Introduction
Recently, tyrosine kinase inhibitors (TKIs) have
been widely developed as targeted anticancer therapeutic drugs.
Tyrosine kinases are protein kinases that modulate various cellular
signaling pathways including proliferation and differentiation.
Overexpression and dysregulation of tyrosine kinases are associated
with several types of cancer. TKIs selectively inhibit the
phosphorylation of tyrosine kinase receptors and thus suppress the
growth, proliferation and differentiation of tumor cells (1).
Although TKIs are thought to be narrowly targeted
and relatively low-toxic agents, they are associated with a range
of adverse effects. Fatigue, diarrhea, weight loss, hypertension,
neurotoxicity, dermatologic and cardiovascular toxicities are
common the side effects associated with TKI agents (2). However, there is little research on
the toxic effects of TKI agents on ovarian function and
reproductive potential and these studies give conflicting results.
One case of primary ovarian insufficiency was reported in a
28-year-old woman treated with imatinib (3), but the cause-and-effect relationship
of this case was speculative (4).
Two mouse models yielded conflicting results on the impact of
imatinib on folliculogenesis (5,6). Thus,
more research is needed on the reproductive toxicity of TKIs in
women.
Several protein kinases, including protein kinase A
(PKA), cyclin-dependent kinase (CDK), mitogen-activated protein
kinase (MAPK), human epidermal growth factor receptor (HER) kinases
and mammalian target of rapamycin (mTOR) are present in the ovary.
Activation or blocking of these protein kinases showed that they
are involved in ovarian cell proliferation and apoptosis, oocyte
maturation, hormone release and response to hormones, and mediating
the action of hormones on ovarian functions (7). As TKIs target protein kinase receptors
in tumor cells and inhibit their phosphorylation, we speculate that
TKIs can also interact with protein kinases in the ovary and
influence ovarian function and reproductive potential. However, few
data are available on the impact and mechanism of TKIs on ovarian
function.
Lapatinib is an oral TKI that selectively binds to
the ATP-binding site of the kinase and prevents phosphorylation of
the EGF receptor and HER2. Combined with trastuzumab therapy,
lapatinib is used in the second-line treatment of advanced or
metastatic HER2-positive breast cancer. Breast cancer is one of the
most frequently diagnosed cancers among women. In 2015, the
incidence of breast cancer among women aged 20 to 39 years was 31.7
per 100,000 women. With advances in systemic therapy, the 5-year
survival rate among young women reached 88.5% between 2010 and 2015
(8). Great attention should be paid
to the preservation of ovarian function and fertility in young
women who are diagnosed with breast cancer and receive anticancer
therapy. As EGF receptors play important roles in oocyte nuclear
maturation, cumulus expansion, and ovulation (9,10), it
is possible that lapatinib treatment may cause fertility toxicity
and have an impact on ovarian function. In vitro culture of
porcine oocyte-cumulus complexes (COCs) indicated that lapatinib
inhibited the meiotic maturation of COCs (11), but there are no in vivo
studies on the effect of lapatinib on ovarian function.
Therefore, we performed in vivo mouse
experiments to investigate whether lapatinib could influence
ovarian function and fertility potential through the inhibition of
EGF receptor tyrosine kinases, and further explored the mechanism
of action of lapatinib both in vivo and in vitro.
Materials and methods
Reagents and antibodies
Lapatinib ditosylate was purchased from Selleck
Chemicals. Antibodies were purchased from Cell Signaling
Technology, Inc.: Phospho-EGFR (Tyr1068, rabbit monoclonal)
(#3777), PI3K (rabbit monoclonal) (#4257), phospho-PI3K
(Tyr458/Tyr199, rabbit polyclonal) (#4228), phospho-PTEN (Ser380,
rabbit polyclonal) (#9551), AKT (rabbit monoclonal) (#4691),
phospho-AKT (Ser473, rabbit monoclonal) (#4060), STAT3 (rabbit
monoclonal) (#4904), phospho-STAT3 (Tyr705, rabbit monoclonal)
(#9145), MEK1/2 (rabbit monoclonal) (#8727), phospho-MEK1/2
(Ser217/221, rabbit monoclonal) (#9154), MAPK1/3 (rabbit
monoclonal) (#4695), phospho-MAPK1/3 (Thr202/Tyr204, rabbit
monoclonal) (#4370). EGFR (rabbit polyclonal) (18986-1-AP) and PTEN
(rabbit polyclonal) (22034-1-AP) were purchased from Proteintech.
