Introduction
Lung cancer is one of the most frequent human
malignancies with the highest global incidence rate and mortality
(1). Approximately 246,220 newly
diagnosed cases and 147,510 mortalities due to respiratory system
cancer are estimated to occur worldwide each year (2). Clinically, non-small cell lung
carcinoma (NSCLC) is the major pathology subtype of lung cancer,
accounting for approximately 75–80% of all lung cancer cases
(3). Approximately 75% of patients
with NSCLC are diagnosed at an advanced stage, which indicates that
most patients are not completely cured after conventional
treatments (4). Despite the
integrated use of surgical resection, radiochemotherapy, and
targeted therapies, the outcomes of patients with NSCLC remain
unsatisfactory (5). The overall
5-year survival rate of patients with NSCLC is approximately 15%,
which has not noticeably improved in the past few decades (6). In view of this, there is a great
demand to better understand NSCLC pathogenesis in order to identify
promising diagnostic methods and therapeutic techniques.
Long noncoding RNAs (lncRNAs) are a group of
noncoding RNA transcripts longer than 200 nucleotides that are not
encoded into proteins (7). They are
identified as important contributors in modulating gene expression
via chromatin modification, transcriptional, post-transcriptional,
and translational regulation (8).
lncRNAs have gained increasing attention due to their roles in
carcinogenesis and cancer progression (9). Several lncRNAs are aberrantly
expressed in NSCLC. MIR503HG (10),
LINC00261 (11), and TOB1-AS1
(12) are downregulated in NSCLC,
whereas SNHG16 (13), FTH1P3
(14), and KCNQ1OT1 (15) are highly expressed. The
pro-oncogenic and anti-oncogenic activities of lncRNAs are known to
be implicated in NSCLC genesis and progression (16,17).
MicroRNAs (miRNAs) are an abundant family of highly
conserved, noncoding, and single-stranded RNA molecules with
lengths ranging from 18 to 25 nucleotides (18). miRNAs post-transcriptionally
regulate gene expression by complementarily binding to the
3′-untranslated region (3′-UTR) of their target mRNAs, which
consequently results in degradation and/or translation suppression
(19). An increasing amount of
evidence highlights that lncRNAs may be capable of directly
interacting with miRNAs by functioning as competitive endogenous
RNAs (ceRNAs) or molecular sponges to regulate their target genes
(20–22). Therefore, an in-depth exploration of
tumor-related lncRNAs and miRNAs in NSCLC, as well as of the
mechanisms underlying their roles, may be of great significance in
cancer diagnosis, therapy, and prevention.
PSMA3 antisense RNA 1 (PSMA3-AS1), an lncRNA, has
been revealed to promote the progression of esophageal squamous
cell carcinoma (23). However,
there are no studies to date that explore the expression or roles
of PSMA3-AS1 in NSCLC. In the present study, the expression profile
and roles of PSMA3-AS1 in NSCLC were determined. It was also
revealed how PSMA3-AS1 regulates the malignant phenotype of NSCLC
cells.
Materials and methods
Sample collection
NSCLC tissues and adjacent normal tissues were
collected from 61 patients (age range, 52–77 years; 32 male and 29
female patients) with NSCLC who were admitted to the Affiliated
Hospital of Beihua University between June 2014 and February 2015.
All participants were diagnosed with primary NSCLC and had not been
treated with preoperative radiotherapy, chemotherapy,
immunotherapy, or targeted therapy. All tissue samples were
immediately frozen in liquid nitrogen after surgical excision and
stored in liquid nitrogen until use. All participants provided
written informed consent, and the present study was approved by the
Ethics Committee of the Affiliated Hospital of Beihua University.
All experimental steps were conducted in accordance with the
Declaration of Helsinki.
Cell lines and culture conditions
The human non-tumorigenic bronchial epithelium cell
line BEAS-2B was acquired from American Type Culture Collection,
and NSCLC cell lines (A549, SK-MES-1, H1703, H460 and H522) were
obtained from Shanghai Academy of Life Sciences, the Chinese
Academy of Sciences. BEAS-2B cells were grown in BEGM™ Bronchial
Epithelial Cell Growth Medium (Lonza Group, Ltd.; Clonetics
Corporation), while SK-MES-1 cells were cultured in Minimum
Essential Medium (Gibco; Thermo Fisher Scientific, Inc.). The other
four NSCLC cell lines were cultured in Roswell Park Memorial
Institute (RPMI)-1640 media (Gibco; Thermo Fisher Scientific,
Inc.). All culture media was supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and a 1%
penicillin/streptomycin mixed solution (TransGen Biotech Co.,
Ltd.). All cells were incubated in humidified air with 5%
CO2 at 37°C.
Cell transfection
Small interfering RNAs (siRNAs) targeting PSMA3-AS1
(si-PSMA3-AS1#1, si-PSMA3-AS1#2, and si-PSMA3-AS1#3) and negative
control (si-NC) were synthesized by Shanghai GenePharma Co., Ltd..
The si-PSMA3-AS1#1 sequence was 5′-CTGATTTTATGGGAAAATTAAGC-3′; the
si-PSMA3-AS1#2 sequence was 5′-AACTTGAAAAGCACATTTTAAAT-3′; the
si-PSMA3-AS1#3 sequence was 5′-TAGTTAAGTTCTGTATTTAGTGA-3′; and the
si-NC sequence was 5′-CACGATAAGACAATGTATTT-3′. miR-493-3p mimic
(cat. no. miR10003161-1-5), scrambled mimic control (cat. no.
miR1N0000001-1-5; miR-NC), miR-493-3p inhibitor (cat. no.
miR20003161-1-5), and negative control inhibitor (cat. no.
miR2N0000001-1-5; NC inhibitor) were acquired from Guangzhou
RiboBio Co., Ltd. The spindlin 1 (SPIN1) overexpression plasmid
(pcDNA3.1/SPIN1) and empty pcDNA3.1 plasmid were designed and
generated by Shanghai GenePharma Co., Ltd.. Cells were inoculated
into 6-well plates with a density of 8×105 cells/well,
and transfected with miRNA mimic (50 nM), miRNA inhibitor (50 nM),
siRNA (100 nM), or plasmid (4 µg) using the Lipofectamine 2000™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Cell Counting
Kit-8 (CCK-8) and cell apoptosis assays were conducted after 24 and
48 h of incubation, respectively. Transwell migration and invasion
assays and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) were performed at 48 h post-transfection.
