Introduction
Photodynamic therapy (PDT) was first introduced over
100 years ago, and causes cell death through interactions of a
photosensitizer, light source of appropriate wavelength, and
oxygen. PDT is widely used in dermatology, pulmonology, urology,
and ophthalmology (1). It is
considered as a safe and effective treatment option for several
dermatological conditions. Furthermore, the applications of PDT in
the treatment of tumors has made great progress in recent years.
However, PDT has certain limitations, such as poor penetration of
light and the risk of adverse reactions such as pain and burns
(2). Therefore, a novel technology
termed sonodynamic therapy (SDT) has been developed in recent
decades. Similar to PDT, SDT involves sensitizers and oxygen;
however, the main difference is that ultrasound, rather than a
laser is used as the energy source to activate the sensitizer.
Ultrasound is more penetrating than light, has fewer side effects
and induces cavitation effects, therefore SDT may have some
advantages in treating tumors (3–5).
As new strategies for cancer treatment, PDT and SDT
result in fewer side effects, and increased selectivity,
adaptability and compliance, compared with traditional methods.
Moreover, the combination of PDT/SDT and surgery, chemoradiotherapy
and immunotherapy may produce synergistic effects (6,7).
However, most of the early generation photosensitizers, such as
Photofrin are mixtures produced with no quality control standards
(8). Side effects such as pain,
burning, and phototoxicity limit the application of these
photosensitizers (9). Therefore,
there is a requirement for the development of safe and effective
sensitizers.
Sinoporphyrin sodium (DVDMS) is a novel
photosensitizer isolated from Photofrin derivatives. The activity
of DVDMS is similar to that of Photofrin at 10% of the dosage.
DVDMS has good water and lipid solubility, and can be activated by
both laser and ultrasound. Preliminary studies have demonstrated
that DVDMS is an effective and safe photosensitizer for PDT and
inhibits antitumor effects both in vitro and in vivo
(10,11). Furthermore, previous studies
revealed that DVDMS can achieve therapeutic effects following
ultrasound activation (12,13).
Glioma is an intractable primary cancer of the
central nervous system that is typically treated with surgery,
drugs, and immunotherapy. However, these treatment modalities have
certain limitations, and the development of new therapeutic
approaches is of great importance to improve patient outcomes
(14–17). Recent studies have indicated that
PDT and SDT are promising new therapeutic modalities for the
treatment of gliomas (18–21). Therefore, the aim of the present
study was to evaluate the antitumor effects of DVDMS-PDT and
DVDMS-SDT using human brain glioma cells in vitro and a
xenograft nude mouse model in vivo. In vivo
ultrasound and fluorescence imaging systems were used to evaluate
the fluorescence of DVDMS in vivo, tumor size, and
therapeutic effects, while FCM, Hoechst 33258 staining and western
blot analysis were performed to reveal the potential underlying
mechanisms.
Materials and methods
Reagents
DVDMS, kindly provided by Professor Qi-Cheng Fang
(Institute of Materia Medica, Chinese Academy of Medical Sciences
and Peking Union Medical College), was dissolved in normal saline
to a concentration of 1,000 µg/ml, stored at 4°C in the dark, and
used within 1 month. U-118 MG and U-87 MG human glioma cells were
purchased from Procell Life Science & Technology Co., Ltd. and
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.).
Animals
A total of 48 BALB/c nude mice (4 weeks old, 16–20
g, 24 male and 24 female), were obtained from Guangdong Medical
Laboratory Animal Center (Foshan, Guangdong, China). The animals
were housed under a 12-h light/dark cycle at 19.2–25.1°C and
relative humidity of 42–59% with ad libitum access to food
and water. Animals were allowed to acclimatize to a specific
pathogen-free environment for 5 days prior to treatment. The study
protocol was approved by the Ethics Committee of Shenzhen PKU-HKUST
Medical Center (SYXK 2015–0106) and was performed in accordance
with the International Guidelines on the Care and Use of Animals
for Scientific Purposes. The animal health and behavior were
monitored every day. Any unnecessary operation was prohibited to
avoid suffering and distress of the animals.
Measurement of the cellular uptake of
DVDMS by U-118 MG cells
Both U-87 MG (cat. no. CL-0238) and U-118 MG (cat.
no. CL-0458) cell lines were purchased from Procell Life Science
& Technology Co. Ltd., and the original preservation
organization was ATCC. The U-87 MG cell line used in this study was
established from glioblastoma of unknown origin. Both the two cell
lines were authenticated using STR profiling (authentication
reports were provided for the review process).
Absorption spectra of DVDMS were measured using a
micro-spectrophotometer (Q5000; Quawell Technology, Inc.). A total
of five absorption peaks at 369, 517, 550, 577 and 631 nm,
respectively, were observed for DVDMS in PBS. U-118 MG cells were
adjusted to a cell density of 1×106 cells/ml and
inoculated 1 ml cell suspension was plated per well in a 6-well
plate. A total of 1 ml DVDMS (10.00 µg/ml) was added to each well
and the plate was incubated for 0, 2, 4, or 6 h. The cells were
subsequently collected and washed with PBS. A single cell
suspension in PBS was analyzed using an Accuri™ C6 Plus flow
cytometer (BD Biosciences). DVDMS uptake was measured using the FL2
channel (585/40 nm).
Effects of DVDMS-mediated PDT and SDT
on cell proliferation and apoptosis
U-118 MG and U-87 MG cells (1×106
cells/ml) were inoculated in culture plates for 24 h and then
divided into three groups: DVDMS, PDT and SDT groups. Each group
was treated with DVDMS at the same concentration. After incubation
for 4 h, cells in the PDT group were exposed to a laser at 630 nm
using a PDT-630 Semiconductor Laser Unit (Guilin Xingda
Optoelectronic Medical Instrument Co., Ltd.) with a luminous flux
of 50 mW/cm2 for 10 min (total energy, 30
J/cm2). Cells in the SDT group were exposed to
ultrasound at a frequency of 1.0 MHz using an AFG3022B Ultrasonic
Signal Generator (Tektronix Inc.) at an intensity of 500
mW/cm2 for 1 min (total energy was 30 J/cm2),
as previously described (11,13).