GAPDH (rabbit polyclonal) and horseradish peroxidase (HRP)-labeled
goat anti-rabbit secondary antibodies were purchased from
Servicebio. Antibodies for immunohistochemical staining were
purchased from ImmunoWay: Stat3 (phospho Tyr705, rabbit polyclonal)
(YP0251) and PI3-kinase p85/p55 (phospho Tyr467/199, rabbit
polyclonal) (YP0224); Abcam: Anti-EGFR (phospho Y1068, rabbit
monoclonal) (ab40815); and Cell Signaling Technology, Inc.:
Phospho-MAPK1/3 (rabbit monoclonal) (#4370).
Animals and treatments
Five-week-old female and 9-week-old male C57BL/6
mice were purchased from Hubei Provincial Center for Disease
Control and Prevention. The animals were provided with ad
libitum access to food and water and housed under SFP
conditions of controlled temperature (20-25°C), humidity (40-70%)
and lighting (12 h light-dark cycle). After acclimatization for 1
week, 76 female mice with regular estrous cyclicity were randomized
into three groups and administered lapatinib (100 mg/kg, n=22 or
200 mg/kg, n=32) or vehicle (distilled water containing 1%
Tween-80, n=22) orally for 4 weeks. The doses for mice was
calculated based on the human dose (1,250 mg/day orally) and these
doses are also commonly used in mouse models (12,13).
In the event that the mice succumbed to the side effects of high
dose lapatinib, more mice were randomized into the 200 mg/kg
lapatinib group. Body weights were monitored before and after
treatment. Then 44 mice (n=12 in control, n=12 in 100 mg/kg
lapatinib, and n=20 in 200 mg/kg lapatinib) were randomly
euthanized and their ovaries were collected for analysis. The
remaining control and treated mice were kept for a fertility trial.
This animal study was approved by the Ethics Committee of Tongji
Hospital, Tongji Medical College, Huazhong University of Science
and Technology Institutional (TJ-A20171206).
Estrous cyclicity
Before treatment, vaginal smears were monitored
daily for 1 week to exclude mice with irregular estrous cyclicity.
At 8:00-9:00 a.m., one drop of PBS was expelled into the vagina,
aspirated, and then transferred to a microscope slide. The vaginal
samples were analyzed for the predominance of lymphocytes,
nucleated epithelial cells or keratinocytes and the estrous cycles
of the mice were deemed as regular, irregular, or prolonged
estrous, as described previously (14). After treatment for 4 weeks, vaginal
smears were again assessed for 1 week to determine the estrous
cyclicity.
Ovary histology
Ovaries were fixed in 4% paraformaldehyde
(Servicebio) overnight and embedded in paraffin. After
deparaffinization, 5-µm-sectioned samples were placed on glass
slides and stained with hematoxylin and eosin. Follicles were
classified using accepted definitions (15). A primordial follicle was defined as
an oocyte surrounded by a single layer of flattened granulosa
cells, a primary follicle was surrounded by a single layer of
cuboidal granulosa cells, a secondary follicle had at least two
layers of cuboidal granulosa cells without an antrum and an antral
follicle had an antrum. Follicles were counted every 6th section
(every 30 µm) throughout the ovary, and then the follicles in each
stage per section were calculated from the total number of
follicles per ovary. To avoid double counting of secondary and
antral follicles, only follicles with an oocyte nucleus in these
stages were counted.
Serum AMH
Blood was collected from the mouse orbital sinus at
the time of death and centrifuged at 1,000 × g for 15 min to
extract the serum. The concentration of AMH was quantified with an
AMH ELISA kit (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions.
Western blotting
Ovarian tissues were lysed in
radioimmunoprecipitation assay (RIPA) buffer (Servicebio)
containing 1% protease inhibitor cocktail (Servicebio) and 1%
phosphorylase inhibitor (Servicebio) for 30 min. Lysates were
centrifuged at 12,000 × g for 10 min at 4°C and the protein was
quantified using a BCA Protein Assay kit (Servicebio). Loading
buffer was added to the protein samples. Then 30 µg proteins in
each lane were separated by 10% SDS-PAGE before being transferred
to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The
membranes were incubated in a blocking buffer (5% nonfat milk in
Tris-buffered saline containing 0.5% Tween-20) for 1 h at 37°C and
then incubated at 4°C with primary antibodies overnight. The
concentrations of primary antibodies were: EGFR (dilution 1:1,000),
phospho-EGFR (Tyr1068, dilution 1:1,000), PI3K (dilution 1:1,000),
phospho-PI3K (Tyr458/Tyr199, dilution 1:1,000), PTEN (dilution
1:1,000), phospho-PTEN (Ser380, dilution 1:1,000), AKT (dilution
1:2,000), phospho-AKT (Ser473, dilution 1:2,000), STAT3 (dilution
1:1,000), phospho-STAT3 (Tyr705, dilution 1:1,000), MEK1/2
(dilution 1:1,000), phospho-MEK1/2 (Ser217/221, dilution 1:1,000),
MAPK1/3 (dilution 1:1,000), phospho-MAPK1/3 (Thr202/Tyr204,
dilution 1:1,000) and GAPDH (dilution 1:1,000). After washing with
TBS-Tween 5 min for three times, the blots were incubated with
HRP-labeled goat anti-rabbit secondary antibody (duration 1:1,000)
for 1 h at 37°C. Proteins were visualized using a chemiluminescent
imager (Syngene) and an ECL kit (Servicebio).