Seventy-two hours later, western blotting was carried out to detect
protein expression.
Subcellular fractionation
A Cytoplasmic and Nuclear RNA Purification Kit
(Norgen Biotek Corp.) was used to isolate nuclear and cytosolic
fractions in NSCLC cells. The relative expression of PSMA3-AS1 in
nuclear and cytosolic fractions was analyzed by RT-qPCR. U6 small
nuclear RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
were used as the nuclear and cytoplasmic controls,
respectively.
RT-qPCR
The extraction of total RNA was conducted using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). To quantify
PSMA3-AS1 and SPIN1, complementary DNA was synthesized by reverse
transcription using a PrimeScript RT Reagent Kit (Takara Bio.). The
temperature protocol for reverse transcription was as follows: 37°C
for 15 min and 85°C for 5 sec. The complementary DNA was then used
as a template to determine the expression of target genes using
SYBR Premix Ex Taq (Takara Bio). The thermocycling conditions for
quantitative PCR were as follows: 5 min at 95°C, followed by 40
cycles at 95°C for 30 sec and 65°C for 45 sec, and 50°C for 30 sec.
The expression of PSMA3-AS1 and SPIN1 was normalized to that of
GAPDH. To determine miR-409-3p expression, total RNA was reverse
transcribed into complementary DNA using a miScript Reverse
Transcription Kit (Qiagen GmbH). The temperature protocols for
reverse transcription were as follows: 37°C for 60 min, 95°C for 5
min and maintenance at 4°C. Quantitative PCR was conducted using a
miScript SYBR Green PCR Kit (Qiagen GmbH). The thermocycling
conditions for quantitative PCR were as follows: 95°C for 2 min,
95°C for 10 sec, 55°C for 30 sec and 72°C for 30 sec, for 40
cycles. U6 was used as the control for miR-409-3p expression. The
2−ΔΔCq method (24) was
applied to calculate relative gene expression.
The primers were designed as follows: PSMA3-AS1,
5′-TTCCTCCAGGACAGCACCTAGT-3′ (forward) and
5′-CGTCTCTGATGTGGCTTATACGA-3′ (reverse); SPIN1,
5′-CCAACATGATGAAGAAGAGGACAT-3′ (forward) and
5′-AGGATTTACAGGCACCTGGTCC-3′ (reverse); miR-409-3p,
5′-TCGGCAGGAGGUUACCCGAGCA-3′ (forward) and
5′-CACTCAACTGGTGTCGTGGA-3′ (reverse); U6,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and
5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse); and GAPDH,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ (forward) and
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ (reverse).
CCK-8 assay
The proliferative ability of NSCLC cells was
evaluated using CCK-8 (Dojindo Molecular Technologies, Inc.).
Transfected cells were collected after 24 h of culture and seeded
into a 96-well plate at a density of 2×103 cells/well.
To assess cell proliferation, 10 µl of the CCK-8 solution was added
into each well, and the plates were incubated in humidified air
with 5% CO2 at 37°C. The optical density at a wavelength
of 450 nm in each well was measured using a microplate reader
(Bio-Rad Laboratories, Inc.). Cellular proliferation was detected
at four time points (0, 1, 2 and 3 days after cell inoculation),
and the growth curves were plotted according to the measurement
data.
Cell apoptosis assay
After 48 h of culture, transfected cells
(~1.5×106 cells) were collected and rinsed with ice-cold
phosphate buffer solution (Gibco, Thermo Fisher Scientific, Inc).
Following centrifugation at 4°C, cells were resuspended in 100 µl
of binding buffer from the Annexin V-Fluorescein Isothiocyanate
(FITC) Apoptosis Detection Kit (Biolegend) and double-stained with
5 µl of FITC-Annexin V and 5 µl of propidium iodide at room
temperature in the dark. The apoptosis rate was examined using a
flow cytometer (FACScan; BD Biosciences). Cell Quest software
(version 5.1; BD Biosciences) was used for data analysis.
Transwell migration and invasion
assays
The migratory ability of NSCLC cells was determined
using Transwell chambers (8 µm; BD Biosciences). The Transwell
invasion assay was performed with experimental steps that were
similar to the migration assay, except that Matrigel (BD
Biosciences) was used to coat the chambers. In brief, 100 µl of
FBS-free culture medium containing 5×104 cells were
added into the upper compartments, while the lower compartments
were covered with culture medium supplemented with 20% FBS, which
was used as a chemoattractant. Following a 24-h incubation, the
non-migrated and non-invaded cells were gently removed with a
cotton swab, whereas the migrated and invaded cells were fixed with
4% paraformaldehyde at room temperature for 20 min and stained with
0.1% crystal violet at room temperature for 20 min. An inverted
light microscope (×200 magnification; Olympus Corporation) was used
to image and count the number of migrated and invaded cells.
Xenograft tumor assay
A short hairpin RNA (shRNA) targeting PSMA3-AS1
(sh-PSMA3-AS1) and negative control (sh-NC) were synthesized by
Shanghai GenePharma Co., Ltd. and used for lentivirus production.
