Introduction
Cancer is a major public health problem worldwide.
Notably, increasing incidences of certain types of cancer, such as
cervical (1–3), breast (4) and lung cancer (5), have been reported in young women.
Although cancer mortality is continuously declining due to progress
in treatment (6), improving
cancer-associated outcomes and quality of life in these patients
remains imperative. Chemotherapeutic agents are well known for
their side effects, such as leukopenia, hepatic insufficiency and
premature ovarian insufficiency (7). Endocrine dysfunction and infertility
induced by ovarian function impairment in patients of reproductive
age can seriously affect their quality of life. The increasing
demand for ovarian function preservation has therefore become a
major challenge for onco-fertility specialists (8).
According to Information Management System (IMS™)
data, the global best-selling chemotherapeutic agent is paclitaxel
(PXL) (9), which is widely used in
cervical (10), breast (11) and lung cancers (12). Mechanistically, PXL functions as an
antineoplastic drug by inhibiting guanosine triphosphate hydrolysis
in the microtubule lattice, inducing microtubule stabilization
(13). Although PXL had been used
for decades, the few studies revealing its effect on the ovaries of
animals have had conflicting results (14–18).
The results of certain clinical trials and meta-analyses that
focused on whether PXL diminishes human fertility were not
reliable, due to lack of an accurate ovarian reserve marker
(19–22). Therefore, it is important to
elucidate how PXL affects ovaries and how long its gonadotoxicity
lasts.
Currently, gonadotropin-releasing hormone
analogues/agonists (GnRHa) are the first and most widely used
agents for ovarian protection during chemotherapy (7). The main mechanism of the protective
effect of GnRHa is reducing follicle-stimulating hormone (FSH)
levels and suppressing follicle growth to maintain ovaries in a
relatively dormant state (7).
However, the evidence of fertility preservation by GnRHa remains
insufficient, since controversial results were reported in several
meta-analyses (23–25). Different chemotherapy regimens used
in each clinical trial were probably responsible for the
contrasting results. It remains unclear whether GnRHa would be
effective in protecting ovaries from the gonadotoxicity caused by
PXL.
The aim of the present study was to clarify the
phenomena and mechanisms through which PXL impairs rodent ovaries,
define the duration of its gonadotoxicity and investigate whether
GnRHa can protect ovaries during PXL treatment, in the hope of
providing laboratory evidence for the clinical application of PXL
and GnRHa more safely in women of reproductive age.
Materials and methods
Animals
Seven-week-old female and 10-week-old male ICR mice
were purchased from the Hubei Provincial Center for Disease Control
and Prevention, China. Mice were kept for 1 week in the animal
husbandry to enable acclimatization to the local conditions in
controlled temperature (20–25°C) and light (12-h light/dark cycle)
with free access to food and tap water. Three to four female mice
were housed in one ventilated cage with wood shavings as bedding
which was changed every 3 days. Each male mouse was separately
housed. The whole experiment lasted 3 months, including 501 female
mice and 12 male mice. All the animals were monitored every day,
and weighed every 5 days. Animals were sacrificed by cervical
dislocation for sample collection following inhalation of 70% v/v
CO2. Euthanasia was confirmed by the cessation of a
heartbeat. Symptoms meeting the NIH guidelines (26) such as abnormal postures, weight
loss, loss of appetite or weakness were set as humane endpoints for
the present study. All experiments were approved by the
Institutional Ethics Committee of Tongji Hospital, Tongji Medical
College, Huazhong University of Science and Technology (approval
no. TJ-A20161101).
PXL and GnRHa treatment
Female ICR mice, weighing 30–35 g, were randomly
assigned to four groups: The vehicle, PXL, GnRHa and PXL+GnRHa
groups. Based on our pre-examination and a previous study (27), the estrous cycle of ICR mice was 5
days on average. Therefore, GnRHa (1 mg/kg, triptorelin acetate;
Ferring Pharmaceuticals) or normal saline was administered
intraperitoneally to mice prior to chemotherapy for 5 days. Next,
animals received a single dose of PXL (30 mg/kg; Pfizer, Inc.) or
vehicle intraperitoneally. One quarter of the mice in each group
were assessed 24 h after chemotherapy. The rest of the mice were
continuously administered GnRHa for another estrous cycle (5 days)
following chemotherapy, and were assessed on days 6, 11 and 16
following chemotherapy (Fig. 1A).
Another set of mice was administerd 30 mg/kg PXL every 3 days, for
a total of 3 doses. GnRHa (1 mg/kg) was also administered prior to,
during and following chemotherapy for a total of 16 days, and mice
were then assessed on days 1, 6, 11 and 16 following chemotherapy
(Fig. 1B). During the experiment,
one mouse in the GnRHa group suffered from weight loss and was
euthanized before the endpoint of our experiment.
 | Figure 1.Experimental scheme. (A) Single-dose
scheme: Before chemotherapy, mice were pretreated with NS or GnRHa
for 5 days (1 estrous cycle). On D0, a single dose of chemotherapy
(PXL) or vehicle was administered. On day 1 following chemotherapy,
a quarter of the mice were assessed (n=24/group). The rest were
continuously administered NS or GnRHa for another 5 days. On day 6,
11 and 16 following chemotherapy, a quarter of the mice were
assessed at each time-point. (B) Multiple-dose scheme: Three doses
of paclitaxel were administered consecutively every 3 days and
GnRHa was administered continuously during chemotherapy. On days 1,
6, 11 and 16 following chemotherapy, a quarter of the mice
(n=10/group) were assessed at each time-point. NS, normal saline;
GnRHa, gonadotropin-releasing hormone agonist; PXL, paclitaxel; V,
vehicle. |
Histology and follicle count
After anesthetizing by 50 mg/kg pentobarbital sodium
(Merck KGaA) intraperitoneally, blood was collected from the eye
orbit for serum anti-müllerian hormone (AMH) analysis
(n=5/group/time-point). Then these mice were euthanized by cervical
dislocation, and mortality was confirmed by the cessation of a
heartbeat. Subsequently, the ovaries were collected. One ovary from
each mouse was fixed in 4% v/v paraformaldehyde (Wuhan Servicebio
Technology Co., Ltd.) for 24 h at 4°C, embedded in paraffin and
sectioned at 5-µm thickness for histology. Hematoxylin and eosin
(H&E) staining was performed using standard methods (28). Follicle counts were conducted on
serially cut sections from every sixth section of entire ovaries.