Immunohistochemical staining
Paraffin sections were rehydrated and treated with
0.3% hydrogen peroxide for 20 min at room temperature to neutralize
endogenous peroxidases. Then sections were immersed in citrate
buffer for antigen retrieval in a microwave oven. After incubation
in blocking buffer (ready-to-use goat serum, Boster Biological
Technology), sections were incubated with primary antibodies at 4°C
overnight and the secondary antibody (Servicebio at room
temperature for 1 h in a wet box. The concentrations of primary
antibodies were: Stat3 (phospho Tyr705, dilution 1:50), PI3-kinase
p85/p55 (phospho Tyr467/199, dilution 1:50), phospho-MAPK1/3
(Thr202/Tyr204, dilution 1:50) and anti-EGFR (phospho Y1068,
dilution 1:100). All sections were visualized using DAB
(Servicebio) and followed by hematoxylin counterstaining. For
negative controls, non-immune rabbit serum (Boster Biological
Technology) and rabbit mAb IgG (Cell Signaling Technology, Inc.) at
the same protein concentration as the primary antibody.
Mating experiments
A total of 32 female mice (n=10 in control, n=10 in
100 mg/kg lapatinib, and n=12 in 200 mg/kg lapatinib) were used for
mating experiments. Two female mice were paired with one proven
fertile male mouse 7 days after treatment with lapatinib or vehicle
treatment as mentioned above. After 1 week of mating, the females
were separated until delivery. Pups were kept with their mothers
for 1 week and then separated. One week after breast-feeding, the
mice were mated again. The duration of the mating experiment was 6
months, with mating intervals of 5–6 weeks.
Ovarian tissue culture
The ovaries from 4-week-old mice were sliced into 3
or 4 pieces using a micro-scissor (Servicebio) under a stereoscopic
microscope (Olympus, Japan). Every 3 or 4 ovary slices were
cultured in a 8.0-µm Transwell culture insert for 24-well plates
(Corning Inc.) under conditions of 5% CO2 and
temperature of 37°C. Each well was filled with 1,000 µl M199
(Boster Biological Technology) supplemented with 5% fetal bovine
serum (FBS) (Tianhang Biotechnology), 1% insulin-transferrin-sodium
(ITS) (Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin G and 100
µg/ml streptomycin (Servicebio), and 100 µIU/ml
follicle-stimulating hormone (FSH) (Livzon). After 24 h of culture,
either vehicle (1‰ DMSO) or 5 µM or 10 µM lapatinib was added to
the medium and the samples were cultured for a further 24 and 48 h.
This dose range covers the human dose of 1,500 mg/day (~5.6 mM)
(11). The tissue culture at each
culture time and drug concentration was replicated for at least 3
times.
Statistical analysis
All data are presented as the means ± standard
deviation (SD) or standard error of the mean (SEM). Statistical
analyses were performed using GraphPad Prism 5.0 (GraphPad
Software, USA) and Statistical Package for Social Sciences 18.0
(SPSS, Inc.). Body weights, follicle numbers, AMH levels and mating
outcomes were analyzed by one-way ANOVA, and pairwise comparisons
were performed using Student-Newman-Keuls (SNK) post hoc
test. Estrous cyclicity was analyzed by Chi-squared test. P<0.05
was considered statistically significant.
Results
Effect of lapatinib on the general
condition of the mice
To evaluate the effects of lapatinib on the general
health of the mice, 76 6-week-old female mice were randomized into
three groups and treated with either lapatinib (100 mg/kg, n=22 or
200 mg/kg, n=32) or vehicle (n=22) for 4 weeks. The mice treated
with 200 mg/kg lapatinib showed a significant decrease in body
weight (Fig. 1A, Table SI) compared to the 100 mg/kg group.
The percentage of irregular estrous cycling was higher in the mice
treated with 200 mg/kg lapatinib than in the control or 100 mg/kg
treatment groups (Fig. 1B and C),
but the difference was not statistically significant.