Cells in the DVDMS group were not exposed to laser or ultrasound,
and served as a control.
Cell proliferation was measured using the Cell
Counting Kit (CCK)-8 assay (Dojindo Molecular Technologies, Inc.)
at 24 h after treatment. The concentrations of DVDMS investigated
were 0, 0.03, 0.06, 0.13, 0.25, 0.50, 1.00, 2.00, 4.00 and 8.00 µM.
After the addition of 10 µl CCK-8 reagent to 100 µl medium, the
cells were cultured at 37°C for 1 h and the optical density of the
cells was determined at an absorbance of 450 nm and a reference
wavelength of 405 nm using a Spectra Max i3× Microplate Reader
(Molecular Devices).
The Hoechst 33258 Staining kit (cat. no. C0003,
Beyotime Institute of Biotechnology) was used to observe the
influence of different treatment times on cell apoptosis. Cells
were divided into 6 groups: The control, laser, ultrasound, DVDMS,
PDT and SDT groups. Cells in the control group were not treated,
while cells in the laser and ultrasound groups were exposed to
laser (50 mW/cm2 for 10 min) and ultrasonic radiation
(500 mW/cm2 for 1 min), respectively. For the DVDMS, PDT
and SDT groups, the concentration of DVDMS was 0.5 µM, and cells in
PDT and SDT group received laser or ultrasound irradiation
aforementioned. At 1, 2, 4, 8 and 24 h after treatment, 0.5 ml
Hoechst 33258 dye solution was used for DNA staining. An IX51
fluorescence microscope (200X, Olympus Corporation) was used to
observe the apoptotic cells.
The PE Annexin V Apoptosis Detection kit I (BD
Pharmingen™) was used to detect the presence of apoptotic cells.
DVDMS was used at a concentration of 0.5 µM. After treatment for 6
h, the cells were washed twice with ice-cold PBS and resuspended in
1X binding buffer at a concentration of 1×106 cells/ml.
A total of 5 µl PE Annexin V and 5 µl 7-AAD were added per 1 ml
cell suspension, and the cells were incubated in the dark at room
temperature for 15 min. Apoptotic cells were subsequently
identified using a flow cytometer. NAC (cat. no. HY-B0215, Med Chem
Express Inc.), a reactive oxygen species (ROS) scavenger (22), was used to observe the effects of
ROS on cell death. A total of 1 µl NAC solution (1,000 mM) was
added to 1 ml cell culture medium, at a final concentration of 1
mM, prior to laser irradiation.
Evaluation of the antitumor effect in
vivo
U-118 MG cells (3×107 cells/ml) were
suspended in PBS containing 50% Matrigel (BD Biocoat™) and
subcutaneously implanted into 4-week-old BALB/c mice as previously
described (23,24). Forty-eight mice were divided into
eight groups (6 in each group): The control, laser, ultrasound,
DVDMS (2.0 mg/kg), low-dose DVDMS-PDT (PDT-L, DVDMS 1.0 mg/kg),
low-dose DVDMS-SDT (SDT-L, DVDMS 1.0 mg/kg), high-dose DVDMS-PDT
(PDT-H, DVDMS 2.0 mg/kg), and high-dose DVDMS-SDT (SDT-H, DVDMS 2.0
mg/kg) groups. At 24 h post DVDMS injection, mice in the laser,
PDT-L and PDT-H groups were exposed to the PDT-630 Laser Unit at a
luminous flux of 150 mW/cm2 for 10 min (total energy,
90.0 J/cm2), while those in the ultrasound, SDT-L and
SDT-H groups received ultrasonic irradiation at a frequency of 1.00
MHz and intensity of 500 mW/cm2 for 3 min (total energy,
90 J/cm2).
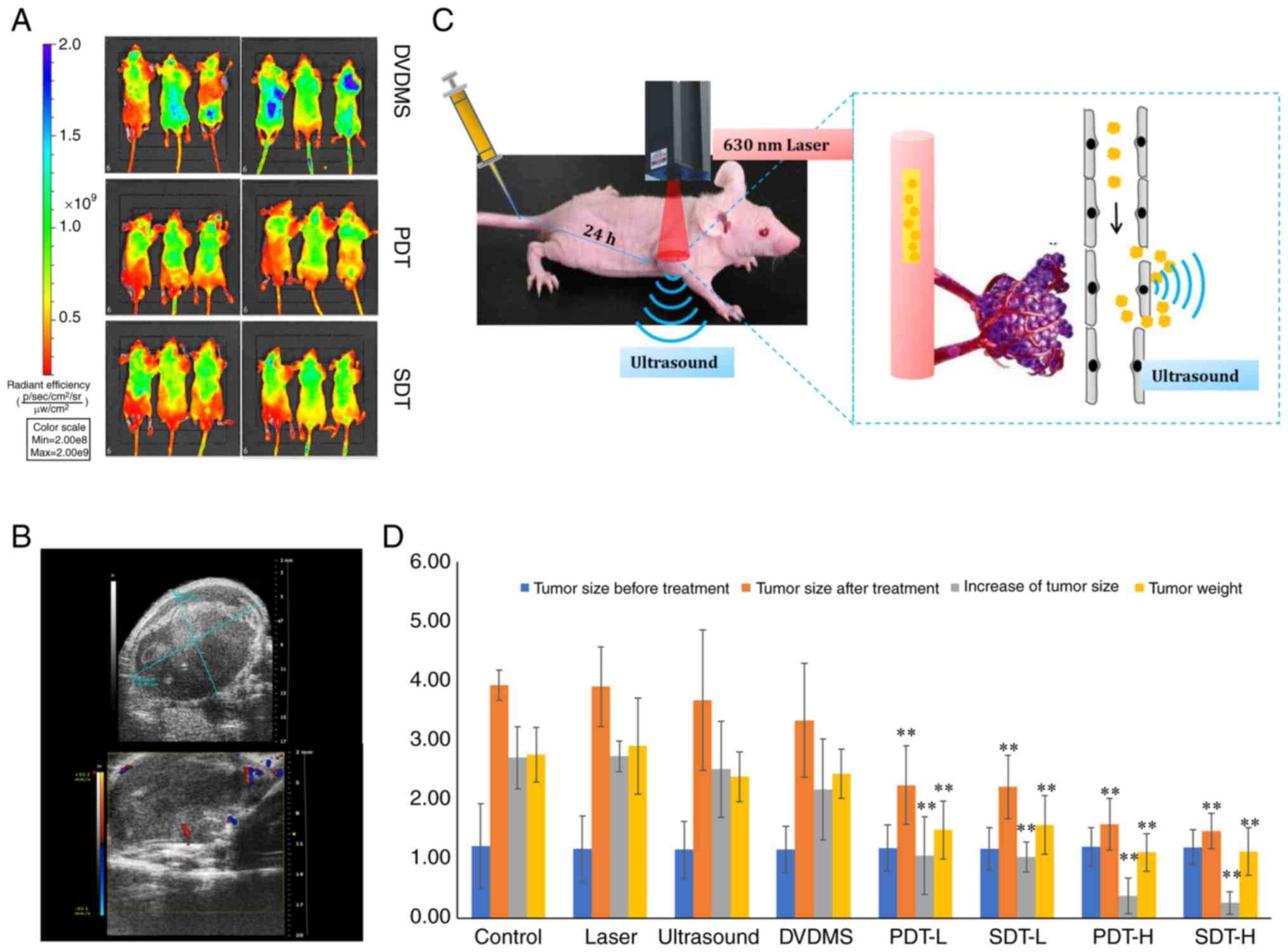
The IVIS® Lumina LT in vivo
Imaging system (Series III; Caliper Life Sciences) was used to
evaluate the in vivo fluorescence of DVDMS after treatment.
The Vevo 2100 Imaging system (FUJIFILM Visual Sonics Inc.) was used
to measure the tumor sizes and evaluate the therapeutic effects.