The follicles were counted at different stages and the mean count
per section was calculated for each stage. The follicle stages were
classified as previously described (29).
AMH measurement
Blood was collected from the orbit and placed at
room temperature for 1 h. Following centrifugation at 3,000 × g,
serum AMH levels were determined using a mouse anti-AMH ELISA kit
(cat. no. CSB-E13156m; Cusabio Technology LLC), following the
manufacturer's instructions.
Oocyte collection and in vitro
maturation
An intraperitoneal injection of 10 IU pregnant mare
serum gonadotropin (PMSG; Beijing Solarbio Science & Technology
Co., Ltd.) was administered, followed by 12 IU human chorionic
gonadotrophin (hCG; Lizhu Pharmaceutical Trading Co., Ltd.) 48 h
after the induction of ovulation in mice. Subsequently, 14–17 h
after hCG, cumulus oocytes were collected in an oviduct ampulla
after mice were scarified by cervical dislocation following
inhalation of 70% v/v CO2. Cumulus cells were removed
using 1% hyaluronidase (Merck KGaA), and denuded metaphase II (MII)
oocytes were prepared for further study. In total, 265 mice were
sacrificed for oocyte collection.
Oocytes with germinal vesicle (GV) were punctured
from ovaries using a 0.5-mm syringe 48 h following PMSG
administration. GV oocytes were cultured in M199 (GE Healthcare
Life Sciences) drops under liquid mineral oil (Vitrolife) at 37°C
in an incubator with 5% CO2 for in vitro
maturation. Oocytes in MI were retrieved after 6 h, and MII oocytes
were retrieved after 12 h. GV, MI and MII oocytes were then
cultured in 1 µM PXL for 5 min at 37°C. GV and MI oocytes were
washed for ongoing culture to gain MII oocytes. The ongoing culture
time of GV and MI oocytes was 12 and 6 h, respectively. MII oocytes
were washed and cultured in normal M199 drops for 4 h for further
study.
Immunofluorescence and confocal
microscopy
Oocytes from each group at each time-point (n=5)
were fixed with 4% v/v paraformaldehyde at 4°C overnight. They were
then transferred to 0.5% v/v Triton X-100 (Beijing Solarbio Science
& Technology Co., Ltd.) for 5 min. Following blocking in 1% w/v
BSA-supplemented (Wuhan Servicebio Technology Co., Ltd.) PBS for 1
h, oocytes were incubated with the monoclonal anti-α-tubulin
antibody produced in mice at a dilution of 1:1,000 (cat. no. T5168;
Merck KGaA) at 4°C overnight. Then specimens were then incubated
with anti-mouse IgG (H+L), F(ab′)2 Fragment (Alexa
Fluor® 488 Conjugate) at a dilution of 1:300 (product
no. 4408S; Cell Signaling Technology, Inc.) for 1 h at 37°C, and
then co-stained with Hoechst 33342 (Merck KGaA) for 5 min at 37°C.
Oocytes were mounted onto glass slides and observed under a
confocal laser-scanning microscope (FV1000; Olympus
Corporation).
Chromosome (CH) spread of oocytes
Oocytes from each group at each time-point (n=4)
were placed in hypotonic solution of 0.9% w/v sodium citrate (Wuhan
Servicebio Technology Co., Ltd.) for 10 min at 37°C and then
exposed to Tyrode's buffer (pH 2.5; Merck KGaA) for ~30 sec at 37°C
to remove the zona pellucida. Oocytes were then fixed in a drop of
1% v/v paraformaldehyde with 0.15% v/v Triton X-100 (pH 9.2) on a
glass slide. Then slides were dried in a humid chamber for over 2 h
at 37°C. CHs were stained with 2% v/v Giemsa (Wuhan Servicebio
Technology Co., Ltd.) for 10 min at room temperature and observed
under a light microscope at a magnification of ×100. The normal
number of MII oocyte CHs (univalents) was 20. Aneuploidy oocytes
had ±20 univalents.
In vitro fertilization
The caudae epididymides of healthy 12-week-old male
mice scarified by cervical dislocation following inhalation of 70%
v/v CO2 were lanced in G-IVF medium (Vitrolife) to
release sperm. Following capacitation, sperm was added to pooled
oocytes from 4 mice in each group, at each time-point, in G-IVF
medium, for 5 h at 37°C and 5% CO2. These oocytes were
then moved to drops of G1 (Vitrolife) medium under mineral oil. The
presence of two pronuclei was considered as successful
fertilization.
Mating protocol
Six female mice in the estrous cycle from each group
at each time-point mated with healthy male mice which had been
demonstrated to be fertile at a ratio of 3:1 for 72 h. No special
treatment was administered to male mice. Female mice were separated
as soon as a plug was observed or after 72 h of mating.
Pup CH analysis
Three pups from each pregnant mouse were selected
randomly for CH analysis. Two-week-old pups were intraperitoneally
injected with 0.5% w/v 0.1 ml colchicine (Jialin) and sacrificed by
cervical dislocation following inhalation of 70% v/v CO2
20 min after injection at room temperature. Bone marrow from the
thighbones was flushed into 0.075 M potassium chloride (Wuhan
Servicebio Technology Co., Ltd.) solution, and then, marrow cells
were fixed with methanol-acetic acid (3:1) fixative for 30 min at
room temperature. CH slides were conventionally stained with 2% v/v
Giemsa solution for 10 min at room temperature. The number of CHs
was counted under a light microscope at magnification of ×100 to
determine aneuploidy.