H460 cells were transfected with lentivirus expressing sh-PSMA3-AS1
or sh-NC, and the stably transfected cells were selected using
puromycin. The sh-PSMA3-AS1 sequence was
5′-CCGGCTAATTTTATGGGAAAATTAAGCCTCGAGGCTTAATTTTCCCATAAAATCAGTTTTTG-3′
and the sh-NC sequence was
5′-CCGGCACGATAAGACAATGTATTTCTCGAGAAATACATTGTCTTATCGTGTTTTTG-3′.
Six-week-old male BALB/c nude mice (total number, 6;
weight, 20 g) were purchased from SLAC Laboratory Animal Co., Ltd.,
and kept under pathogen-free conditions at 25°C with 50% humidity,
with a 10/14-h light/dark cycle and ad libitum food/water
access. Animal experiments were performed under the approval of the
Animal Ethics Committee of the Affiliated Hospital of Beihua
University. H460 cells that stably expressed sh-PSMA3-AS1 or sh-NC
were subcutaneously injected into the flank of nude mice. After 8
days, tumor size was recorded every 5 days and used for calculating
the tumor volume with a simplified equation (tumor volume=length ×
width2 ×0.5). All mice were euthanized by cervical
dislocation at 4 weeks post-injection, and tumor xenografts were
excised and weighed.
Bioinformatics analysis
StarBase version 3.0 (http://starbase.sysu.edu.cn/) was used to predict the
target miRNA(s) of PSMA3-AS1.
RNA immunoprecipitation (RIP)
The Magna-RIP RNA Binding Immunoprecipitation Kit
(EMD Millipore) was used to verify the interaction between
PSMA3-AS1 and miR-409-3p. NSCLC cells were collected and lysed in
radioimmunoprecipitation assay (RIPA) buffer that was supplemented
with a protease inhibitor cocktail and RNase inhibitor. Cell
lysates were collected and incubated with magnetic beads coated
with Anti-Argonaute2 (Anti-Ago2) or IgG (both from EMD Millipore)
at 4°C overnight. The coprecipitated RNA was extracted and used in
RT-qPCR to determine the expression of PSMA3-AS1 and
miR-409-3p.
Luciferase reporter assay
The sequences of wild-type (WT) PSMA3-AS1 containing
miR-409-3p binding sequences and mutant (MUT) PSMA3-AS1 fragments
were synthesized by Shanghai GenePharma Co., Ltd. and inserted into
a pmirGLO luciferase reporter vector (Promega Corporation),
generating WT-PSMA3-AS1 and MUT-PSMA3-AS1. NSCLC cells were seeded
into 24-well plates with a density of 1.5×105
cells/well. After overnight culture at 37°C, cells were
co-transfected with WT-PSMA3-AS1 (1.6 µg) or MUT-PSMA3-AS1 (1.6 µg)
and miR-409-3p mimic (50 nM) or miR-NC (50 nM) using Lipofectamine™
2000. Following 48 h of culture, a Dual-Luciferase Reporter assay
system (Promega Corporation) was used to assess luciferase
activity. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Western blotting
Total protein was isolated from cultured cells using
RIPA buffer (Beyotime Institute of Biotechnology). After
quantification by the bicinchoninic acid assay (Beyotime Institute
of Biotechnology), equal amounts of protein (30 µg) were separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and subsequently transferred onto polyvinylidene difluoride
membranes. The membranes were then blocked with 5% non-fat milk
powder at room temperature for 2 h and incubated with primary
antibodies overnight at 4°C. Rabbit anti-human SPIN1 (product code
ab118784) and rabbit anti-human GAPDH (product code ab181603; both
from Abcam) antibodies were used with a dilution of 1:1,000.
Membranes were then incubated with a goat anti-rabbit horseradish
peroxidase-conjugated antibody (product code ab205718; 1:5,000
dilution; Abcam), after which the protein signals were detected
using an Enhanced Chemiluminescence Western Blotting Detection
Reagent (GE Healthcare Life Sciences). Quantity One software
(version 4.62; Bio-Rad Laboratories, Inc.) was utilized for
densitometric analysis.
Statistical analysis
All data are presented as the means ± standard
deviation and analyzed with SPSS version 13.0 software (SPSS,
Inc.). Pearson's correlation coefficient analysis was performed to
examine the correlation between PSMA3-AS1 and miR-409-3p in NSCLC
tissues. The χ2 test was used to test the association
between PSMA3-AS1 expression and clinicopathological factors in
patients with NSCLC. Student's t-test was performed to analyze the
differences between two groups, whereas one-way analysis of
variance with Tukey's post hoc test was used for comparing the data
among multiple groups. Survival curves were plotted using the
Kaplan-Meier method, after which the survival curves were compared
with the log-rank test. A P-value <0.05 (P<0.05) was
considered to indicate a statistically significant difference.
Results
PSMA3-AS1 is upregulated in NSCLC and
negatively associated to the survival of patients
First, RT-qPCR was performed to determine PSMA3-AS1
expression in 61 pairs of NSCLC tissues and adjacent normal
tissues. The data confirmed the higher expression of PSMA3-AS1 in
NSCLC tissues relative to that in adjacent normal tissues (Fig. 1A). In addition, PSMA3-AS1 expression
in a panel of NSCLC cell lines (A549, SK-MES-1, H1703, H460 and
H522) and a human non-tumorigenic bronchial epithelium cell line
BEAS-2B was investigated via RT-qPCR. PSMA3-AS1 expression was
significantly increased in the five examined NSCLC cell lines
compared with in BEAS-2B cells (Fig.
1B).
The median value of PSMA3-AS1 expression in NSCLC
tissues was regarded as the cut-off line, and all enrolled patients
with NSCLC were accordingly classified into either low-PSMA3-AS1
(n=30) or high-PSMA3-AS1 (n=31) groups. Increased PSMA3-AS1
expression was significantly associated with tumor-node-metastasis
(TNM) stage (P=0.040), and lymph node metastasis (P=0.018) in the
61 patients with NSCLC (Table I).