Effect of lapatinib on ovarian reserve
and fertility
To investigate the effect of lapatinib on ovarian
reserve, a total of 44 mice in three groups (n=12 in control, n=12
in 100 mg/kg lapatinib, and n=20 in 200 mg/kg lapatinib) were
sacrificed after treatment and ovarian morphology and
anti-Müllerian hormone (AMH) were measured. Ten ovarian samples in
each group were randomly selected for ovary histology assessment.
No apparent difference was observed in the histological morphology
of the ovaries (Fig. 2A). Follicle
numbers of the different stages were similar among the three groups
(Fig. 2B, Table SII), which indicated that lapatinib
did not influence oocyte maturation, follicle activation, or
follicle apoptosis. Serum AMH levels showed a slight decrease in
the lapatinib-treated groups compared with the control group,
however, the data were not statistically significant (Fig. 2C, Table
SIII). The remaining 32 female mice were kept for mating
experiment. During this procedure, 6 mice died and the reason was
unknown (may be the side effects of drugs or gavage). Ultimately 26
living mice were included in the analysis (n=7 in control, n=9 in
100 mg/kg lapatinib, and n=10 in 200 mg/kg lapatinib). The results
showed that the pregnancy rate was not significantly influenced by
the lapatinib treatment (Fig. 2D,
Table SIV). Although the average
number of pups showed a decrease in the lapatinib-treated groups,
the data were not statistically significant (Fig. 2E, Table
SV).
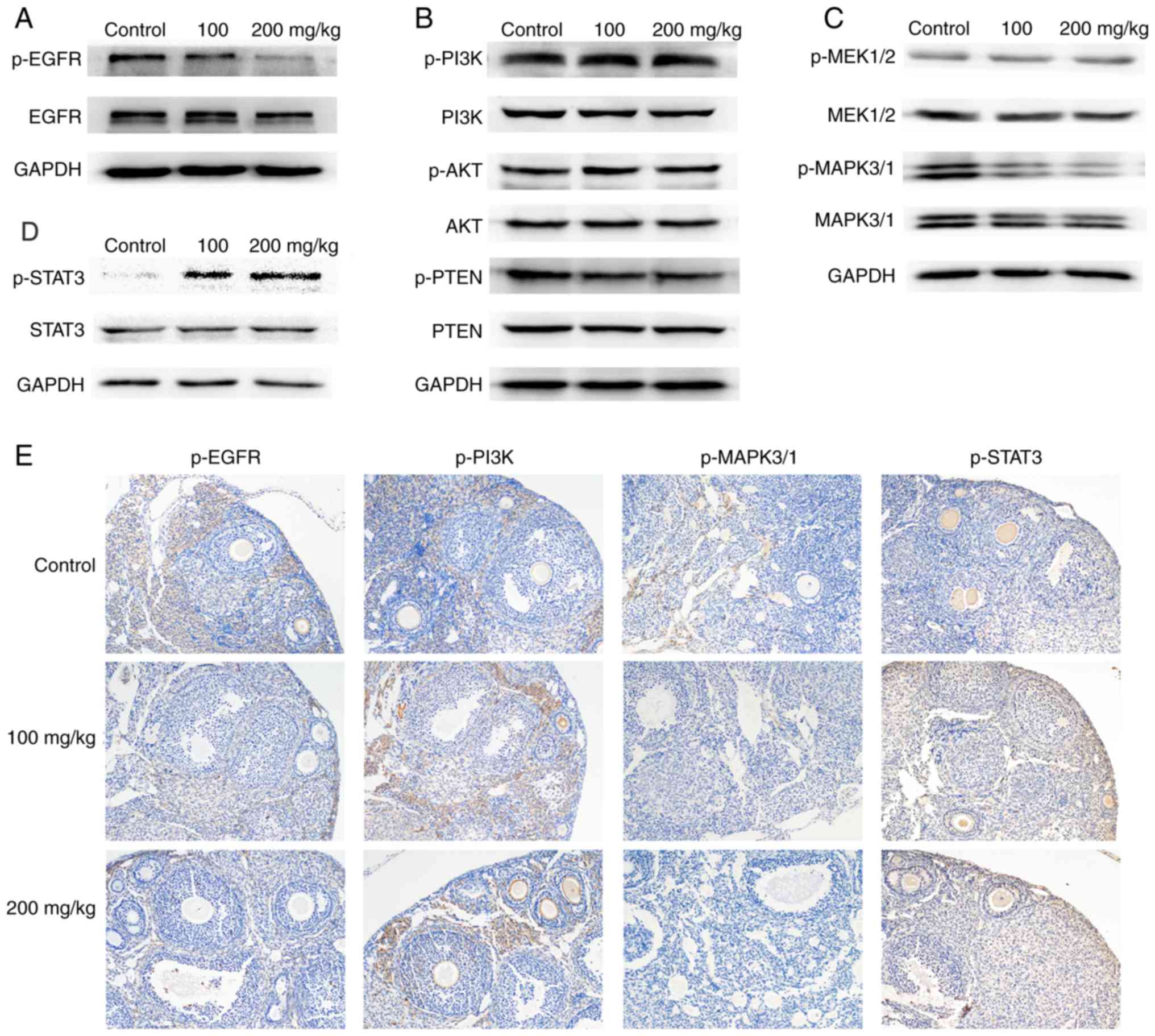
Effect of lapatinib on the EGF
receptor signaling pathway
Theoretically, the EGFR and HER2 inhibitor lapatinib
can influence ovarian function and reproductive potential.