Statistical analysis
The results are presented as the mean ± SEM. The
data were analyzed by one-way ANOVA followed by LSD post hoc or
χ2 test using SPSS 17.0 (SPSS, Inc.). A P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
PXL only damages antral follicles in a
transient manner and does not jeopardize ovarian reserve
In order to determine the impact of PXL on ovarian
reserve, histological analysis of the ovaries was performed and the
serum AMH of mice was evaluated. On day 1 following the injection
of PXL, only antral follicles were significantly reduced in the PXL
group, as compared with the control group (3.35±0.30 vs. 5.90±1.27;
P<0.05). The antral follicles in the GnRHa group (3.07±0.47 vs.
5.90±1.27; P<0.05) and PXL+GnRHa (2.60±0.75 vs. 5.90±1.27;
P<0.05) groups were also reduced, due to the suppressing effect
of GnRHa. Atretic follicles were significantly increased in the PXL
group as compared with the control group (30.67±4.27 vs.
17.95±1.35; P<0.01). Co-administration of GnRHa plus PXL tended
to abrogate the increase of atretic follicles caused by PXL, but
not significantly (23.35±3.44 vs. 30.67±4.27; P=0.09). Primordial,
primary and secondary follicles were compared between groups
(Fig. 2A and B). After 1 estrous
cycle, on day 6 after chemotherapy, the follicle counts between the
four groups had already exhibited no difference in each follicular
stage, except that antral follicles in the GnRHa group were still
less than the control (3.10±1.09 vs. 5.60±1.53, P<0.05)
(Fig. 2A). On days 11 and 16
following chemotherapy, there was no difference in follicle counts
between the four groups. Histological analysis revealed that PXL
destroyed antral follicles in a transient way and had no effect on
primordial follicles. To further confirm the mild effect of PXL on
ovarian reserve, serum AMH was assessed in mice, and the result
revealed a stationary trend of AMH without any significant
difference between the groups at each time-point (Fig. 2C).
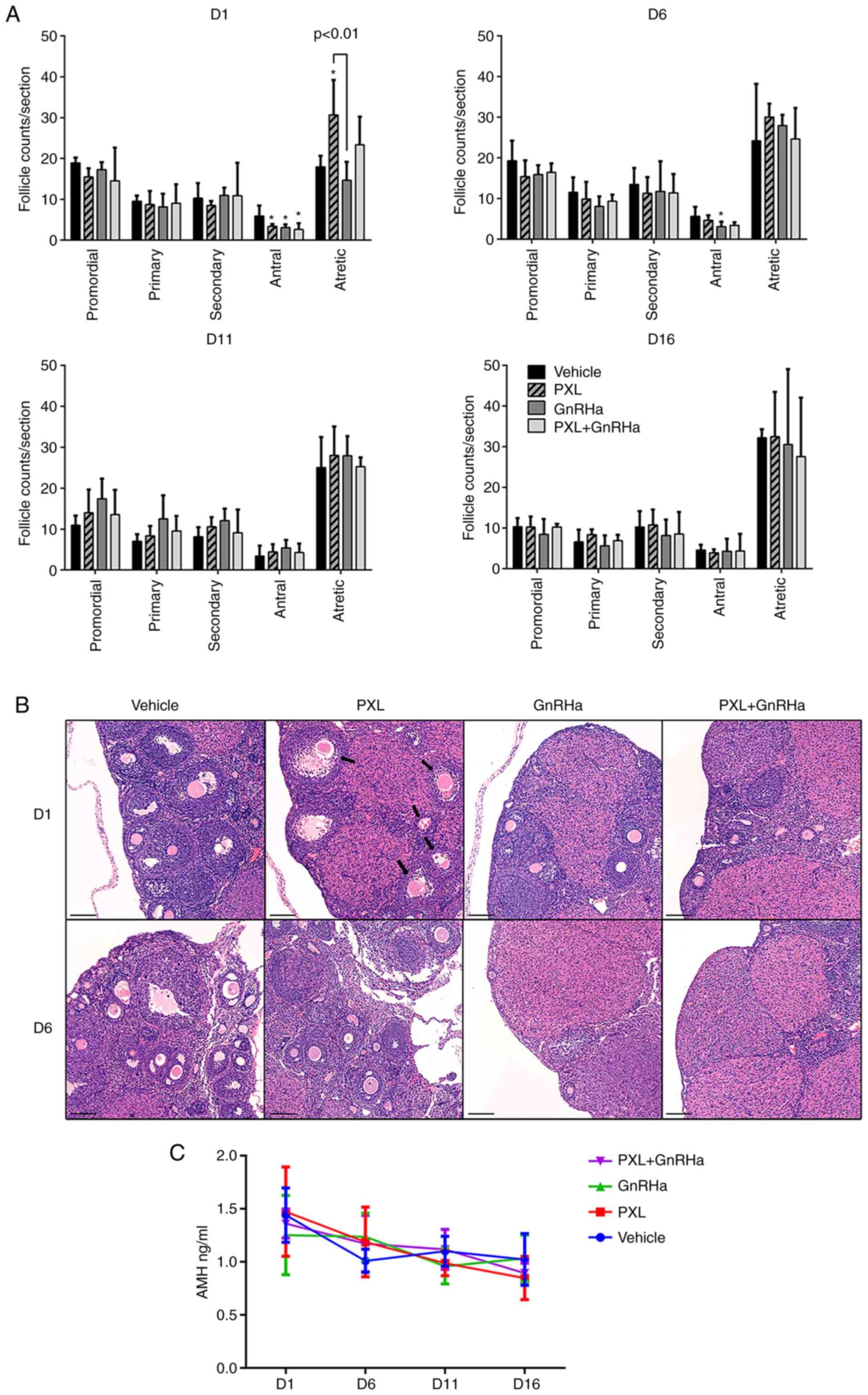 | Figure 2.Damage caused by PXL on follicles
only lasts for 1 estrous cycle, and GnRHa tends to abrogate this
damage. (A) Number of primordial, primary, secondary, antral and
atretic follicles on days 1–16 following chemotherapy. Data are
expressed as the mean ± SEM. Statistical analysis was performed by
one-way ANOVA followed by LSD test. *P<0.05 vs. the control
group (vehicle). (B) H&E-stained sections revealing
representative histological fields in the four groups on days 1–6
following chemotherapy (original magnification, ×10). The arrows
indicate atretic follicles, which were mainly from antral
follicles. Scale bars, 100 µm. (C) Serum AMH levels in the 4 groups
on day 1–16 following chemotherapy. Data are expressed as the mean
± SEM. PXL, placlitaxel; GnRHa, gonadotropin-releasing hormone
agonist; H&E, hematoxylin and eosin; AMH, anti-müllerian
hormone. |
PXL induces meiotic arrest in oocytes
in metaphase but not in GV stage in vitro
The antral follicles were damaged by PXL where
oocytes resumed meiosis, experienced germinal vesicle breakdown
(GVBD) and M I, and then stopped at MII, waiting for fertilization.