Furthermore, patients with NSCLC with high PSMA3-AS1 expression had
a relatively shorter overall survival than the patients with low
PSMA3-AS1 expression (Fig. 1C;
P=0.0357). These results indicated that PSMA3-AS1 was upregulated
in NSCLC and negatively associated with the clinical outcomes of
patients.
 | Table I.Association between PSMA3-AS1
expression and clinicopathological factors in NSCLC. |
Table I.
Association between PSMA3-AS1
expression and clinicopathological factors in NSCLC.
|
| PSMA3-AS1 |
|
|---|
|
|
|
|
|---|
| Factors | High (n=31) | Low (n=30) | P-value |
|---|
| Sex |
|
| 0.164 |
|
Male | 17 | 15 |
|
|
Female | 14 | 15 |
|
| Age (years) |
|
| 0.779 |
|
<55 | 11 | 13 |
|
|
≥55 | 20 | 17 |
|
| Smoking
history |
|
| 0.267 |
|
Smokers | 14 | 12 |
|
|
Non-smokers | 17 | 18 |
|
| Tumor size
(cm) |
|
| 0.073 |
|
<3 | 10 | 17 |
|
| ≥3 | 21 | 13 |
|
| TNM stage |
|
| 0.040 |
|
I–II | 13 | 21 |
|
|
III–IV | 18 | 9 |
|
| Lymph node
metastasis |
|
| 0.018 |
|
Negative | 14 | 23 |
|
|
Positive | 17 | 7 |
|
PSMA3-AS1 depletion inhibits
proliferation, migration, and invasion and increases apoptosis in
NSCLC cells
Since A549 and H460 cell lines exhibited the most
significant upregulation of PSMA3-AS1 expression among the five
NSCLC cell lines, these two cell lines were selected for subsequent
functional assays. To investigate the precise roles of PSMA3-AS1 in
NSCLC, three siRNAs targeting PSMA3-AS1 (si-PSMA3-AS1#1,
si-PSMA3-AS1#2, and si-PSMA3-AS1#3) were designed and transfected
into A549 and H460 cells. The transfection efficiencies were
evaluated by RT-qPCR analysis. A substantial decrease in PSMA3-AS1
was confirmed in A549 and H460 cells after transfection with
si-PSMA3-AS1 (Fig. 2A).
si-PSMA3-AS1#1 was the most effective at silencing PSMA3-AS1
expression and therefore was renamed si-PSMA3-AS1 and used in the
loss-of-function experiments.
The CCK-8 assay was conducted to evaluate the
proliferation of A549 and H460 cells after PSMA3-AS1 knockdown.
Transfection with si-PSMA3-AS1 significantly inhibited the
proliferative ability of A549 and H460 cells relative to that in
si-NC-transfected cells (Fig. 2B).
In addition, knockdown of PSMA3-AS1 resulted in increased apoptosis
of A549 and H460 cells compared with the si-NC group (Fig. 2C), as evidenced by flow cytometric
analysis. Furthermore, the migration and invasion abilities of
NSCLC cells were analyzed by Transwell assays, which revealed that
knockdown of PSMA3-AS1 caused a significant decrease in the
migratory (Fig. 2D) and invasive
(Fig. 2E) abilities of A549 and
H460 cells. To summarize, PSMA3-AS1 downregulation inhibited the
aggressive phenotype of NSCLC cells.
PSMA3-AS1 acts as a molecular sponge
for miR-409-3p and negatively regulates its levels in NSCLC
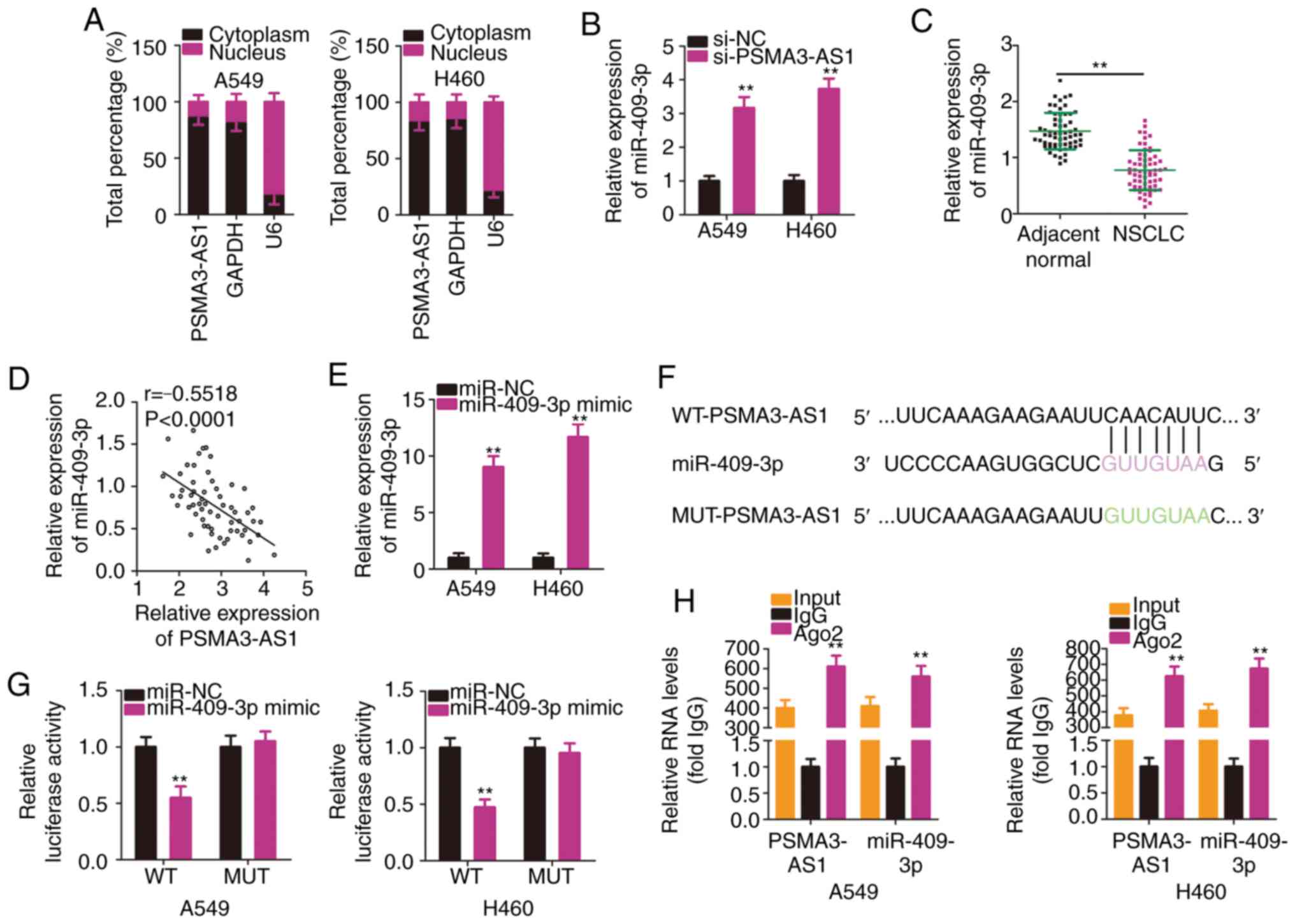
To reveal the molecular mechanism of PSMA3-AS1 in
NSCLC, subcellular fractionation was used to analyze the expression
distribution of PSMA3-AS1 in A549 and H460 cells. PSMA3-AS1 was
mostly distributed in the cytoplasm of A549 and H460 cells
(Fig. 3A). Mechanistically, it is
widely accepted that cytoplasmic lncRNAs are implicated in the
regulation of target genes by sponging miRNAs (25). Accordingly, it was presumed that
PSMA3-AS1 may function as a ceRNA or molecular sponge for specific
miRNAs in NSCLC cells.
To test the aforementioned hypothesis,