Therefore, to explore the mechanism for the lack of reproduction
toxicity of lapatinib in our experiments, ovaries of the sacrificed
mice after the 4-week treatment were collected to evaluate the
expression levels of the EGF receptor and its downstream signaling
pathways using western blotting and immunohistochemical staining.
We found that the phosphorylation site of the EGFR receptor, the
target of lapatinib, showed significantly decreased phosphorylation
in the lapatinib-treated groups compared to the control group
(Fig. 3A and E). We also found
decreased phosphorylation of MAPK3/1 and increased phosphorylation
of STAT3 in the lapatinib-treated groups compared to the control
group (Fig. 3C, D and E). However,
no obvious variation was noted in the phosphorylation of the
PI3K/AKT pathway (Fig. 3B). This
result indicated that lapatinib was able to inhibit the activation
of the EGF receptor and further inhibit its downstream MAPK/ERK
pathway in mouse ovary. However, activation of the STAT3 pathway
may counteract these inhibitory effects. Therefore, no apparent
effect was observed in either the estrous cyclicity or the ovarian
reserve of the mice.
In vitro effect of lapatinib on
ovarian tissue and oocytes
As the targets of lapatinib are found throughout the
body, to exclude the global effects of lapatinib on the whole body
and to confirm the direct effects of lapatinib on the ovaries and
oocytes, in vitro experiments were performed using ovarian
tissue slices. Ovarian tissues were cultured with or without
lapatinib (5 and 10 µM) for 24 and 48 h. There were no obvious
differences in the morphological assessment (Fig. 4A) and follicle counting (Fig. 4B) of the ovarian slices after
treatment. Western blotting experiments to examine the EGF receptor
and the downstream pathways showed similar results as those noted
with the in vivo experiments (Fig. 4C and D). The phosphorylation of
EGFR, MEK1/2, MAPK3/1, PI3K and AKT decreased in the
lapatinib-treated groups, and phosphorylation of STAT3 increased in
the lapatinib-treated groups compared to the control group. These
results indicated that the inhibition of the EGFR and the PI3K and
MAPK pathways by lapatinib may be counteracted by the activation of
the STAT3 signaling pathway.
Discussion
Lapatinib is an oral dual tyrosine kinase inhibitor
that selectively targets the tyrosine kinase domain of the
epidermal growth factor (EGF) and human epidermal growth factor
receptor-2 (HER2) receptors. Commonly reported side effects of
lapatinib include diarrhea, skin rash, and headache (16), but the toxic effects on gonadal
function and fertility are uncertain. Our study is the first to
investigate the effects of lapatinib on ovarian function and
reproductive potential in a mouse model. We performed in
vivo experiments to study the estrous cyclicity, ovarian
reserve and fertility potential of female mice after a 4-week
lapatinib treatment, and the results showed that lapatinib was well
tolerated and had little effect on ovarian function and fertility
in mice. Western blotting indicated that while lapatinib inhibited
ovarian EGF receptors, the activation of the signal transducers and
activators of transcription (STAT)3 signaling pathway may
counteract this inhibitory effect.
In the present study, mice were treated with either
lapatinib or vehicle. The body weight of the mice treated with 200
mg/kg lapatinib was significantly lower than that of the mice
treated with 100 mg/kg or the control mice. Weight loss is a side
effect commonly noted in tyrosine kinase inhibitors (TKIs)
(2). Although we observed slight
decreases in estrous cycling, serum AMH levels and the average
number of pups in the lapatinib-treated groups, there were no
statistical differences among the three groups. These results
demonstrated that lapatinib had little effect on the endocrine
system, oocyte maturation, ovarian reserve or fertility of female
mice.
To the best of our knowledge, there are no studies
on the influence of lapatinib on human ovarian function.
Nevertheless, some in vitro animal studies have shown a
relationship between EGFR inhibitors and oocyte maturation. In
vitro culture of bovine oocyte-cumulus complexes (COCs) showed
that the EGFR inhibitor AG1478 promoted oocyte arrest at the GV
stage (17). In rat follicles,
AG1478 blocked the LH stimulation of EGFR and inhibited oocyte
maturation and cumulus expansion (18). The inhibitory effect of lapatinib on
porcine COCs was similar to the effect of AG1478, which inhibited
oocyte maturation and reduced cumulus expansion (11). It is therefore interesting that
lapatinib showed no inhibitory effects on the ovary in our in
vivo study.