Following exposure to PXL and washing, GV oocytes were continuously
cultured. The results revealed that PXL did not affect the GVBD
(75.17±1.81% vs. 77.45±2.85%; P>0.05; Fig. S1A) or maturation rate (43.65±4.81%
vs. 41.02±4.13%; P>0.05; Fig.
S1A), which suggested that GV oocytes were either away from or
resistant to the damage produced by PXL. MI oocytes were also
administered PXL, which was then washed off. Following ongoing
maturation, the oocytes exposed to PXL barely extruded the polar
body, and the maturation rate was markedly lower (27.84±10.00% vs.
86.80±4.40%, P<0.005; Fig.
S1B). Immunostaining revealed that the spindle apparatuses of
these arrested MI oocytes that were exposed to PXL were mostly
damaged, while the majority of MI oocytes without PXL
administration exhibited normal spindle organization and CH
alignment (spindle, 3.70±11.33% vs. 69.84±20.78%, P<0.05; CH,
0.00±0.00% vs. 73.01±27.74%; P<0.05; Fig. S1B). Similarly, PXL-exposed MII
oocytes had a clearly disordered spindle organization and CH
alignment, as compared with the controls (spindle, 29.72±3.47% vs.
71.28%±17.99; CH, 26.62±6.50% vs. 60.72±14.97%; P<0.05; Fig. S1C). As a result, the fertilization
rate of PXL-exposed MII oocytes was markedly reduced (9.49±6.41%
vs. 46.35±8.72%, P<0.01; Fig.
S1C), while nearly half of the control oocytes were able to be
fertilized and develop into 2-cell embryos. It was confirmed that
only oocytes in metaphase and not earlier-stage oocytes were
affected by PXL through the destruction of spindle apparatuses.
PXL-induced impairment of MII oocytes
lasts for 2 estrous cycles in vivo, and GnRHa can protect
oocytes
To confirm the effect of PXL on mouse oocytes in
vivo, the amount and morphology of oocytes retrieved following
ovarian stimulation in mice was observed. On day 1 following
chemotherapy, retrieved MII oocytes in the PXL group were
significantly less than those in the vehicle group (2.50±1.50 vs.
29.75±5.65; P<0.005 Fig. 3B),
and the percentage of MII in retrieved oocytes had also decreased
(12.22±14.8% vs. 41.40±4.7%; P=0.096; Fig. 3C). At this time-point, GnRHa did not
rescue the number of PXL-exposed oocytes, since the MII oocytes
retrieved in the PXL+GnRHa group were not more than those retrieved
in the PXL group (6.0±6.0 vs. 2.50±1.50; P>0.05). On day 6
following chemotherapy, the MII oocyte number and percentage in the
PXL group started to recover, but was still slightly lower than
that in the control (18.00±6.49 vs. 32.40±4.39: P>0.05)
(37.1±4.2% vs 52.00±7.3%; P>0.05). In addition, mice co-treated
with GnRHa took less time to completely recover. In the PXL+GnRHa
group, the number and percentage of MII oocytes were totally
similar to those of the controls (38.25±8.35 vs. 32.40±4.39;
P>0.05) (49.85±6.9% vs. 52.00±7.3%; P>0.05), and the number
of MII oocytes was slightly higher than that in the PXL group,
although without significance (38.25±8.35 vs. 18.00±6.49; P=0.058).
On days 11 and 16, the number and percentage of MII oocytes were
similar among the four groups (Fig.
3).
Although some oocytes developed into MII oocytes, we
cannot say that all of them were normal. Spindle morphology, CH
alignment, karyotype analysis and in vitro fertilization
were used to evaluate the quality of MII oocytes. Spindle apparatus
immunostaining revealed that, on day 1 following chemotherapy, most
oocytes collected from the control or GnRHa groups had typical
barrel-like spindles with CHs located on the equatorial plate
(Fig. 4C). By contrast, a majority
of disorganized spindles and misaligned CHs were observed in the
PXL group (spindle, 25.32±22.69% vs. 69.64±7.76%; P<0.05; CH,
25.21±22.69% vs. 63.21±6.84%; P<0.05; Fig. 4A). Co-treatment of GnRHa tended to
protect spindle organization, although without significance
(spindle, 57.50±18.12% vs. 25.32±22.69%; P>0.05; CH, 47.5±29.18%
vs. 25.21%±22.69%; P>0.05; Fig.