Mice were anesthetized using isoflurane (inhalation anesthesia, the
induction and maintenance dose was 3 and 1.5%, respectively) before
the PDT/SDT treatment, measurements and imaging observations to
minimize suffering and distress. Treatment was performed twice
(once per week). Two weeks later, when the treatment was over, the
mice were euthanized using pentobarbital sodium (120 mg/kg,
intraperitoneal injection) and the tumors were collected. The sizes
and weights of the tumors were measured and H&E staining was
performed to show the significant changes in vascular obstruction
and cell apoptosis in the PDT and SDT groups.
Effects of DVDMS-mediated PDT and SDT
on protein expression and phosphorylation
Western blot analysis was used to detect protein
expression and phosphorylation levels. Briefly, U-118 MG cells were
inoculated in 6-well plates (1 ml/well) for 24 h. Cells in the
DVDMS, PDT and SDT groups were treated with DVDMS (0, 0.03, 0.13
and 0.5 µM) for 4 h. The cells were subsequently treated as
aforementioned. At 6 h after treatment, the cells were collected,
washed three times with ice-cold PBS, and lysed with lysis buffer
(80 µl/well; Thermo Fisher Scientific, Inc.) containing 1 mM phenyl
methane sulfonyl fluoride (Thermo Fisher Scientific, Inc.), 1 mM
Na3VO4 (Sigma-Aldrich; Merck KGaA), and 20 mM
NaF (Sigma-Aldrich; Merck KGaA). The mixture was incubated at 4°C
for 30 min and then centrifuged at 12,900 × g for 30 min. Protein
concentrations were measured using a micro-spectrophotometer and
adjusted to 15.00 µg/ml. Then, 10.00 µl aliquots of the samples
were loading into the wells of polyacrylamide gels (spacer gel, 6%;
separation gel, 12%) and separated by electrophoresis at 80–120 V,
with a constant current of 330 mA for 90 min. The separated
proteins were transferred onto PVDF membranes (0.22 µm), which were
blocked for 1 h at room temperature. The membranes were washed
three times with Tris-buffered saline containing Tween (10 min per
wash), and incubated with primary antibodies overnight at 4°C
(dilution 1:1,000; PCNA, cat. no. PA5-27214; Bcl-xL, cat. no.
PA5-104974; Bax, cat. no. MA5-32031; p-PI3K, cat. no. PA5-104853;
p-AKT, cat. no. PA5-95669; p-mTOR, cat. no. 44–1125G; p-p70s6K,
cat. no. PA5-104841; p-rps6, cat. no. A300-584A; p-4EBP-1, cat. no.
700238; p-eIF4E, cat. no. 44-528G; p62, cat. no. PA5-20839; LC3,
cat. no. PA1-16931; Invitrogen; Thermo Fisher Scientific, Inc.) and
then horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG 1 h
at room temperature (dilution 1:5,000; cat. no. 31460; Invitrogen;
Thermo Fisher Scientific, Inc.). The protein bands were detected
using a chemiluminescence detector (Tanon 5220S; Guangzhou Ewell
Bio-technology Co., Ltd.). The gray values of the protein bands
were measured using the built-in analysis software of the
chemiluminescence detector, and all normalized to the housekeeper
control, for phosphorylated antibodies, the protein bands were then
normalized to the corresponding unphosphorylated ones. NAC was used
to observe the effects of ROS on protein expression and
phosphorylation changes induced by DVDMS-mediated PDT and SDT.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 23.0; IBM Corp.). Data are expressed as the means
± standard deviation. The one-way analysis of variance (Tukey's,
Dunnett's) was used to compare inter-group differences. P<0.05
was considered to indicate a statistically significant
difference.