To investigate the mechanisms behind the lack of
in vivo inhibition of ovarian function in our study, we
conducted western blotting and immunohistochemical staining of EGFR
and its downstream signaling pathways in samples from our control
and lapatinib-treated ovaries. Activation of EGFR promotes several
signaling pathways, including phosphatidylinositol-3 kinase
(PI3K)/protein kinase B (AKT), mitogen-activated protein kinases
(MAPK)/extracellular regulated kinase (ERK) and Janus kinase
(JAK)/signal transducers and activators of transcription (STAT)
pathways (9). Our study showed that
lapatinib targeted ovarian EGF receptors and inhibited the
phosphorylation of EGFR proteins, which further downregulated the
MAPK/ERK signaling pathway. However, the STAT3 pathway was
upregulated following lapatinib treatment. To exclude the potential
effects of lapatinib on other tissues and organs in the body and
verify the outcome, we cultured ovary slices in vitro with
or without lapatinib. The western blotting results of the ovary
slices also showed inhibition of EGFR and the PI3K and MAPK
pathways, and activation of the STAT3 pathway following lapatinib
treatment.
Previous evidence has shown that both the MAPK/ERK
and JAK/STAT pathways are necessary for oocyte maturation and
function in the mouse. Disruption of MAPK3/1 in mouse granulosa
cells resulted in complete infertility in vivo: No oocytes
matured, no cumulus-oocyte complex (COC) expanded, and no ovulation
occurred (19). Inhibition of
MAPK3/1 further demonstrated the role of the MAPK pathway in oocyte
meiosis, cumulus expansion and ovulation in mice (20,21).
Similarly, STAT3 has been proven to be essential for the formation
and function of follicles (22).
Activation of p-STAT3 was found to be involved in meiotic spindle
assembly and chromosome segregation in mouse oocytes (23), and STAT3 activation was able to
stimulate the progression of meiosis (24). In vitro maturation of COCs
supplemented with STAT3 inhibitor stattic was found to block
cumulus expansion (25). Based on
these findings, we speculated that the inhibition of EGFR and
MAPK1/3 in the ovary had been negated by the upregulation of STAT3,
thus no effects of lapatinib treatment were noted on ovarian
reserve or fertility potential in vivo (Fig. 5).
In fact, STAT3 signaling upregulation has been shown
in clinical and laboratory tests to be one of the mechanisms of
resistance in some anti-EGFR therapeutics in several cancer types
(26). Clinical evidence showed
that patients who are resistant to EGFR therapeutics such as
gefitinib, cetuximab and lapatinib showed increased activity of
STAT3 in their tumor tissues (26–28).
Cell line studies of hepatoma, colon and head and neck cancers also
showed that the level of STAT3 phosphorylation was correlated with
the efficacy of anti-EGFR agents (27,29,30).
Furthermore, many studies have shown that combining anti-EGFR
agents with agents that block STAT3 activity could overcome this
drug resistance (31–34). These results strongly suggest that
STAT3 signaling counteracts the therapeutic effects of EGFR
inhibition in tumors. However, to the best of our knowledge, there
are no data on STAT3 counteractive effects in nontumor tissues. Our
study is the first to show that the inhibitory effects of EGFR may
be counteracted by the STAT3 signaling pathway in healthy mouse
ovaries. The mechanism of the STAT3 regulation of EGFR may be
similar to that noted in tumor resistance to TKIs, or it may be due
to some other kinase-substrate relationships in mouse ovary. The
exact mechanism needs to be further explored.
Still, our study has some limitations. The major one
was that there was no experimental data on the molecular mechanism
between the phosphorylation of STAT3 and ovarian reserve. As this
molecular mechanism has been demonstrated in the literature, we
gave less effort on this and we mainly focused on the impact of
lapatinib on ovary and fertility. Further analysis is needed to
make our conclusion more reliable.
In conclusion, our study demonstrated that although
lapatinib can target EGF receptors and inhibit downstream pathways
in ovaries, the inhibitory effects of lapatinib may be counteracted
by activation of the STAT3 signaling pathway. Thus, there was
little effect of lapatinib on oocyte maturation, follicle count,
ovarian reserve or reproductive function in lapatinib-treated mice.
Our data are valuable to young women who receive lapatinib therapy
and wish to preserve their fertility. However, large scale clinical
data are still needed to confirm that lapatinib does not impair
fertility.