4A). A significantly higher frequency of aneuploid oocytes of
±20 univalents was found in the PXL group than in the control or
GnRHa groups (79.17±5.15% vs. 24.44±21.76% vs. 18.75±8.11%;
P<0.05; Fig. 4B) on day 1
following chemotherapy. Co-treatment with PXL and GnRHa tended to
reduce the aneuploidy rate (79.17±5.15% vs. 45.00±5.03%; P>0.05;
Fig. 4B). The fertilization rate
was also clearly decreased in the PXL group, as compared with the
control (27.27% vs. 75.51%; P<0.05; Fig. 4D; Table
SI) on day 1 following chemotherapy. The fertilization ability
of the PXL+GnRHa group was also partially restored (64.29 vs.
27.27%; P>0.05). After 1 estrous cycle, on day 6 following
chemotherapy, the normal spindle morphology, as well as CH
alignment, aneuploidy and fertilization rates of the oocytes in the
PXL group had all reached the same level as those of the control
group (Fig. 4).
PXL-induced adverse reproductive
outcomes last for 1 estrous cycle and GnRHa reverses this
outcome
A mating experiment was carried out to study whether
damage to oocytes caused by PXL could affect reproductive outcomes.
After mating on day 1 following chemotherapy, animals treated with
GnRHa produced fewer litters (Table
I; Fig. 5B), as 4/6 mice were
pregnant in the control and PXL group, but only 2–3 mice in the
GnRHa or PXL+GnRHa groups. No significant difference in live pups
per litter was observed among the four groups. However, markedly,
the PXL group delivered a total of 18 dead pups, which was more
than those delivered in the control group (P=0.05) and the GnRHa
group (P=0.046). There was no stillbirth in the PXL+GnRHa group but
since only 2 mother mice delivered, no statistical difference was
identified. (Table I; Fig. 5A and C). On day 6 and 11 following
chemotherapy, the percentage of pregnant mothers in mice treated
with GnRHa remained low. But on day 16, the pregnancy rate of mice
treated with PXL dropped. As for live pups/litters and stillbirths,
1 estrous cycle after chemotherapy, the live pups/litters delivered
in each group were similar, with stillbirth seldom happening
(Table I; Fig. 5A and C). To ensure that the
offspring of PXL-exposed animals had no potential genetic defects,
we examined the karyotypes of 3 random pups from every pregnant
mother. The results revealed that every live pup had a euploid
karyotype (Tables SII–SV).
 | Table I.Reproductive outcomes of mice mated
during the 1st estrous cycle after chemotherapy. |
Table I.
Reproductive outcomes of mice mated
during the 1st estrous cycle after chemotherapy.
| Groups | N | Litters (%) | Total pups (live
pups) | Live
pups/littera | P-value | Stillbirths |
Stillbirths/littera | P-value |
|---|
| Vehicle | 6 | 4 (66.7) | 50(48) | 12.00±2.16 |
| 2 | 0.50±0.50 |
|
| PXL | 6 | 4 (66.7) | 39 (21) | 5.25±3.20 | 0.066 | 18 |
4.50±2.10b | 0.050 |
| GnRHa | 6 | 3 (50.0) | 28 (28) | 9.33±1.20 | 0.464 | 0 |
0.00±0.00b | 0.799 (0.042) |
| PXL+GnRHa | 6 | 2 (33.3) | 22 (22) | 11.00±0.00 | 0.806 | 0 | 0.00±0.00 | 0.822 |
Multiple doses of PXL do not cause
permanent damage to mouse fertility
In order to mimic the clinical usage of PXL, 3
subsequent doses of PXL were administered to mice (1 dose per 3
days). Since it was determined that GnRHa had no adverse effect on
mouse fertility in the single-dose experiment, in order to reduce
mice sacrificing in our study, we canceled this group in the
multiple-dose experiment. The GnRHa was administered 1 estrous
cycle before, during and continuing with one more estrous cycle
following chemotherapy (Fig. 1B).
The follicle counts were similar to those of the single-dose-PXL
experiment. On day 1 following 3 doses of chemotherapy, the antral
follicles in the PXL and PXL+GnRHa groups were significantly
reduced (7.00±1.00 vs. 2.00±0.00 vs. 2.70±0.30; P<0.05). The
primordial, primary and secondary follicles were all similar
between groups. The administration of GnRHa during chemotherapy
significantly reduced the PXL-induced atretic follicles (30.60±5.00
vs. 63.80±4.00; P<0.05). On days 6 and 11 following
chemotherapy, antral follicles in the PXL group were still less
than those in the control, but co-treatment with PXL and GnRHa
helped antral follicles recover on day 11. The increase in atretic
follicles in the PXL group also lasted until day 11, but the
PXL+GnRHa group maintained a similar amount of atretic follicles to
that of the control group. On day 16 following chemotherapy, no
significant difference in follicles of all stages was identified
among groups (Fig. 6A). To further
estimate the duration of the gonadotoxicity caused by multiple
doses of PXL administration, ovarian stimulation was also
performed. On days 1 and 6 following the last dose of PXL,
significantly less MII oocytes were collected in the PXL group than
in the control (D1, 1.00±0.00 vs. 30.40±5.27; P<0.001; D6,
17.20±4.25 vs. 31.33±4.67; P<0.05; Fig. 6B). With the protection of GnRHa,
even more MII oocytes were retrieved than in the control
(46.80±3.44 vs. 31.33±4.67; P<0.05; Fig. 6B) on day 6 following chemotherapy.
On days 11 and 16, the amount of MII oocytes was similar in all
groups.