bioinformatics analysis was performed to identify putative miRNAs
harboring binding site(s) in PSMA3-AS1. Table SI indicates all the putative miRNAs
harboring binding sites within PSMA3-AS1. miR-409-3p is known to
exhibit cancer-inhibiting roles in NSCLC (26), and therefore, it was selected for
subsequent experiments. The RT-qPCR results revealed that
miR-409-3p expression was significantly increased in A549 and H460
cells upon PSMA3-AS1 knockdown (Fig.
3B). In addition, miR-409-3p was significantly downregulated in
NSCLC tissues compared with in adjacent normal tissues (Fig. 3C). Furthermore, an inverse
correlation between the levels of miR-409-3p and PSMA3-AS1 in the
61 NSCLC tissues was demonstrated by Pearson's correlation
coefficient analysis (Fig. 3D;
r=−0.5518, P<0.0001).
To conduct follow-up assays, miR-409-3p mimic was
used to overexpress miR-409-3p in A549 and H460 cells, which was
confirmed by RT-qPCR analysis (Fig.
3E). A luciferase reporter assay was then conducted to confirm
the binding interaction between miR-409-3p and PSMA3-AS1 in NSCLC
cells. The WT and MUT binding sequences between miR-409-3p and
PSMA3-AS1 are presented in Fig. 3F.
The results of the luciferase reporter assays demonstrated that
exogenous miR-409-3p expression suppressed the luciferase activity
of WT-PSMA3-AS1-treated A549 and H460 cells, whereas the luciferase
activity of MUT-PSMA3-AS1 was unaffected following miR-409-3p
overexpression (Fig. 3G). According
to the results of the RIP assay, PSMA3-AS1 and miR-409-3p were
substantially enriched in Ago2-containing beads in A549 and H460
cells (Fig. 3H). This indicated
that PSMA3-AS1 and miR-409-3p co-exist in an RNA-induced silencing
complex and that PSMA3-AS1 and miR-409-3p directly interact in
NSCLC cells. In short, these results demonstrated that PSMA3-AS1
acted as a molecular sponge for miR-409-3p in NSCLC cells.
PSMA3-AS1 modulates SPIN1 expression
in NSCLC cells by sponging miR-409-3p
Considering that PSMA3-AS1 acts as a molecular
sponge, it was hypothesized that PSMA3-AS1 may regulate the
expression of SPIN1, a direct target of miR-409-3p (26), in NSCLC cells via sponging
miR-409-3p. The expression levels of SPIN1 mRNA and protein in
PSMA3-AS1-deficient A549 and H460 cells were determined by RT-qPCR
and western blotting. Reduced PSMA3-AS1 expression substantially
decreased the expression of SPIN1 mRNA (Fig. 4A) and protein (Fig. 4B) in A549 and H460 cells. To perform
rescue experiments, miR-409-3p inhibitor or NC inhibitor was
transfected into A549 and H460 cells. After confirming the
inhibition of miR-409-3p expression by the miR-409-3p inhibitor
(Fig. 4C), rescue experiments were
conducted. A549 and H460 cells were transfected with miR-409-3p
inhibitor or NC inhibitor in the presence of si-PSMA3-AS1. SPIN1
mRNA (Fig. 4D) expression was
significantly downregulated by PSMA3-AS1 knockdown, while this
reduction was abrogated in A549 and H460 cells by co-transfection
with the miR-409-3p inhibitor. The impact of miR-409-3p inhibitor
on SPIN1 mRNA expression was determined via RT-qPCR, and the
results indicated that miR-409-3p inhibitor transfection resulted
in a significant increase of SPIN1 mRNA expression in A549 and H460
cells (Fig. 4E). Additionally, the
si-PSMA3-AS1-mediated downregulation of SPIN1 protein expression
was restored in A549 and H460 cells after miR-409-3p inhibitor
co-transfection (Fig. 4F).