Results
DVDMS uptake by U-118 MG cells
As shown in Fig. 1A,
five absorption peaks at 369, 517, 550, 577 and 631 nm,
respectively were observed for DVDMS in PBS. The cellular uptake of
DVDMS increased with the incubation time. Approximately 50% of the
U-118 MG cells contained DVDMS 2 h after incubation, which
increased to 80% at 4 h. Subsequently, the cellular uptake began to
decrease to ~5% at 6 h (Fig. 1B).
These results suggested that the optimum uptake of DVDMS by U-118
MG cells occurred at an incubation time of 4 h.
DVDMS-mediated PDT and SDT inhibit the
proliferation of glioma cells and induce apoptosis
The CCK-8 assay showed that DVDMS-mediated PDT and
SDT inhibited the proliferation of U-118 MG cells (Fig. 2A) and U-87 MG cells (Fig. S1A). Hoechst 33258 staining showed
that, the proportion of apoptotic cells increased with increasing
time after PDT or SDT (Fig. 2B and
C). Annexin V-positive cells were considered to be apoptotic.
PDT and SDT increased the number of apoptotic cells compared with
the control, and NAC partially abrogated this effect (Fig. 2D).
 | Figure 2.Effects of DVDMS-mediated PDT and SDT
on proliferation and apoptosis of U-118 MG cells. (A) Inhibition of
cell proliferation, *P<0.05, **P<0.01, ***P<0.001 vs.
DVDMS. (B) Hoechst 33258 staining of apoptotic cells:
(B1) control group, (B2) laser group,
(B3) ultrasound group, (B4) DVDMS group,
(B5) PDT group, (B6) SDT group; original
magnification was ×100, and the yellow bar indicates 100 µm. (C)
Effects of different treatment times on apoptosis, **P<0.01 vs.
the control group. (D) Effects of NAC on PDT- and SDT-induced cell
apoptosis: (D1) control group, (D2) DVDMS
group, (D3) PDT group, (D4) PDT+NAC group,
(D5) SDT group, (D6) SDT+NAC group. DVDMS,
sinoporphyrin sodium; PDT, photodynamic therapy; SDT, sonodynamic
therapy; NAC, N-acetyl-L-cysteine. |
DVDMS-mediated PDT and SDT inhibit
tumor development in U-118 MG xenograft models
The duration of the animal experiment lasted for 6
weeks, including 4 weeks of tumor cell inoculation and 2 weeks PDT
and SDT treatment period. There was no mouse found dead during the
whole experiment cycle. All mice were euthanized using
pentobarbital sodium (120 mg/kg) when the experiment was over
avoiding the pain inducing by tumor growth. The death of mice was
verified as follows: The breath, heartbeat and circulation were
permanently stopped and the onset of rigor mortis was confirmed.
According to previous studies, the maximum concentration of DVDMS
in tumors occurs at 24 h after intravenous injection through the
tail vein (10,11). Therefore, PDT and SDT were performed
at this time in the present study (Fig.
3C). Ultrasound results in sonoporation, which increases cell
clearance and membrane permeability, and may increase delivery of
drugs into tumor cells (25,26)
(Fig. 3C). In vivo
fluorescence revealed that the fluorescence intensity of DVDMS was
reduced in the PDT and SDT groups compared with the DVDMS group,
indicating that photobleaching occurred after PDT and SDT (Fig. 3A). DVDMS-PDT and DVDMS-SDT produced
biological effects in the tumors. Ultrasound images were taken
using the Vevo 2100 Imaging system, which showed that after 4 weeks
of tumor cell inoculation, low-echo masses were characteristically
elliptical in shape with clear boundaries. The shapes of certain
tumors were irregular with uneven internal echoes. Color Doppler
flow imaging of peripheral and blood flow signals is presented in
Fig. 3B. These findings indicated
that ultrasound images could be used as an index to evaluate
therapeutic effects. There were no significant differences in the
tumor sizes among all eight groups before treatment. However, after
treatment, the tumor size of the PDT and SDT groups was
significantly decreased compared with the control group
(P<0.01). Tumor cell proliferation and tumor weight were
significantly decreased in the PDT and SDT groups compared with the
control group (P<0.01). Individual application of the laser,
ultrasound or DVDMS had no therapeutic effects compared with the
control group (Fig. 3D). H&E
staining suggested that PDT and SDT induced significant apoptosis
and vascular obstruction of the cancer tissue (Fig. S1B).
Effects of DVDMS-mediated PDT and SDT
on protein expression and phosphorylation
Western blotting results showed that PDT and SDT
enhanced DVDMS suppression of PCNA and Bcl-xL, however, the levels
of Bax were not significantly different in the 3 groups (Fig. 4A-C). PCNA is a member of the DNA
sliding clamp family of proteins that are involved in DNA
replication, repair and the cell cycle. PCNA protein expression is
a well-accepted marker of proliferation (27). Suppression of PCNA may inhibit
glioma cells in vitro. Bcl-xL is a 30-kDa anti-apoptotic
protein that belong to the Bcl-2 family, and it can prevent
apoptosis through two different mechanisms: i) Heterodimerization
with an apoptotic protein, thereby inhibiting the apoptotic effect;
and ii) formation of mitochondrial outer membrane pores that help
maintain a normal membrane state under stressful conditions
(28). Therefore, suppression of
Bcl-xL by DVDMS-mediated PDT and SDT may lead to cell apoptosis.
Furthermore, the suppression of PCNA and Bcl-xL induced by PDT and
SDT was partly reversed by NAC, as well as the enhanced
cleaved-caspase 3 levels (Fig.