Discussion
In the present study, it was determined that PXL
only induced loss of antral follicles and increased atretic
follicles without causing any loss of primordial, primary or
secondary follicles. Notably, after 1–2 estrous cycles for
recovery, the follicle counts of each stage tended to be the same
among groups. In addition, AMH examination verified that the
ovarian reserve was not affected by PXL. PXL only affected antral
follicles. That was consistent with the results of former studies,
which reported that PXL acted on cells with active division,
irrespective of their malignancy (30), since the development from secondary
to antral follicles is a process in which granulosa cells, theca
cells and vessels proliferate rapidly (31). The transient impact of PXL may be
due to its short plasma half-time (32–34)
and limited effect on pre-antral follicles. When pre-antral
follicles grew into antral follicles in the next estrous cycle, the
plasma concentration of PXL had already decreased. As a result,
after one estrous cycle, the morphology of follicles was able to
recover.
To understand which stage of oocytes in
antral-preovulatory follicles would be affected by PXL, an in
vitro experiment was carried out. The results revealed that,
following exposure to PXL, mice GV oocytes were capable of
maturation, which suggested that GV oocytes were insensitive to
PXL, since microtubules had not assembled into specific forms at
this stage (35,36). However, oocytes in MI or MII were
very sensitive to PXL, as their development depends on the assembly
of microtubules (37,38) and the formation of a spindle
apparatus, which were destroyed by exposure to PXL.
To confirm the effect of PXL on oocytes in
vivo, an animal model was designed to estimate the time mice
required to recover normal ovulation. This was measured through
ovarian stimulation of mice at different time-points following
chemotherapy. It was determined that PXL caused acute oocyte
damage. Following the administration of PMSG in mice 1 day after
chemotherapy, when oocytes were in antral-preovulatory follicles
and directly exposed to PXL, the number and quality of oocytes was
markedly decreased. Following 1 estrous cycle, MII oocytes
collected in the PXL group had a similar quality to those of the
control group. Two estrous cycles after chemotherapy, the MII
oocytes in the PXL and control groups were totally comparable.
These results were consistent with the transient effect of PXL on
antral follicles, and supplemented the findings of a previous
study, which stated that PXL caused a meiotic maturation delay and
spindle defects in mouse oocytes on hCG trigger day (14).
The mating experiment indicated that, although PXL
did not disrupt copulation, it could cause stillbirths, which was
consistent with a previous study (18). Following 1 estrous cycle, the
recovery of litter size and disappearance of stillbirth in the PXL
group was consistent with the effect of PXL on antral follicles and
oocytes, further verifying the transient effect of PXL in
vivo. Of note, there was 1 stillbirth on day 11 in the PXL
group, which may have been a random incident, since there was no
other stillbirth in any subsequent mating; 3 stillbirths were
recorded in the control group on day 1. The karyotypes of every
live pup delivered in all four groups were normal, which indicated
that PXL caused lethal impairment in oocytes. On day 16, a slope in
the pregnancy rate was observed in the PXL group, following
repeated experiments. However, on days 30 and 45 following
chemotherapy, the pregnancy rate and litter size in the PXL group
were similar to those of the control group. This result suggested
that the reduced pregnancy rate in the PXL group on day 16 was not
due to the decrease in ovarian reserve, but other unknown reasons
that require further exploration.
GnRHa have been studied as protective agents of
chemotherapy-induced ovarian failure for decades. The mechanism of
GnRHa was considered to be decreasing FSH levels and suppressing
follicle growth through the pituitary-gonadal axis (39), as well as upregulating
anti-apoptotic molecules (40) and
decreasing the exposure of primordial follicles to cytotoxic agents
(41). However due to contradictory
results of clinical trials (23,42),
to date, GnRHa has not been recommended as a regular ovarian
protective agent (43,44). In the present study, the follicle
count results revealed that GnRHa suppressed follicle maturation
effectively. GnRHa reduced atretic follicles in the PXL+GnRHa
group, which suggested that pretreatment with GnRHa kept ovaries in
a relatively quiescent condition to avoid damage induced by PXL to
antral follicles. It was speculated that the growing follicles were
suppressed due to the administration of GnRHa before they became
sensitive to PXL. During PMSG or mating-induced ovulation when the
plasma concentration of PXL decreased rapidly, exposure to PXL at
lower concentrations led to the better-quality of oocytes. In the
mating experiment, mice that received a GnRHa injection delivered
fewer litters, since GnRHa disrupted copulation by suppressing
reproductive hormones. In combination, the protective effect of
GnRHa was not obvious, possibly due to the short duration of the
effect of PXL on ovaries.
Multiple-dose administration of PXL had a similar
effect on ovaries to that of the single-dose experiment, suggesting
that there was no cumulative effect or follicle exhaustion caused
by PXL. This mouse model mimicked the common clinical chemotherapy
regimen with PXL included [i.e., TP regimen in ovarian cancer
(45) and AC-T regimen in breast
cancer (11), in which multiple
courses of PXL treatment are required]. Notably, ovulation in the
PXL+GnRHa group was higher than that of the control 1 estrous cycle
after the last dose of PXL. This may be due to the controlled
ovarian hyperstimulation effect of GnRHa followed by gonadotrophin
PMSG. This suggested that the temporary destruction of the oocytes
was more likely associated with the last dose of PXL, which once
again underlined the transient effect of PXL on ovaries.
In clinical practice, physicians may face several
challenges, such as what the required duration of contraceptive
method administration is following chemotherapy and whether GnRHa
should be used to protect ovarian function. Physicians used to
recommend 6 months of contraceptives based on experience. The
present study revealed that PXL had no impact on ovarian reserve
and only a transient one on oocytes. The present laboratory
evidence provided the possibility for shortening contraceptive
method administration following PXL chemotherapy. The present study
also revealed that GnRHa has a protective effect on ovaries in PXL
chemotherapy, and provided a scheme of GnRHa administration for
reproductive protection, which suggests that the application of
GnRHa should be encouraged in PXL-based chemotherapeutic regimens.