Furthermore, SPIN1 was upregulated in NSCLC tissues (Fig. 4G) and was positively associated with
PSMA3-AS1 expression (Fig. 4H;
r=0.5220, P<0.0001). Collectively, these results clearly
demonstrated that PSMA3-AS1 positively regulated SPIN1 expression
in NSCLC cells by competitively binding to miR-409-3p.
PSMA3-AS1-knockdown inhibitory
activities in NSCLC cells are counteracted by miR-409-3p inhibition
or SPIN1 upregulation
To further investigate whether the miR-409-3p/SPIN1
axis mediates the oncogenic actions of PSMA3-AS1 in NSCLC cells,
rescue experiments were designed and conducted. Before these
experiments, SPIN1 expression was determined to be increased in
A549 and H460 cells following transfection with pcDNA3.1/SPIN1
(Fig. 5A). Subsequently,
PSMA3-AS1-depleted A549 and H460 cells were co-transfected with
pcDNA3.1/SPIN1 or an empty pcDNA3.1 plasmid. PSMA3-AS1 knockdown
significantly inhibited A549 and H460 cell proliferation (Fig. 5B) and promoted cell apoptosis
(Fig. 5C), whereas pcDNA3.1/SPIN1
co-transfection abolished these effects. In addition, restoring
SPIN1 expression attenuated PSMA3-AS1 knockdown-induced suppression
of A549 and H460 cell migration (Fig.
5D) and invasion (Fig. 5E).
Furthermore, PSMA3-AS1 deficiency-induced inhibition of cell
proliferation (Fig. 5F) and
enhancement of cell apoptosis (Fig.
5G) were rescued by miR-409-3p inhibition. Similarly, the
impaired migratory (Fig. 5H) and
invasive (Fig. 5I) abilities
following PSMA3-AS1 knockdown were restored with the
co-transfection of the miR-409-3p inhibitor. Collectively,
PSMA3-AS1 promoted the malignant characteristics of NSCLC cells via
the miR-409-3p/SPIN1 axis.
 | Figure 5.Overexpression of SPIN1 or inhibition
of miR-409-3p counteracts PSMA3-AS1 depleted-induced inhibition of
NSCLC cell proliferation, migration, and invasion, as well as the
increase in cell apoptosis. (A) Western blotting was used to
determine the SPIN1 protein level in A549 and H460 cells after
pcDNA3.1/SPIN1 or pcDNA3.1 transfection. (B and C) A549 and H460
cells were co-transfected with pcDNA3.1/SPIN1 or pcDNA3.1 and
si-PSMA3-AS1. The assessment of cell proliferation and apoptosis
was performed via the CCK-8 assay and flow cytometric analysis,
respectively. (D and E) The evaluation of migration and invasion in
the cells described above was conducted by Transwell migration and
invasion assays. (F-I) The miR-409-3p inhibitor or NC inhibitor,
along with si-PSMA3-AS1, was transfected into A549 and H460 cells.
The proliferation, apoptosis, migration, and invasion were
determined by the CCK-8 assay, flow cytometric analysis, and
Transwell migration and invasion assays, respectively. *P<0.05
and **P<0.01. SPIN1, spindlin 1; miR-409-3p, microRNA-409-3p;
PSMA3-AS1, PSMA3 antisense RNA 1; NSCLC, non-small cell lung
carcinoma; CCK-8, Cell Counting Kit-8. |
Interference of PSMA3-AS1 impedes
NSCLC tumor growth in vivo
A xenograft tumor assay was performed to reveal the
role of PSMA3-AS1 in NSCLC tumor growth in vivo. H460 cells
infected with lentivirus expressing sh-PSMA3-AS1 or sh-NC were
subcutaneously inoculated into nude mice. The growth of tumor
xenografts was reduced in the sh-PSMA3-AS1 group compared with that
in the sh-NC group (Fig. 6A and B).
The average weight of the tumor xenografts stably transfected with
sh-PSMA3-AS1 was also decreased (Fig.
6C). Furthermore, the molecular analysis indicated that the
expression of PSMA3-AS1 remained low (Fig. 6D), whereas miR-409-3p expression was
increased (Fig. 6E) in the tumor
xenografts collected from the sh-PSMA3-AS1 group. In addition, the
tumor xenografts derived from sh-PSMA3-AS1 stably transfected H460
cells exhibited a significantly lower SPIN1 protein level than in
the sh-NC group (Fig. 6F), as
revealed by western blotting. Collectively, the downregulation of
PSMA3-AS1 inhibited NSCLC tumor growth in vivo by regulating
the miR-409-3p/SPIN1 axis.
Discussion
The dysregulation of lncRNAs in various types of
cancer is a growing concern among researchers (27,28).
Emerging studies have revealed that several lncRNAs are aberrantly
expressed in NSCLC, and they have been recognized to perform
oncogenic or anti-oncogenic activities during NSCLC progression
(16,29,30).
Therefore, the identification of cancer-related lncRNAs and
elucidation of the underlying mechanisms by which lncRNAs promote
the aggressive phenotype of cancer cells is essential for the
development of effective targets for anticancer therapies. The
present study detected the expression profile of PSMA3-AS1 in NSCLC
and determined its clinical relevance among patients with NSCLC. In
addition, extensive experimental exploration was performed to
investigate the detailed functions of PSMA3-AS1 during NSCLC
progression. Furthermore, the potential mechanisms underlying the
tumor-promoting actions of PSMA3-AS1 in NSCLC cells were elucidated
through a series of mechanistic experiments.