Supplementary Material
Supporting Data
Acknowledgements
We thank the Laboratory Animal Center of Tongji
Hospital for the animal handling. We thank the Department of
Gynecology and Obstetrics of Tongji Hospital for providing a good
experimental platform.
Funding
This study was supported by the National Natural
Science Foundation of China (81802896), Natural Science Foundation
of Hubei Province (2017CFB800), Hubei Province Health and Family
Planning Scientific Research Project (WJ2017Z013, WJ2019M127) and
National Key Research and Development Program (2018YFC1002103,
2019YFC1005200 and 2019YFC1005202).
Availability of data and materials
The datasets supporting the current study are
available on request: Please contact tjkeke@126.com or jihuiai@tjh.tjmu.edu.cn.
Authors' contributions
QL and XF performed the experiments, carried out
data analysis, and drafted the article. XL, GC, JC and BY
coordinated the research and experiments. KL and JA were
responsible for the study design and manuscript revision. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Our procedures were performed in accordance with the
Guide for the Care and Use of Laboratory Animals. The experiment
was proved by the Ethics Committee of Tongji Hospital, Tongji
Medical College, Huazhong University of Science and Technology
Institutional (TJ-A20171206).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGF
|
epidermal growth factor
|
|
EGFR
|
epidermal growth factor receptor
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
COCs
|
oocyte-cumulus complexes
|
|
AMH
|
anti-Müllerian hormone
|
|
PI3K
|
phosphatidylinositol-3 kinase
|
|
AKT
|
protein kinase B
|
|
PTEN
|
phosphatase and tensin homologue
deleted on chromosome ten
|
|
MAPK
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular regulated kinase
|
|
JAK
|
Janus kinase
|
|
STAT
|
signal transducers and activators of
transcription
|
|
PKA
|
protein kinase A
|
|
CDK
|
cyclin-dependent kinase
|
References
|
1
|
Johnson LN: Protein kinase inhibitors:
Contributions from structure to clinical compounds. Q Rev Biophys.
42:1–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dy GK and Adjei AA: Understanding,
recognizing, and managing toxicities of targeted anticancer
therapies. CA Cancer J Clin. 63:249–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christopoulos C, Dimakopoulou V and Rotas
E: Primary ovarian insufficiency associated with imatinib therapy.
N Engl J Med. 358:1079–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malozowski S, Nelson L and Calis KA: More
on ovarian insufficiency with imatinib. N Engl J Med. 358:26482008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schultheis B, Nijmeijer BA, Yin H, Gosden
RG and Melo JV: Imatinib mesylate at therapeutic doses has no
impact on folliculogenesis or spermatogenesis in a leukaemic mouse
model. Leuk Res. 36:271–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asadi-Azarbaijani B, Santos RR,
Jahnukainen K, Braber S, van Duursen MBM, Toppari J, Saugstad OD,
Nurmio M and Oskam IC: Developmental effects of imatinib mesylate
on follicle assembly and early activation of primordial follicle
pool in postnatal rat ovary. Reprod Biol. 17:25–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sirotkin AV, Makarevich AV and Grosmann R:
Protein kinases and ovarian functions. J Cell Physiol. 226:37–45.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo F, Kuo YF, Shih YCT, Giordano SH and
Berenson AB: Trends in breast cancer mortality by stage at
diagnosis among young women in the United States. Cancer.
124:3500–3509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richani D and Gilchrist RB: The epidermal
growth factor network: Role in oocyte growth, maturation and
developmental competence. Hum Reprod Update. 24:1–14. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conti M, Hsieh M, Park JY and Su YQ: Role
of the epidermal growth factor network in ovarian follicles. Mol
Endocrinol. 20:715–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagyova E, Nemcova L, Mlynarcikova A,
Scsukova S and Kalous J: Lapatinib inhibits meiotic maturation of
porcine oocyte-cumulus complexes cultured in vitro in
gonadotropin-supplemented medium. Fertil Steril. 99:1739–1748.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Z, Parris AB, Xiao Z, Howard EW,
Kosanke SD, Feng X and Yang X: Short-term early exposure to
lapatinib confers lifelong protection from mammary tumor
development in MMTV-erbB-2 transgenic mice. J Exp Clin Cancer Res.
36:62017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spector NL, Robertson FC, Bacus S,
Blackwell K, Smith DA, Glenn K, Cartee L, Harris J, Kimbrough CL,
Gittelman M, et al: Lapatinib plasma and tumor concentrations and
effects on HER receptor phosphorylation in tumor. PLoS One.
10:e01428452015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelson JF, Felicio LS, Randall PK, Sims C
and Finch CE: A longitudinal study of estrous cyclicity in aging
C57BL/6J mice: I. Cycle frequency, length and vaginal cytology.