Further research should be carried out to determine whether the
impact of PXL on human ovaries and oocytes is consistent with its
impact on mouse ovaries and oocytes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Technology R&D Program of China (grant no. 2019YFC1005200,
2019YFC1005202 and 2018YFC1002103), National Natural Science
Foundation of China (grant no. 81802896), the Natural Science
Foundation of Hubei Province (grant no. 2017CFB800) and the Hubei
Province Health and Family Planning Scientific Research Project
(grant nos. WJ2017Z013 and WJ2019M127).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NM performed the experiments, analyzed and
interpreted the data and wrote the manuscript. GeC contributed to
the execution of the oocyte experiment. JC performed the hormone
assays and assisted in data analysis. MC performed the animal
experiments and acquired the data. YY performed the histological
analyses of ovaries. QL, MT and XF assisted in the animal
experiments. XL contributed to the designing and drafting the
article and discussed the results. SZ contributed to the analysis
and interpretation of data. DM and GaC conceived the study,
discussed the results and supervised the study. KL conceived the
study, designed the experiments, conducted study, discussed the
results, contributed to the drafting of the article, submission and
revision. JA conceived the study, designed the experiments,
discussed the results and supervised the study. All the authors
have read and approved the final article.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Ethics Committee of Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (approval no.
TJ-A20161101).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Olorunfemi G, Ndlovu N, Masukume G,
Chikandiwa A, Pisa PT and Singh E: Temporal trends in the
epidemiology of cervical cancer in South Africa (1994–2012). Int J
Cancer. 143:2238–2249. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brinton LA, Sherman ME, Carreon JD and
Anderson WF: Recent trends in breast cancer among younger women in
the United States. J Natl Cancer Inst. 100:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Z, Zheng Y, Wen W, Wu C, Bao P, Wang
C, Zhong W, Gao YT, Jin F, Xiang YB, et al: Incidence and mortality
of gynaecological cancers: Secular trends in urban Shanghai, China
over 40 years. Eur J Cancer. 63:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomez SL, Von Behren J, McKinley M, Clarke
CA, Shariff- Marco S, Cheng I, Reynolds P and Glaser SL: Breast
cancer in Asian Americans in California, 1988–2013: Increasing
incidence trends and recent data on breast cancer subtypes. Breast
Cancer Res Treat. 164:139–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aareleid T, Zimmermann ML, Baburin A and
Innos K: Divergent trends in lung cancer incidence by gender, age
and histological type in Estonia: A nationwide population-based
study. BMC Cancer. 17:5962017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roness H, Kashi O and Meirow D: Prevention
of chemotherapy-induced ovarian damage. Fertil Steril. 105:20–29.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donnez J and Dolmans MM: Fertility
preservation in women. N Engl J Med. 377:1657–1665. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sofias AM, Dunne M, Storm G and Allen C:
The battle of ‘nano’ paclitaxel. Adv Drug Deliv Rev. 122:20–30.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HK, Chu C, Clark R, Cohn D, et al: Cervical
cancer, version 3.2019, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 17:64–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ettinger DS, Wood DE, Aggarwal C, Aisner
DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac
LR, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 1.2020. J Natl Compr Canc Netw. 17:1464–1472. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alushin GM, Lander GC, Kellogg EH, Zhang
R, Baker D and Nogales E: High-resolution microtubule structures
reveal the structural transitions in αβ-tubulin upon GTP
hydrolysis. Cell. 157:1117–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mailhes JB, Carabatsos MJ, Young D, London
SN, Bell M and Albertini DF: Taxol-induced meiotic maturation
delay, spindle defects, and aneuploidy in mouse oocytes and
zygotes. Mutat Res. 423:79–90. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozcelik B, Turkyilmaz C, Ozgun MT, Serin
IS, Batukan C, Ozdamar S and Ozturk A: Prevention of paclitaxel and
cisplatin induced ovarian damage in rats by a
gonadotropin-releasing hormone agonist. Fertil Steril.
93:1609–1614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gücer F, Balkanli-Kaplan P, Doganayl, Yüce
MA, Demiralay E, Sayin NC and Yardim T: Effect of paclitaxel on
primordial follicular reserve in mice. Fertil Steril. 76:628–629.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tarumi W, Suzuki N, Takahashi N, Kobayashi
Y, Kiguchi K, Sato K and Ishizuka B: Ovarian toxicity of paclitaxel
and effect on fertility in the rat. J Obstet Gynaecol Res.
35:414–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kai S, Kohmura H, Hiraiwa E, Koizumi S,
Ishikawa K, Kawano S, Kuroyanagi K, Hattori N, Chikazawa H, Kondoh
H, et al: Reproductive and developmental toxicity studies of
paclitaxel. (I)-Intravenous administration to rats prior to and in
the early stages of pregnancy. J Toxicol Sci. 19 (Suppl 1):S57–S67.
1994.(In Japanese). View Article : Google Scholar
|
|
19
|
Reh A, Oktem O and Oktay K: Impact of
breast cancer chemotherapy on ovarian reserve: A prospective
observational analysis by menstrual history and ovarian reserve
markers. Fertil Steril. 90:1635–1639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Liu J, Chen K, Li S, Wang Y, Yang
Y, Deng H, Jia W, Rao N, Liu Q and Su F: What lies behind
chemotherapy-induced amenorrhea for breast cancer patients: A
meta-analysis. Breast Cancer Res Treat. 145:113–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamy AS, Porcher R, Eskenazi S, Cuvier C,
Giacchetti S, Coussy F, Hocini H, Tournant B, Perret F, Bonfils S,
et al: Anti-mullerian hormone in breast cancer patients treated
with chemotherapy: A retrospective evaluation of subsequent
pregnancies. Reprod Biomed Online. 32:299–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sukumvanich P, Case LD, Van Zee K,
Singletary SE, Paskett ED, Petrek JA, Naftalis E and Naughton MJ:
Incidence and time course of bleeding after long-term amenorrhea
after breast cancer treatment: A prospective study. Cancer.