PSMA3-AS1 has been revealed to be highly expressed
in esophageal squamous cell carcinoma (23). High PSMA3-AS1 expression has been
demonstrated to be significantly associated with tumor size,
distant metastasis, and poor prognosis in patients with esophageal
squamous cell carcinoma (23). In
terms of its functions in esophageal squamous cell carcinoma,
exogenous PSMA3-AS1 expression has been revealed to facilitate cell
growth and metastasis in vitro (23). However, there are few studies that
focus on PSMA3-AS1 expression and its detailed involvement in
NSCLC. The results of the present study revealed that PSMA3-AS1 was
upregulated in NSCLC tissues and cell lines. Its overexpression was
closely associated with TNM stage and lymph node metastasis among
patients with NSCLC. Patients with NSCLC with high PSMA3-AS1
expression had shorter overall survival compared with patients with
low PSMA3-AS1 expression. In addition, knockdown of PSMA3-AS1
resulted in a decrease in cell proliferation, migration, and
invasion in vitro, as well as an increase in cell apoptosis.
Furthermore, knockdown of PSMA3-AS1 impaired NSCLC tumor growth
in vivo. Herein, it was revealed that si-PSMA3-AS1
transfection resulted in a reduction of NSCLC cell proliferation at
72 h, and migration and invasion at 24 h, whereas its knockdown
induced apoptosis at 48 h post-transfection. Difference in timing
observed for each effect may be attributed to the different effects
of PSMA3-AS1 silencing on function-related protein expression. We
aim to address this issue further in future studies.
Mechanistically, ceRNAs have been identified as an
important group of post-transcriptional regulators that contribute
to carcinogenesis and cancer progression by regulating their target
genes through a miRNA-mediated mechanism (31). Accumulating evidence has revealed
that lncRNAs localized in the cytoplasm function as molecular
sponges to sequester miRNAs and consequently release their
downstream target mRNAs to regulate cancer progression (25). To identify the molecular events
underlying the oncogenic activities of PSMA3-AS1 in NSCLC, its
subcellular localization was first evaluated. It was confirmed that
PSMA3-AS1 was mainly localized in the cytoplasm of NSCLC cells,
indicating that PSMA3-AS1 may function as a miRNA molecular
sponge.
Next, bioinformatics analysis was performed to
identify the miRNAs that potentially interact with PSMA3-AS1.
miR-409-3p has been reported to perform tumor-suppressive roles in
NSCLC (26). Therefore, miR-409-3p
was selected for subsequent experimental verification. The RT-qPCR
analysis confirmed that the downregulation of PSMA3-AS1 increased
the expression of miR-409-3p in NSCLC cells. In addition,
miR-409-3p was expressed at low levels in NSCLC, which was
consistent with previous findings (26). The direct binding interaction
between miR-409-3p and PSMA3-AS1 in NSCLC cells was verified by
luciferase reporter and RIP assays. SPIN1 was identified as a
downstream gene of miR-409-3p (26); therefore, it was hypothesized that
PSMA3-AS1 could regulate SPIN1 expression in NSCLC cells by
competitively sponging miR-409-3p, thereby driving the malignant
properties of NSCLC cells. In agreement with our hypothesis, the
expression level of SPIN1 was positively regulated by PSMA3-AS1 in
NSCLC cells. Additional studies demonstrated that SPIN1 was highly
expressed in NSCLC cells and positively correlated with PSMA3-AS1
expression. Rescue experiments further validated that the sponging
of miR-409-3p was essential for the regulation of SPIN1 expression
by PSMA3-AS1 in NSCLC cells. Collectively, a novel ceRNA molecular
pathway involving PSMA3-AS1, miR-409-3p, and SPIN1 was identified
in NSCLC cells.
miR-409-3p has been revealed to be downregulated in
NSCLC and significantly related to histological stage, tumor size,
pleural invasion, and metastasis (26). Notably, the overall survival and
disease-free survival of patients with NSCLC with low miR-409-3p
expression were shorter than that of patients with high miR-409
expression (26). SPIN1, a member
of the SPIN/SSTY gene family, is known to be a direct target of
miR-409-3p in NSCLC cells (26). It
has emerged as an essential regulator of cancer initiation and
progression (32,33). The results of the present study
indicated that PSMA3-AS1 acted as a ceRNA for miR-409-3p and
thereby enhanced SPIN1 expression. Rescue experiments demonstrated
that decreasing miR-409-3p or increasing SPIN1 abrogated the
effects of PSMA3-AS1-knockdown in NSCLC cells. Collectively, the
present results revealed that PSMA3-AS1 executed its oncogenic
activities in NSCLC cells by sponging miR-409-3p to increase SPIN1
expression. Therefore, the PSMA3-AS1/miR-409-3p/SPIN1 pathway
(Fig. 7) may contribute to the
progression of NSCLC.
In conclusion, it was demonstrated that PSMA3-AS1
functioned as an oncogenic lncRNA in NSCLC. PSMA3-AS1 sponged
miR-409-3p to increase SPIN1 expression and promote the aggressive
characteristics of NSCLC cells. The present study suggested that
the PSMA3-AS1/miR-409-3p/SPIN1 pathway could be an attractive
target for NSCLC therapies.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Jilin
Provincial Department of Health Research Project (grant nos.
2018J084 and 2014Q004) and the Jilin Science and Technology Bureau
Medical and Health Key Projects (grant no. 201830461).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LWa and JP designed the study. LWa, LWu and JP
performed all the experiments. LWa and JP wrote the manuscript. All
authors reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All participants provided written informed consent,
and this study was approved by the Ethics Committee of the
Affiliated Hospital of Beihua University. All experimental steps
were conducted in accordance with the Declaration of Helsinki.