Biol Reprod. 27:327–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedersen T and Peters H: Proposal for a
classification of oocytes and follicles in the mouse ovary. J
Reprod Fertil. 17:555–557. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Voigtlaender M, Schneider-Merck T and
Trepel M: Lapatinib. Recent Results Cancer Res. 211:19–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
da Rosa PRA, De Cesaro MP, Pereira Dau AM,
Duggavathi R, Bordignon V and Gonçalves PBD: Reversible meiotic
arrest of bovine oocytes by EGFR inhibition and follicular
hemisections. Theriogenology. 99:53–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashkenazi H, Cao X, Motola S, Popliker M,
Conti M and Tsafriri A: Epidermal growth factor family members:
Endogenous mediators of the ovulatory response. Endocrinology.
146:77–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan HY, Liu Z, Shimada M, Sterneck E,
Johnson PF, Hedrick SM and Richards JS: MAPK3/1 (ERK1/2) in ovarian
granulosa cells are essential for female fertility. Science.
324:938–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siddappa D, Beaulieu É, Gévry N, Roux PP,
Bordignon V and Duggavathi R: Effect of the transient
pharmacological inhibition of Mapk3/1 pathway on ovulation in mice.
PLoS One. 10:e01193872015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su YQ, Denegre JM, Wigglesworth K, Pendola
FL, O'Brien MJ and Eppig JJ: Oocyte-dependent activation of
mitogen-activated protein kinase (ERK1/2) in cumulus cells is
required for the maturation of the mouse oocyte-cumulus cell
complex. Dev Biol. 263:126–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sobinoff AP, Sutherland JM and Mclaughlin
EA: Intracellular signalling during female gametogenesis. Mol Hum
Reprod. 19:265–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haraguchi S, Ikeda M, Akagi S and Hirao Y:
Dynamic changes in pStat3 are involved in meiotic spindle assembly
in mouse oocytes. Int J Mol Sci. 21:12202020. View Article : Google Scholar
|
|
24
|
Lee HS, Kim KH, Kim EY, Lee SY, Ko JJ and
Lee KA: Obox4-silencing-activated STAT3 and MPF/MAPK signaling
accelerate nuclear membrane breakdown in mouse oocytes.
Reproduction. 151:369–378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tscherner A, Brown AC, Stalker L, Kao J,
Dufort I, Sirard MA and LaMarre J: STAT3 signaling stimulates
miR-21 expression in bovine cumulus cells during in vitro oocyte
maturation. Sci Rep. 8:115272018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zulkifli AA, Tan FH, Putoczki TL, Stylli
SS and Luwor RB: STAT3 signaling mediates tumour resistance to EGFR
targeted therapeutics. Mol Cell Endocrinol. 451:15–23. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dobi E, Monnien F, Kim S, Ivanaj A,
N'Guyen T, Demarchi M, Adotevi O, Thierry-Vuillemin A, Jary M,
Kantelip B, et al: Impact of STAT3 phosphorylation on the clinical
effectiveness of anti-EGFR-based therapy in patients with
metastatic colorectal cancer. Clin Colorectal Cancer. 12:28–36.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haura EB, Sommers E, Song L, Chiappori A
and Becker A: A pilot study of preoperative gefitinib for
early-stage lung cancer to assess intratumor drug concentration and
pathways mediating primary resistance. J Thorac Oncol. 5:1806–1814.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Zhang D, Chen X, He L, Li T, Xu X
and Li M: Nuclear PKM2 contributes to gefitinib resistance via
upregulation of STAT3 activation in colorectal cancer. Sci Rep.
5:160822015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonner JA, Yang ES, Trummell HQ, Nowsheen
S, Willey CD and Raisch KP: Inhibition of STAT-3 results in greater
cetuximab sensitivity in head and neck squamous cell carcinoma.
Radiother Oncol. 99:339–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dowlati A, Nethery D and Kern JA: Combined
inhibition of epidermal growth factor receptor and JAK/STAT
pathways results in greater growth inhibition in vitro than single
agent therapy. Mol Cancer Ther. 3:459–463. 2004.PubMed/NCBI
|
|
32
|
Sen M, Joyce S, Panahandeh M, Li C, Thomas
SM, Maxwell J, Wang L, Gooding WE, Johnson DE and Grandis JR:
Targeting stat3 abrogates EGFR inhibitor resistance in cancer. Clin
Cancer Res. 18:4986–4996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R and Yim JH:
Synergistic anti-tumor effect of combined inhibition of EGFR and
JAK/STAT3 pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ and
Settleman J: Drug resistance via feedback activation of Stat3 in
oncogene-addicted cancer cells. Cancer Cell. 26:207–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|