116:3102–3111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silva C, Caramelo O, Almeida-Santos T and
Ribeiro Rama AC: Factors associated with ovarian function recovery
after chemotherapy for breast cancer: A systematic review and
meta-analysis. Hum Reprod. 31:2737–2749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Senra JC, Roque M, Talim MCT, Reis FM and
Tavares RLC: Gonadotropin-releasing hormone agonists for ovarian
protection during cancer chemotherapy: Systematic review and
meta-analysis. Ultrasound Obstet Gynecol. 51:77–86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munhoz RR, Pereira AA, Sasse AD, Hoff PM,
Traina TA, Hudis CA and Marques RJ: Gonadotropin-releasing hormone
agonists for ovarian function preservation in premenopausal women
undergoing chemotherapy for early-stage breast cancer: A systematic
review and meta-analysis. JAMA Oncol. 2:65–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Institutes of Health (NIH),
Animal Research Advisory Committee, . NIH Guidelines for Endpoints
in Animal Study Proposals. NIH; Bethesda, MD: 2016
|
|
27
|
Wang XN, Roy SK and Greenwald GS: In vitro
DNA synthesis by isolated preantral to preovulatory follicles from
the cyclic mouse. Biol Reprod. 44:857–863. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardiff RD, Miller CH and Munn RJ: Manual
hematoxylin and eosin staining of mouse tissue sections. Cold
Spring Harb Protoc. 2014:655–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Myers M, Britt KL, Wreford NG, Ebling FJ
and Kerr JB: Methods for quantifying follicular numbers within the
mouse ovary. Reproduction. 127:569–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tinwell H and Ashby J: Genetic toxicity
and potential carcinogenicity of taxol. Carcinogenesis.
15:1499–1501. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oktem O and Urman B: Understanding
follicle growth in vivo. Hum Reprod. 25:2944–2954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rezazadeh M, Emam J, Mostafavi A, Rostami
M, Hassanzadeh F, Sadeghi H, Minaiyan M and Lavasanifar A: A rapid
and sensitive HPLC method for quantitation of paclitaxel in
biological samples using liquid-liquid extraction and UV detection.
Application to pharmacokinetics and tissues distribution study of
paclitaxel loaded targeted polymeric micelles in tumor bearing
mice. J Pharm Pharm Sci. 18:647–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JE and Park YJ: Paclitaxel-loaded
hyaluronan solid nanoemulsions for enhanced treatment efficacy in
ovarian cancer. Int J Nanomedicine. 12:645–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anwar M, Akhter S, Mallick N, Mohapatra S,
Zafar S, Rizvi MMA, Ali A and Ahmad FJ: Enhanced anti-tumor
efficacy of paclitaxel with PEGylated lipidic nanocapsules in
presence of curcumin and poloxamer: In vitro and in vivo studies.
Pharmacol Res. 113:(Pt A). 146–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coticchio G, Dal Canto M, Mignini Renzini
M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV and Fadini R:
Oocyte maturation: Gamete-somatic cells interactions, meiotic
resumption, cytoskeletal dynamics and cytoplasmic reorganization.
Hum Reprod Update. 21:427–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oktem O and Oktay K: The ovary: Anatomy
and function throughout human life. Ann N Y Acad Sci. 1127:1–9.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Capalbo A, Hoffmann ER, Cimadomo D, Ubaldi
FM and Rienzi L: Human female meiosis revised: New insights into
the mechanisms of chromosome segregation and aneuploidies from
advanced genomics and time-lapse imaging. Hum Reprod Update.
23:706–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun QY, Lai L, Wu GM, Park KW, Day BN,
Prather RS and Schatten H: Microtubule assembly after treatment of
pig oocytes with taxol correlation with chromosomes, gamma-tubulin,
and MAP Kinase. Mol Reprod Dev. 60:481–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blumenfeld Z and von Wolff M:
GnRH-analogues and oral contraceptives for fertility preservation
in women during chemotherapy. Hum Reprod Update. 14:543–552. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meirow D, Dor J, Kaufman B, Shrim A,
Rabinovici J, Schiff E, Raanani H, Levron J and Fridman E: Cortical
fibrosis and blood-vessels damage in human ovaries exposed to
chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod.
22:1626–1633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blumenfeld Z: How to preserve fertility in
young women exposed to chemotherapy? The role of GnRH agonist
cotreatment in addition to cryopreservation of embrya, oocytes, or
ovaries. Oncologist. 12:1044–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elgindy EA, El-Haieg DO, Khorshid OM,
Ismail EI, Abdelgawad M, Sallam HN and Abou-Setta AM: Gonadatrophin
suppression to prevent chemotherapy-induced ovarian damage: A
randomized controlled trial. Obstet Gynecol. 121:78–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ethics Committee of American Society for
Reproductive Medicine, . Fertility preservation and reproduction in
patients facing gonadotoxic therapies: A committee opinion. Fertil
Steril. 100:1224–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Loren AW, Mangu PB, Beck LN, Brennan L,
Magdalinski AJ, Partridge AH, Quinn G, Wallace WH and Oktay K;
American Society of Clinical Oncology, : Fertility preservation for
patients with cancer: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 31:2500–2510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morgan RJ Jr, Armstrong DK, Alvarez RD,
Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA,
DeRosa M, Dorigo O, et al: Ovarian cancer, version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:1134–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|




