Animal experiments were performed under the approval of the Animal
Ethics Committee of the Affiliated Hospital of Beihua
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avelino CU, Cardoso RM, Aguiar SS and
Silva MJ: Assessment of quality of life in patients with advanced
non-small cell lung carcinoma treated with a combination of
carboplatin and paclitaxel. J Bras Pneumol. 41:133–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keskin S, Kutluk AC and Tas F: Prognostic
and predictive role of angiogenic markers in non-small cell lung
cancer. Asian Pac J Cancer Prev. 20:733–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin P and Leighl NB: Review of the use
of pretest probability for molecular testing in non-small cell lung
cancer and overview of new mutations that may affect clinical
practice. Ther Adv Med Oncol. 9:405–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Li P, Zhang N, Cao L, Gao YF and
Ping F: Long non-coding RNA MIR503HG serves as a tumor suppressor
in non-small cell lung cancer mediated by wnt1. Eur Rev Med
Pharmacol Sci. 23:10818–10826. 2019.PubMed/NCBI
|
|
11
|
Wang Z, Zhang J, Yang B, Li R, Jin L, Wang
Z, Yu H, Liu C, Mao Y and You Q: Long intergenic noncoding RNA
00261 acts as a tumor suppressor in non-small cell lung cancer via
regulating miR-105/FHL1 axis. J Cancer. 10:6414–6421. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shangguan WJ, Liu HT, Que ZJ, Qian FF, Liu
LS and Tian JH: TOB1-AS1 suppresses non-small cell lung cancer cell
migration and invasion through a ceRNA network. Exp Ther Med.
18:4249–4258. 2019.PubMed/NCBI
|
|
13
|
Yu L, Chen D and Song J: LncRNA SNHG16
promotes non-small cell lung cancer development through regulating
EphA2 expression by sponging miR-520a-3p. Thorac Cancer.
11:603–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z and Wang Y: Long non-coding RNA
FTH1P3 promotes the metastasis and aggressiveness of non-small cell
lung carcinoma by inducing epithelial-mesenchymal transition. Int J
Clin Exp Pathol. 12:3782–3790. 2019.PubMed/NCBI
|
|
15
|
Kang Y, Jia Y, Wang Q, Zhao Q, Song M, Ni
R and Wang J: Long noncoding RNA KCNQ1OT1 promotes the progression
of non-small cell lung cancer via regulating miR-204-5p/ATG3 axis.
Onco Targets Ther. 12:10787–10797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu T, Wang Y, Chen D, Liu J and Jiao W:
Potential clinical application of lncRNAs in non-small cell lung
cancer. Onco Targets Ther. 11:8045–8052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Ma L, Xu F, Zhai W, Dong S, Yin L,
Liu J and Yu Z: Role of long non-coding RNA in drug resistance in
non-small cell lung cancer. Thorac Cancer. 9:761–768. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Y, Zhang C, Ma MH and Dai DQ:
Identification and prediction of novel non-coding and coding
RNA-associated competing endogenous RNA networks in colorectal
cancer. World J Gastroenterol. 24:5259–5270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu BQ, Lin XH, Ye XD, Huang W, Pei X,
Xiong D, Long X, Zhu SQ, Lu F, Lin K, et al: Long non-coding RNA
PSMA3-AS1 promotes malignant phenotypes of esophageal cancer by
modulating the miR-101/EZH2 axis as a ceRNA. Aging (Albany NY).
12:1843–1856. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdollahzadeh R, Daraei A, Mansoori Y,
Sepahvand M, Amoli MM and Tavakkoly-Bazzaz J: Competing endogenous
RNA (ceRNA) cross talk and language in ceRNA regulatory networks: A
new look at hallmarks of breast cancer. J Cell Physiol.
234:10080–10100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song Q, Ji Q, Xiao J, Li F, Wang L, Chen
Y, Xu Y and Jiao S: miR-409 inhibits human non-small-cell lung
cancer progression by directly targeting SPIN1. Mol Ther Nucleic
Acids. 13:154–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abildgaard C, Do Canto LM, Steffensen KD
and Rogatto SR: Long non-coding RNAs involved in resistance to
chemotherapy in ovarian cancer. Front Oncol. 9:15492020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng
X, Xiong F, Guo C, Wu X, Li Y, et al: Emerging role of
tumor-related functional peptides encoded by lncRNA and circRNA.
Mol Cancer. 19:222020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang L, Li Z and Wang R: Long noncoding
RNAs in lung cancer: Regulation patterns, biologic function and
diagnosis implications (Review). Int J Oncol. 55:585–596.
2019.PubMed/NCBI
|
|
30
|
Li L, Wang Y, Song G, Zhang X, Gao S and
Liu H: HOX cluster-embedded antisense long non-coding RNAs in lung
cancer. Cancer Lett. 450:14–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shuwen H, Qing Z, Yan Z and Xi Y:
Competitive endogenous RNA in colorectal cancer: A systematic
review. Gene. 645:157–162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang JX, Zeng Q, Chen L, Du JC, Yan XL,
Yuan HF, Zhai C, Zhou JN, Jia YL, Yue W and Pei XT: SPINDLIN1
promotes cancer cell proliferation through activation of WNT/TCF-4
signaling. Mol Cancer Res. 10:326–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Drago-Ferrante R, Pentimalli F, Carlisi D,
De Blasio A, Saliba C, Baldacchino S, Degaetano J, Debono J,
Caruana-Dingli G, Grech G, et al: Suppressive role exerted by
microRNA-29b-1-5p in triple negative breast cancer through SPIN1
regulation. Oncotarget. 8:28939–28958. 2017. View Article : Google Scholar : PubMed/NCBI
|