633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant tumours. John Wiley & Sons,
Inc.; Hoboken, New Jersey: 2017
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Pan J, Zhang L, Wei Y and Wang C:
Long non-coding RNA CRNDE sponges miR-384 to promote proliferation
and metastasis of pancreatic cancer cells through upregulating
IRS1. Cell Prolif. 50:e123892017. View Article : Google Scholar
|
|
11
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vlodavsky I, Singh P, Boyango I,
Gutter-Kapon L, Elkin M, Sanderson RD and Ilan N: Heparanase: From
basic research to therapeutic applications in cancer and
inflammation. Drug Resist Updat. 29:54–75, 20165. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rabelink TJ, van den Berg BM, Garsen M,
Wang G, Elkin M and van der Vlag J: Heparanase: Roles in cell
survival, extracellular matrix remodelling and the development of
kidney disease. Nat Rev Nephrol. 13:201–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv Q, Wu K, Liu F, Wu W, Chen Y and Zhang
W: Interleukin17A and heparanase promote angiogenesis and cell
proliferation and invasion in cervical cancer. Int J Oncol.
53:1809–1817. 2018.PubMed/NCBI
|
|
15
|
Lv B, Zhang B, Hu XY and Zeng QD:
Heparanase regulates in vitro VEGF-C expression and its clinical
significance to pancreatic ductal cell adenocarcinoma. Oncol Lett.
11:1327–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Zhou ZH, Li W, Zhang SK, Li J, Zhou
MJ and Song JW: Heparanase promotes tumor growth and liver
metastasis of colorectal cancer cells by activating the p38/MMP1
Axis. Front Oncol. 9:2162019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Jiang W, Yue C, Zhang W, Tong C,
Dai D, Cheng B, Huang C and Lu L: Heparanase contributes to
trans-endothelial migration of hepatocellular carcinoma cells. J
Cancer. 8:3309–3317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CL, Huang SS and Huang JS: Cellular
heparan sulfate negatively modulates transforming growth
factor-beta1 (TGF-beta1) responsiveness in epithelial cells. J Biol
Chem. 281:11506–11514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pearlman RL, Montes de Oca MK, Pal HC and
Afaq F: Potential therapeutic targets of epithelial-mesenchymal
transition in melanoma. Cancer Lett. 391:125–140. 2017. View Article : Google Scholar : PubMed/NCBI
|