S1C). These results indicated that ROS was the main factor for
DVDMS-mediated PDT and SDT. This supported the flow cytometry
results demonstrating that NAC reduced PDT and SDT-induced
apoptosis in vitro.
 | Figure 4.DVDMS-mediated PDT and SDT inhibition
of the PI3K/AKT/mTOR signaling pathway. (A) PCNA, (B) Bcl-xL, (C)
Bax, (D) p-PI3K, (E) p-AKT, (F) p-mTOR, (G) p-p70s6K, (H) p-rps6,
(I) p-4EBP-1, (J) p-eIF4E, (K) p62, (L) LC3-II/LC3-I.
Concentrations of DVDMS in the three groups were 0, 0.03, 0.13 and
0.5 µM. PDT and SDT were performed 4 h after co-incubation, n=3,
*P<0.05, **P<0.01 vs. DVDMS. DVDMS, sinoporphyrin sodium;
PDT, photodynamic therapy; SDT, sonodynamic therapy; PCNA,
proliferating cell nuclear antigen; Bcl-xL, B-cell lymphoma-extra
large; Bax, BCL2-associated X; p-PI3K, phospho-phosphatidylinositol
3 kinase; p-AKT, phospho-protein kinase B; p-mTOR,
phospho-mammalian target of rapamycin; p-p70s6k, phospho-p70
ribosomal s6 kinase; p-rps6, phospho-ribosomal protein s6; p-4EBP1,
phospho-4E binding protein 1; p-eIF4E, phospho-protein synthesis
initiation factor 4E; SQSTM1/p62, sequestosome 1; LC3, light chain
3. |
The phosphorylation cascades of the PI3K/AKT/mTOR
signaling pathway can be suppressed by ROS to induce autophagy
(29). The results showed that the
phosphorylation levels of PI3K, AKT, mTOR, p70s6K, rps6 and eIF4E
were more significantly inhibited by both PDT and SDT, compared
with DVDMS alone; however, the phosphorylation levels of 4EBP-1
were significantly increased in the PDT and SDT groups than that in
DVDMS group (Fig. 4D-J). p62 and
LC3 are markers of autophagy. During autophagy, LC3-I is converted
to LC3-II through lipidation by a ubiquitin-like system. Lysosomal
degradation of autophagosomes leads to a decrease in p62 levels
during autophagy; conversely, autophagy inhibitors stabilize p62
levels (30,31). In the present study, both PDT and
SDT significantly inhibited the p62 levels (Fig. 4K), however, only PDT promoted the
conversion of LC3-I to LC3-II (Fig.
4L).
Discussion
Sinoporphyrin sodium (DVDMS) is a novel sensitizer
that is activated by both laser and ultrasound. Previous studies
have confirmed that DVDMS-mediated photodynamic therapy (PDT) and
sonodynamic therapy (SDT) are effective against several types of
tumors through mechanisms such as ROS generation, DNA damage,
matrix metallic proteinase downregulation, collapse of F-actin
filaments and cell apoptosis (32–34).
The present study demonstrated the antitumor effects of DVDMS-PDT
and DVDMS-SDT in U-118 MG cells in vitro and in vivo.
The results showed that both DVDMS-PDT and DVDMS-SDT significantly
inhibited proliferation and induced apoptosis of U-118 MG cells
in vitro. Moreover, in a nude mouse xenograft model, the
fluorescence intensity of DVDMS was lower in both the PDT and SDT
groups compared with the DVDMS group, while tumor proliferation and
weight were lower in the PDT and SDT groups compared with the
control group. These results indicated that both DVDMS-PDT and
DVDMS-SDT may serve as potential strategies for glioma treatment.
Burns and pain are the most common adverse reactions caused by PDT,
resulting in decreased patient compliance. These side effects are
mainly caused by the photothermal effects of the laser. In the
present study, a low power laser with a long irradiation time
showed good antitumor effects, indicating that a low-power laser
could be considered in future studies.
The present study had certain limitations. Future
studies investigating an orthotopic transplantation model should be
used to validated the therapeutic effects observed, as the
environment of tumor cells in the cranium may differ to that under
the skin. Furthermore, the blood-brain barrier (BBB) may block the
entry of DVDMS to the brain, therefore, drug delivery systems, such
as microbubbles, should be considered to increase treatment
efficiency. Recently, Pi et al (35) used focused ultrasound with
microbubbles to open the BBB to enhance the delivery of DVDMS to
intracranial U-87 MG tumors, and then used another ultrasound to
active DVDMS. The results revealed that DVDMS-SDT significantly
inhibited glioma in vivo. Liu et al (36) encapsulated DVDMS chelated with
manganese ions into nanoliposomes and demonstrated enhanced
antitumor effects, suggesting that this approach may be a promising
strategy for glioma treatment. Sun et al (37) used ultrasound-targeted microbubble
destruction combined with iRGD-modified DVDMS liposomes to treat
glioma, and found that the orthotopically implanted C6 gliomas were
significantly suppressed. The aforementioned studies provide
potential strategies for the treatment of brain gliomas with
DVDMS-SDT.
As the laser will not penetrate the skull, DVDMS-PDT
may be more challenging than SDT. However, there may be some
potential uses of DVDMS and its light sensitivity. Since glioma is
an invasive growth with unclear boundaries, it is difficult to
completely remove by surgery and patients are prone to recurrence.
DVDMS can be used as a fluorescent indicator to display the tumor
boundaries and guide surgical resection, on the other hand,
postoperative DVDMS-PDT may inhibit tumor recurrence. Yan et
al (38) developed a novel
photo-theranostic agent based on DVDMS-loaded PEGylated graphene
oxide with improved fluorescence properties for enhanced optical
imaging guided PDT. Photoacoustic imaging (PAI) is a novel
non-invasive biomedical imaging method developed in recent years.
As DVDMS is activated by both laser and ultrasound, it may be used
in PAI. DVDMS and PAI may be used in image-guided therapy for
glioma surgery, or to dynamically monitor the therapeutic effect.
Furthermore, PDT and SDT may enhance the therapeutic effects. An
in vivo PAI system may be used with an orthotopic
transplantation model to validate the aforementioned effects.
Previous studies have revealed that DVDMS has good
water solubility and stability, is widely applicable in PDT and
SDT, and shows good therapeutic effects against tumors, bacteria
and skin diseases (11,39). Preclinical safety evaluation showed
that DVDMS as a good safety profile in SD rats and beagles
(10,40). Furthermore, there are two registered
phase I clinical trials investigating DVDMS for the treatment of
esophageal cancer and advanced solid tumors (no. CTR20150690 and
CTR20150725, respectively). The results of the present study showed
that DVDMS-mediated PDT and SDT demonstrated good inhibition of
glioma, indicating that DVDMS may be a promising sensitizer for
both PDT and SDT.
The majority of the cell-damaging effects induced by
PDT and SDT are generally attributed to ROS. Sun et al
(41) found that ROS were major
triggering intermediates during the DVDMS-SDT treatment of human
glioma U-373 MG cells, and that the increase in apoptosis was
accompanied by an increase in cleaved-caspase 3 level and DNA
fragmentation. Our study indicated that DVDMS-mediated PDT and SDT
significantly inhibited the expression levels of PCNA and Bcl-xL,
and enhanced cleaved-caspase 3. The ROS scavenger NAC partly
reversed these changes, and reduced cell apoptosis induced by
DVDMS-mediated PDT and SDT, as demonstrated by the flow cytometry
results. These results indicated that ROS may play an important
role in inhibition of cell proliferation and induction of cell
apoptosis by DVDMS-mediated PDT and SDT.
The PI3K/AKT/mTOR signaling pathway is important in
the onset and progression of cancer and it can be regulated by ROS
(42). Recent studies have shown
that both PDT and SDT inhibit the PI3K/AKT/mTOR signaling pathway.
Han et al (43) found that
PDT mediated by fluorescent nanoparticles encapsulating Chlorin e6
suppressed p-mTOR and p-AKT via ROS generation in
macrophage-derived foam cells. Zheng et al (44) revealed that SDT mediated by hydroxyl
acetylated curcumin induced PI3K/AKT/mTOR pathway-dependent
autophagy, and the mitochondrial-caspase pathway was crucial in
apoptosis. The results of the present study demonstrated that
DVDMS-mediated PDT and SDT efficiently inhibited the PI3K/AKT/mTOR
signaling pathway.
The results of the present study revealed that the
phosphorylation of p70s6K, rps6 and eIF4E, downstream of mTORC1
were decreased, and the phosphorylation of 4EBP1 was increased,
which is generally thought to be upregulated when this signaling
pathway is inhibited (45), and it
may therefore require further investigation. Recent studies have
revealed that autophagy pathways are activated in response to PDT
or SDT. The activation of autophagy pathways was also associated
with the PI3K/AKT/mTOR signaling pathway. However, the effects of
autophagy activation and antitumor effects in PDT and SDT remain
controversial (46–49). In the present study, we found that
both DVDMS-PDT and DVDMS-SDT significantly inhibited the p62
levels, however, only PDT promoted the conversion from LC3-I to
LC3-II. Interestingly, unlike PCNA, Bcl-xL, and caspase-3, changes
in LC3 induced by PDT were not reversed by NAC, whereas in SDT, NAC
induced a ignificant increase in LC3II/I (Fig. S1C), highlighting the differences
between PDT- and SDT-induced autophagy. The role of autophagy in
PDT- and SDT-induced cell damage is very complex. Shi et al
(34) found that DVDMS-PDT induced
apoptosis and autophagy in esophageal cancer cells via ROS
generation, while inhibiting autophagy reduced DVDMS-PDT-triggered
apoptosis. However, Zhu et al (50) found that inhibiting autophagy with
chloroquine enhanced DVDMS-PDT-induced apoptosis in colorectal
cancer cells. To the best of our knowledge, the present study was
the first to reveal the influences of DVDMS-SDT on autophagy;
however, the association between autophagy and apoptosis remain
unclear. Further studies are warranted to explore the mechanisms of
autophagy in regulating glioma cell apoptosis induced by
DVDMS-mediated PDT and SDT.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Professor Qi-Cheng Fang for
kindly providing the DVDMS.
Funding
This study was financially supported by the Science
and Technology Planning Project of Shenzhen (grant no.
JCYJ20180302153611416), the National Natural Science Fund of
China-Henan Joint Fund (grant no. U1804187) and the National
Science and Technology Major Projects of China for ‘Major New Drugs
Innovation Development’ (grant no. 2018ZX09201017-005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YWA and HQL were the main conductors of the
experiments. FW designed the in vivo experiments. HTJ
reviewed the data and drafted the manuscript. HQL was responsible
for submission and answering to reviewers' comments. GYJ, ZWL and
ZQZ conducted the western blotting, PDT and SDT, respectively. JCW
carried out the statistics. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shenzhen PKU-HKUST Medical Center (SYXK
2015-0106).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
PDT
|
photodynamic therapy
|
|
SDT
|
sonodynamic therapy
|
|
DVDMS
|
sinoporphyrin sodium
|
|
PBS
|
phosphate-buffered saline
|
|
FCM
|
flow cytometry
|
|
H&E
|
hematoxylin and eosin
|
|
NAC
|
N-acetyl-L-cysteine
|
|
ROS
|
reactive oxygen species
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bcl-xL
|
B-cell lymphoma-extra large
|
|
Bax
|
Bcl-2-associated X
|
|
p-PI3K
|
phospho-phosphatidylinositol 3
kinase
|
|
p-AKT
|
phospho-protein kinase B
|
|
p-mTOR
|
phospho-mammalian target of
rapamycin
|
|
p-p70s6k
|
phospho-p70 ribosomal s6 kinase
|
|
p-rps6
|
phospho-ribosomal protein s6
|
|
p-4EBP1
|
phospho-4E binding protein 1
|
|
p-eIF4E
|
phospho-protein synthesis initiation
factor 4E
|
|
SQSTM1/p62
|
sequestosome 1
|
|
LC3
|
light chain 3
|
|
ATCC
|
American Type Culture Collection
|
|
STR
|
short tandem repeat
|
References
|
1
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ang JM, Riaz IB, Kamal MU, Paragh G and
Zeitouni NC: Photodynamic therapy and pain: A systematic review.
Photodiagnosis Photodyn Ther. 19:308–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McHale AP, Callan JF, Nomikou N, Fowley C
and Callan B: Sonodynamic therapy: Concept, mechanism and
application to cancer treatment. Adv Exp Med Biol. 880:429–450.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fitzmaurice S and Eisen DB: Daylight
photodynamic therapy: What is known and what is yet to be
determined. Dermatol Surg. 42:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rkein AM and Ozog DM: Photodynamic
therapy. Dermatol Clin. 32:415–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chilakamarthi U and Giribabu L:
Photodynamic therapy: Past, present and future. Chem Rec.
17:775–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Jia Y, Wang P, Liu Q and Zheng H:
Current status and future perspectives of sonodynamic therapy in
glioma treatment. Ultrason Sonochem. 37:592–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abrahamse H and Hamblin MR: New
photosensitizers for photodynamic therapy. Biochem J. 473:347–364.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ibbotson SH: Adverse effects of topical
photodynamic therapy. Photodermatol Photoimmunol Photomed.
27:116–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin N, Li C, Wang Z, Zhang J, Ye X, Gao W,
Wang A, Jin H and Wei J: A safety study of a novel photosensitizer,
sinoporphyrin sodium, for photodynamic therapy in Beagle dogs.
Photochem Photobiol Sci. 14:815–832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi R, Li C, Jiang Z, Li W, Wang A and Wei
J: Preclinical study of antineoplastic sinoporphyrin sodium-PDT via
in vitro and in vivo models. Molecules. 22:1122017. View Article : Google Scholar
|
|
12
|
Xiong W, Wang P, Hu J, Jia Y, Wu L, Chen
X, Liu Q and Wang X: A new sensitizer DVDMS combined with multiple
focused ultrasound treatments: An effective antitumor strategy. Sci
Rep. 5:174852015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Wang P, Liu Q and Wang X:
Sinoporphyrin sodium triggered sono-photodynamic effects on breast
cancer both in vitro and in vivo. Ultrason Sonochem. 31:437–448.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Q, Xu R, Xu H, Wang G, Shen X and
Jiang H: Extracranial metastases of high-grade glioma: The clinical
characteristics and mechanism. World J Surg Oncol. 15:1812017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGranahan T, Li G and Nagpal S: History
and current state of immunotherapy in glioma and brain metastasis.
Ther Adv Med Oncol. 9:347–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uhm JH and Porter AB: Treatment of glioma
in the 21st century: An exciting decade of postsurgical treatment
advances in the molecular era. Mayo Clin Proc. 92:995–1004. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long W, Yi Y, Chen S, Cao Q, Zhao W and
Liu Q: Potential new therapies for pediatric diffuse intrinsic
pontine glioma. Front Pharmacol. 8:4952017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zavadskaya ТS: Photodynamic therapy in the
treatment of glioma. Exp Oncol. 37:234–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noske DP, Wolbers JG and Sterenborg HJ:
Photodynamic therapy of malignant glioma. A review of literature.
Clin Neurol Neurosurg. 93:293–307. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao D, Song Y, Che Z and Liu Q: Calcium
overload and in vitro apoptosis of the C6 glioma cells mediated by
sonodynamic therapy (hematoporphyrin monomethyl ether and
ultrasound). Cell Biochem Biophys. 70:1445–1452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nonaka M, Yamamoto M, Yoshino S, Umemura
S, Sasaki K and Fukushima T: Sonodynamic therapy consisting of
focused ultrasound and a photosensitizer causes a selective
antitumor effect in a rat intracranial glioma model. Anticancer
Res. 29:943–950. 2009.PubMed/NCBI
|
|
22
|
Halasi M, Wang M, Chavan TS, Gaponenko V,
Hay N and Gartel AL: ROS inhibitor N-acetyl-L-cysteine antagonizes
the activity of proteasome inhibitors. Biochem J. 454:201–208.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Y, Cho G, Suh JY, Lee CK, Kim YR, Kim
YJ and Kim JK: Dynamic contrast-enhanced MRI for monitoring
antiangiogenic treatment: Determination of accurate and reliable
perfusion parameters in a longitudinal study of a mouse xenograft
model. Korean J Radiol. 14:589–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi H, Gillespie DL, Berg S, Rice C,
Couldwell S, Gu J, Colman H, Jensen RL and Huang LE: Intermittent
induction of HIF-1alpha produces lasting effects on malignant
progression independent of its continued expression. PLoS One.
10:e01251252015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu H and Xu L: Cell experimental studies
on sonoporation: State of the art and remaining problems. J Control
Release. 174:151–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muleki Seya P, Fouqueray M, Ngo J, Poizat
A, Inserra C and Béra JC: Sonoporation of adherent cells under
regulated ultrasound cavitation conditions. Ultrasound Med Biol.
41:1008–1019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goodlad RA: Quantification of epithelial
cell proliferation, cell dynamics, and cell kinetics in vivo. Wiley
Interdiscip Rev Dev Biol. 6:2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levesley J, Steele L, Brüning-Richardson
A, Davison A, Zhou J, Ding C, Lawler S and Short SC: Selective
BCL-XL inhibition promotes apoptosis in combination with MLN8237 in
medulloblastoma and pediatric glioblastoma cells. Neuro Oncol.
20:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Liu P, Feng X and Ma C:
Salidroside suppressing LPS-induced myocardial injury by inhibiting
ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol
Med. 21:3178–3189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang P and Mizushima N: LC3- and
p62-based biochemical methods for the analysis of autophagy
progression in mammalian cells. Methods. 75:13–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu J, Wang X, Liu Q, Zhang K, Xiong W, Xu
C, Wang P and Leung AW: Antitumor effect of sinoporphyrin
sodium-mediated photodynamic therapy on human esophageal cancer
Eca-109 cells. Photochem Photobiol. 90:1404–1412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao J, Gao W, Wang Y, Wang L, Diabakte K,
Li J, Yang J, Jiang Y, Liu Y, Guo S, et al: Sonodynamic therapy
suppresses neovascularization in atherosclerotic plaques via
macrophage apoptosis-induced endothelial cell apoptosis. JACC Basic
Transl Sci. 5:53–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Y, Zhang B, Feng X, Qu F, Wang S, Wu
L, Wang X, Liu Q, Wang P and Zhang K: Apoptosis and autophagy
induced by DVDMs-PDT on human esophageal cancer Eca-109 cells.
Photodiagn Photodyn Ther. 24:198–205. 2018. View Article : Google Scholar
|
|
35
|
Pi Z, Huang Y, Shen Y, Zeng X, Hu Y, Chen
T, Li C, Yu H, Chen S and Chen X: Sonodynamic therapy on
intracranial glioblastoma xenografts using sinoporphyrin sodium
delivered by ultrasound with microbubbles. Ann Biomed Eng.
47:549–562. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Zhou M, Sheng Z, Chen Y, Yeh CK,
Chen W, Liu J, Liu X, Yan F and Zheng H: Theranostic
nanosensitizers for highly efficient MR/fluorescence imaging-guided
sonodynamic therapy of gliomas. J Cell Mol Med. 22:5394–5405. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun Y, Wang H, Wang P, Zhang K, Geng X,
Liu Q and Wang X: Tumor targeting DVDMS-nanoliposomes for an
enhanced sonodynamic therapy of gliomas. Biomater Sci. 7:985–994.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan X, Niu G, Lin J, Jin AJ, Hu H, Tang Y,
Zhang Y, Wu A, Lu J, Zhang S, et al: Enhanced fluorescence imaging
guided photodynamic therapy of sinoporphyrin sodium loaded graphene
oxide. Biomaterials. 42:94–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mai B, Wang X, Liu Q, Leung AW, Wang X, Xu
C and Wang P: The antibacterial effect of sinoporphyrin sodium
photodynamic therapy on Staphylococcus aureus planktonic and
biofilm cultures. Lasers Surg Med. 48:400–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi R, Lin X, Zhang J, Jin H, Wang A and
Wei J: Safety evaluation of repeated intravenous infusion of
sinoporphyrin with and without PDT in rats. Photochem Photobiol
Sci. 15:1366–1376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Wang H, Zhang K, Liu J, Wang P,
Wang X and Liu Q: Sonodynamic therapy induces oxidative stress, DNA
damage and apoptosis in glioma cells. RSC Adv. 8:36245–36256. 2018.
View Article : Google Scholar
|
|
42
|
Wang X, Fu YF, Liu X, Feng G, Xiong D, Mu
GF and Chen FP: ROS promote Ox-LDL-induced platelet activation by
up-regulating autophagy through the inhibition of the PI3K/AKT/mTOR
pathway. Cell Physiol Biochem. 50:1779–1793. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han XB, Li HX, Jiang YQ, Wang H, Li XS,
Kou JY, Zheng YH, Liu ZN, Li H, Li J, et al: Upconversion
nanoparticle-mediated photodynamic therapy induces autophagy and
cholesterol efflux of macrophage-derived foam cells via ROS
generation. Cell Death Dis. 8:e28642017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng L, Li Y, Li X, Kou J, Zhong Z, Jiang
Y, Liu Z, Tian Y and Yang L: Combination of hydroxyl acetylated
curcumin and ultrasound induces macrophage autophagy with
anti-apoptotic and anti-lipid aggregation effects. Cell Physiol
Biochem. 39:1746–1760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang CI, Wang CC, Tai TS, Hwang TZ, Yang
CC, Hsu CM and Su YC: eIF4E and 4EBP1 are prognostic markers of
head and neck squamous cell carcinoma recurrence after definitive
surgery and adjuvant radiotherapy. PLoS One. 14:e02255372019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reiners JJ Jr, Agostinis P, Berg K,
Oleinick NL and Kessel D: Assessing autophagy in the context of
photodynamic therapy. Autophagy. 6:7–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiong L, Liu Z, Ouyang G, Lin L, Huang H,
Kang H, Chen W, Miao X and Wen Y: Autophagy inhibition enhances
photocytotoxicity of Photosan-II in human colorectal cancer cells.
Oncotarget. 8:6419–6432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dewaele M, Martinet W, Rubio N, Verfaillie
T, de Witte PA, Piette J and Agostinis P: Autophagy pathways
activated in response to PDT contribute to cell resistance against
ROS damage. J Cell Mol Med. 15:1402–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li X, Zhang X, Zheng L, Kou J, Zhong Z,
Jiang Y, Wang W, Dong Z, Liu Z, Han X, et al: Hypericin-mediated
sonodynamic therapy induces autophagy and decreases lipids in THP-1
macrophage by promoting ROS-dependent nuclear translocation of
TFEB. Cell Death Dis. 7:e25272016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu B, Li S, Yu L, Hu W, Sheng D, Hou J,
Zhao N, Hou X, Wu Y, Han Z, et al: Inhibition of autophagy with
chloroquine enhanced sinoporphyrin sodium mediated photodynamic
therapy-induced apoptosis in human colorectal cancer cells. Int J
Biol Sci. 15:12–23. 2019. View Article : Google Scholar : PubMed/NCBI
|